Abstract
Aims
The aim of the study was to compare the influence of gap junctional communication (GJC) in osteoclastogenesis from bone marrow (BM) and peripheral blood (PB) monocytes. These widely used sources differ in purity, since BM cultures contain a significant number of stromal cells. We studied whether stimulation of GJC in BM monocyte/stromal cell cultures differs from the effect in pure PB monocyte cultures. We compared the differentiation also in acidosis, which is a known inducer of bone resorption.
Main methods
Human BM and PB monocytes were isolated from BM aspirates or whole blood samples. The cells were cultured on human bone slices with osteoclastogenic growth factors and a GJC modulator, antiarrhythmic peptide AAP10, at physiological and acidic pH.
Key findings
Both BM and PB monocytes differentiated into osteoclasts. Acidosis increased resorption in both cultures but stimulated cell fusion only in BM cultures, which demonstrates the role of stromal cells in osteoclastogenesis. At physiological pH, AAP10 increased the number of multinuclear cells and bone resorption in both BM and PB cultures indicating that GJC is involved in differentiation in both of these osteoclastogenesis assays. Interestingly, in PB cultures at pH 6.5 the stimulation of GJC with AAP10 inhibited both osteoclastogenesis and bone resorption suggesting a different role of GJC in BM and PB monocytes at stressed environment.
Significance
The study is conducted with primary human tissue samples and adds new knowledge on factors affecting osteoclastogenesis from different monocyte sources.
Keywords: Cell biology, Developmental biology, Stem cell research
1. Introduction
Osteoclasts are multinuclear bone-resorbing cells differentiated from the monocyte/macrophage lineage [1, 2]. In addition to bone marrow (BM) peripheral blood (PB) has also been shown to contain cells capable of fusion into bone-resorbing polykaryons [3, 4, 5, 6]. Osteoclasts can be generated in vitro from BM or PB monocytes with receptor activator for nuclear factor κB ligand (RANKL) and macrophage colony-stimulating factor (M-CSF) [7, 8]. The addition of transforming growth factor beta (TGF-β) and dexamethasone can enhance osteoclastogenesis and the activity of osteoclasts [9, 10]. However, contradictory effects of TGF-β on osteoclastogenesis have been reported [11], and the outcome is also affected by the presence of stromal cells in the culture [12, 13].
The main osteoclast precursors have been shown to be the monocyte subpopulation CD14+CD16− cells [14, 15, 16, 17, 18]. The major monocyte type in PB is the classical (CD14++CD16−) monocyte, whereas BM contains mostly intermediate (CD14++CD16+) monocytes [19, 20]. It has been shown that after initial differentiation in hematopoietic tissues, the cell cycle-arrested quiescent osteoclast precursors (QOPs) circulate in the bloodstream before homing to bone surfaces for final osteoclastogenesis [21, 22]. The minor monocyte subpopulations in PB, the non-classical (CD14+CD16++) and intermediate monocytes, have also been shown to differentiate into osteoclasts [19, 23]. However, the osteoclasts differentiated from distinct monocyte subsets seem to be functionally different. Sprangers et al. suggest that the main source of osteoclasts under physiological conditions are the classical monocytes, whereas the intermediate monocytes differentiate into osteoclasts with an increased bone resorption ability under inflammatory conditions [23].
Cell fusion in osteoclastogenesis is a multifactorial process involving various fusion proteins as well as gap junctional communication (GJC). Gap junctions are comprised of two connexon hemichannels, which allow the intercellular transfer of small molecules. Hemichannels can also act as unopposed channels without gap junction formation in mediating communication between the cell and the extracellular environment. The hemichannels/connexons consist of six connexin (Cx) subunits, of which Cx43 is the most abundant in bone tissue. Gap junctions are widely expressed in bone, and GJC has been shown to be important in the overall maintenance of bone homeostasis, as well as more specifically in osteoclastogenesis, bone resorption and osteoclast survival [24, 25, 26, 27]. Several studies have shown that blocking of GJC during osteoclastogenesis leads to a decreased number of osteoclasts [24, 25, 26, 28, 29]. In addition, Pacheco-Costa et al. [30] showed that osteoclasts in mice lacking Cx37 are smaller and have fewer nuclei, indicating that Cx37 is needed for the proper fusion of the cells. The precise mechanisms behind this are not known, but GJC has been suggested to be involved especially in the fusion of the mononuclear osteoclast precursors, since Cx43 mRNA expression was shown to be time-dependently downregulated in the course of differentiation [26]. In addition to the regulation of osteoclastogenesis directly by the osteoclast gap junctions, it is possible that GJC in other bone cells affects the differentiation. Zhang et al. [31] provided evidence that Cx43 ablation in osteocytes leads to an increased RANKL/osteoprotegerin (OPG) ratio supporting osteoclastogenesis, and Watkins et al. [32] have shown that altered GJC in osteoblasts can have indirect effects on osteoclasts.
GJC can be enhanced with a group of synthetic antiarrhythmic peptides (AAP). Their proposed mechanism of action is increasing total Cx expression and promotion of PKCα-dependent phosphorylation of Cx43 [33, 34, 35, 36]. In bone tissue, rotigaptide, one of the improved and more stable AAPs, has been shown to increase GJC in human osteoblasts and to prevent ovariectomy-induced bone loss in rats [37]. We have previously shown that GJC is involved in osteoclastogenesis in mouse cell cultures, and that GJC in these cultures can be selectively affected with a group of novel AAPs [28].
In regard to the clinical background of the study, several Cx mutations have been identified that lead to either minor symptoms or to severe diseases, such as oculodentodigital dysplasia [38]. In addition, inflammation and GJC are linked to each other [39]. Inflammation causes defective GJC, or on the contrary, certain pathogens can utilize GJC to spread the inflammation in the central nervous system [40]. This is an interesting point considering that the functions of bone cells and the immune system are tightly coupled [41]. Further, aging is well known to cause bone loss, and from this point of view Genetos et al. [42] have provided an interesting observation of age-related defects in GJC and in the formation of functional gap junctions in osteoblasts. Therefore, with the global aging of the population, it is important to study the relationships of these issues thoroughly. While antiarrhythmic peptides can rescue GJC in heart [43, 44, 45], a similar approach in cellular coupling in bone could be valuable in skeletal disorders as well.
It is known that GJC influences bone remodeling and the coupling of bone cells but it is unclear whether the influence on osteoclasts is direct or indirect. Here we investigated osteoclasts generated from circulating or bone marrow derived human monocytes. The BM in vitro culture also contains stromal cells but the PB model is deficient of cells other than monocytes. Inflammation is usually associated with low pH [46] that stresses cells and inhibits GJC [47]. Thus we studied whether acidosis or pharmacological stimulation of GJC could have variable effects in pure circulating monocytes compared to cultures containing both monocytes and stromal cells.
2. Materials and methods
2.1. Osteoclastogenesis from human BM mononuclear cells
The isolation and culture methods were modified from Susa et al. [9]. BM samples were obtained from hip replacement surgery patients in Oulu University Hospital. The patients were 79–85 year-old men and women who all gave a written informed consent, and the Ethical Committee of The Northern Ostrobothnia Hospital District approved the study. All experiments in this study were performed in accordance with the relevant guidelines and regulations. A BM sample was first maintained in α-MEM (Corning Life Sciences, Tewksbury, MA) containing 10% FBS, 100 IU/ml penicillin and 100 μg/ml streptomycin and 24 mM Hepes buffer (Sigma-Aldrich, St. Louis, MO) at +37 °C (5% CO2, 95% air) for 1–2 days. After pre-culture, media containing the non-adherent cells were collected and isolation was continued with Ficoll-Paque Premium (GE Healthcare, Little Chalfont, UK) following the manufacturer's protocol. The cell suspension was diluted 1:1 in PBS, layered over Ficoll-Paque Premium solution (1:1) and centrifuged at 400 x g for 35 minutes. The mononuclear cell layer was collected and washed 2 times by centrifugation at 190 x g for 10 minutes in PBS. The cells were suspended in α-MEM (Sigma-Aldrich) and the cell number was calculated with a hemocytometer. 9.4 × 105 cells/cm2 were cultured on sonicated human cortical bone slices (0.28 cm2) in 96-well plates (Costar; Corning Life Sciences). Anonymous bone samples were acquired from the clinical bone bank held in Oulu University Hospital, Finland. The Special National Supervisory Authority for Welfare and Health (Valvira) granted permission for the use of aged cadaver specimens for research purposes, decision 8.5.2009, diary number 2240/05.01.00.06/2009. The cells were maintained in α-MEM containing 10% FBS, 2 mM L-glutamine, 100 IU/ml penicillin and 100 μg/ml streptomycin (Sigma-Aldrich). Differentiation to osteoclasts was induced with 50 ng/ml RANKL (PeproTech EC, London, UK), 25 ng/ml M-CSF (R&D Systems, Minneapolis, MN), 5 ng/ml TGF-β (R&D Systems) and 1 μM dexamethasone (Sigma-Aldrich). Acidosis was induced with a dropwise addition of 0.5 M HCl into the culture medium in order to lower the pH of the growth medium to 6.5 instead of physiological pH 7.4. The final concentration of HCl was between 4.5 and 10 mM. To maintain the pH, Hepes (for pH 7.4) or Pipes (for pH 6.5) buffer was added to the culture medium to 20 mM final concentration. Acidification was done in the beginning of the culture and when refreshing the medium. At the one-week time point, all the medium was refreshed (200 μl/well), but otherwise only half (100 μl) was refreshed every 3–4 days. Cells were cultured at +37 °C (5% CO2, 95% air) for 12 days and fixed with 4% PFA in PBS.
2.2. Osteoclastogenesis from human PB mononuclear cells
PB mononuclear cells were collected from whole blood samples after written informed consent from healthy or patient donors using Ficoll-Paque Premium gradient centrifugation as described earlier. The patients were 28–75 year-old men and women. Cell culture was done in a similar way to the BM sample, and the same number of cells were cultured on the human bone slices. Differentiation to osteoclasts was induced with 20 ng/ml RANKL (PeproTech EC) and 10 ng/ml M-CSF (R&D Systems). Acidification of the culture medium and medium changes were done as described earlier. Cells were cultured at +37 °C (5% CO2, 95% air) for 14 days and fixed with 4% PFA in PBS.
2.3. Gap junction modulation
Gap junctions were treated with 100 μM gap junction modulator peptide AAP10 (H2N-Gly-Ala-Gly-4Hyp-Pro-Tyr-CONH2; Zealand Pharma, Copenhagen, Denmark). The peptide was dissolved in water and added to the cultures once daily.
2.4. Analysis of cell fusion: counting of multinuclear cells
The actin cytoskeleton of the cells was stained with Alexa 488-conjugated phalloidin (200 U/ml stock diluted 1:100 in PBS; Invitrogen Europe, Paisley, UK) for 20 minutes at +37 °C. The nuclei were stained with Hoechst 33258 (1 mg/ml stock diluted 1:800 in PBS; Sigma-Aldrich) for 10 minutes at room temperature. The osteoclast-specific enzyme tartrate resistant acidic phosphatase (TRACP) was stained with a commercial acid phosphatase leukocyte kit (Sigma-Aldrich) for 20 minutes at +37 °C. The samples were mounted in 70% glycerol-PBS and viewed in a Nikon Eclipse E600 fluorescence microscope with Plan 10x/0.25 objective (Tokyo, Japan). Multinuclear cells with three or more nuclei were counted. Images were taken with the Nikon Eclipse E600 fluorescence microscope and Plan 20x/0.5 objective with QImaging MicroPublisher 5.0 RTV camera and QCapture 2.90.1 software (QImaging, Surrey, Canada). For demonstration of the number of stromal cells in the two culture types the cells were grown on glass slides and stained with Alexa 488-conjugated phalloidin as described earlier. Samples were mounted in Immu-Mount (Thermo Fisher Scientific, Waltham, MA) and viewed in an LSM 510 META confocal microscope combined with an Axiovert 200 M inverted microscope (Carl Zeiss, Oberkochen, Germany) with Plan Neofluar 10x/0.5 objective (Carl Zeiss).
2.5. Field Emission Scanning Electron Microscopy and measurement of resorption pit areas
Resorption pit areas were compared from Field Emission Scanning Electron Microscopy (FESEM) images with Merz grid analysis. Cells were wiped away from the bone slices. Samples were dehydrated in an ascending ethanol series and dried with critical point drying equipment K850 (Quorum technologies, UK). The samples were sputter coated with 5 nm platinum with a Q150T ES sputter coater (Quorum Technologies) and viewed with a Sigma HD VP FE-SEM (Carl Zeiss Microscopy GmbH, Germany). FESEM images were taken from three fields (voltage 5.0 kV, magnification 50x, area 0.035 cm2) from each bone slice, n = 3. The morphometric analysis of the resorption pits was performed with ImageJ 1.49t software (NIH, USA) by superimposing a Merz grid over the image. 80–88 points from semicircular lines were counted and the proportion of resorption pits versus intact bone surface was determined.
2.6. Analysis of the effects of AAP10 on GJC with Fluorescence Recovery After Photobleaching (FRAP)
Osteoblast lineage MC3T3-E1 cells were obtained from LGC Standards (Teddington, UK). The cells were cultured in α-MEM containing 10% FBS, 2 mM L-glutamine, 100 IU/ml penicillin and 100 μg/ml streptomycin (Sigma-Aldrich) in T25 cell culture flasks and passaged every 2–3 days. For the FRAP experiment, 3.95 × 104 cells/cm2 were cultured in 4 compartment glass bottom cell culture dishes (Cellview; Greiner Bio-One, Kremsmünster, Austria) in 500 μl α-MEM per compartment. 100 μM AAP10 was added to the cultures once daily and finally after staining with calcein red-orange. The cells were cultured for 3 days, after which the media were removed and replaced with phenol red free D-MEM (Gibco/Thermo Fisher Scientific, Waltham, MA) containing 5 μM calcein red-orange, AM (Molecular Probes/Thermo Fisher Scientific). Staining was performed at +37 °C for 30 minutes after which the cells were washed 3 times with phenol red free D-MEM. FRAP experiments were performed with a Zeiss LSM 780 confocal microscope using a Plan-Apochromat 20x/0.8 objective (Carl Zeiss Microscopy GmbH, Germany). The excitation wavelength was 561 nm and emission was collected between 566 nm - 670 nm. The pixel size was set to 354 μm × 354 μm. The time-lapse imaging interval was set to 5 seconds and recovery of the fluorescence was followed for 280 seconds. All measurements were performed at +37 °C and 5% CO2 maintained by an OkoLab bold line top stage incubator (OkoLab, Italy). Before analyzing the results, the fluorescence signals were corrected for acquisition bleaching and normalized by scaling the pre-bleach intensity values to 1, and the post-bleach values were scaled to 0.
2.7. Statistical analysis
All cell culture experiments were done with groups of n ≥ 3 and repeated at least three times. Statistical analyses were performed using the SPSS statistics program version 22 (SPSS Inc., Chicago, IL). The normality of the response variables was tested by the Kolmogorov-Smirnov test and histogram visualization. This analysis revealed that the response variables were not normally distributed. Statistical differences between the test groups were evaluated using the Kruskal-Wallis test, and comparison between specific groups was done with the Mann-Whitney U-test. The graphical presentation of the results was created with OriginPro 9.1 software (OriginLab, Northampton, MA). p < 0.05 was considered significant. The data are shown as means ± SEM.
3. Results
3.1. The effects of AAP10 on multinuclear cell number generated from human BM and PB monocytes
Osteoclastogenesis from BM and PB monocytes was analyzed both at physiological pH 7.4 and acidic pH 6.5 since AAPS have been assumed to be more active during acidosis or other metabolic stress [37, 48, 49].
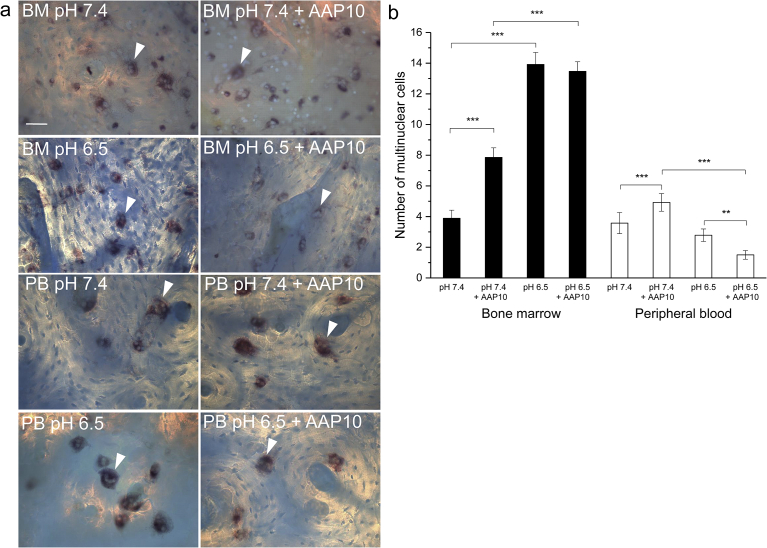
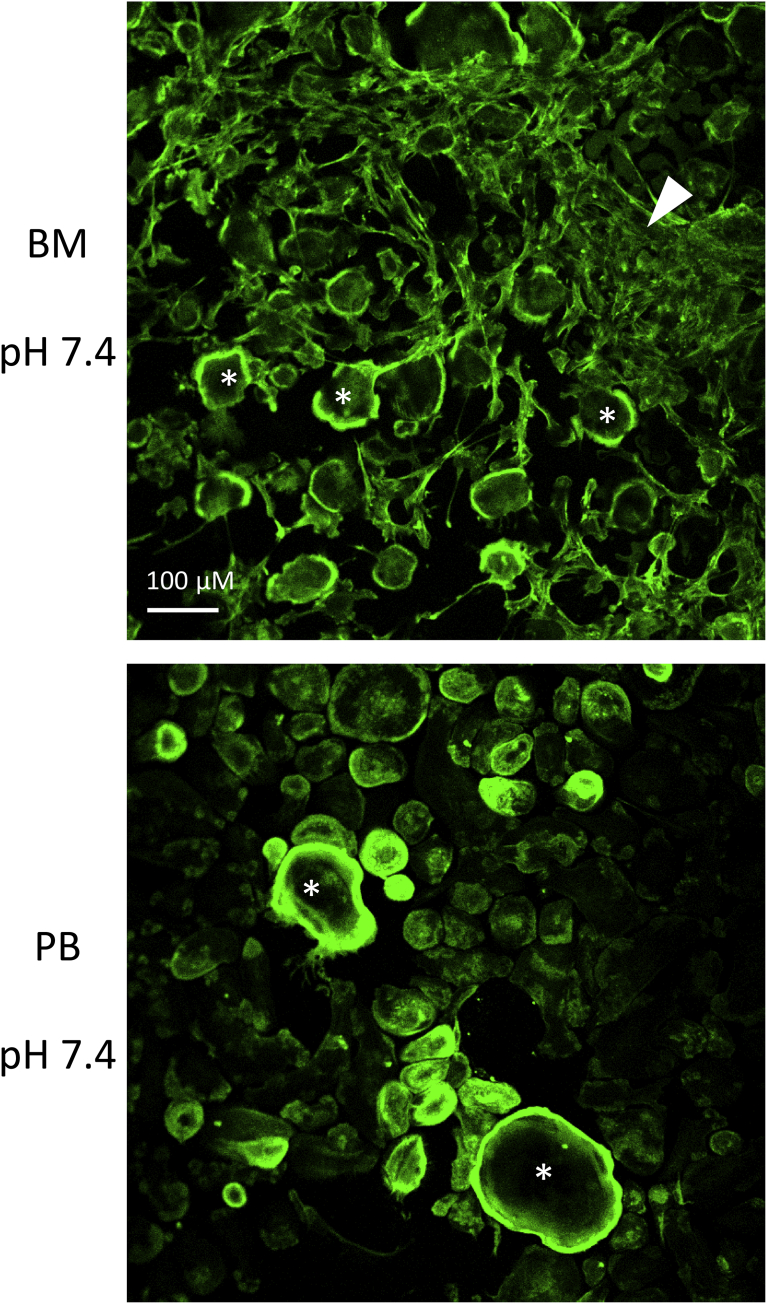
Multinuclear cells in both cell cultures were TRACP positive (Fig. 1a). In addition, some mononuclear TRACP positive cells existed in both cultures. The number of stromal cells present in the two culture types are shown in Fig. 2. The BM cultures contain always a large number of fibroblast-like stromal cells growing in patches in certain areas, whereas the PB cultures contain very few fibroblast-like cells. The actin rings of the multinuclear cells can be seen in the images. Neither acidosis nor AAP10 had an effect on the number of stromal cells in the cultures.
Fig. 1.
The effect of AAP10 on the number of multinuclear cells. Multinuclear TRACP positive cells (arrow heads) formed in both BM and PB cultures (a). The cells were grown on human bone slices and stained with Hoechst 33258 (blue) and an acid phosphatase leukocyte kit to visualize TRACP (red). Acidosis increased multinuclear cell number in BM cultures, but did not have an effect in PB cultures (b). In BM cultures AAP10 increased multinuclear cell number at physiological pH 7.4, but no effect was seen when compared to control in acidic pH 6.5. The effect was similar in PB cultures at physiological pH 7.4, as AAP10 increased the multinuclear cell number, but in acidosis, AAP10 decreased the number of multinuclear cells. The data are shown as mean ± SEM. AAP10: 100 μM AAP10 in the cultures. **p < 0.01, ***p < 0.001. Scale bar 50 μm.
Fig. 2.
The number of stromal cells in BM and PB cultures. Mononuclear cells isolated from human BM or PB samples were grown on glass slides and stained with Alexa 488-conjugated phalloidin for actin (green). A large number of fibroblast-like stromal cells (arrow head) growing in patches are present in BM samples, whereas the PB samples contain very few stromal cells among the multinuclear cells (asterisks). Acidosis and AAP10 had no effect on the number of the stromal cells.
Culturing the BM cells in medium at acidic pH 6.5 instead of physiological pH 7.4 had a dramatic effect on the amount of multinuclear cells (Fig. 1b). The acidic pH increased the number of multinuclear cells in the control group significantly (p < 0.001). In PB cultures the acidic pH decreased slightly but not significantly the number of multinuclear cells.
The effects of AAP10 on the number of multinuclear cells are shown in Fig. 1b. In both cell types AAP10 increased the number of multinuclear cells at physiological pH 7.4, and the effect was statistically significant (p < 0.001). In acidic conditions AAP10 had no effect on the BM cultures, but decreased the number of multinuclear cells in PB cultures (p < 0.01).
3.2. The effects of AAP10 on bone resorption of human BM and PB osteoclasts
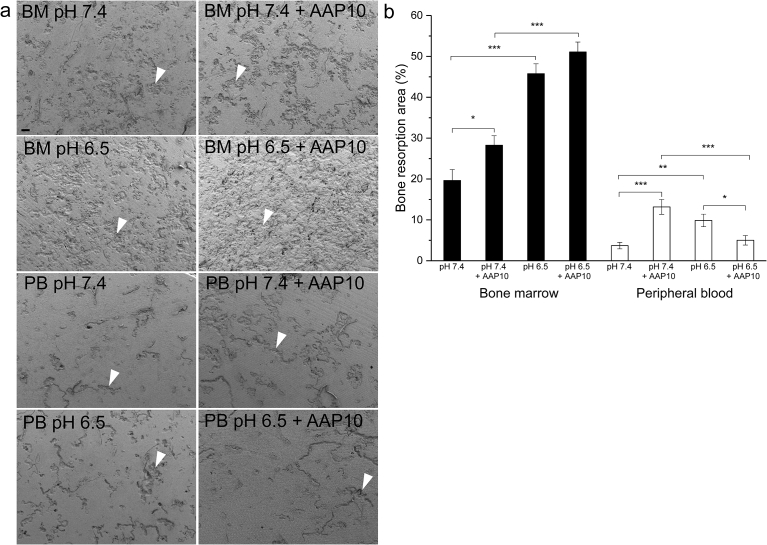
Both BM and PB derived osteoclasts resorbed bone actively (Fig. 3a). Acidosis increased the resorbed area in both cultures (p < 0.001 in BM and p < 0.01 in PB culture) (Fig. 3b). Also AAP10 increased resorption pit area in cultures at pH 7.4 (p < 0.05 in BM and p < 0.001 in PB cultures). In BM cultures at pH 6.5, AAP10 did not have a significant effect on resorption pit area, but in PB cultures under acidic conditions, AAP10 decreased the resorbed area (p < 0.05).
Fig. 3.
The effect of AAP10 on bone resorption. Both BM and PB-derived multinuclear cells formed resorption pits (arrow heads) on human bone slices (a). BM-derived multinuclear cells were more active in bone resorption. Acidosis caused an increase in the resorbed area in both cultures, and also AAP10 increased the resorbed area at physiological pH 7.4 (b). In BM cultures AAP10 did not have a significant effect in pH 6.5, but in PB cultures AAP10 decreased the resorbed area in pH 6.5. The data are shown as mean ± SEM. Images were taken with FESEM with 50x magnification. AAP10: 100 μM AAP10 in the cultures. *p < 0.05, **p < 0.01, ***p < 0.001. Scale bar 100 μm.
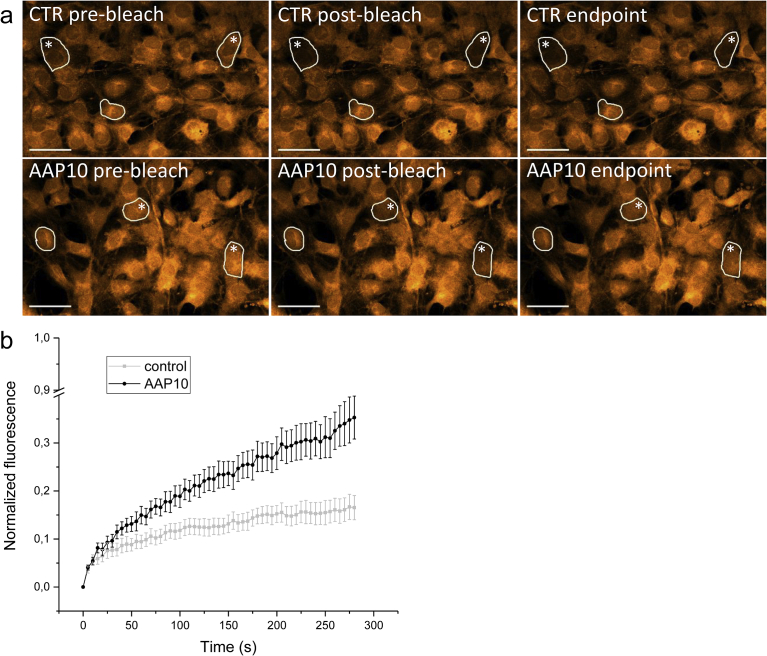
3.3. The effects of AAP10 on GJC in osteoblast lineage MC3T3 cells
MC3T3 cells grown on glass slides appeared as a confluent cell layer having connections between the cells (Fig. 4a). The figure shows the effect of photobleaching in selected cells (asterisks) and the recovery of fluorescence at the endpoint. The FRAP experiment revealed that treatment of MC3T3 cell cultures with AAP10 increased the recovery of fluorescence in the cells after bleaching (Fig. 4b). The result was statistically significant compared to the control group (p < 0.01). This indicates that AAP10 affected GJC in bone cells, since the recovery of fluorescence is based on the diffusion of fluorescent calcein molecules to the bleached cell from adjacent cells via gap junctions.
Fig. 4.
The effect of AAP10 on GJC in MC3T3 cells. MC3T3 cells (a) were cultured for 3 days and GJC between the cells was analyzed with the FRAP technique. The bleached cells are marked with an asterisk. The recovery of fluorescence after photobleaching was increased in MC3T3 cells treated with AAP10 compared to the control group (p < 0.01) (b). The pre-bleach values of fluorescence intensity were normalized to 1 and the post-bleach values to 0. The recovery of the fluorescence was followed for 280 seconds (endpoint). The data are shown as mean ± SEM. AAP10: 100 μM AAP10 in the cultures. Scale bar 50 μm.
4. Discussion
Regardless of the differing monocyte populations in BM and PB, they differentiate into multinuclear bone resorbing osteoclasts. Here we show for the first time that stimulation of GJC by the peptide AAP10 increases osteoclastogenesis at physiological pH in both human BM and PB monocyte cultures. This suggests that gap junctions are involved in human osteoclastogenesis, and that both the BM and PB monocytes utilize gap junctions in cell fusion, although GJC is not indispensable in the fusion process.
It is well known that acidosis promotes osteoclast formation and survival [50, 51] and increases osteoclastic bone resorption [52, 53]. In our study acidification of the cell culture medium resulted in a threefold increase in osteoclastogenesis and a twofold increase in bone resorption in BM cultures. Acidification did not have an effect on osteoclastogenesis in PB cultures, but it increased bone resorption twofold. A possible explanation to this could be that the effects of acidosis are partly mediated by the stromal cells present in the bone marrow cultures. Osteoblast precursors have been shown to express proton receptors [54], and based on this, Fuller and Chambers [55] remarked that the direct effects of acidosis on osteoclasts could be derived from the contaminating stromal cells in the BM cultures.
Acidosis is a typical stress which might close gap junctions [47]. This could explain our results in acidosis, where cell fusion was not affected with AAP10 in BM cultures. We suggest that acidosis causes the closure of gap junction channels, and AAP10 is not able to restore their open state. In this regard AAP10 is not as effective as another AAP, ZP123, also termed rotigaptide, which has been shown to attenuate gap junction closure during acidosis. There are also other studies supporting the idea that the AAPs are active only during metabolic stress [37, 49, 56, 57]. We believe that although the gap junction channels are closed during acidosis, the normal level of osteoclastogenesis observed in BM cultures occurs due to other fusion mechanisms, probably also with the help of stromal cells present in the BM cultures, which might secrete substances that promote osteoclastogenesis. This signaling between osteoblasts, osteoclasts and their precursors is well studied [58], and it can lead to either increased or decreased osteoclastogenesis. Watkins et al. [32] have shown that GJC modulation in osteoblasts can regulate osteoclastogenesis indirectly by paracrine signaling. The schematic representation of the effects of AAP10 on osteoclastogenesis from human BM and PB monocytes is shown in Fig. 5. The decreased osteoclastogenesis observed in PB cultures may result from the lack of stromal cells and their complementary effect on osteoclastogenesis.
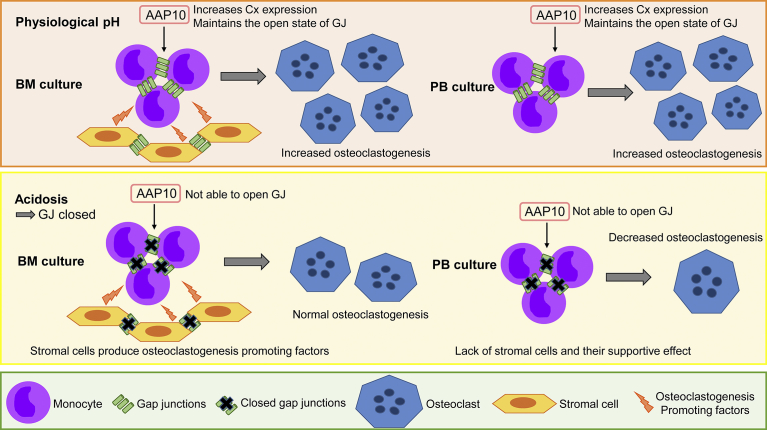
Fig. 5.
Schematic representation of the effects of AAP10 on osteoclastogenesis from human BM and PB monocytes. At physiological pH (7.4) AAP10 increases connexin expression and maintains the gap junctions in the open state in both BM and PB monocytes. The increased GJC leads to increased osteoclastogenesis since the monocytes are able to fuse more effectively. BM cultures contain also stromal cells, which produce osteoclastogenesis promoting factors and thus contribute to the differentiation. AAP10 also affects gap junctions in the stromal cells. Acidosis (pH 6.5) leads to closure of gap junctions in both BM and PB monocytes as well as stromal cells, which decreases osteoclastogenesis. However, osteoclastogenesis is not totally inhibited due to other fusion mechanisms. In acidosis AAP10 is not able to restore the open state of the gap junctions. In BM cultures the osteoclastogenesis promoting factors produced by stromal cells maintain osteoclastogenesis at normal levels regardless of the gap junction closure. PB cultures lack the large number of stromal cells and thus their supportive effect in osteoclastogenesis, which leads to a decrease in the number of osteoclasts in the cultures.
FRAP analysis showed that AAP10 improves GJC in bone cells. The effect was shown with the osteoblast lineage MC3T3 cells which form a confluent, even layer of cells connected to each other, thus being suitable for the FRAP experiment and for the evaluation of the diffusion of the dye from cell to cell through gap junctions. We also tried FRAP with the BM and PB cultures, but due to the heterogeneous nature of these cultures, it was difficult to find the proper cells for bleaching. In addition, the osteoclast cultures in our study were conducted for 12 or 14 days and knowing the exact times of the cell fusions when GJC occurs during osteoclastogenesis turned out to be challenging. As mentioned earlier, GJC in osteoclastogenesis is not the main mechanism of fusion, therefore, finding the cells connected with gap junctions was difficult.
It is unclear whether the effects seen in our study are conducted between docked gap junctions or unopposed hemichannels. We also cannot exclude the possibility that the effects are caused solely by altered PKC-signaling, which has been proposed to be the main mechanism of action of AAPs. One explanation could be that only hemichannels are activated with AAP10, and this improves signaling between the cell and the extracellular environment, where also stromal cells contribute to the effects seen. The relatively high (100 μM) concentration needed to achieve our results has been noted also in other studies with gap junction modulators. Ponsaerts et al. [59] also used a concentration of 100 μM for modulation of Cx43 hemichannels. Iyyathurai et al. [60] suggest that the high concentration is needed for the cell-entry and even distribution of the peptide modulators inside the cell, and likely also reflect their low affinity in binding to their target regions. We performed also dose-response experiments with lower concentrations, but did not observe any effects with concentrations lower than 100 μM.
We have identified a possible problem in that the patients in our study do not match in age. The group donating the blood samples was younger on average compared to the group donating the bone marrow sample. Age might affect osteoclastogenesis, therefore, ideally, the study should have been made with age and sex matched groups. We would like to emphasize that the study is conducted with the valuable primary human samples from femur bone marrow requiring a highly invasive procedure, which limits the number of available samples.
BM and PB derived osteoclasts differ in morphology and the ability to resorb bone. Sprangers et al. have shown that the three PB monocyte subsets (classical, non-classical and intermediate monocytes) can differentiate into osteoclasts with distinct features and differing bone resorption activity [23]. In our study, the BM-derived osteoclasts were extremely robust in bone resorption compared to the PB-derived osteoclasts. We believe that our results support their hypothesis of classical monocytes from PB being the main source of osteoclasts, whereas intermediate monocytes from the BM would be activated in acidosis and to have an increased bone-resorbing capacity.
These BM and PB cultures differ in concentration of RANKL. RANKL has been shown to activate bone resorption in a concentration-dependent manner [61, 62, 63]. However, in studies conducted with mouse and rat cells, the resorption activity was saturated already at 10–20 ng/ml concentration and addition of 100 ng/ml [61] or even 500 ng/ml [63] RANKL did not further increase resorption. It has also been demonstrated that osteoclastogenesis and bone resorption are regulated by different factors [55]. Although it is well known that RANKL induces osteoclastogenesis and osteoclast survival, little is known about the effects of RANKL on mature osteoclasts. Fuller and Chambers demonstrated increased RANKL-induced resorption in mature mouse osteoclasts harvested from plastic substrate [55], but since their protocol differs greatly from ours, direct conclusions from mouse experiments cannot be made concerning human osteoclasts. We have noticed that while RANKL is essential in osteoclastogenesis from human monocytes, its influence in increasing bone resorption is less clear. Based on these facts, we chose to use two different culture protocols optimized for the monocytes derived from BM and PB. The RANKL concentrations in our cultures (50 ng/ml in BM and 20 ng/ml in PB) are at a level sufficient for osteoclastogenesis, but on the contrary not too apart to cause dramatic differences on bone resorption. However, further experiments are needed to discover the effects of RANKL on resorbing osteoclasts and their activation.
It is possible that for the final differentiation into bone-resorbing osteoclasts the precursors require molecules secreted by the stromal cells, which are present in the BM environment. Since we used TGF-β and dexamethasone in addition to RANKL and M-CSF only in BM cultures, the differences between the osteoclast types might also indicate the pronounced effect of these additional growth factors on osteoclastogenesis. Several studies have shown that RANKL and M-CSF alone are sufficient to induce osteoclastogenesis from PB monocytes [17, 18, 64], but other studies highlight the importance of TGF-β as a costimulating agent in osteoclastogenesis both from PB and BM monocytes [9, 10]. Susa et al. [9] presented the idea that RANKL and M-CSF induce mainly the differentiation of macrophage polykaryons rather than active osteoclasts, which could also be the explanation for our results.
5. Conclusion
This study shows that although the osteoclast cultures from human BM and PB have different features, GJC is presumably involved in osteoclastogenesis in both cultures. Modulation of GJC with AAP10 increases osteoclastogenesis in both cultures at physiological pH, but differences in the effects appear at low pH. This indicates that the monocytes from BM and PB respond differently to environmental changes, which might be explained by the presence of the osteoclastogenesis supporting stromal cells in BM cultures.
Declarations
Author contribution statement
Elina Kylmäoja, Veli-Pekka Ronkainen: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Miho Nakamura, Hanna Kokkonen-Puuperä, Juha Tuukkanen: Conceived and designed the experiments; Analyzed and interpreted the data; Wrote the paper.
Petri Lehenkari: Analyzed and interpreted the data; Wrote the paper.
Funding statement
This work was supported by the Sigrid Juselius Foundation, Finland, and the Oulu University Scholarship Foundation, Finland.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
The following persons are gratefully acknowledged for their contribution to this work: Miia Vierimaa and Minna Savilampi for the help with the bone marrow sample pre-culture. Biocenter Oulu electron microscopy core facility, a member of Biocenter Finland, is acknowledged for their assistance with electron microscopic analysis.
References
- 1.Teitelbaum S.L., Ross F.P. Genetic regulation of osteoclast development and function. Nat. Rev. Genet. 2003;4:638–649. doi: 10.1038/nrg1122. [DOI] [PubMed] [Google Scholar]
- 2.Xing L., Schwarz E.M., Boyce B.F. Osteoclast precursors, RANKL/RANK, and immunology. Immunol. Rev. 2005;208:19–29. doi: 10.1111/j.0105-2896.2005.00336.x. [DOI] [PubMed] [Google Scholar]
- 3.Udagawa N. Origin of osteoclasts: mature monocytes and macrophages are capable of differentiating into osteoclasts under a suitable microenvironment prepared by bone marrow-derived stromal cells. Proc. Natl. Acad. Sci. U. S. A. 1990;87:7260–7264. doi: 10.1073/pnas.87.18.7260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Quinn J.M., Sabokbar A., Athanasou N.A. Cells of the mononuclear phagocyte series differentiate into osteoclastic lacunar bone resorbing cells. J. Pathol. 1996;179:106–111. doi: 10.1002/(SICI)1096-9896(199605)179:1<106::AID-PATH535>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 5.Matsuzaki K. Osteoclast differentiation factor (ODF) induces osteoclast-like cell formation in human peripheral blood mononuclear cell cultures. Biochem. Biophys. Res. Commun. 1998;246:199–204. doi: 10.1006/bbrc.1998.8586. [DOI] [PubMed] [Google Scholar]
- 6.Shalhoub V. Characterization of osteoclast precursors in human blood. Br. J. Haematol. 2000;111:501–512. doi: 10.1046/j.1365-2141.2000.02379.x. [DOI] [PubMed] [Google Scholar]
- 7.Quinn J.M., Elliott J., Gillespie M.T., Martin T.J. A combination of osteoclast differentiation factor and macrophage-colony stimulating factor is sufficient for both human and mouse osteoclast formation in vitro. Endocrinology. 1998;139:4424–4427. doi: 10.1210/endo.139.10.6331. [DOI] [PubMed] [Google Scholar]
- 8.Yasuda H. Osteoclast differentiation factor is a ligand for osteoprotegerin/osteoclastogenesis-inhibitory factor and is identical to TRANCE/RANKL. Proc. Natl. Acad. Sci. U. S. A. 1998;95:3597–3602. doi: 10.1073/pnas.95.7.3597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Susa M., Luong-Nguyen N.H., Cappellen D., Zamurovic N., Gamse R. Human primary osteoclasts: in vitro generation and applications as pharmacological and clinical assay. J. Transl. Med. 2004;2:6. doi: 10.1186/1479-5876-2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fuller K., Lean J.M., Bayley K.E., Wani M.R., Chambers T.J. A role for TGFbeta(1) in osteoclast differentiation and survival. J. Cell Sci. 2000;113:2445–2453. doi: 10.1242/jcs.113.13.2445. PMID: 10852823. [DOI] [PubMed] [Google Scholar]
- 11.Houde N., Chamoux E., Bisson M., Roux S. Transforming growth factor-beta1 (TGF-beta1) induces human osteoclast apoptosis by up-regulating Bim. J. Biol. Chem. 2009;284:23397–23404. doi: 10.1074/jbc.M109.019372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Karst M., Gorny G., Galvin R.J., Oursler M.J. Roles of stromal cell RANKL, OPG, and M-CSF expression in biphasic TGF-beta regulation of osteoclast differentiation. J. Cell. Physiol. 2004;200:99–106. doi: 10.1002/jcp.20036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Quinn J.M. Transforming growth factor beta affects osteoclast differentiation via direct and indirect actions. J. Bone Miner. Res. 2001;16:1787–1794. doi: 10.1359/jbmr.2001.16.10.1787. [DOI] [PubMed] [Google Scholar]
- 14.Massey H.M., Flanagan A.M. Human osteoclasts derive from CD14-positive monocytes. Br. J. Haematol. 1999;106:167–170. doi: 10.1046/j.1365-2141.1999.01491.x. [DOI] [PubMed] [Google Scholar]
- 15.Nicholson G.C. Induction of osteoclasts from CD14-positive human peripheral blood mononuclear cells by receptor activator of nuclear factor kappaB ligand (RANKL) Clin. Sci. (Colch) 2000;99:133–140. [PubMed] [Google Scholar]
- 16.Komano Y., Nanki T., Hayashida K., Taniguchi K., Miyasaka N. Identification of a human peripheral blood monocyte subset that differentiates into osteoclasts. Arthritis Res. Ther. 2006;8:R152. doi: 10.1186/ar2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Costa-Rodrigues J., Fernandes A., Fernandes M.H. Spontaneous and induced osteoclastogenic behaviour of human peripheral blood mononuclear cells and their CD14(+) and CD14(−) cell fractions. Cell Prolif. 2011;44:410–419. doi: 10.1111/j.1365-2184.2011.00768.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hemingway F. In vitro generation of mature human osteoclasts. Calcif. Tissue Int. 2011;89:389–395. doi: 10.1007/s00223-011-9530-0. [DOI] [PubMed] [Google Scholar]
- 19.Wong K.L. Gene expression profiling reveals the defining features of the classical, intermediate, and nonclassical human monocyte subsets. Blood. 2011;118:e16–e31. doi: 10.1182/blood-2010-12-326355. [DOI] [PubMed] [Google Scholar]
- 20.Mandl M., Schmitz S., Weber C., Hristov M. Characterization of the CD14++CD16+ monocyte population in human bone marrow. PLoS One. 2014;9:e112140. doi: 10.1371/journal.pone.0112140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mizoguchi T. Identification of cell cycle-arrested quiescent osteoclast precursors in vivo. J. Cell Biol. 2009;184:541–554. doi: 10.1083/jcb.200806139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kotani M. Systemic circulation and bone recruitment of osteoclast precursors tracked by using fluorescent imaging techniques. J. Immunol. 2013;190:605–612. doi: 10.4049/jimmunol.1201345. [DOI] [PubMed] [Google Scholar]
- 23.Sprangers S., Schoenmaker T., Cao Y., Everts V., de Vries T.J. Different blood-borne human osteoclast precursors respond in distinct ways to IL-17A. J. Cell. Physiol. 2016;231:1249–1260. doi: 10.1002/jcp.25220. [DOI] [PubMed] [Google Scholar]
- 24.Ilvesaro J., Vaananen K., Tuukkanen J. Bone-resorbing osteoclasts contain gap-junctional connexin-43. J. Bone Miner. Res. 2000;15:919–926. doi: 10.1359/jbmr.2000.15.5.919. [DOI] [PubMed] [Google Scholar]
- 25.Ilvesaro J., Tavi P., Tuukkanen J. Connexin-mimetic peptide Gap 27 decreases osteoclastic activity. BMC Musculoskelet. Disord. 2001;2:10. doi: 10.1186/1471-2474-2-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schilling A.F. Gap junctional communication in human osteoclasts in vitro and in vivo. J. Cell. Mol. Med. 2008;12:2497–2504. doi: 10.1111/j.1582-4934.2008.00275.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Plotkin L.I., Stains J.P. Connexins and pannexins in the skeleton: gap junctions, hemichannels and more. Cell. Mol. Life Sci. 2015;72:2853–2867. doi: 10.1007/s00018-015-1963-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kylmaoja E. Osteoclastogenesis is influenced by modulation of gap junctional communication with antiarrhythmic peptides. Calcif. Tissue Int. 2013;92:270–281. doi: 10.1007/s00223-012-9680-8. [DOI] [PubMed] [Google Scholar]
- 29.Matemba S.F., Lie A., Ransjo M. Regulation of osteoclastogenesis by gap junction communication. J. Cell. Biochem. 2006;99:528–537. doi: 10.1002/jcb.20866. [DOI] [PubMed] [Google Scholar]
- 30.Pacheco-Costa R. High bone mass in mice lacking Cx37 because of defective osteoclast differentiation. J. Biol. Chem. 2014;289:8508–8520. doi: 10.1074/jbc.M113.529735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang Y. Enhanced osteoclastic resorption and responsiveness to mechanical load in gap junction deficient bone. PLoS One. 2011;6:e23516. doi: 10.1371/journal.pone.0023516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Watkins M. Osteoblast connexin43 modulates skeletal architecture by regulating both arms of bone remodeling. Mol. Biol. Cell. 2011;22:1240–1251. doi: 10.1091/mbc.E10-07-0571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Weng S. Pharmacological modification of gap junction coupling by an antiarrhythmic peptide via protein kinase C activation. FASEB J. 2002;16:1114–1116. doi: 10.1096/fj.01-0918fje. [DOI] [PubMed] [Google Scholar]
- 34.Axelsen L.N. Identification of ischemia-regulated phosphorylation sites in connexin43: a possible target for the antiarrhythmic peptide analogue rotigaptide (ZP123) J. Mol. Cell. Cardiol. 2006;40:790–798. doi: 10.1016/j.yjmcc.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 35.Stahlhut M., Petersen J.S., Hennan J.K., Ramirez M.T. The antiarrhythmic peptide rotigaptide (ZP123) increases connexin 43 protein expression in neonatal rat ventricular cardiomyocytes. Cell Commun. Adhes. 2006;13:21–27. doi: 10.1080/15419060600631375. [DOI] [PubMed] [Google Scholar]
- 36.Easton J.A., Petersen J.S., Martin P.E. The anti-arrhythmic peptide AAP10 remodels Cx43 and Cx40 expression and function. N. Schmied. Arch. Pharmacol. 2009;380:11–24. doi: 10.1007/s00210-009-0411-2. [DOI] [PubMed] [Google Scholar]
- 37.Jorgensen N.R. The antiarrhythmic peptide analog rotigaptide (ZP123) stimulates gap junction intercellular communication in human osteoblasts and prevents decrease in femoral trabecular bone strength in ovariectomized rats. Endocrinology. 2005;146:4745–4754. doi: 10.1210/en.2004-1414. [DOI] [PubMed] [Google Scholar]
- 38.Pfenniger A., Wohlwend A., Kwak B.R. Mutations in connexin genes and disease. Eur. J. Clin. Invest. 2011;41:103–116. doi: 10.1111/j.1365-2362.2010.02378.x. [DOI] [PubMed] [Google Scholar]
- 39.Willebrords J. Connexins and their channels in inflammation. Crit. Rev. Biochem. Mol. Biol. 2016;51:413–439. doi: 10.1080/10409238.2016.1204980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Castellano P., Eugenin E.A. Regulation of gap junction channels by infectious agents and inflammation in the CNS. Front. Cell. Neurosci. 2014;8:122. doi: 10.3389/fncel.2014.00122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Arboleya L., Castaneda S. Osteoimmunology: the study of the relationship between the immune system and bone tissue. Reumatol. Clin. 2013;9:303–315. doi: 10.1016/j.reuma.2013.02.008. [DOI] [PubMed] [Google Scholar]
- 42.Genetos D.C., Zhou Z., Li Z., Donahue H.J. Age-related changes in gap junctional intercellular communication in osteoblastic cells. J. Orthop. Res. 2012;30:1979–1984. doi: 10.1002/jor.22172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Clarke T.C., Thomas D., Petersen J.S., Evans W.H., Martin P.E. The antiarrhythmic peptide rotigaptide (ZP123) increases gap junction intercellular communication in cardiac myocytes and HeLa cells expressing connexin 43. Br. J. Pharmacol. 2006;147:486–495. doi: 10.1038/sj.bjp.0706631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dhein S. Improving cardiac gap junction communication as a new antiarrhythmic mechanism: the action of antiarrhythmic peptides. N. Schmied. Arch. Pharmacol. 2010;381:221–234. doi: 10.1007/s00210-009-0473-1. [DOI] [PubMed] [Google Scholar]
- 45.Sun B., Jiang J.F., Zhao C.M., Hu C.H. Effects of antiarrhythmic peptide 10 on acute ventricular arrhythmia. Asian Pac. J. Trop. Med. 2015;8:229–233. doi: 10.1016/S1995-7645(14)60321-7. [DOI] [PubMed] [Google Scholar]
- 46.Arnett T.R. Acidosis, hypoxia and bone. Arch. Biochem. Biophys. 2010;503:103–109. doi: 10.1016/j.abb.2010.07.021. [DOI] [PubMed] [Google Scholar]
- 47.Spray D.C., Harris A.L., Bennett M.V. Gap junctional conductance is a simple and sensitive function of intracellular pH. Science. 1981;211:712–715. doi: 10.1126/science.6779379. [DOI] [PubMed] [Google Scholar]
- 48.Eloff B.C., Gilat E., Wan X., Rosenbaum D.S. Pharmacological modulation of cardiac gap junctions to enhance cardiac conduction: evidence supporting a novel target for antiarrhythmic therapy. Circulation. 2003;108:3157–3163. doi: 10.1161/01.CIR.0000101926.43759.10. [DOI] [PubMed] [Google Scholar]
- 49.Kjolbye A.L., Knudsen C.B., Jepsen T., Larsen B.D., Petersen J.S. Pharmacological characterization of the new stable antiarrhythmic peptide analog Ac-D-Tyr-D-Pro-D-Hyp-Gly-D-Ala-Gly-NH2 (ZP123): in vivo and in vitro studies. J. Pharmacol. Exp. Ther. 2003;306:1191–1199. doi: 10.1124/jpet.103.052258. [DOI] [PubMed] [Google Scholar]
- 50.Kato K., Morita I. Acidosis environment promotes osteoclast formation by acting on the last phase of preosteoclast differentiation: a study to elucidate the action points of acidosis and search for putative target molecules. Eur. J. Pharmacol. 2011;663:27–39. doi: 10.1016/j.ejphar.2011.04.062. [DOI] [PubMed] [Google Scholar]
- 51.Ahn H., Kim J.M., Lee K., Kim H., Jeong D. Extracellular acidosis accelerates bone resorption by enhancing osteoclast survival, adhesion, and migration. Biochem. Biophys. Res. Commun. 2012;418:144–148. doi: 10.1016/j.bbrc.2011.12.149. [DOI] [PubMed] [Google Scholar]
- 52.Arnett T.R. Extracellular pH regulates bone cell function. J. Nutr. 2008;138:415S–418S. doi: 10.1093/jn/138.2.415S. [DOI] [PubMed] [Google Scholar]
- 53.Meghji S., Morrison M.S., Henderson B., Arnett T.R. pH dependence of bone resorption: mouse calvarial osteoclasts are activated by acidosis. Am. J. Physiol. Endocrinol. Metab. 2001;280:E112–E119. doi: 10.1152/ajpendo.2001.280.1.E112. [DOI] [PubMed] [Google Scholar]
- 54.Ludwig M.G. Proton-sensing G-protein-coupled receptors. Nature. 2003;425:93–98. doi: 10.1038/nature01905. [DOI] [PubMed] [Google Scholar]
- 55.Fuller K., Kirstein B., Chambers T.J. Murine osteoclast formation and function: differential regulation by humoral agents. Endocrinology. 2006;147:1979–1985. doi: 10.1210/en.2005-1340. [DOI] [PubMed] [Google Scholar]
- 56.Haugan K. The antiarrhythmic peptide analog ZP123 prevents atrial conduction slowing during metabolic stress. J. Cardiovasc. Electrophysiol. 2005;16:537–545. doi: 10.1111/j.1540-8167.2005.40687.x. [DOI] [PubMed] [Google Scholar]
- 57.Wang R., Zhang C., Ruan Y., Liu N., Wang L. Changes in phosphorylation of connexin43 in rats during acute myocardial hypoxia and effects of antiarrhythmic peptide on the phosphorylation. J. Huazhong Univ. Sci. Technol. Med. Sci. 2007;27:241–244. doi: 10.1007/s11596-007-0306-8. [DOI] [PubMed] [Google Scholar]
- 58.Phan T.C., Xu J., Zheng M.H. Interaction between osteoblast and osteoclast: impact in bone disease. Histol. Histopathol. 2004;19:1325–1344. doi: 10.14670/HH-19.1325. [DOI] [PubMed] [Google Scholar]
- 59.Ponsaerts R. Intramolecular loop/tail interactions are essential for connexin 43-hemichannel activity. FASEB J. 2010;24:4378–4395. doi: 10.1096/fj.09-153007. [DOI] [PubMed] [Google Scholar]
- 60.Iyyathurai J. Peptides and peptide-derived molecules targeting the intracellular domains of Cx43: gap junctions versus hemichannels. Neuropharmacology. 2013;75:491–505. doi: 10.1016/j.neuropharm.2013.04.050. [DOI] [PubMed] [Google Scholar]
- 61.Lacey D.L. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell. 1998;93:165–176. doi: 10.1016/s0092-8674(00)81569-x. [DOI] [PubMed] [Google Scholar]
- 62.Burgess T.L. The ligand for osteoprotegerin (OPGL) directly activates mature osteoclasts. J. Cell Biol. 1999;145:527–538. doi: 10.1083/jcb.145.3.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Li J. RANK is the intrinsic hematopoietic cell surface receptor that controls osteoclastogenesis and regulation of bone mass and calcium metabolism. Proc. Natl. Acad. Sci. U. S. A. 2000;97:1566–1571. doi: 10.1073/pnas.97.4.1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sørensen M.G. Characterization of osteoclasts derived from CD14+ monocytes isolated from peripheral blood. J. Bone Miner. Metab. 2007;25:36–45. doi: 10.1007/s00774-006-0725-9. [DOI] [PubMed] [Google Scholar]