Supplemental Digital Content is available in the text
Keywords: CAP, cART, HIV monoinfection, liver injury, NAFLD
Abstract
Prevalence and risk factors for hepatic steatosis (HS) in the human immunodeficiency virus (HIV)-positive population of western countries are controversially discussed and potentially confounded by coinfection with viral hepatitis. Significant HS (more than 10% of hepatocytes) can be accurately assessed using controlled attenuation parameter (CAP) determination. Aim of this study was to assess prevalence and factors associated with significant HS in HIV monoinfected patients.
A total of 364 HIV-infected patients (289 monoinfected) were included in this prospective, cross-sectional study. All patients underwent CAP determination. Steatosis was classified as S1 (significant steatosis) with CAP > 238 dB/m, S2 with CAP > 260 dB/m, and S3 with CAP > 292 dB/m. Multivariable logistic regression analyses were performed to assess the factors associated with HS in this cohort.
Significant HS was detected in 118 monoinfected patients (149 in the total cohort). In the total cohort as well as in the monoinfected patients alone, HS grade distribution showed a similar pattern (S1:29%, S2:34%, and S3:37%). Interestingly, patients with HS had a longer history of HIV infection and combined antiretroviral therapy (cART). Interalia, age, gender, ethnicity, and metabolic factors were strongly associated with HS, while body mass index (BMI), triglyceride, and glycated hemoglobin (HbA1c) levels were independently associated with significant HS.
HS is highly prevalent among HIV monoinfected patients. Although metabolic risk factors, such as obesity and poorly controlled diabetes, are independently associated with HS in HIV monoinfected patients, cART and control of HIV seem to play an indirect role in the development of HS, probably through the return-to-health effect.
1. Introduction
Present day combined antiretroviral therapy (cART), leading to permanent suppression of human immunodeficiency virus (HIV) replication, has dramatically improved survival of HIV-infected patients. Chronic liver disease has emerged as an increasingly significant contributor to nonacquired immune deficiency syndrome related morbidity and mortality in the HIV-infected population.[1] Independent of coinfection with hepatotropic viruses, increased rates of liver fibrosis and steatosis have been observed.[5–7] On the one hand, HIV induces metabolic changes; on the other hand, long-term exposure to antiretroviral drugs may exert toxicities (in particular mitochondrial toxicity, dyslipidemia, and insulin resistance)[2–4,33], which may further contribute to the development of clinically relevant liver disease.[8–10] Hepatic steatosis (HS) may then progress to nonalcoholic steatohepatitis (NASH), an emerging cause of hepatocellular carcinoma.[11] Furthermore, liver steatosis may predispose to acute liver failure, especially when those patients are exposed to additional hepatic risk factors.[12]
Little information is available on the prevalence and potential risk factors for liver steatosis among HIV monoinfected patients. Indeed, most of the currently existing data on HS solely derives from HIV/hepatitis C virus (HCV) coinfected patients. In these coinfected patients, the prevalence rate for HS ranges between 20% and 80%.[6,11,13,14] In a recently published, large Canadian study with more than 22% coinfected patients, no difference in the grade of steatosis was observed between co- and monoinfected patients.[15] Importantly, coinfection with HCV is a strong confounder for HS, not only due to changes in the measurement as a result of inflammation and fibrosis, but also because it is dependent on the HCV genotype.
With regard to risk factors promoting steatosis in HIV infection, little is known and this issue is controversially discussed.[16–22] Steatosis is mostly associated with metabolic risk factors (obesity, diabetes, and dyslipidemia) in the general population and might lead to severe liver disease. In HIV-positive population, the metabolic risk factors still play a role for the development of steatosis, the impact of long-term exposure to antiretroviral regimes for liver injury and specifically for steatosis has also been discussed. A recently published meta-analysis demonstrated that body mass index (BMI), waist circumference, diabetes mellitus (DM), hypertension, triglycerides, and cluster of differentiation 4 (CD4) cell count were associated with nonalcoholic fatty liver disease (NAFLD), whereas HIV specific parameters like HIV viral load, duration of HIV, duration of cART, and nadir CD4 count were not.[23]
This study aimed to examine in more detail the prevalence and risk factors for severe HS in a large cohort of HIV monoinfected patients from the Infectious Diseases outpatient clinic at Bonn University.
2. Methods
2.1. Patients
This cross-sectional prospective study included 364 HIV-infected patients followed at the Infectious Diseases outpatient clinic at Bonn University. Patients with confirmed HIV infection and self-reported alcohol intake of no more than 30 g/day (male participants) or 20 g/day (female participants) were eligible for inclusion. The use of anabolics or hormons was not reported by patients but would have exclusion criteria. To assess the potential effect of concomitant HCV or hepatitis B virus coinfection, as well as to minimize the potential effect of confounders, analyses were performed on the total study population and the group of 289 HIV monoinfected patients (Fig. 1A). Blood collection and controlled attenuation parameter (CAP) measurement were performed during a regular scheduled visit at our outpatient clinic. Informed consent was obtained from each patient included in the study and the study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in the approval by the institution's human research committee (279/14).
Figure 1.
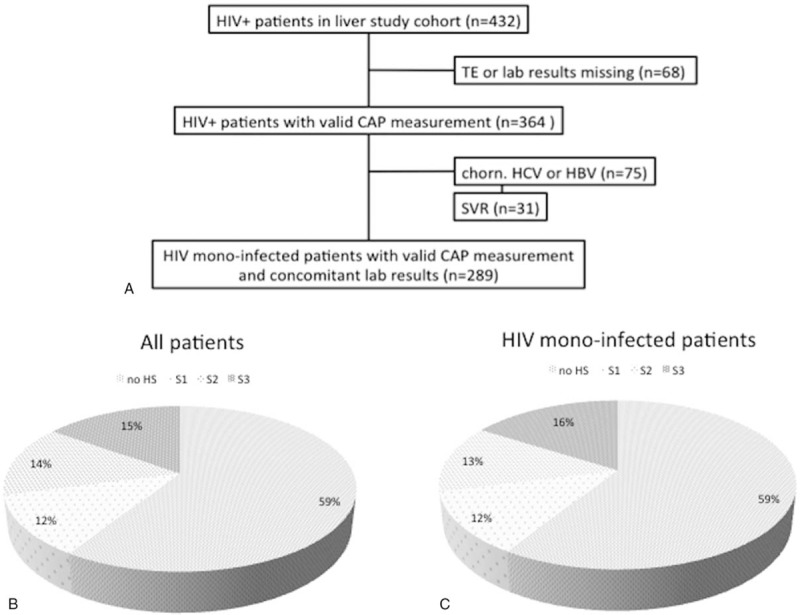
Flow chart displaying selection of study participants (A). Prevalence and distribution of hepatic steatosis in all patients (B) and in HIV monoinfected patients (C).
2.2. Data acquisition and follow-up
Laboratory work-up of metabolic (glycated hemoglobin [HbA1c], cholesterol, low-density lipoprotein, high-density lipoprotein [HDL], triglycerides, transaminases, bilirubin, creatinine, platelets, albumin, and thyroid-stimulating hormone), inflammatory/immunologic (c-reactive protein, CD4, and CD8), and HIV-relevant disease markers (viral load) was performed at the same time as transient elastography. Demographic and clinical characteristics as well as data on health trajectory of HIV infection and antiretroviral treatments were collected from the patient's health record and our department's database. Current comedication, alcohol consumption, and smoking status were recorded and the actual BMI was assessed.
2.3. Hepatic steatosis measurement
CAP measures the degree of ultrasound attenuation by hepatic fat at the central frequency of the FibroScan M probe, simultaneously with liver stiffness measurement. The technique can establish presence of HS affecting more than 10% of the hepatocytes in the liver biopsy[24] and has been validated in different groups of patients.[25–27]
CAP measurement was assessed using FibroScan 502 touch (ECHOSENS, France) performed by experienced and trained operators. According to standards, examinations with at least 10 valid measurements, a low variability defined as an interquartile range (IQR/M) smaller than 30% of the median value, and a successful acquisition rate of at least 60% were considered reliable and were approved for analysis.[28]
The optimal threshold of CAP for the detection of HS is unknown in NAFLD. With values above 238 dB/m, we selected a conservative cut-off to define beginning significant HS (S1).[24,25] As a solid criteria for the prevalence of clinically apparent, advanced steatosis, cut-off values of 260 dB/m (S2) and 292 dB/m (S3) were applied.
2.4. Statistical analysis
Demographic, clinical, and laboratory characteristics stratified by severity of HS were compared. Differences in continuous variables, expressed as medians, and 1st and 3rd quartiles were assessed using either nonparametric Mann–Whitney test or Kruskal–Wallis test. Categorical variables, expressed as absolute frequencies and percentages, were compared using Pearson chi squared test or Fisher exact tests. A correlation analysis estimated possible impacts on HS. Uni- and multivariable analyses were performed using logistic and Cox-regression models. SPSS version 22 (IBM Corporation, Armonk, NY) was used for statistical analysis.
3. Results
3.1. General characteristics of the cohort
In total, 364 patients were included in the study. The majority was male (78%), Caucasian (76%), with a mean age of 46 (range, 20–75) years. The baseline characteristics of the study subjects are shown in Table 1.
Table 1.
Baseline characteristics.
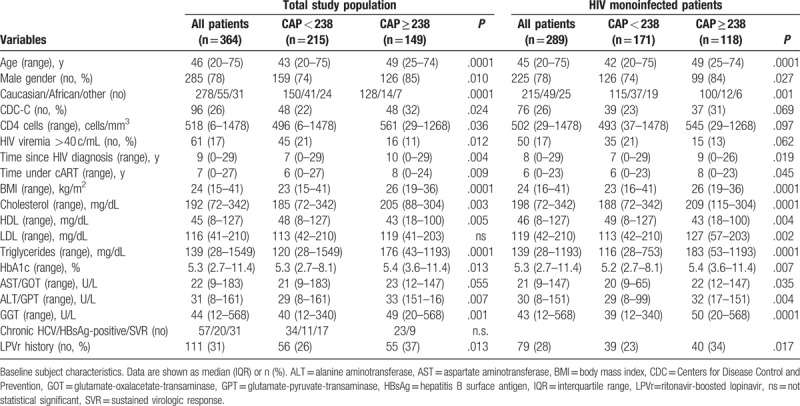
Two hundred and eighty seven patients (79%) were HIV monoinfected, 20 (6%) patients were hepatitis B surface antigen-positive, 57 (16%) were anti-HCV-positive, of which 31 (54%) had achieved sustained virologic response, 17 (31%) were HCV therapy naïve, and 9 (15%) either relapsed or were nonresponder to previously administered HCV treatments. Most of the patients (95%) were treated with cART and showed undetectable plasma HIV ribonucleic acid (83%). Ninety six (26%) patients were classified as Centers for Disease Control and Prevention category C. The mean CD4+ T cell count was 546 (range, 6–1478) cells/mm3.
Overweight, defined by a BMI of at least 25 and less than 30 kg/m2, was observed in 93 (32%) and obesity, defined by a BMI of at least 30 kg/m2, in 16 (6%) HIV monoinfected individuals (Supplemental Table 2). Twelve (4%) HIV monoinfected patients had the diagnosis of DM, with 9 (75%) receiving antidiabetic treatment and 10 (4%) presenting HbA1c levels greater than 6.5%. Blood triglycerides of at least 150 mg/dL were detected in 125 (43%), and blood HDL cholesterol levels less than 40 mg/dL were observed in 63 (22%) HIV monoinfected patients.
3.2. Prevalence of hepatic steatosis
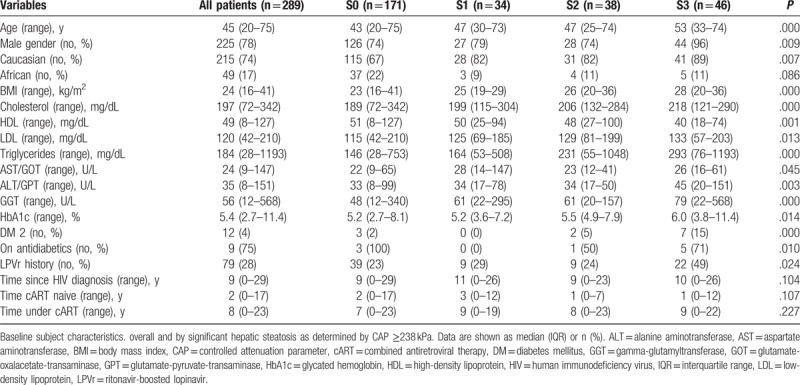
Median CAP was 230 (range, 100–367) dB/m and 229 dB/m when including coinfected patients. Significant HS, that is, CAP ≥238 dB/m, was observed in 118 (41%) patients, of whom 34 (29%) had S1, 38 (32%) S2, and 46 (39%) S3. No significant difference in prevalence and distribution (41%, and 29%, 34%, 37%, respectively) of HS was observed in the coinfected patients (Table 2, Supplementary Table 1).
Table 2.
HIV monoinfected population.
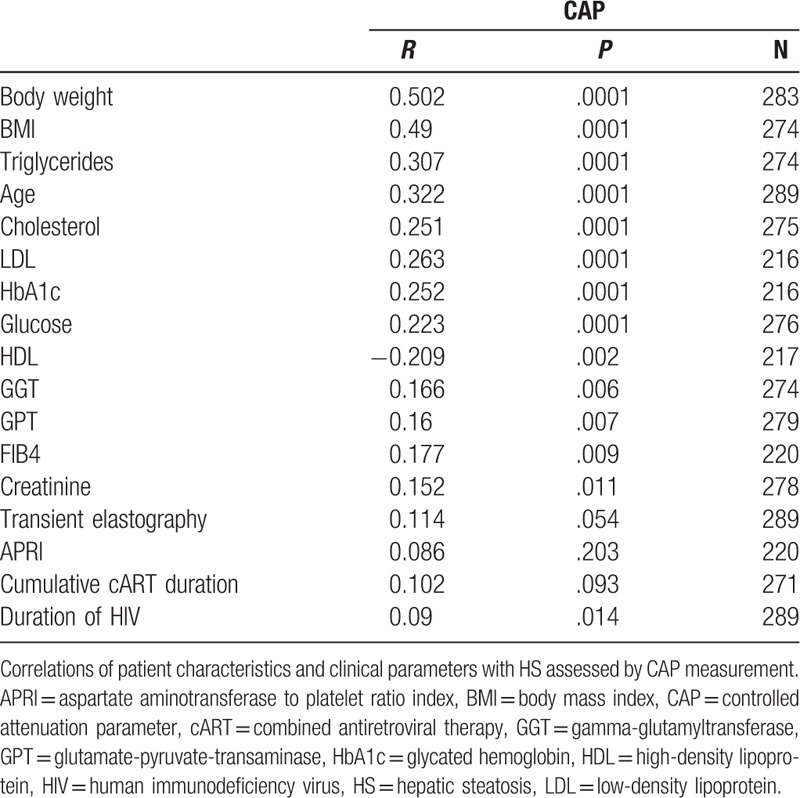
Age, metabolic conditions (BMI, cholesterol, HDL, LDL, levels of triglyceride, glucose, and HbA1c), and markers of liver injury (alanine aminotransferase, gamma-glutamyltransferase) correlated with altered CAP measurement, whereas HIV viral load showed an inverse trend. Interestingly, the total duration of HIV infection and the duration of cART showed positive correlations with HS (Table 3). Similar findings were obtained when considering the whole cohort (Supplemental Table 3).
Table 3.
Associations of CAP with different variables in HIV monoinfected patients.
As expected, patients with significant steatosis showed higher mean HbA1c levels (5.4 [3.6–11.4] vs5.2 [2.7–8.1] %; P = .007), higher mean BMI (26 [19–36] vs 23 [16–41]kg/m2; P < .001), higher triglyceride levels (183 [53–1193] vs 116 [28–753]mg/dL; P < .001), and lower HDL cholesterol (43 [18–100] vs 49 [8–127] mg/dL; P = .004) compared to patients without steatosis. DM, gender, and ethnicity revealed a significant impact on HS (Table 1). Interestingly, apart from gender, ethnicity also revealed a significant difference in median CAP values (Fig. 2A–D), with higher levels in males and in Caucasians.
Figure 2.
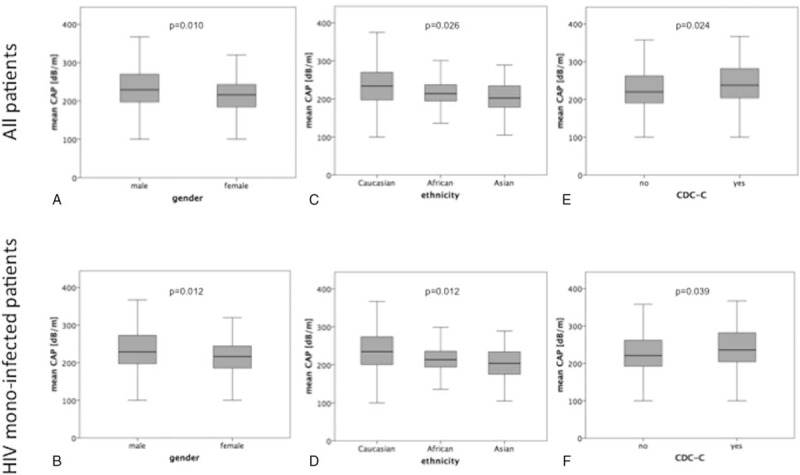
Association between hepatic steatosis and gender, ethnicity, and CDC-C in all patients (A, C, E, respectively) and in HIV-monoinfected patients (B, D, F, respectively). Data are presented as box plots. CDC = centers for disease control and prevention.
More severe steatosis was observed in patients with longer duration of known HIV infection (9 [0–26] vs 7 [0–29] years; P = .019], although uncontrolled HIV infection (viral load ≥40 copies/mL) did not have a statistically significant influence on CAP values (Table 2). However, patients in Centers for Disease Control and Prevention class C had higher HS levels than patients without acquired immune deficiency syndrome-defining events (P = .039) (Fig. 1E, F).
In contrast to liver fibrosis development, no significant differences in HS were observed regarding anti-HCV positivity (222 dB/m), sustained virologic response (238 dB/m), hepatitis B surface antigen positivity (239 dB/m), and HIV monoinfected patients (229 dB/m).
3.3. Factors associated with hepatic steatosis
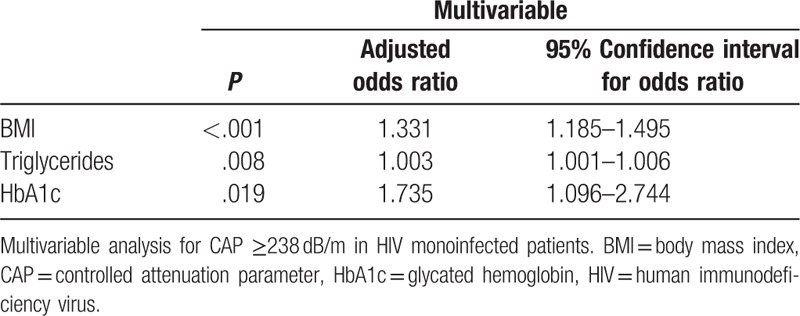
Logistic regression analysis revealed that many demographic (age, gender, and ethnicity), metabolic (BMI, triglyceride, DM type 2, HbA1c, cholesterol, HDL, and LDL), and hepatic parameters (aminotransferase and gamma-glutamyltransferase levels), but not time since HIV diagnosis, were significantly associated with significant HS. More parameters were found when considering the whole cohort, suggesting that coinfection might bias the analysis of risk factors for the development of significant steatosis in HIV infection (Supplemental Table 4). Subsequent multivariable analysis identified only BMI, HbA1c, and triglyceride levels as independently associated with significant HS (Table 4).
Table 4.
Logistic regression.
Regarding antiretroviral therapy only ritonavir-boosted lopinavir (LPV/r) was associated with the development of liver steatosis. In the current study, no other PIs nor other antiretroviral drug classes could be identified to have an impact on the severity of steatosis. The effect of the duration of HIV infection and cART duration mainly seems to depend on metabolic changes (BMI, HbA1c, and triglycerides), which in HIV monoinfected patients, are strongly and independently associated with HS, while in the whole population, age also seems to play an important role (Supplemental Table 4).
4. Discussion
Our findings outline that significant HS is highly prevalent in HIV-infected patients. Using CAP-derived data from a large and unselected real-life cohort, this cross-sectional study is likely to be more accurate than previous estimations.
Most published data on the prevalence of HS in HIV-infected patients were obtained from liver biopsy studies in HIV/HCV coinfected patients.[11,13,14] Even recently published data from a prospective cohort holds a bias by including HCV coinfected patients, as depending on the genotype, HCV might or might not predispose to HS.[29] Usually, these patients have severe fibrosis, which impairs the CAP measurement.[30] Our study clearly focused on HIV monoinfected patients to make the cohort less heterogeneous than previous studies. Although this study is not primarily designed to compare mono- and coinfected patients, we found evidence that many HIV-specific factors, which significantly correlate with CAP, are found only in the coinfected patients, such as transient elastography, aspartate aminotransferase to platelet ratio index score, HIV viral load, and duration of cART. These factors are strongly associated with liver fibrosis and inflammation, which is another bias present in our previous studies.[7,31]
Other studies using liver biopsy, which is an invasive diagnostic tool and not applicable for screening purpose, hold a significant selection bias. Steatosis involving at least 10% of hepatocytes can be accurately detected applying a CAP cut-off of 238 dB/m.[24,25] Significant steatosis was found in 41% of our study population, with an emphasis on S2 and S3 steatosis. This is in line with 2 previously published CAP-based studies rendering the prevalence of significant HS in HIV-infected patients to about 30% to 40%.[15,17,18] Nevertheless, these data were either derived from a much smaller cohort or included 20% to 50% patients with concomitant chronic HCV infection. The 60% prevalence rate of HS determined in a liver biopsy study, including 14 HIV monoinfected patients with persistent liver enzyme elevations, seems overestimated. Nevertheless, it showed that 26% of HIV-infected patients without viral hepatitis, DM, or alcohol use fulfilled the histological criteria for NASH.[22]
In the general population, increased BMI, insulin resistance, and dyslipidemia are the main risk factors for NAFLD and NASH.[32] NAFLD is characterized by greater hepatic uptake rates of plasma fatty acids as a consequence of an increased release from an expanded mass of adipose tissue with diminished insulin responsiveness. Furthermore, hyperinsulinemia promotes the upregulation of de novo lipogenesis in the liver.[32] In our study, metabolic factors were strongly related to steatosis development. Nevertheless, we observed higher rates of significant HS compared to the general population, where prevalence amounts up to 25%.[32] HIV itself induces metabolic changes, while long-term exposure to antiretroviral drugs may also exert toxicities, which could further contribute to the development of clinically relevant liver disease.[8–10] Nucleoside analogue reverse transcriptase inhibitors were in particular associated with mitochondrial toxicity.[34] Impaired beta-oxidation of fatty acids leads to increased hepatic lipid accumulation. Existing studies have found no relationship between HS and individual antiretroviral drugs.[6,13–16] However, our data show that LPV/r has a significant impact on the severity of steatosis. Whether this is due to a direct drug effect or a secondary effect by the metabolic changes induced by PIs cannot be determined with certainty.
Importantly, HIV-infected patients show an increase in BMI shortly after starting cART. Especially those with a lower nadir CD4 T-cell count, gained more lean mass and fat during the first 96 weeks of cART.[35] This is consistent with our findings, namely that a longer time since HIV diagnosis and longer periods of cART are associated with presence of HS. However, these were not independent associations but could predispose the patients to metabolic changes, which finally were independent and strongly associated with presence of HS. Possibly, the return-to-health effect of cART is one of the reasons for the presence of HS in HIV monoinfected patients. This is also supported by our previous data, showing that cART and control of HIV replication is protective for liver fibrosis and liver injury in HIV monoinfected patients. However, in these patients, HS might be induced by other mechanisms and not be the result of direct hepatic effects of HIV or cART. This may be supported by the finding of a gender and ethnicity influence on CAP-value, despite the fact that these patient groups were randomly distributed among the population.
The main limitations of this study are its cross-sectional design, which limits the ability to evaluate change in variables of interest over time, and the presence of a nonrandomized study population. Homeostasis model assessment index to quantify insulin resistance and dual-energy X-ray absorptiometry or magnetic resonance tomography imaging to quantify body composition changes were not available. Alcohol consumption as well as anabolic use was self-reported by the patients, for example, binge drinking behavior may have been underestimated. No drug and anabolic screening tests were performed. Finally, while liver biopsy remains the gold standard for the assessment of HS, the acceptability and potential risks of biopsy make its application difficult for a large study population.
5. Conclusions
In conclusion, we found that prevalence of HS in HIV-infected patients is higher than in the general population. Overweight, dyslipidemia, and poorly controlled diabetes were independent factors for HS in HIV monoinfected patients. Suppression of viral replication might contribute to the development of steatosis. Controlling HIV replication might not condition liver disease by causing metabolic disorders, instead, this may be due to the return-to-health effect.
Acknowledgments
The authors thank Arite Eicker, Svetlana Hass, and Nadine Owsiany for excellent technical assistance and Sabine Dentler for critical reading.
Author contributions
Study concept and design, acquisition of data, analysis and interpretation of data, drafting of the manuscript, statistical analysis: Raphael Mohr.
Acquisition of data, analysis and interpretation of data, drafting of the manuscript, statistical analysis: Christoph Boesecke
Acquisition of data, analysis and interpretation of data, critical revision of the manuscript regarding important intellectual content: Leona Dold.
Acquisition of data, analysis and interpretation of data, critical revision of the manuscript regarding important intellectual content: Robert Schierwagen.
CS: acquisition of data, analysis and interpretation of data, critical revision of the manuscript regarding important intellectual content: Carolynne Schwarze-Zander.
JCW: acquisition of data, analysis and interpretation of data, critical revision of the manuscript regarding important intellectual content: Jan-Christian Wasmuth.
Acquisition of data, analysis and interpretation of data, critical revision of the manuscript regarding important intellectual content: Insa Weisensee.
Study concept and design, acquisition of data, analysis and interpretation of data, critical revision of the manuscript regarding important intellectual content, funding recipient, study supervision: Jürgen Kurt Rockstroh.
Study concept and design, acquisition of data, analysis and interpretation of data, drafting of the manuscript, critical revision of the manuscript regarding important intellectual content, funding recipient, administrative, technical and material support, study supervision: Jonel Trebicka.
Data curation: Raphael Mohr, Robert Schierwagen, Carolynne Schwarze-Zander, Jan-Christian Wasmuth, Insa Weisensee, Jürgen Kurt Rockstroh.
Formal analysis: Raphael Mohr, Christoph Boesecke, Leona Dold, Robert Schierwagen, Carolynne Schwarze-Zander, Jan-Christian Wasmuth, Insa Weisensee.
Investigation: Raphael Mohr, Leona Dold, Carolynne Schwarze-Zander, Jan-Christian Wasmuth, Insa Weisensee.
Writing – original draft: Raphael Mohr, Jonel Trebicka.
Writing – review & editing: Raphael Mohr, Christoph Boesecke, Leona Dold, Robert Schierwagen, Carolynne Schwarze-Zander, Jan-Christian Wasmuth, Jürgen Kurt Rockstroh.
Validation: Christoph Boesecke, Jürgen Kurt Rockstroh.
Methodology: Leona Dold, Robert Schierwagen, Carolynne Schwarze-Zander, Jan-Christian Wasmuth, Insa Weisensee.
Supervision: Insa Weisensee, Jürgen Kurt Rockstroh, Jonel Trebicka.
Conceptualization: Jürgen Kurt Rockstroh, Jonel Trebicka.
Funding acquisition: Jürgen Kurt Rockstroh, Jonel Trebicka.
Resources: Jürgen Kurt Rockstroh, Jonel Trebicka.
Software: Jürgen Kurt Rockstroh.
Project administration: Jonel Trebicka.
Supplementary Material
Footnotes
Abbreviations: CAP = controlled attenuation parameter, cART = combined antiretroviral therapy, CD4 = cluster of differentiation 4, DM = diabetes mellitus, HbA1c = glycated hemoglobin, HCV = hepatitis C virus, HDL = high-density lipoprotein, HIV = human immunodeficiency virus, HS = hepatic steatosis, NAFLD = nonalcoholic fatty liver disease, NASH = nonalcoholic steatohepatitis.
JKR and JT shared last coauthorship.
Ethics approval and consent to participate: Informed consent was obtained from each patient included in the study and the study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in the approval by the Ethics Committee of the University of Bonn (279/14).
Availability of data and materials: The datasets are available from the corresponding author on reasonable request.
Funding/support: The study was supported by the German Center for Infection Research (DZIF to JKR), Deutsche Forschungsgemeinschaft (SFB TRR57 P18 to JT), and Cellex Foundation (to JT).
The authors have no conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
References
- [1].Palella FJ, Baker RK, Moorman AC, et al. Mortality in the highly active antiretroviral therapy era: changing causes of death and disease in the HIV outpatient study. J Acquir Immune Defic Syndr 2006;43:27–34. [DOI] [PubMed] [Google Scholar]
- [2].Hernando V, Perez-Cachafeiro S, Lewden C, et al. All-cause and liver-related mortality in HIV positive subjects compared to the general population: differences by HCV co-infection. J Hepatol 2012;57:743–51. [DOI] [PubMed] [Google Scholar]
- [3].Alejos B, Hernando V, López-Aldeguer J, et al. Overall and cause-specific mortality in HIV-positive subjects compared to the general population. J Int AIDS Soc 2014;17:19711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Weber R, Sabin CA, Friis-Møller N, et al. Liver-related deaths in persons infected with the human immunodeficiency virus: the D:A:D study. Arch Intern Med 2006;166:1632–41. [DOI] [PubMed] [Google Scholar]
- [5].Mallet VO, Sultanik PS, Vallet-Pichard A, et al. HIV-associated obliterative portopathy (HIV-OP) underlies cryptogenic liver disease in HIV-seropositive patients. HIV Med 2010;11:540–1. [DOI] [PubMed] [Google Scholar]
- [6].Machado MV, Oliveira AG, Cortez-Pinto H. Hepatic steatosis in patients coinfected with human immunodeficiency virus/hepatitis C virus: a meta-analysis of the risk factors. Hepatology 2010;52:71–8. [DOI] [PubMed] [Google Scholar]
- [7].Mohr R, Schierwagen R, Schwarze-Zander C, et al. Liver fibrosis in HIV patients receiving a modern cART: which factors play a role? Medicine 2015;94:e2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Maida I, Núñez M, Ríos MJ, et al. Severe liver disease associated with prolonged exposure to antiretroviral drugs. J Acquir Immune Defic Syndr 2006;42:177–82. [DOI] [PubMed] [Google Scholar]
- [9].Bongiovanni M, Tordato F. Steatohepatitis in HIV-infected subjects: pathogenesis, clinical impact and implications in clinical management. Curr HIV Res 2007;5:490–8. [DOI] [PubMed] [Google Scholar]
- [10].Guaraldi G, Squillace N, Stentarelli C, et al. Nonalcoholic fatty liver disease in HIV-infected patients referred to a metabolic clinic: prevalence, characteristics, and predictors. Clin Infect Dis 2008;47:250–7. [DOI] [PubMed] [Google Scholar]
- [11].Macías J, Berenguer J, Japón MA, et al. Hepatic steatosis and steatohepatitis in human immunodeficiency virus/hepatitis C virus-coinfected patients. Hepatology 2012;56:1261–70. [DOI] [PubMed] [Google Scholar]
- [12].Kahraman A, Miller M, Gieseler RK, et al. Non-alcoholic fatty liver disease in HIV-positive patients predisposes for acute-on-chronic liver failure: two cases. Eur J Gastroenterol Hepatol 2006;18:101–5. [DOI] [PubMed] [Google Scholar]
- [13].Gaslightwala I, Bini EJ. Impact of human immunodeficiency virus infection on the prevalence and severity of steatosis in patients with chronic hepatitis C virus infection. J Hepatol 2006;44:1026–32. [DOI] [PubMed] [Google Scholar]
- [14].Woreta TA, Sutcliffe CG, Mehta SH, et al. Incidence and risk factors for steatosis progression in adults coinfected with HIV and hepatitis C virus. Gastroenterology 2011;140:809–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Pembroke T, Deschenes M, Lebouche B, et al. Hepatic steatosis progresses faster in HIV mono-infected than HIV/HCV co-infected patients and is associated with liver fibrosis. J Hepatol 2017;67:801–8. [DOI] [PubMed] [Google Scholar]
- [16].Crum-Cianflone N, Dilay A, Collins G, et al. Nonalcoholic fatty liver disease among HIV-infected persons. J Acquir Immune Defic Syndr 2009;50:464–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Macías J, Gonzalez J, Tural C, et al. Prevalence and factors associated with liver steatosis as measured by transient elastography with controlled attenuation parameter in HIV-infected patients. AIDS 2014;28:1279–87. [DOI] [PubMed] [Google Scholar]
- [18].Sulyok M, Makara M, Rupnik Z, et al. Hepatic steatosis in individuals living with HIV measured by controlled attenuation parameter: a cross-sectional study. Eur J Gastroenterol Hepatol 2015;27:679–85. [DOI] [PubMed] [Google Scholar]
- [19].Sonderup MW, Wainwright H, Hall P, et al. A clinicopathological cohort study of liver pathology in 301 patients with human immunodeficiency virus/acquired immune deficiency syndrome. Hepatology 2015;61:1721–9. [DOI] [PubMed] [Google Scholar]
- [20].Sebastiani G, Rollet-Kurhajec KC, Pexos C, et al. Incidence and predictors of hepatic steatosis and fibrosis by serum biomarkers in a large cohort of human immunodeficiency virus mono-infected patients. Open Forum Infect Dis 2015;2:ofv015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Hoffmann CJ, Hoffmann JD, Kensler C, et al. Tuberculosis and hepatic steatosis are prevalent liver pathology findings among HIV-infected patients in South Africa. PLoS One 2015;10:e0117813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Sterling RK, Smith PG, Brunt EM. Hepatic steatosis in human immunodeficiency virus: a prospective study in patients without viral hepatitis, diabetes, or alcohol abuse. J Clin Gastroenterol 2013;47:182–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Maurice JB, Patel A, Scott AJ, et al. Prevalence and risk factors of nonalcoholic fatty liver disease in HIV-monoinfection. AIDS 2017;31:1621–32. [DOI] [PubMed] [Google Scholar]
- [24].Sasso M, Miette V, Sandrin L, et al. The controlled attenuation parameter (CAP): a novel tool for the noninvasive evaluation of steatosis using Fibroscan. Clin Res Hepatol Gastroenterol 2012;36:13–20. [DOI] [PubMed] [Google Scholar]
- [25].De Lédinghen V, Vergniol J, Foucher J, et al. Noninvasive diagnosis of liver steatosis using controlled attenuation parameter (CAP) and transient elastography. Liver Int 2012;32:911–8. [DOI] [PubMed] [Google Scholar]
- [26].Myers RP, Pollett A, Kirsch R, et al. Controlled Attenuation Parameter (CAP): a noninvasive method for the detection of hepatic steatosis based on transient elastography. Liver Int 2012;32:902–10. [DOI] [PubMed] [Google Scholar]
- [27].Friedrich-Rust M, Romen D, Vermehren J, et al. Acoustic radiation force impulse-imaging and transient elastography for noninvasive assessment of liver fibrosis and steatosis in NAFLD. Eur J Radiol 2012;81:e325–31. [DOI] [PubMed] [Google Scholar]
- [28].Castera L, Forns X, Alberti A. Noninvasive evaluation of liver fibrosis using transient elastography. J Hepatol 2008;48:835–47. [DOI] [PubMed] [Google Scholar]
- [29].European Association for the Study of the Liver. EASL Recommendations on Treatment of Hepatitis C 2016. J Hepatol 2017;66:153–94. [DOI] [PubMed] [Google Scholar]
- [30].Petta S, Wong VW, Cammà C, et al. Improved noninvasive prediction of liver fibrosis by liver stiffness measurement in patients with nonalcoholic fatty liver disease accounting for controlled attenuation parameter values. Hepatology 2017;65:1145–55. [DOI] [PubMed] [Google Scholar]
- [31].Austermann C, Schierwagen R, Mohr R, et al. MicroRNA-200a: a stage-dependent biomarker and predictor of steatosis and liver cell injury in human immunodeficiency virus patients. Hepatol Commun 2017;1:36–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Bellentani S. The epidemiology of non-alcoholic fatty liver disease. Liver Int 2017;37(Suppl 1):81–4. [DOI] [PubMed] [Google Scholar]
- [33].Kawano Y, Cohen DE. Mechanisms of hepatic triglyceride accumulation in non-alcoholic fatty liver disease. J Gastroenterol 2013;48:434–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Venhoff N, Setzer B, Melkaoui K, et al. Mitochondrial toxicity of tenofovir, emtricitabine and abacavir alone and in combination with additional nucleoside reverse transcriptase inhibitors. Antivir Ther 2007;12:1075–85. [PubMed] [Google Scholar]
- [35].Grant PM, Kitch D, McComsey GA, et al. Long-term body composition changes in antiretroviral-treated HIV-infected individuals. AIDS 2016;30:2805–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


