Abstract
Purpose
The primary purposes of this study were to examine the effects of hearing loss and respondent type (self- vs. parent-proxy report) on subjective fatigue in children. We also examined associations between child-specific factors and fatigue ratings.
Method
Subjective fatigue was assessed using the Pediatric Quality of Life Inventory Multidimensional Fatigue Scale (PedsQL-MFS; Varni, Burwinkle, Katz, Meeske, & Dickinson, 2002). We compared self- and parent-proxy ratings from 60 children with hearing loss (CHL) and 43 children with normal hearing (CNH). The children ranged in age from 6 to 12 years.
Results
School-age CHL experienced more overall and cognitive fatigue than CNH, although the differences were smaller than previously reported. Parent-proxy report was not strongly associated with child self-report, and parents tended to underestimate their child's fatigue, particularly sleep/rest fatigue. Language ability was also associated with subjective fatigue. For CHL and CNH, as language abilities increased, cognitive fatigue decreased.
Conclusions
School-age CHL experience more subjective fatigue than CNH. The poor association between parent-proxy and child reports suggests that the parent-proxy version of the PedsQL-MFS should not be used in isolation when assessing fatigue in school-age children. Future research should examine how language abilities may modulate fatigue and its potential academic consequences in CHL.
This special issue contains papers from the 2016 Hearing Across the Lifespan (HEAL) conference held in Cernobbio, Italy.
Mild, transient fatigue is something most people have experienced in their everyday lives. This type of fatigue generally resolves with rest or breaks and has a minimal impact on everyday life. However, for some individuals, especially those with disabilities and chronic health conditions, fatigue can be more frequent and severe. This type of fatigue can have a broad, profound impact on quality of life (Dinges et al., 1997; Eddy & Cruz, 2007; Evans & Wickstrom, 1999; Flechtner & Bottomley, 2003; Gaba & Howard, 2002; Hockenberry-Eaton et al., 1999; Kramer, Kapteyn, & Houtgast, 2006; Van Dongen & Dinges, 2000).
Despite the omnipresence of fatigue and its adverse consequences in persons with chronic conditions, empirical research examining hearing loss and fatigue is sparse (for a review, see Hornsby, Naylor, & Bess, 2016). To date, most research examining hearing loss and fatigue has focused on the adult population, and results suggest that adults with hearing loss may be at increased risk for fatigue-related issues (Alhanbali, Dawes, Lloyd, & Munro, 2017; Hornsby & Kipp, 2016; Kramer et al., 2006). Studies examining fatigue in children with hearing loss (CHL) are even more limited; however, growing empirical evidence suggests that CHL may also be at increased risk for fatigue (Bess et al., 2016; Bess & Hornsby, 2014a; Hornsby, Werfel, Camarata, & Bess, 2014; Werfel & Hendricks, 2016). Parents and teachers of CHL, audiologists, and speech-language pathologists have long speculated that the additional attention and concentration needed for listening and processing speech could result in increased effort, stress, and fatigue for CHL. Such an outcome might compromise their ability to learn in a noisy classroom environment—potentially increasing the risk for CHL to experience learning problems in school and highlighting the potential importance of this research. In the sections to follow, we will introduce the construct and measurement of subjective fatigue and discuss its potential association with hearing loss.
Subjective Fatigue
A detailed discussion of the complexity and multifaceted nature of fatigue is beyond the scope of this article; the interested reader is referred elsewhere for more information (Bess & Hornsby, 2014a; Hornsby et al., 2016; McGarrigle et al., 2014). Briefly, fatigue is a complex construct with manifestations in both the physical and mental domains. While a universally accepted definition does not exist, fatigue is often defined as a subjective feeling or mood state—characterized by feelings of weariness, tiredness, a lack of energy, and/or a limited desire to continue with a task. Subjective fatigue can develop for a variety of reasons, including high levels of sustained physical or mental effort, recurring emotional distress, poor sleep, and various physical or mental conditions (Hockey, 2013; Hornsby & Kipp, 2016; Hornsby et al., 2016; Lieberman, 2007). This diversity of mechanisms for eliciting fatigue has led some to describe fatigue as a multidimensional construct, although the validity of this is under debate (e.g., Michielsen, De Vries, Van Heck, Van de Vijver, & Sijtsma, 2004). Common domains identified to characterize the fatigue experience include energy and vigor, fatigue-associated sleep or rest issues, cognitive or mental fatigue, physical fatigue, emotional fatigue, and general fatigue (Hornsby et al., 2016).
Self-Report Fatigue Scales
Self-report questionnaires are commonly used to assess feelings and the perceived impact of fatigue. Numerous self-report scales/questionnaires have been developed to assess mental and physical fatigue in adults; fewer are available for children (Bess & Hornsby, 2014a; Christodoulou, 2005; Hockenberry et al., 2003; McGarrigle et al., 2014). Fatigue scales may be generic or disease specific (see Dittner, Wessely, & Brown, 2004 and Whitehead, 2009 for reviews). Of importance is the fact that no fatigue scales specific to hearing loss have been developed for children or adults.
Self- Versus Proxy-Report
Some fatigue measures for children have parent/provider-proxy versions. Proxy-reports are common when assessing subjective experiences (e.g., quality of life, depression, fatigue) and behaviors (e.g., social or emotional behavior problems in school) of children and are considered useful for a variety of reasons. For example, a proxy-report would be required if a very young child with an illness was unable to participate in an evaluation or if the validity of the child's direct responses was questionable (Meeske, Katz, Palmer, Burwinkle, & Varni, 2004). Proxy-reports can be obtained from multiple sources including parents, medical providers, teachers, or other caregivers. Given the differing viewpoints of the child and proxy, it is not surprising that discrepancies between respondents, sometimes referred to as cross-informant variance (Varni, Katz, Colegrove, & Dolgin, 1996), are commonly observed (De Los Reyes & Kazdin, 2005; Drotar, 2014; Eiser & Varni, 2013; Koot & Wallander, 2014; Upton, Lawford, & Eiser, 2008; Verhulst & Ende, 1992).
While proxy–child discrepancies are common, there is substantial between-study variability in terms of the magnitude and direction of discrepancies. Some studies show fair-to-good agreement, whereas others show poor agreement. The magnitude of proxy–child discrepancies can vary on the basis of many factors. For example, disagreements between self- and parent proxy-reports tend to be larger for adolescent children compared with those younger in age (De Los Reyes & Kazdin, 2005; Verhulst & Ende, 1992). In addition, although results vary across studies, parents of typically developing children tend to underestimate the problems their child is experiencing, whereas parents of children with significant health issues (e.g., cancer) tend to overestimate the problems their children are experiencing (Upton et al., 2008). Finally, proxy–child discrepancies appear to be more common when examining agreement on internal, subjective feelings and experiences (e.g., fatigue, sadness, pain) compared with examining agreement for more externalized, overt, and physical behaviors (e.g., aggression, walking, running; see De Los Reyes & Kazdin, 2005 and Upton et al., 2008 for reviews). Research examining agreement between parent–child fatigue ratings in CHL is limited to a single, relatively small (N = 19), study of cochlear implant (CI) users (Werfel & Hendricks, 2016). The results of this study are described in the next section. In addition, given the diverse findings in the literature, there is a clear need for additional research in this area.
Subjective Fatigue in CHL
As previously mentioned, although fatigue can develop for a variety of reasons, it is commonly associated with high levels of sustained mental or physical effort (e.g., Earle, Hockey, Earle, & Clough, 2015; Hockey, 2013). This recurring finding provides a rationale for why adults and CHL may be at increased risk for fatigue. We have proposed a simple conceptual model linking the effort and stress associated with repeated communication breakdowns experienced by adults and CHL to the development of fatigue (Bess & Hornsby, 2014b). Substantial research has shown that, compared with individuals without hearing loss, adults and CHL must increase their listening effort (i.e., allocate more attentional resources) when processing speech (Alhanbali et al., 2017; Hicks & Tharpe, 2002; McCoy et al., 2005; Zekveld, Kramer, & Festen, 2011). The consequences of this sustained, high level of effort can be significant. For example, Hétu, Riverin, Lalande, Getty, and St-Cyrm, (1988) found that adults with hearing loss compensated for their hearing difficulties by maintaining a high level of concentration and attention at work, which, in turn, led to increased reports of stress, tension, and fatigue. Likewise Kramer et al. (2006) found that adults with hearing loss were almost four times more likely to take a sick day due to fatigue, strain, or burnout compared with age-matched adults without hearing loss. Although similar work in CHL is limited, it seems clear that the listening demands of school-age CHL may be equally, if not more, challenging—also putting this group at increased risk for fatigue.
Recent physiologic work by Bess et al. (2016) supports the hypothesis that the listening demands of CHL may put them at increased risk for stress and its negative effects, including fatigue. The authors measured salivary cortisol levels, a biomarker of stress, throughout a “typical” school day in a group of CHL and children without hearing loss. Consistent with the hypothesis that CHL experience increased stress, they observed elevated cortisol levels upon awakening and a reduced cortisol awakening response (a shallower increase in cortisol levels upon awakening) in the CHL compared with a control group. While not true markers of fatigue, elevated cortisol levels and abnormal cortisol awakening response patterns have been reported in other groups (e.g., working adults experiencing job burnout) with an increased risk of fatigue (De Vente, Olff, Van Amsterdam, Kamphuis, & Emmelkamp, 2003; Grossi et al., 2005).
Research focusing on subjective fatigue in CHL is also scarce. Bess, Dodd-Murphy, and Parker (1998) examined fatigue-related outcomes in a group of children with minimal hearing loss using the Dartmouth Cooperative Information Project (COOP) Adolescent Chart Method, an office-based screener for subjective functional health status (Nelson, Wasson, Johnson, & Hays, 1996; Nelson et al., 1987). The authors found that children with minimal hearing loss experienced greater dysfunction than children with normal hearing (CNH) on two COOP subtests, stress and energy, which are conceptually associated with fatigue. In contrast, Hicks and Tharpe (2002) failed to find differences in fatigue-related issues between CHL and CNH when using the COOP. The many methodological differences between these studies (e.g., number of participants, degree of hearing loss), however, make a direct comparison between studies difficult. In addition, the divergent outcomes might also reflect the fact that the COOP was not designed as a primary measure of fatigue. Thus, its sensitivity to fatigue effects is unclear.
Hornsby et al. (2014) were the first to examine subjective fatigue in CHL using a standardized measure. In a preliminary study, they used the Pediatric Quality of Life Inventory Multidimensional Fatigue Scale (PedsQL-MFS; Varni et al., 2002) to examine fatigue in a small, heterogeneous group of CHL and a control group of age-matched CNH. The sample of CHL included individuals with unilateral and bilateral hearing losses ranging from mild to profound; all children used unilateral or bilateral hearing aids or cochlear implants (CIs). The PedsQL-MFS is a standardized self-report measure, which assesses three fatigue domains (general, sleep/rest, and cognitive) and provides an overall fatigue score. Results of this pilot study showed that CHL experienced significantly more fatigue across all domains than the control group. A somewhat unexpected finding was that the CHL reported more fatigue on the PedsQL-MFS than children with other health conditions commonly associated with fatigue, such as cancer, rheumatoid arthritis, diabetes, and obesity (Varni et al., 2002; Varni, Burwinkle, & Szer, 2004; Varni, Limbers, Bryant, & Wilson, 2009, 2010). While Hornsby et al. provided empirical support for the idea that CHL are at increased risk for fatigue, the preliminary study had several limitations. The small sample size and heterogeneous population of the CHL are obvious limitations. In addition, the children's fatigue was based only on self-report. Parent-proxy ratings were not obtained. Given the young age of some of the children (i.e., 30% of their sample was younger than 8 years), parent-proxy report may have provided additional insight into the fatigue experienced by the CHL.
Werfel and Hendricks (2016) also used the PedsQL-MFS when they examined subjective fatigue in children who used CIs. They found that children who used CIs reported fatigue levels that were similar to those reported by the diverse group of CHL in Hornsby et al. (2014). However, unlike Hornsby et al., Werfel and Hendricks also obtained parent-proxy reports using the parent version of the PedsQL-MFS. Discrepancies were noted between the children's perceptions of their fatigue and parental reports of the child's fatigue. Parents of children who use CIs tended to underestimate their child's fatigue, although the difference was significant only for the general and sleep/rest fatigue domains. Unfortunately, the study did not include a control group, so it remains unclear whether parents of children who use CIs (Werfel & Hendricks, 2016), or parents of CHL (the current study), underestimate their child's fatigue more so than a control group of parents of CNH.
Factors Influencing Subjective Fatigue in Listeners With Hearing Loss
While there is growing evidence that CHL are at increased risk for fatigue, factors that modulate or mediate this increased risk are unclear. Factors such as age, degree of hearing loss, and other child-specific variables could influence the likelihood and severity of fatigue in CHL. For instance, some work suggests that the child's age may play a role, with typically developing older children and adolescents reporting greater general fatigue than younger children (Gordijn, Cremers, Kaspers, & Gemke, 2011). Consistent with a potential age-related risk of fatigue for CHL, Bess et al. (2016) found that cortisol levels increased with age in CHL, whereas no such age-related changes were found in a control group of CNH. As mentioned previously, abnormal cortisol patterns have been observed in groups at increased risk for fatigue, such as those with chronic fatigue syndrome or work-related burnout (Grossi et al., 2005; Jerjes, Cleare, Wessely, Wood, & Taylor, 2005). An age-related change in subjective fatigue has not yet been examined in CHL.
Degree of hearing loss is another factor that could potentially influence fatigue ratings in CHL. One might speculate that children with the most hearing loss would experience more communication difficulties, potentially leading to greater listening-related fatigue than children with milder hearing losses. However, research examining this question in adults with hearing loss suggests this is not the case (Alhanbali et al., 2017; Hornsby & Kipp, 2016). For example, despite finding a high prevalence of severe fatigue and vigor deficits, Hornsby and Kipp (2016) found no association between degree of hearing loss and fatigue or vigor ratings in a group of adults (N = 149) seeking help for hearing difficulties. A similar finding was reported by Alhanbali et al. (2017). They found no association between degree of hearing loss and subjective fatigue ratings in a group of 50 adult hearing-aid users. To date, the relationship (or lack thereof) between degree of hearing loss and subjective fatigue has not been examined in children.
Finally, recent research suggests a potential association between language ability and fatigue. Werfel and Hendricks (2016) found an association between poor language skills and several fatigue domains in children with profound hearing loss who use CIs. Werfel and Hendricks suggested a directional relationship whereby the increased fatigue experienced by CHL limits the cognitive resources available for academic learning during the school day—potentially leading to poorer language skills. It is also possible that an opposite relationship exists. Specifically, a relationship where the repeated stress and strain of struggling to understand auditory information, commonly experienced by CHL, may lead to fatigue, regardless of whether the children have mild-to-severe losses or are CI users. The poor language abilities of some CHL will add to these common difficulties, potentially increasing their risk for fatigue. It is also possible that the association between language ability and fatigue is circular, or bidirectional, in nature. Regardless, the relationship between language skills and fatigue in children with mild-to-severe hearing loss has not been explored. Given that the majority of CHL has mild-to-moderate/severe losses (Bess et al., 1998), this remains an important area of study.
In this article, we expand on our previous work (Hornsby et al., 2014) by examining subjective ratings of fatigue in a larger, more homogenous population of CHL and their parents using the PedsQL-MFS. The specific goals of this study were to address the following questions:
Do children with mild-to-severe hearing loss report greater subjective fatigue on the PedsQL-MFS than a control group of CNH? If so, do variations between groups differ across fatigue domains (general, sleep/rest, cognitive, overall) or respondent (self- vs. proxy-report)? On the basis of pilot data from Hornsby et al. (2014), we predicted significant, and similar, between-groups differences across all domains, with CHL reporting more fatigue than a control group.
Do CHL rate their fatigue, on the basis of PedsQL-MFS responses, differently than their parent or guardian? If so, do variations between children and their parents differ across fatigue domains or between those with and without hearing loss? On the basis of extant literature showing poor concordance between child and parent/guardian-proxy reports, we expected a similar finding with our groups.
Finally, are individual factors such as age, degree of loss, or language ability associated with fatigue ratings of CHL and CNH? On the basis of extant literature, we expected no association between degree of loss and fatigue ratings. In contrast, fatigue ratings were expected to increase with age and decrease as language ability increased.
Method
These data were obtained in the course of a more extensive study designed to examine the effects of listening effort and fatigue in CHL (Bess et al., 2016; Bess, Gustafson, & Hornsby, 2014; Hornsby et al., 2014). The Vanderbilt University Institutional Review Board approved the study procedures. Informed parental consent and child assent were obtained from all participants before the study procedures began.
Participants
Participants included 60 CHL, 43 CNH, and one parent or guardian of each child. All child participants were between 6.0 and 12.9 years of age and had no diagnosis of learning disability or cognitive impairment as reported by the parent or guardian. Cognitive ability was also assessed in all children using the Test of Nonverbal Intelligence–Fourth Edition (TONI-4; Brown, Sherbenou, & Johnson, 2010). Participants were recruited using a variety of methods. CNH were primarily recruited through an advertisement in a local parenting magazine, through word of mouth, and through the Vanderbilt Kennedy Center's StudyFinder website. CHL were recruited from the Vanderbilt Audiology Clinics and from school districts throughout Middle Tennessee. Children were excluded from this study on the basis of factors known to affect fatigue. This criterion resulted in the exclusion of (a) children who were bilingual or whose primary language in the home was not listening and spoken language, (b) children with autism spectrum disorder, (c) children with a linear metabolic or endocrine disorder (e.g., diabetes or hypothyroidism), (d) children with a chronic medical condition, and (e) children who utilized stimulant medications. Ten CNH and five CHL who participated in our preliminary study (Hornsby et al., 2014) met the inclusion criteria for the current study, and their data were included in the current study. Of the 60 CHL that participated, all but one was fitted with hearing aids.
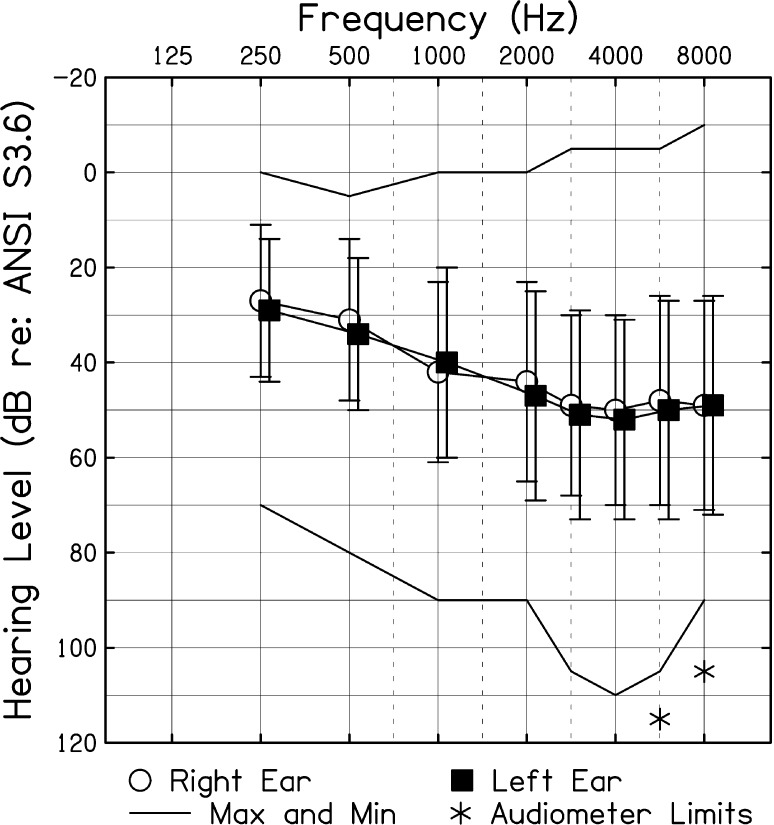
Upon entry into the study, all children received an audiological assessment. CNH received a standard hearing screening at 15 dB HL for octave frequencies ranging from 0.25 to 8.0 kHz, bilaterally. For CHL, hearing thresholds were obtained bilaterally via air and bone conduction over the frequency range of 0.25 to 8.0 kHz (Carhart & Jerger, 1959). CHL had bilateral hearing loss that was at least mild in magnitude in their better-hearing ear (audiometric data from one child were missing). We defined mild hearing loss as a pure-tone average (PTA; thresholds at 0.5, 1.0, and 2.0 kHz) between 20 and 40 dB HL (n = 26) or thresholds greater than 25 dB HL at two or more frequencies above 2.0 kHz (n = 9). Moderate-to-severe hearing loss was defined as a PTA of 41–70 dB HL in the better ear (n = 24). Children exhibiting a conductive component were included (n = 3) in the data set as long as the sensorineural loss fit the above criterion and the hearing loss was stable (not fluctuating). In contrast to Hornsby et al. (2014), children with CIs and children with unilateral hearing loss were not included in the data set. The mean better-ear PTA for CHL included in this study was 35.9 dB HL (range = 5.0–68.3 dB HL). Figure 1 shows a composite audiogram for the CHL.
Figure 1.
Mean pure-tone thresholds for children with hearing loss (dB re: American National Standards Institute, 2010). Error bars = 1 SD. Solid lines show recorded minimum and maximum thresholds. Asterisks represent thresholds that were recorded as no responses at the limits of the audiometer.
Upon entry into the study, children also completed a standardized measure of language ability, the Clinical Evaluation of Language Fundamentals–Fourth Edition (CELF-4; Semel, Wiig, & Secord, 2003). The core language index of the CELF-4 provides a reliable norm-referenced measure of language performance by age. Demographic information obtained from the parent/guardian as well as information about language and cognitive abilities are shown in Table 1. The CHL were slightly, but significantly, older than the control sample, t(101) = −2.06, p = .042. The age-adjusted overall language level for our sample of CHL was significantly lower than the control sample, t(98) = 4.65, p < .001. Mean IQ scores, on the basis of the TONI-4, were within normal limits for both CNH and CHL. However, as seen in Table 1, mean IQ scores were significantly higher for the CNH compared with the CHL, t(101) = 2.91, p = .004.
Table 1.
Participant characteristics.
| Group | CNH | CHL |
|---|---|---|
| Number of participants | 43 | 60 |
| Males/Females | 26/17 | 31/29 |
| Homeschool/General ed. | 6/37 | 6/54 |
| Age (years) | 9.1 (2.32)* | 9.96 (1.92)* |
| Language a | 109.0 (10.5)** | 92.1 (21.8)** |
| Nonverbal IQ b | 108.8 (10.3)*** | 102.1 (12.3)*** |
Note. Means (±1 SD) for children with hearing loss (CHL) and children with normal hearing (CNH).
Standard score on the core language index of the Comprehensive Evaluation of Language Fundamentals–Fourth Edition (CELF-4).
Standard score on the Test of Nonverbal Intelligence–Fourth Edition (TONI-4).
p = .042.
p < .001.
p = .004.
Fatigue Measure
The PedsQL-MFS was completed by children and their parent/guardian to assess self-reported perceptions of fatigue for CHL and CNH. The PedsQL-MFS is a comprehensive fatigue scale that has been validated for use with children from 5 to 18 years of age (Varni et al., 2002, 2004; Varni & Limbers, 2008). The 18-item PedsQL-MFS is a standardized fatigue measure comprised of three subscales, each containing six items: (1) general fatigue—items in this subscale ask about general feelings of tiredness or weakness, regardless of the cause; (2) sleep/rest fatigue—items in this subscale ask specifically about sleep/rest-related tiredness; and (3) cognitive fatigue—items in this subscale ask specifically about fatigue-related cognitive difficulties. An overall (composite) fatigue score is also calculated by combining scores from the subscales. The instrument uses a 5-point Likert scale, which is transformed into a scale from 0 to 100 (0 = 100, 1 = 75, 2 = 50, 3 = 25, 4 = 0). Higher scores indicate less fatigue symptoms. The children are asked how much of a problem each item has been over the past month, or the past few weeks for children 5–7 years old. Response options for younger children (5–7 years) are limited to a 3-point (0, 2, 4) Likert scale, which is also transformed into a scale from 0 to 100 (100 = Not at all, 50 = Sometimes, 0 = A lot), and include simple pictures of faces to help the child differentiate severity. The PedsQL-MFS reportedly possesses good internal consistency, reliability, and construct validity (Varni et al., 2002; Varni, Burwinkle, Limbers, & Szer, 2007; Varni et al., 2004). Parent versions of the PedsQL-MFS are also available. The PedsQL-MFS was developed for children and adolescents with different chronic conditions such as cancer, rheumatoid arthritis, diabetes, cerebral palsy, and obesity (Varni et al., 2006, 2002, 2004, 2009, 2010). The test was not developed for CHL and does not include items weighted for fatigue potentially associated with sustained listening, attending, concentrating, or processing speech in difficult listening conditions.
Procedure
Participants completed audiologic and language testing during the initial study visit. During this visit, CHL, CNH, and their parent/guardian also completed subjective ratings of fatigue using the PedsQL-MFS. At the start of the visit, a trained research assistant administered the age-appropriate PedsQL-MFS: Young Child (ages 6–7) or Child (ages 8–12). For the Young Child form, the research assistant read each item aloud and asked the child to point to the corresponding happy, neutral, or sad face for their response. For the Child form, the research assistant read each item aloud and asked the child to circle their response. The PedsQL-MFS was self-administered for the parent/guardian.
Analyses
Because some of the distributions of the PedsQL-MFS overall and subscale scores were skewed (Shapiro–Wilk's test) and sample sizes were small (see Table 2), nonparametric rank-based methods were selected to answer the following primary research questions: (a) Are there differences in PedsQL-MFS ratings between groups (i.e., a main effect of hearing loss)? (b) Are there differences in PedsQL-MFS ratings as a function of respondent type (i.e., a main effect of respondent parent vs. child)? (c) Does the effect of hearing loss vary between respondent types (i.e., a Group × Respondent interaction)? These questions were investigated by examining PedsQL-MFS overall fatigue scores and scores in each of the three subscales, that is, general fatigue, sleep/rest fatigue, and cognitive fatigue.
Table 2.
Pediatric Quality of Life Inventory Multidimensional Fatigue Scale (PedsQL-MFS) descriptive results.
| Domain | General |
Sleep/Rest |
Cognitive |
Overall |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Respondent | Parent report |
Child report |
Parent report |
Child report |
Parent report |
Child report |
Parent report |
Child report |
||||||||
| CNH | CHL | CNH | CHL | CNH | CHL | CNH | CHL | CNH | CHL | CNH | CHL | CNH | CHL | CNH | CHL | |
| Mean | 73.3 | 71.2 | 73.4 | 71.7 | 79.4 | 75.8 | 63.7 | 61.3 | 68.8 | 55.9 | 58.6 | 52.6 | 73.8 | 67.6 | 65.2 | 61.9 |
| Median | 70.8 | 70.8 | 75.0 | 70.8 | 83.3 | 75.0 | 66.7 | 66.7 | 66.7 | 58.3 | 58.3 | 50.0 | 73.6 | 68.8 | 66.7 | 58.3 |
| SD | 17.8 | 16.8 | 16.6 | 17.8 | 16.9 | 15.0 | 19.2 | 18.2 | 23.0 | 23.1 | 21.9 | 26.1 | 16.8 | 15.4 | 16.3 | 16.1 |
| Minimum | 29.2 | 25.0 | 29.2 | 29.2 | 29.2 | 20.8 | 4.2 | 16.7 | 12.5 | 0.0 | 0.0 | 8.3 | 31.9 | 20.8 | 11.1 | 25.0 |
| Maximum | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 95.8 | 95.8 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 93.1 | 95.8 | 88.9 |
| Skewness | −0.31 | −0.44 | −0.98 | −0.43 | −1.09 | −1.21 | −0.86 | −0.24 | −0.28 | −0.42 | −0.35 | 0.16 | −0.56 | −0.64 | −0.73 | 0.18 |
| Kurtosis | −0.46 | −0.17 | 0.89 | −0.40 | 1.24 | 2.76 | 1.50 | −0.62 | −0.65 | −0.06 | 0.37 | −0.88 | 0.12 | 0.73 | 1.70 | −0.67 |
| Shapiro–Wilk | 0.110 | 0.170 | 0.009* | 0.080 | 0.002* | < 0.001* | 0.039* | 0.210 | 0.044* | 0.240 | 0.490 | 0.031* | 0.120 | 0.090 | 0.150 | 0.014* |
Note. CNH = children with normal hearing; CHL = children with hearing loss.
p < .05.
The null hypotheses of no main effect of hearing loss or respondent and no interaction effect between hearing loss group and respondent were tested using two nonparametric statistics, namely, the Wald-type statistics and the analysis of variance–type statistics. These analyses were completed using the R package nparLD (Noguchi, Gel, Brunner, & Konietschke, 2012). The results from each statistical analysis were similar and directly replicated; hence, only the analysis of variance–type statistics results are reported herein. Hearing loss group (CHL vs. CNH) was a between-subjects factor in the analyses. Because both parent and child respondents provided estimates of child fatigue levels, respondent was analyzed as a within-subject factor.
Agreement between child and parent-proxy report was examined using intraclass correlation coefficients (ICCs; McGraw & Wong, 1996). ICCs were calculated using the R package “irr” (Gamer, Lemon, Fellows, & Singh, 2012) using Spearman's mean rank correlations between child and parent raters. Previous researchers have suggested that ICCs of ≤ 0.40 reflect poor-to-fair agreement, ICCs of 0.41 to 0.60 reflect moderate agreement, ICCs ranging from 0.61 to 0.80 reflect good agreement, and ICCs between 0.81 and 1.00 reflect excellent agreement (Varni et al., 2009; Wilson, Dowling, Abdolell, & Tannock, 2000). Finally, associations between PedsQL-MFS scores and individual participant factors (i.e., age, degree of hearing loss, and language ability) were explored using Spearman's nonparametric rank correlations. Bonferroni corrections were applied when appropriate to control for potential increases in Type 1 errors due to a familywise error arising from multiple comparisons.
Results
Parent-proxy and child PedsQL-MFS results, including means, medians, and other descriptive characteristics of the data set, are shown in Table 2. Results of the nonparametric analyses revealed significant effects for hearing loss group and respondent type. These differences varied across fatigue domains (see Table 3). Of importance is that significant Group × Respondent interactions were not observed for any domain, suggesting that the main effect of hearing status did not vary as a function of respondent and that the main effect of respondent type did not vary with hearing status. We describe these results in detail below.
Table 3.
Summary results from nonparametric analyses of Pediatric Quality of Life Inventory Multidimensional Fatigue Scale (PedsQL-MFS) ratings showing analysis of variance (ANOVA)-type statistics on the basis of analyses of main effects, Group-G (normal hearing [NH] vs. hearing loss [HL] collapsed across respondent type) and Respondent-R (parent vs. child collapsed across hearing group), and interaction effect (Group × Respondent; G × R).
| Measure | Null hypothesis | ANOVA-type statistic |
||
|---|---|---|---|---|
| Statistic | df | p value | ||
| General | Main effect (Group-G) | 0.59 | 1 | 0.440 |
| Main effect (Respondent-R) | 0.11 | 1 | 0.740 | |
| Interaction effect (G × R) | 0.00 | 1 | 0.990 | |
| Sleep/Rest | Main effect (Group-G) | 1.70 | 1 | 0.200 |
| Main effect (Respondent-R) | 44.90 | 1 | < 0.001* | |
| Interaction effect (G × R) | 0.18 | 1 | 0.670 | |
| Cognitive | Main effect (Group-G) | 6.80 | 1 | 0.009* |
| Main effect (Respondent-R) | 5.10 | 1 | 0.024* | |
| Interaction effect (G × R) | 0.78 | 1 | 0.380 | |
| Overall | Main effect (Group-G) | 4.30 | 1 | 0.037* |
| Main effect (Respondent-R) | 14.10 | 1 | < 0.001* | |
| Interaction effect (G × R) | 0.22 | 1 | 0.640 | |
p < .05.
A Comparison Between PedsQL-MFS Ratings of CNH and CHL
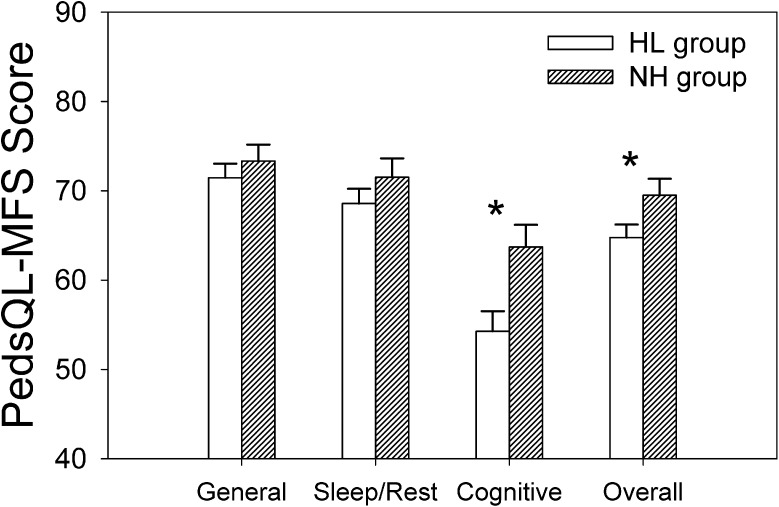
Our previous, preliminary, findings from a diverse sample (Hornsby et al., 2014) indicated that CHL experienced more fatigue than CNH. Thus, a primary question for the current study was whether PedsQL-MFS scores differed between a control group of CNH and our CHL in this larger, more homogenous, sample. Mean results, collapsed across respondent type, are shown in Figure 2. 1 Recall that lower PedsQL-MFS scores reflect more fatigue. Therefore, Figure 2 shows that CHL reported more fatigue (lower scores) than the control group across all domains, although the magnitude of the differences varied across domains. Differences were largest in the cognitive fatigue domain (9.4 points) and for overall fatigue (4.8 points). Differences between groups were smaller, and not statistically significant, in the general (1.9 points) and sleep/rest domains (2.9 points). Results of nonparametric analyses are shown in Table 3.
Figure 2.
Mean Pediatric Quality of Life Inventory Multidimensional Fatigue Scale (PedsQL-MFS) scores for the hearing loss (HL) and normal hearing (NH) groups, collapsed across respondent type (parent-proxy and child). Lower values reflect more fatigue. Open and dashed bars reflect HL and NH group responses, respectively. Asterisks show significant between-groups differences (p < .05).
Effect of Respondent Type (Parent-Proxy Versus Child) on PedsQL-MFS Ratings
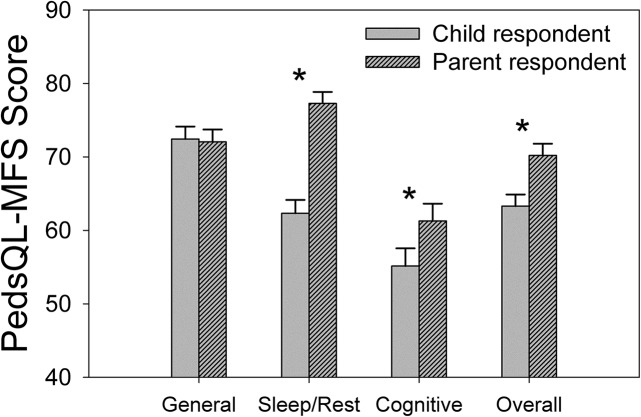
A second primary question was whether parental report could serve as an accurate proxy for the child when assessing fatigue using the PedsQL-MFS in this sample. Our analyses, collapsed across hearing status group (hearing loss [HL] vs. normal hearing [NH]), revealed significant differences between the parent-proxy and child reports across all domains except general fatigue (−0.4 point difference; see Figure 3 and Table 3). Parents consistently underestimated the fatigue of their children across the remaining domains, with the largest discrepancy in the sleep/rest domain (15 points). Smaller significant differences were observed in the cognitive domain and for overall fatigue ratings as well (6.1 and 6.9 points, respectively). There were no significant interactions observed for any fatigue domain, suggesting that the parent–child differences were not dissimilar for the HL and NH groups.
Figure 3.
Mean Pediatric Quality of Life Inventory Multidimensional Fatigue Scale (PedsQL-MFS) scores for our child and parent-proxy respondents, collapsed across hearing loss groups (hearing loss and no hearing loss). Lower values reflect more fatigue. Solid gray and dashed gray bars reflect child and parent-proxy group responses, respectively. Asterisks show significant between-groups differences (p < .05).
In addition to looking at mean differences between parent-proxy and child report, we were also interested in whether associations between the parent and child report might allow parent ratings to be used as a proxy for the child report. We used ICCs to examine associations between the parent-proxy and child reports for each subscale domain and for overall fatigue ratings. Given that these were a priori domain-specific comparisons, Bonferroni corrections were not applied. The observed associations between the parent-proxy and child reports were poor to fair, which is consistent with prior research. The only significant finding was on ratings of overall fatigue between parent-proxies and their CHL (ICC = 0.34, p = .009). Table 4 shows results for our NH and HL groups separately.
Table 4.
Intraclass correlations (ICCs) using mean rank between parent and child Pediatric Quality of Life Inventory Multidimensional Fatigue Scale (PedsQL-MFS) ratings for normal hearing (NH) and hearing loss (HL) groups.
| Fatigue domain | NH group |
|||
|---|---|---|---|---|
| General | Sleep/Rest | Cognitive | Overall | |
| ICC | 0.21 | 0.03 | 0.05 | 0.12 |
|
z |
1.30 (0.193) |
0.22 (0.828) |
0.29 (0.766) |
0.74 (0.459) |
|
HL group |
||||
| ICC | 0.24 | 0.17 | 0.24 | 0.34* |
| z | 1.78 (0.075) | 1.31 (0.190) | 1.81 (0.071) | 2.96 (0.009)* |
Note. Spearman's rho and z are reported. P values (two-tailed) are shown in parentheses.
p < .05.
Associations Between Individual Factors and PedsQL-MFS Ratings
Finally, we were interested in individual factors that may be associated with variations in fatigue ratings in our sample of children. We used a series of Spearman's nonparametric rank correlations to test associations between PedsQL-MFS ratings and age, degree of hearing loss (CHL and parents of CHL only), and language ability. These analyses were conducted separately for each participant group (i.e., for CHL, CNH, parents of CHL, and parents of CNH). Degree of hearing loss was quantified as the better-ear PTA (mean of thresholds at 0.5, 1.0, and 2.0 kHz). Associations were examined for each subscale domain and overall fatigue separately. We used a Bonferroni correction to adjust for multiple comparisons in each domain resulting in an adjusted significance level of .025 (p = .05/2 comparisons for NH groups, age and language ability) or .017 (p = .05/3 comparisons within the HL groups, age, degree of hearing loss, and language ability). Results of these analyses are shown in Table 5. Child age was not associated with any PedsQL-MFS subscale or with the total score. No significant associations between degree of hearing loss and self-reported fatigue ratings in any domain were observed for CHL. A weak association between degree of hearing loss and general fatigue was observed for the parent-proxy report (Spearman's r = .30, p = .024), but it did not survive the Bonferroni correction. No association was found between degree of hearing loss and any other domain or with the overall scores reported by parent-proxy (see Table 5). Similar analyses were conducted using various measures of better- and poorer-ear PTA (i.e., 0.5, 1.0, 2.0, and 4.0 kHz; 1.0, 2.0, and 4.0 kHz) and asymmetry (difference in PTA) between ears, but no significant associations were observed (data not shown). Finally, to account for variations in the impact of hearing loss on frequency regions important for speech processing, we also looked at associations between fatigue ratings and better- and poorer-ear aided Speech Intelligibility Index (American National Standards Institute, 1997) values obtained at input levels of 55, 65, and 75 dB SPL. However, again, no significant associations were observed (data not shown).
Table 5.
Spearman's rank correlations between Pediatric Quality of Life Inventory Multidimensional Fatigue Scale (PedsQL-MFS) ratings for normal hearing (NH) and hearing loss (HL) groups, age, degree of hearing loss, and language abilities (Comprehensive Evaluation of Language Fundamentals–Fourth Edition [CELF-4] scores).
| Fatigue domain | Child data |
|||||||
|---|---|---|---|---|---|---|---|---|
| NH group |
HL group |
|||||||
| General | Sleep/Rest | Cognitive | Overall | General | Sleep/Rest | Cognitive | Overall | |
| Age | 0.14 (0.390) | 0.11 (0.480) | 0.02 (0.910) | 0.10 (0.520) | −0.03 (0.820) | −0.06 (0.650) | −0.12 (0.350) | −0.12 (0.380) |
| Degree of hearing loss | 0.02 (0.910) | −0.17 (0.190) | −0.07 (0.610) | −0.08 (0.560) | ||||
| Language ability |
0.11 (0.450) |
0.11 (0.490) |
0.36 (0.021)
* |
0.22 (0.170) |
0.21 (0.110) |
0.48 (0.720) |
0.32 (0.013)** |
0.30 (0.025) |
|
Fatigue domain |
Parent data |
|||||||
|
NH group |
HL group |
|||||||
|
General |
Sleep/Rest |
Cognitive |
Overall |
General |
Sleep/Rest |
Cognitive |
Overall |
|
| Age | −0.15 (0.350) | −0.15 (0.350) | −0.05 (0.750) | −0.11 (0.480) | −0.21 (0.120) | −0.15 (0.260) | 0.08 (0.510) | −0.09 (0.520) |
| Degree of hearing loss | 0.30 (0.024) | 0.07 (0.620) | 0.14 (0.290) | 0.21 (0.110) | ||||
| Language ability | 0.03 (0.840) | 0.23 (0.150) | 0.05 (0.770) | 0.06 (0.720) | −0.09 (0.510) | −0.06 (0.640) | −0.06 (0.650) | −0.08 (0.550) |
Note. Degree of hearing loss is defined as the better-ear pure-tone average threshold at 0.5, 1.0, and 2.0 kHz. P values (two-tailed) are shown in parentheses. Bolded data are significant after using a Bonferroni correction to adjust for multiple comparisons.
p < .025.
p < .017.
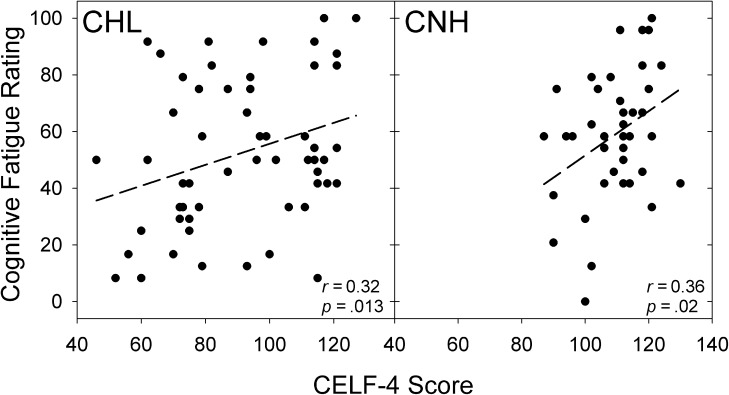
No significant associations between language ability (CELF-4 scores) and parent-proxy fatigue ratings for CNH or CHL were observed in any domain or for the overall fatigue score. However, there were significant associations between language ability and ratings of cognitive fatigue for both the CHL (Spearman's r = 0.32, p = .013) and for the CNH (Spearman's r = 0.36, p = .021). Specifically, children with poorer language abilities across the CHL and CNH groups had lower PedsQL-MFS cognitive fatigue scores—reflecting an increase in reported fatigue (recall that lower PedsQL-MFS scores reflect more fatigue). Figure 4 shows scatter plots of the association between language ability and cognitive fatigue for our CHL and CNH. A similar association between language abilities and overall fatigue scores was observed for the CHL only, although the association was not significant following the Bonferroni correction (Spearman's r = 0.30, p = .025). No other significant associations were observed.
Figure 4.
Scatter plot showing the association between Comprehensive Evaluation of Language Fundamentals–Fourth Edition (CELF-4) scores and Pediatric Quality of Life Inventory Multidimensional Fatigue Scale (PedsQL-MFS) cognitive fatigue scores provided by children with hearing loss (CHL) and children with normal hearing (CNH).
Discussion
Subjective Ratings of Fatigue in Children With and Without Hearing Loss and Their Comparison to Prior Work
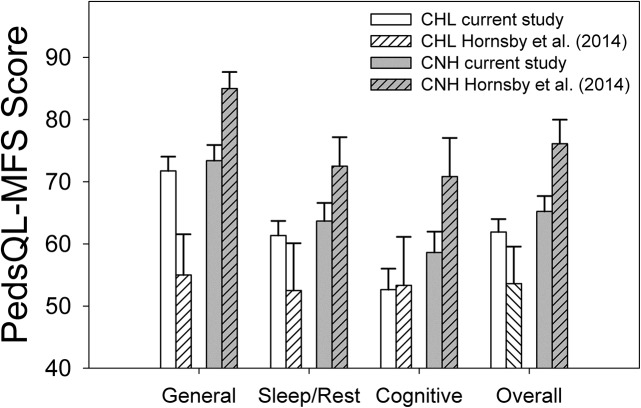
In this article, we follow up on previous pilot work (Hornsby et al., 2014) examining subjective fatigue in CHL. Hornsby et al. found that subjective ratings of fatigue were substantially greater in a small, diverse sample of CHL compared with an age-matched control group of CNH. A primary question of this study was whether these preliminary results would hold in a larger, more homogenous group of children with mild-to-severe hearing loss. Our results suggest that CHL do experience increased fatigue compared with CNH, at least in some domains. This difference was significant on the basis of the total (overall) fatigue score and for the cognitive subscale (see Figure 2). However, group differences in fatigue ratings were smaller than those observed in the preliminary work of Hornsby et al. Between-groups differences for CHL and CNH in Hornsby et al. were substantial, ranging from 17 to 30 percentage points across domains. In contrast, differences in self-reported fatigue by CHL and CNH in the current study were much smaller, ranging from two to six percentage points (see Table 2). While the reasons for the smaller between-groups differences in the current study are unclear, they appear to reflect not only differences in self-reported fatigue by the CHL but also differences in self-reported fatigue between the study control groups. Specifically, except for the cognitive fatigue domain, CHL in the current study reported less fatigue (higher PedsQL-MFS scores) than those in the preliminary study. In contrast, across all domains and for overall fatigue, CNH in the current study reported more fatigue (lower PedsQL-MFS scores) than the CNH in the preliminary study. These differences are shown graphically in Figure 5.
Figure 5.
Pediatric Quality of Life Inventory Multidimensional Fatigue Scale (PedsQL-MFS) subscale and overall scores for children with hearing loss (CHL) and children with normal hearing (CNH) from the current study (means for both groups are based on child data only) and a preliminary study (Hornsby et al., 2014). Data from the current study are shown by the white (CHL) and gray (CNH) unfilled vertical bars. Data from the preliminary study are shown by the white (CHL) and gray (CNH) striped bars. Error bars = 1 SE.
Children With Normal Hearing
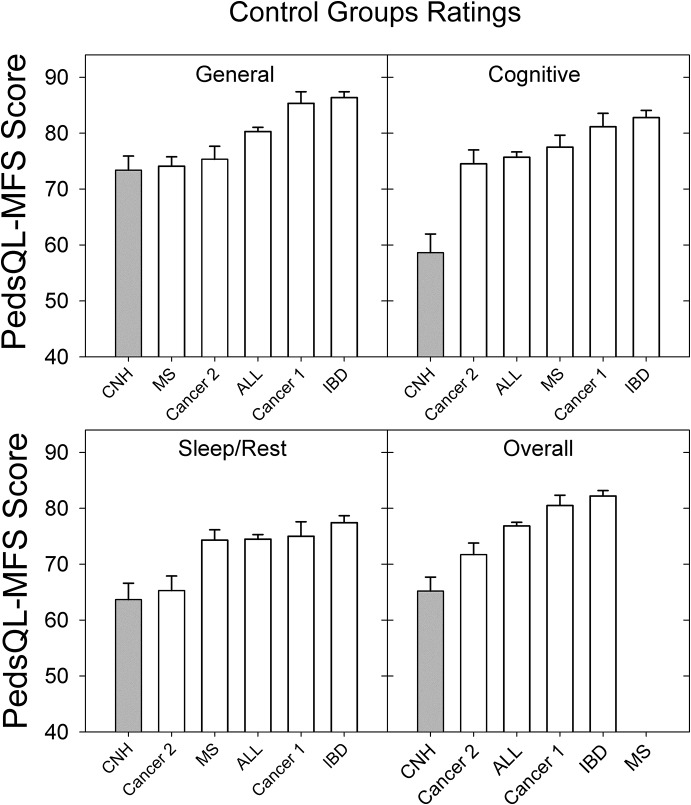
The large increase in fatigue reported by the control group in the current study was particularly surprising given (a) that the preliminary and current study excluded children on the basis of factors known to affect fatigue and (b) that ratings from CNH in the preliminary study appeared to be in line with other reports from typically developing children in the literature (Hornsby et al., 2014). To investigate this finding, we reviewed the extant literature to identify studies that included a control group of children when assessing fatigue in children with a chronic health condition using the PedsQL-MFS.
We identified five studies that included ratings from a unique control group of healthy children and ratings from children with a chronic health condition (see Figure 6). Several other studies from the Varni et al. research group were identified but not included because their control groups were the same as, or subsets of, the control group children shown in Figure 6. In contrast to the current study's sample, which consisted of younger children (6–12 years of age), mean fatigue ratings from the literature also include ratings from adolescent children (13–17 years old), and some included ratings from young adults (18–20 years old). Gordijn et al. (2011) found that adolescents (13–18 years) reported more fatigue than did younger children (aged 5–12 years). Given that our sample of CNH did not include adolescents, we might predict that children in the current study would report less fatigue (higher scores) compared with data from the literature; however, the reverse was true. Figure 6 shows the rank order of studies from the literature and the current study on the basis of the mean fatigue rating for each subscale and the overall score for the child. These comparisons show that fatigue ratings from CNH in the current study were, in fact, increased compared with all control group data reported in the literature.
Figure 6.
Comparison of mean control group Pediatric Quality of Life Inventory Multidimensional Fatigue Scale (PedsQL-MFS) subscale and overall scores from four existing studies (open bars) and the control group of CNH (gray bars) from the current study. (Cancer 1, Varni et al., 2002; Cancer 2, Daniel et al., 2013; IBD [Inflammatory Bowel Disease], Marcus et al., 2009; and MS [Multiple Sclerosis], Goretti et al., 2012). Error bars = 1 SE.
The reasons for the consistently higher levels of fatigue (lower scores) reported by our control group, compared with results from the literature, are unclear; however, it is possible that self-selection bias may have played some role. Participants in the current study were recruited as part of a larger study examining listening effort and fatigue in children. Participation in the larger study involved a significant time commitment including three to four visits. Each visit required several hours to complete standardized tests or prolonged listening tasks. In addition, as part of the larger study, parents also collected saliva samples at home, multiple times a day on multiple days. In contrast, participation in the other studies shown in Figure 6 required only that control group participants complete the PedsQL-MFS once or twice. It is possible that the type of parents and children who were willing to commit to the time-intensive demands required for participation in our larger study might also be the kind of parents who are active and involved, and who also involve their children, in many other activities resulting in busier and more hectic lifestyles. This could potentially increase their risk for fatigue, compared with the control group participants from other studies in Figure 6. While not an unreasonable hypothesis, it is not clear why similar high levels of fatigue in the control group data were not observed in our preliminary study. Although, given the small sample size (n = 10) of Hornsby et al. (2014), between-study differences could simply reflect sampling variability.
Children With Hearing Loss
In addition to the higher levels of fatigue reported by our control group, CHL in the current study reported relatively less fatigue (higher PedsQL-MFS scores), except in the cognitive domain, when compared with Hornsby et al. (2014). The reason for this difference is also unclear, although a variety of factors could have played some role. Potential contributing factors include differences in degree of hearing loss, age, language ability, or other systematic differences between the small diverse sample in the preliminary study and the larger sample in the current study. The potential impact of some of these factors is discussed later in this section.
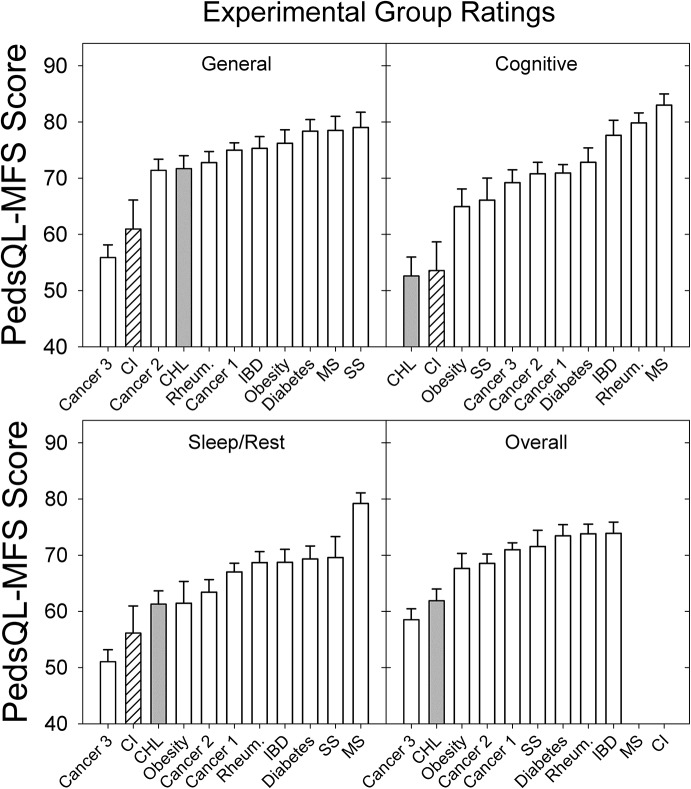
Despite the between-study variations, the current results are consistent with our preliminary findings suggesting that CHL are at increased risk for fatigue, particularly in the cognitive domain, compared with CNH. We highlight the increased risk of fatigue for CHL by comparing PedsQL-MFS ratings from the current study to ratings from children with other chronic conditions as reported in the literature. In addition to the five studies mentioned earlier, we identified four other studies examining fatigue using the PedsQL-MFS in five different health conditions for which fatigue is a common complaint (i.e., rheumatic diseases, Varni et al., 2004; type 1 diabetes, Varni et al., 2009; active cancer treatment, Varni et al., 2009; obesity, Varni et al., 2010; and short stature, Varni, Limbers, Bryant, & Wilson, 2012). PedsQL-MFS ratings from these children, from the CHL from the current study, and from children with CIs (Werfel & Hendricks, 2016) are shown in Figure 7.
Figure 7.
Comparison of mean experimental group Pediatric Quality of Life Inventory Multidimensional Fatigue Scale (PedsQL-MFS) subscale and overall scores from nine studies of children with a chronic health condition (open bars), the CHL from the current study (gray bars), and children with cochlear implants (CIs; striped bars; Werfel & Hendricks, 2016). (Cancer 1, Varni et al., 2002; Cancer 2, Daniel et al., 2013; Cancer 3, Varni, et al., 2009; IBD [Inflammatory Bowel Disease], Marcus et al., 2009; MS [Multiple Sclerosis], Goretti et al., 2012; Diabetes, Varni et al., 2009; Rheumatology, Varni et al., 2004; Obesity, Varni et al., 2010; and SS [Short Stature], Varni et al., 2012). Error bars = 1 SE.
Across all fatigue domains and for the overall fatigue score, CHL from the current study reported similar or more fatigue than almost all other groups (see Figure 7, upper panels). Specifically, CHL reported more (lowest scores) cognitive fatigue than any of the other groups, regardless of their health condition. Note that the CHL who use CIs (Werfel & Hendrcks, 2016) also reported more cognitive fatigue than any of the other groups, except the CHL from the current study. Similarly, for sleep/rest, general, and overall fatigue, CHL (including children who use CIs) reported more fatigue (lower scores) than all other groups except for select studies examining children undergoing active cancer treatment including chemotherapy and radiation (labeled “Cancer 3” in Figure 7).
To summarize, CHL in the current study report fewer problems with fatigue compared with our preliminary study (Hornsby et al., 2014); however, their reported fatigue is still similar to or greater than fatigue ratings reported by children with most other chronic health conditions, especially in the cognitive domain.
Subjective Ratings of Fatigue in Children With and Without Hearing Loss: Self- Versus Parent-Proxy Report
A second focus of this study was to examine associations and agreement between child and parent-proxy reports of fatigue in our sample of CHL and CNH. Consistent with past work, we found relatively poor concordance between parent-proxy and child ratings (ICCs ranging from 0.03 to 0.34 across comparisons; see Table 4). The weak association between fatigue ratings provided by parents and CHL highlights the importance of obtaining information from both parties whenever possible. Although agreement between child and parent reports was generally poor, some systematic differences were observed. Except for the general fatigue domain (where there was close agreement in means), parents of CHL in the current study tended to underestimate the fatigue of their children (see Table 2).
These findings replicate, and contrast with, results from the literature. Data from our control group are consistent with the broader, qualitative research literature discussed earlier, which suggests that parents of typically developing children tend to underestimate their child's health and behavior problems (De Los Reyes & Kazdin, 2005; Upton et al., 2008), including fatigue (Daniel, Brumley, & Schwartz, 2013; Goretti et al., 2012; Marcus et al., 2009). Except for the general fatigue domain, we saw this pattern of underestimation of fatigue by our control group parents as well (i.e., control group parent PedsQL-MFS ratings were higher than their child's).
In contrast, the broader extant literature suggests that parents of children with a chronic health condition tend to overestimate their child's problems (De Los Reyes & Kazdin, 2005; Upton et al., 2008). However, data from our CHL and their parents show an opposite trend. Except for the general fatigue domain, parents of CHL underestimated their child's fatigue. The discrepancies are most apparent in the sleep/rest domain and are similar in magnitude for our HL and NH (typically developing) groups (see Table 2). Interestingly, Werfel and Hendricks (2016) reported a similar pattern of parents underestimating the fatigue reported by their children who use CIs, particularly in the sleep/rest domain. This underestimation of sleep/rest fatigue by parents of CHL was unexpected, as anecdotal reports from these parents commonly included examples of their child's fatigue-related behaviors, particularly in the sleep/rest domain (e.g., parents might describe their child as so worn out after school that he or she would need a nap). Given that a similar pattern of parent underestimation of fatigue was seen in another sample of CHL (Werfel & Hendricks, 2016), additional research is needed to better understand the reasons for, and implications of, such discrepancies.
Factors Associated With Subjective Fatigue in CHL
In addition to examining between-groups effects of hearing loss, we also investigated associations between individual characteristics (age, degree of hearing loss, and language ability) and fatigue ratings in CHL. With regard to age effects, we found no relationship between child age and child or parent-proxy scores on the PedsQL-MFS for CNH or CHL between the ages of 6 and 12 years. This finding is consistent with previous studies that have found differences in PedsQL-MFS ratings between younger children (aged 5–12 years) and adolescents (13–18 years old) but not between children aged 5–7 and 8–12 years old (Gordijn et al., 2011).
The lack of association between degree of hearing loss and self- or parent-proxy reported fatigue is consistent with prior literature in adults (Alhanbali et al., 2017; Hornsby & Kipp, 2016). However, given the significant between-groups differences (HL vs. NH) in fatigue ratings seen in the current study and in the related adult literature, the lack of association between fatigue ratings and degree of hearing loss remains counterintuitive. The findings of this study and others (Alhanbali et al., 2017; Hornsby & Kipp, 2016; Hornsby et al., 2014; Werfel & Hendricks, 2016) suggest that any degree of loss—mild to profound, unilateral or bilateral— may increase one's risk for fatigue. Thus, factors other than auditory sensitivity clearly play a role in self-reported fatigue. For example, Hornsby and Kipp found that fatigue ratings were strongly associated with an individual's perceived hearing difficulties. The question of whether children's perception of hearing difficulty has a similar effect on their reports of fatigue is an area for future study. In addition, it seems possible that a nonlinear relationship between various factors (e.g., degree of loss, language ability) and subjective fatigue may exist. For example, some small degree of hearing loss or language deficit might increase the listening demands of a situation enough to be fatiguing for someone actively engaged in the setting. As the degree of loss or language difficulty increases, we might predict some additional increase in fatigue. However, at some point, the hearing and/or language difficulties may become so great that the individual disengages from the task, in part, to limit fatigue effects. This sort of nonlinear relationship is consistent with a motivation-based model of fatigue (Hockey, 2013).
CNH and CHL in this study who had poorer language abilities reported greater cognitive fatigue. This finding is consistent with that of Werfel and Hendricks (2016). They found a strong relationship between language ability and general, and sleep/rest, fatigue in children who use CIs. Compared with the moderate relationship found in the current study, the stronger relationship reported in Werfel and Hendricks could be due to the lower language levels of the children who use CIs compared with the CHL in the current study (i.e., means and standard deviations of 83.5 (25.8) and 92.1 (21.8) for children with CIs and CHL, respectively). Despite these differences in magnitude, the findings of the current study combined with those of Werfel and Hendricks suggest that children with poor language abilities, regardless of hearing status, are at risk for cognitive fatigue. This finding is not surprising given that children with poor language skills will likely experience communication problems, which, in turn, leads to potential confusion and/or incomplete understanding of concepts. It is worth reiterating that although there appears to be an association between language abilities and subjective fatigue, the direction of the association remains unclear.
Finally, factors other than those examined here may have an effect on subjective fatigue in CNH and CHL. Previous studies have also shown associations between fatigue ratings and child-specific variables such as immigrant status, family structure (one- vs. two-parent homes), parent education, speech perception, and literacy level (Gordijn et al., 2011; Werfel & Hendricks, 2016). Socioeconomic status, another factor known to have a significant impact on many aspects of quality of life (e.g., von Rueden, Gosch, Rajmil, Bisegger, & Ravens-Sieberer, 2006), might also influence subjective fatigue. However, to our knowledge, there is no work specifically examining this association in children. Unfortunately, we did not obtain a direct measure of socioeconomic status in our study. Future studies should consider these additional child-specific variables when examining fatigue in CHL.
Study Limitations
Several limitations pertain to the current study that could influence the generalizability of our findings. First, these data are cross-sectional and prohibit the exploration of temporal trends in fatigue ratings. For example, we were unable to determine if the children's ratings became more (or less) like the parent-proxy ratings with increasing age. In addition, the sample size, while adequate for our study purpose, was relatively small, and the age range of our children was restricted to 6–12 years. While having a homogenous group accentuates our ability to detect between-groups differences, a much larger, more diverse sample of CHL is desirable to allow for generalization of results. Another important limitation to this study is the use of a fatigue scale (PedsQL-MFS) that does not include items weighted for listening-related fatigue. A hearing-specific scale that probes such areas as sustained listening, speech processing, attending, and concentrating could be more sensitive to the listening effort and fatigue problems typically experienced by CHL. Moreover, such an instrument might provide more compatible (or diverse) fatigue ratings between CHL and their parent-proxies. In addition, it is important to consider participant selection bias as a possible limitation in our study. As noted earlier, our children were participating in an extensive study on listening effort and fatigue that required attending multiple and lengthy visits. It is possible that participation in such a rigorous study might influence a child's fatigue ratings.
Conclusions and Future Research Needs
Consistent with our hypotheses, the school-age CHL in this study appear to experience more subjective fatigue than a control group of CNH, although the magnitude of the effect is reduced compared with previously reported results (Hornsby et al., 2014). The risk for fatigue appears to be increased for children with poor language abilities. While parent-proxy reports can be useful, given the discrepancy with child reports of fatigue, our results suggest that the parent-proxy version of the PedsQL-MFS should not be used exclusively when assessing fatigue in school-age CHL. The need for additional research on subjective fatigue in CHL and CNH seems obvious. Future research studies might include the development of a hearing-related and age-specific fatigue instrument, the use of longitudinal designs to explore self-report fatigue in large diverse samples of CHL, and further exploration of parent–child differences including factors that influence agreement (i.e., impact of proxy gender in relation to child gender). Finally, future studies are needed to explore the impact of fatigue on academic performance and to examine in more detail the relationship between specific language abilities and fatigue.
Acknowledgments
The research reported here was supported by the Institute of Education Sciences, U.S. Department of Education Grant R324A110266, awarded to Bess, PI of Vanderbilt University; by National Institute of Child Health & Human Development Grant P30HD15052, awarded to the Vanderbilt Kennedy Center for Research on Human Development; by Vanderbilt Institute for Clinical and Translational Research Grant UL1 TR000445 from the National Center for Advancing Translational Sciences/National Institutes of Health; and by the Dan and Margaret Maddox Charitable Fund. We gratefully acknowledge the children and parents who participated in this study as well as the many Vanderbilt undergraduate and graduate students who generously assisted with this research. The opinions expressed are those of the authors and do not represent views of the Institute of Education Sciences, the National Institutes of Health, or the U.S. Department of Education.
Funding Statement
The research reported here was supported by the Institute of Education Sciences, U.S. Department of Education Grant R324A110266, awarded to Bess, PI of Vanderbilt University; by National Institute of Child Health & Human Development Grant P30HD15052, awarded to the Vanderbilt Kennedy Center for Research on Human Development; by Vanderbilt Institute for Clinical and Translational Research Grant UL1 TR000445 from the National Center for Advancing Translational Sciences/National Institutes of Health; and by the Dan and Margaret Maddox Charitable Fund.
Footnote
Note that outcomes from the nonparametric analyses are based on rank data; however, we chose to show means, rather than medians or rank means, in figures highlighting results. The rationale for this representation is that mean scores allow the reader to more easily compare the magnitude of differences in PedsQL-MFS scores between groups and domains. Furthermore, because the existing literature provides mean scores rather than aggregate ranks, this presentation allows for a comparison with the existing literature.
References
- Alhanbali S., Dawes P., Lloyd S., & Munro K. J. (2017). Self-reported listening-related effort and fatigue in hearing-impaired adults. Ear and Hearing, 38(1), e39–e48. [DOI] [PubMed] [Google Scholar]
- American National Standards Institute. (1997). American National Standard Methods for the calculation of the Speech Intelligibility Index (ANSI S3.5-1997). New York, NY: Author. [Google Scholar]
- American National Standards Institute. (2010). American National Standard Specification for Audiometers (ANSI S3.6-2010). New York, NY: Author. [Google Scholar]
- Bess F. H., Dodd-Murphy J., & Parker R. A. (1998). Children with minimal sensorineural hearing loss: Prevalence, educational performance, and functional status. Ear and Hearing, 19(5), 339–354. [DOI] [PubMed] [Google Scholar]
- Bess F. H., Gustafson S. J., Corbett B. A., Lambert E. W., Camarata S. M., & Hornsby B. W. (2016). Salivary cortisol profiles of children with hearing loss. Ear and Hearing, 37(3), 334–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bess F. H., Gustafson S. J., & Hornsby B. W. (2014). How hard can it be to listen? Fatigue in school-age children with hearing loss. Journal of Educational Audiology, 20, 1–14. [Google Scholar]
- Bess F. H., & Hornsby B. W. (2014a). Commentary: Listening can be exhausting—Fatigue in children and adults with hearing loss. Ear and Hearing, 35(6), 592–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bess F. H., & Hornsby B. W. (2014b). The complexities of fatigue in children with hearing loss. SIG 9 Perspectives on Hearing and Hearing Disorders in Childhood, 24(2), 25–39. [Google Scholar]
- Brown L., Sherbenou R. J., & Johnson S. K. (2010). Test of Nonverbal Intelligence–Fourth Edition: TONI-4. Austin, TX: Pro-Ed. [Google Scholar]
- Carhart R., & Jerger J. (1959). Preferred method for clinical determination of pure-tone thresholds. Journal of Speech and Hearing Disorders, 24(4), 330–345. [Google Scholar]
- Christodoulou C. (2005). The assessment and measurement of fatigue. In DeLuca J. (ed.), Fatigue as a Window to the Brain. (pp. 19–35). Cambridge, MA: MIT Press. [Google Scholar]
- Daniel L. C., Brumley L. D., & Schwartz L. A. (2013). Fatigue in adolescents with cancer compared to healthy adolescents. Pediatric Blood & Cancer, 60(11), 1902–1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Los Reyes A., & Kazdin A. E. (2005). Informant discrepancies in the assessment of childhood psychopathology: A critical review, theoretical framework, and recommendations for further study. Psychological Bulletin, 131(4), 483–509. [DOI] [PubMed] [Google Scholar]
- De Vente W., Olff M., Van Amsterdam J., Kamphuis J., & Emmelkamp P. (2003). Physiological differences between burnout patients and healthy controls: Blood pressure, heart rate, and cortisol responses. Occupational and Environmental Medicine, 60(Suppl. 1), i54–i61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinges D. F., Pack F., Williams K., Gillen K. A., Powell J. W., Ott G. E., … Pack A. I. (1997). Cumulative sleepiness, mood disturbance and psychomotor vigilance performance decrements during a week of sleep restricted to 4–5 hours per night. Sleep, 20(4), 267–277. [PubMed] [Google Scholar]
- Dittner A. J., Wessely S. C., & Brown R. G. (2004). The assessment of fatigue: A practical guide for clinicians and researchers. Journal of Psychosomatic Research, 56(2), 157–170. [DOI] [PubMed] [Google Scholar]
- Drotar D. (2014). Measuring health-related quality of life in children and adolescents: Implications for research and practice. London, United Kingdom: Psychology Press. [Google Scholar]
- Earle F., Hockey B., Earle K., & Clough P. (2015). Separating the effects of task load and task motivation on the effort–fatigue relationship. Motivation and Emotion, 39(4), 1–10. [Google Scholar]
- Eddy L., & Cruz M. (2007). The relationship between fatigue and quality of life in children with chronic health problems: A systematic review. Journal for Specialists in Pediatric Nursing, 12(2), 105–114. [DOI] [PubMed] [Google Scholar]
- Eiser C., & Varni J. W. (2013). Health-related quality of life and symptom reporting: Similarities and differences between children and their parents. European Journal of Pediatrics, 172(10), 1299–1304. [DOI] [PubMed] [Google Scholar]
- Evans E. J., & Wickstrom B. (1999). Subjective fatigue and self-care in individuals with chronic illness. Medsurg Nursing, 8(6), 363–369. [PubMed] [Google Scholar]
- Flechtner H., & Bottomley A. (2003). Fatigue and quality of life: Lessons from the real world. Oncologist, 8(Suppl. 1), 5–9. [DOI] [PubMed] [Google Scholar]
- Gaba D. M., & Howard S. K. (2002). Fatigue among clinicians and the safety of patients. New England Journal of Medicine, 347(16), 1249–1255. [DOI] [PubMed] [Google Scholar]
- Gamer M., Lemon J., Fellows I., & Singh P. (2012). irr: Various coefficients of interrater reliability and agreement. Retrieved from https://cran.r-project.org/package=irr [Google Scholar]
- Gordijn M. S., Cremers E. M., Kaspers G. J., & Gemke R. J. (2011). Fatigue in children: Reliability and validity of the Dutch PedsQLTM Multidimensional Fatigue Scale. Quality of Life Research, 20(7), 1103–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goretti B., Portaccio E., Ghezzi A., Lori S., Moiola L., Falautano M., … Pozzilli C. (2012). Fatigue and its relationships with cognitive functioning and depression in paediatric multiple sclerosis. Multiple Sclerosis Journal, 18(3), 329–334. [DOI] [PubMed] [Google Scholar]
- Grossi G., Perski A., Ekstedt M., Johansson T., Lindström M., & Holm K. (2005). The morning salivary cortisol response in burnout. Journal of Psychosomatic Research, 59(2), 103–111. [DOI] [PubMed] [Google Scholar]
- Hétu R., Riverin L., Lalande N., Getty L., & St-Cyr C. (1988). Qualitative analysis of the handicap associated with occupational hearing loss. British Journal of Audiology, 22(4), 251–264. [DOI] [PubMed] [Google Scholar]
- Hicks C. B., & Tharpe A. M. (2002). Listening effort and fatigue in school-age children with and without hearing loss. Journal of Speech, Language, and Hearing Research, 45(3), 573–584. [DOI] [PubMed] [Google Scholar]
- Hockenberry M. J., Hinds P. S., Barrera P., Bryant R., Adams-McNeill J., Hooke C., … Manteuffel B. (2003). Three instruments to assess fatigue in children with cancer: The child, parent and staff perspectives. Journal of Pain and Symptom Management, 25(4), 319–328. [DOI] [PubMed] [Google Scholar]
- Hockenberry-Eaton M., Hinds P., Howard V., Gattuso J., O'Neill J. B., Alcoser P., … Euell K. (1999). Developing a conceptual model for fatigue in children. European Journal of Oncology Nursing, 3(1), 5–11. [Google Scholar]
- Hockey R. (2013). The psychology of fatigue: Work, effort and control. Cambridge, United Kingdom: Cambridge University Press. [Google Scholar]
- Hornsby B. W., & Kipp A. M. (2016). Subjective ratings of fatigue and vigor in adults with hearing loss are driven by perceived hearing difficulties not degree of hearing loss. Ear and Hearing, 37(1), e1–e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornsby B. W., Naylor G., & Bess F. H. (2016). A taxonomy of fatigue concepts and their relation to hearing loss. Ear and Hearing, 37, 136S–144S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornsby B. W., Werfel K., Camarata S., & Bess F. H. (2014). Subjective fatigue in children with hearing loss: Some preliminary findings. American Journal of Audiology, 23(1), 129–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerjes W. K., Cleare A. J., Wessely S., Wood P. J., & Taylor N. F. (2005). Diurnal patterns of salivary cortisol and cortisone output in chronic fatigue syndrome. Journal of Affective Disorders, 87(2), 299–304. [DOI] [PubMed] [Google Scholar]
- Koot H., & Wallander J. (2014). Quality of life in child and adolescent illness: Concepts, methods and findings. Abingdon, United Kingdom: Routledge. [Google Scholar]
- Kramer S. E., Kapteyn T. S., & Houtgast T. (2006). Occupational performance: Comparing normally-hearing and hearing-impaired employees using the Amsterdam Checklist for Hearing and Work. International Journal of Audiology, 45(9), 503–512. [DOI] [PubMed] [Google Scholar]
- Lieberman H. R. (2007). Cognitive methods for assessing mental energy. Nutritional Neuroscience, 10(5–6), 229–242. [DOI] [PubMed] [Google Scholar]
- Marcus S. B., Strople J. A., Neighbors K., Weissberg-Benchell J., Nelson S. P., Limbers C., … Alonso E. M. (2009). Fatigue and health-related quality of life in pediatric inflammatory bowel disease. Clinical Gastroenterology and Hepatology, 7(5), 554–561. [DOI] [PubMed] [Google Scholar]
- McCoy S. L., Tun P. A., Cox L. C., Colangelo M., Stewart R. A., & Wingfield A. (2005). Hearing loss and perceptual effort: Downstream effects on older adults' memory for speech. The Quarterly Journal of Experimental Psychology Section A, 58(1), 22–33. [DOI] [PubMed] [Google Scholar]
- McGarrigle R., Munro K. J., Dawes P., Stewart A. J., Moore D. R., Barry J. G., & Amitay S. (2014). Listening effort and fatigue: What exactly are we measuring? A British Society of Audiology Cognition in Hearing Special Interest Group “white paper”. International Journal of Audiology, 53(7), 433–445. [DOI] [PubMed] [Google Scholar]
- McGraw K. O., & Wong S. P. (1996). Forming inferences about some intraclass correlation coefficients. Psychological Methods, 1(1), 30. [Google Scholar]
- Meeske K., Katz E. R., Palmer S. N., Burwinkle T., & Varni J. W. (2004). Parent proxy–reported health‐related quality of life and fatigue in pediatric patients diagnosed with brain tumors and acute lymphoblastic leukemia. Cancer, 101(9), 2116–2125. [DOI] [PubMed] [Google Scholar]
- Michielsen H. J., De Vries J., Van Heck G. L., Van de Vijver F. J., & Sijtsma K. (2004). Examination of the dimensionality of fatigue: The construction of the Fatigue Assessment Scale (FAS). European Journal of Psychological Assessment, 20(1), 39–48. [Google Scholar]
- Nelson E. C., Wasson J., Johnson D., & Hays R. D. (1996). Dartmouth COOP Functional Health Assessment Charts: Brief measures for clinical practice. In Spilker B. (Ed.), Quality of Life and Parmacoeconomics in Clinical Trials (2nd ed, pp. 161–168). Philadelphia, PA: Lippencott-Raven. [Google Scholar]
- Nelson E., Wasson J., Kirk J., Keller A., Clark D., Dietrich A., … Zubkoff M. (1987). Assessment of function in routine clinical practice: Description of the COOP Chart method and preliminary findings. Journal of Chronic Diseases, 40, 55S–63S. [DOI] [PubMed] [Google Scholar]
- Noguchi K., Gel Y. R., Brunner E., & Konietschke F. (2012). nparLD: An R software package for the nonparametric analysis of longitudinal data in factorial experiments. Journal of Statistical Software, 50(12), 1–23.25317082 [Google Scholar]
- Semel E., Wiig E., & Secord W. (2003). Clinical evaluation of language fundamentals-IV. Marickville: Harcourt Assessment. [Google Scholar]
- Upton P., Lawford J., & Eiser C. (2008). Parent–child agreement across child health-related quality of life instruments: A review of the literature. Quality of Life Research, 17(6), 895–913. [DOI] [PubMed] [Google Scholar]
- Van Dongen H. P., & Dinges D. F. (2000). Circadian rhythms in fatigue, alertness, and performance. Principles and Practice of Sleep Medicine, 20, 391–399. [Google Scholar]
- Varni J. W., Burwinkle T. M., Berrin S. J., Sherman S. A., Artavia K., Malcarne V. L., & Chambers H. G. (2006). The PedsQL in pediatric cerebral palsy: Reliability, validity, and sensitivity of the Generic Core Scales and Cerebral Palsy Module. Developmental Medicine and Child Neurology, 48(6), 442–449. [DOI] [PubMed] [Google Scholar]
- Varni J. W., Burwinkle T. M., Katz E. R., Meeske K., & Dickinson P. (2002). The PedsQLTM in pediatric cancer. Cancer, 94(7), 2090–2106. [DOI] [PubMed] [Google Scholar]
- Varni J. W., Burwinkle T. M., Limbers C. A., & Szer I. S. (2007). The PedsQLTM as a patient-reported outcome in children and adolescents with fibromyalgia: An analysis of OMERACT domains. Health and Quality of Life Outcomes, 5, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varni J. W., Burwinkle T. M., & Szer I. S. (2004). The PedsQL Multidimensional Fatigue Scale in pediatric rheumatology: Reliability and validity. The Journal of Rheumatology, 31(12), 2494–2500. [PubMed] [Google Scholar]
- Varni J. W., Katz E. R., Colegrove R. Jr., & Dolgin M. J. (1996). Adjustment of children with newly diagnosed cancer: Cross-informant variance. Journal of Psychosocial Oncology, 13(4), 23–38. [Google Scholar]
- Varni J. W., & Limbers C. A. (2008). The PedsQLTM Multidimensional Fatigue Scale in young adults: Feasibility, reliability and validity in a University student population. Quality of Life Research, 17(1), 105–114. [DOI] [PubMed] [Google Scholar]
- Varni J. W., Limbers C. A., Bryant W. P., & Wilson D. P. (2009). The PedsQLTM Multidimensional Fatigue Scale in type 1 diabetes: Feasibility, reliability, and validity. Pediatric Diabetes, 10(5), 321–328. [DOI] [PubMed] [Google Scholar]
- Varni J. W., Limbers C. A., Bryant W. P., & Wilson D. P. (2010). The PedsQLTM Multidimensional Fatigue Scale in pediatric obesity: Feasibility, reliability and validity. International Journal of Pediatric Obesity, 5(1), 34–42. [DOI] [PubMed] [Google Scholar]
- Varni J. W., Limbers C. A., Bryant W. P., & Wilson D. P. (2012). Assessment of fatigue in pediatric patients with short stature utilizing the PedsQLTM Multidimensional Fatigue Scale. Children's Health Care, 41(2), 162–181. [Google Scholar]
- Verhulst F. C., & Ende J. (1992). Agreement between parents' reports and adolescents' self‐reports of problem behavior. Journal of Child Psychology and Psychiatry, 33(6), 1011–1023. [DOI] [PubMed] [Google Scholar]
- von Rueden U., Gosch A., Rajmil L., Bisegger C., & Ravens-Sieberer U. (2006). Socioeconomic determinants of health related quality of life in childhood and adolescence: Results from a European study. Journal of Epidemiology and Community Health, 60(2), 130–135. https://doi.org/10.1136/jech.2005.039792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werfel K. L., & Hendricks A. E. (2016). The relation between child versus parent report of chronic fatigue and language/literacy skills in school-age children with cochlear implants. Ear and Hearing, 37(2), 216–224. [DOI] [PubMed] [Google Scholar]
- Whitehead L. (2009). The measurement of fatigue in chronic illness: A systematic review of unidimensional and multidimensional fatigue measures. Journal of Pain and Symptom Management, 37(1), 107–128. [DOI] [PubMed] [Google Scholar]
- Wilson K. A., Dowling A. J., Abdolell M., & Tannock I. F. (2000). Perception of quality of life by patients, partners and treating physicians. Quality of Life Research, 9(9), 1041–1052. [DOI] [PubMed] [Google Scholar]
- Zekveld A. A., Kramer S. E., & Festen J. M. (2011). Cognitive load during speech perception in noise: The influence of age, hearing loss, and cognition on the pupil response. Ear and Hearing, 32(4), 498–510. https://doi.org/10.1097/AUD.0b013e31820512bb [DOI] [PubMed] [Google Scholar]