Abstract
Background:
Recent study has reported that risedronate was effective in reducing periprosthesis bone loss after total hip arthroplasty (THA). The meta-analysis was performed to compare the clinical outcomes of THA with oral risedronate versus placebo.
Methods:
Electronic databases: PubMed (1950–March 2018), EMBASE (1974–March 2018), the Cochrane Central Register of Controlled Trials, Web of Science (1950–March 2018) were systematically searched. Two authors independently graded the methodological quality of each eligible study using the Cochrane Collaboration tool and extracted relevant data. Statistical heterogeneity among the trials were evaluated with chi-square and I-square tests. This meta-analysis was performed using STATA 14.0.
Results:
A total of 4 randomized controlled trials (RCTs) published between 2006 and 2015 were included in our study. The meta-analysis demonstrated that risedronate was associated with a significantly reduction of periprosthetic bone mineral density after THA. No increased postoperative complications were observed.
Conclusion:
Oral risedronate might reduce the periprosthetic bone resorption after cementless THA. Additionally, no severe adverse effects were observed. High-quality RCTs with large sample size were still required.
Keywords: bisphosphonate, bone mineral density, meta-analysis, risedronate, total hip arthroplasty
1. Introduction
Total hip arthroplasty (THA) has become main-stream method for hip osteoarthritis and femoral neck fracture.[1] It is effective in reconstructing joint function, and improving quality of life for elderly, patients. Bone remodeling around the femoral stem after THA will cause periprosthesis bone loss, and periprosthetic fracture, which mainly, occurs in the first post-operative year.[2,3] Besides, aseptic loosening will increase the risk of revision hip arthroplasty.[4] Internal fixator is stiffer than bone tissue, and bears the majority of the load, thus bone is stress-shielded, resulting disuse atrophy.[5]
Currently, no guideline has been proposed to prevent the periprosthesis bone loss after THA. Published articles have shown that bisphosphonates appeared to be associated with an improved outcome in bone metabolism.[6,7] Cankaya et al[8] showed the evidence from preclinical animal that perioperative treatment with bisphosphonates improved the bone mineral density (BMD) around the stems and implant stability. Gao et al[9] reported that intravenous administration of zoledronic acid could significantly reduce periprosthetic BMD loss after THA without severe adverse events. Risedronate is an orally, administered bisphosphonate used for the prevention of osteoporosis, and Paget's disease.[10,11] Recent study has reported that risedronate was effective in reducing periprosthesis bone loss and periprosthetic fracture.[12] However, some studies demonstrated no beneficial effects of risedronate on BMD or bone remodeling.[13] The clinical application of risedronate in THA was limited due to the small published articles.
Based on the current clinical studies with risedronate, we tried to pool the results from randomized controlled trials (RCTs) in a meta-analysis to identify whether the use of risedronate was associated with reduced periprosthetic bone resorption. Our hypothesis was that risedronate was effective in reducing femoral periprosthetic BMD loss in THA.
2. Methods
The meta-analysis was designed, and conducted according to the preferred reporting items for systematic reviews, and meta-analyses (PRISMA) guidelines.[14] No primary personal data will be collected; therefore no additional ethical approval needs to be obtained.
2.1. Search strategy and study selection
Electronic databases: PubMed (1950–March 2018), EMBASE (1974–March 2018), the Cochrane Central Register of Controlled Trials, Web of Science (1950–March 2018) were systematically searched. The search algorithm was structured by different combination of keywords. The details of the search strategy were shown in Fig. 1. (RCTs or trial or placebo or controlled or random) and (THA or replacement) and (bisphosphonate or risedronate). There were no restrictions on language and publication date. Relevant review studies and reference lists were also searched for additional relevant studies.
Figure 1.
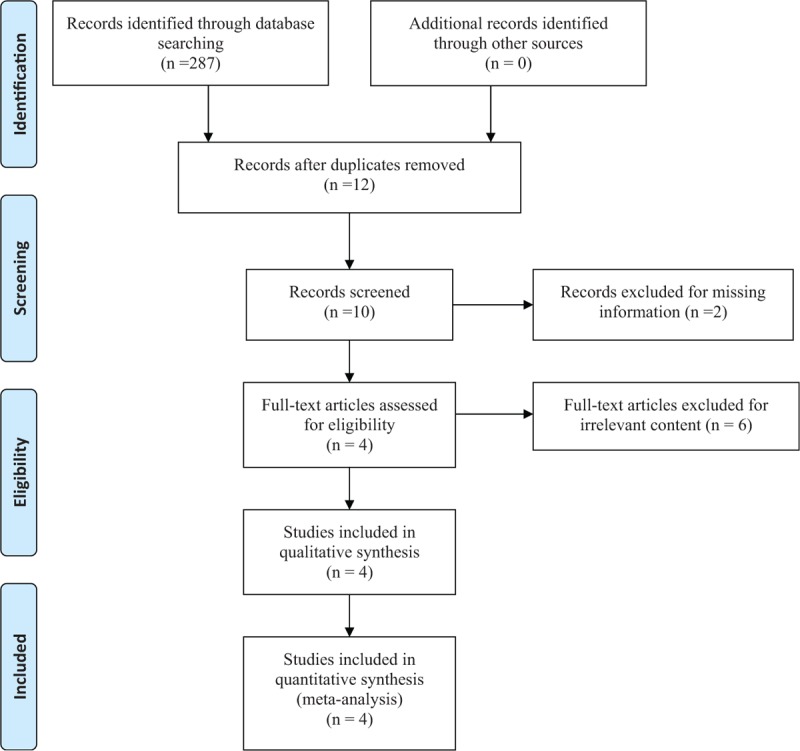
Selection process.
2.2. Inclusion and exclusion criteria
The study was included in this meta-analysis if it was firstly, prospective RCT; secondly, it compared the clinical outcomes of risedronate versus placebo for the bone metabolism in THA; thirdly, it was with a follow-up term of at least 6 months. Exclusion criteria were as follows: firstly, retrospective study, case series, case report, and review articles; secondly, follow-up less than 6 months; thirdly, duplicated publications from the same hospital or research center.
2.3. Data extraction and outcome measures
Two authors independently, extracted the author, publication year, the number of patients in intervention groups, and control groups, the proportion of male patients, and the mean age of the patients, the dose of risedronate, outcomes, and duration of follow-up. The disagreement was resolved by discussion. The primary outcomes were periprosthetic BMD in Gruen zone (Fig. 2). The secondary outcomes were post-operative complications, and length of stay. If the data were not reported numerically, weextracted values from the diagrams using the “GetData Graph Digitizer (Microsoft, Washington)” software as needed.
Figure 2.
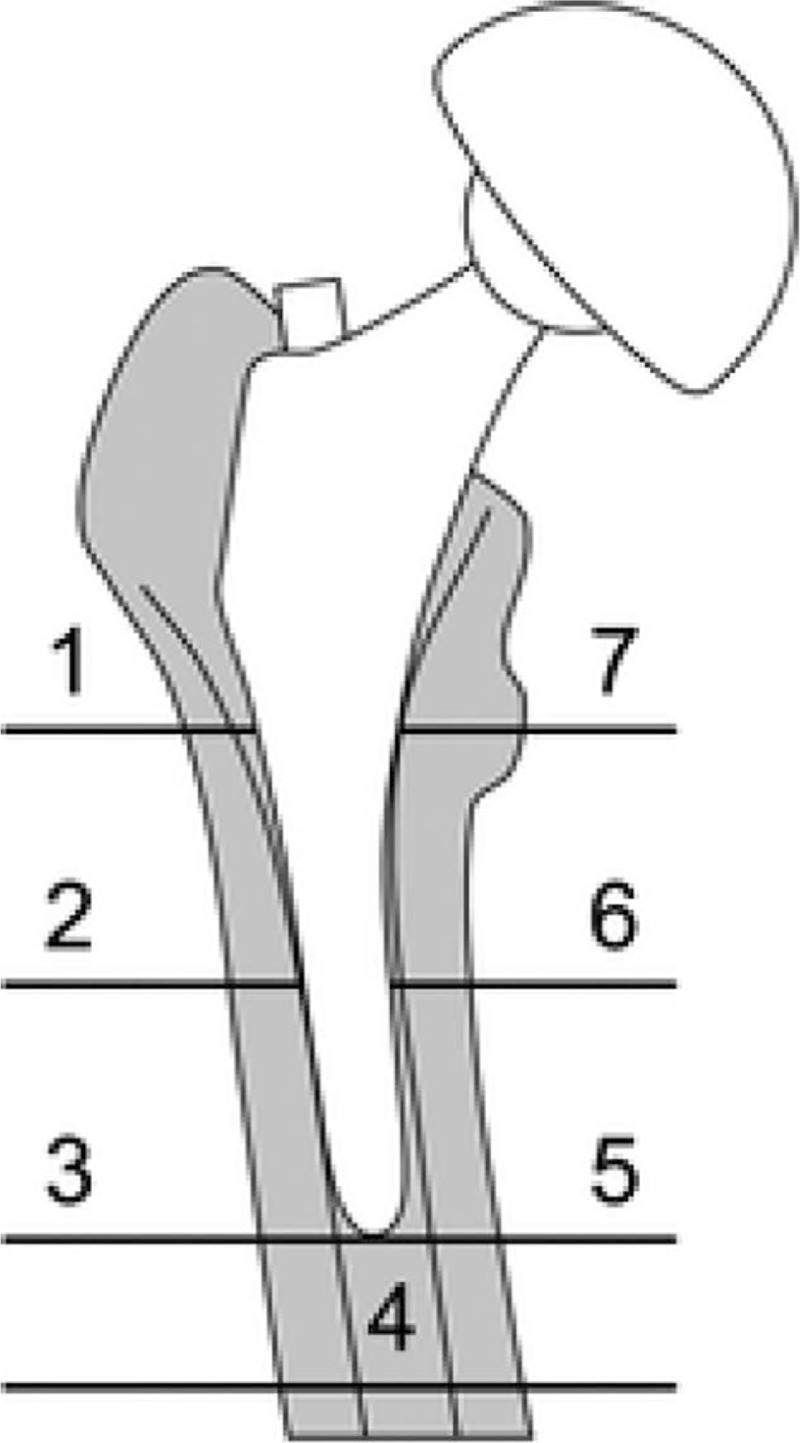
The 7 regions of interest based on Gruen zones.
2.4. Quality assessment of included studies
Cochrane Handbook for Systematic Reviews of Interventions (version 5.1.0) (Cochrane Collaboration's software, Ohio) was used for assessing the risk of bias of the included studies by 2 authors independently. The content for the assessment including the following items: random sequence generation, allocation concealment, blinding, incomplete outcome data, selective outcome reporting, and other sources of bias. Based on the information provided from included studies, each item was recorded by “Yes (low risk of bias),” “No (high risk of bias),” or “Unclear (lack of information or unknown risk of bias).”
2.5. Statistical analysis
This meta-analysis was performed using STATA 14.0 (The Cochrane Collaboration, Oxford, UK) with a significance threshold of P ≤ .05. The heterogeneity was assessed with Chi-square test and I-square statistic. When if I2 < 50% or P > .1, a fixed-effect model was used, otherwise a random-effect model was used. For continuous outcomes, we calculated the mean difference (MD) with 95% confidence interval (CI). The risk difference (RD) with 95% CI was calculated for discontinuous data. Sensitivity analyses and subgroup analyses also used to explore the heterogeneity.
3. Results
3.1. Search results and study characteristics
In the initial research, a total of 287 articles were identified, and 12 articles were reviewed after the removal of duplicates. Then 2 articles were excluded because the date was incomplete, and has no response of e-mail sent to first author for obtaining data. Six studies were excluded for irrelevant content. No gray literature was included. A total of 4 RCTs[15–18] published between 2006 to 2015 were included in the present meta-analysis.
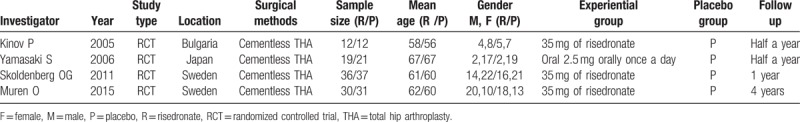
All included studies randomized patients to risedronate groups, and had a follow-up period of at least half a year. All of the articles were published in English. The included studies were conducted in 3 countries (Bulgaria, Japan, and Sweden), and involved 198 patients (97 patients treated with risedronate, 101 patients treated with placebo) aged between 56 to 67 years. The detailed baseline characteristics of the included studies were presented in Table 1.
Table 1.
Characteristics of included trials showing general patient information.
3.2. Quality assessment
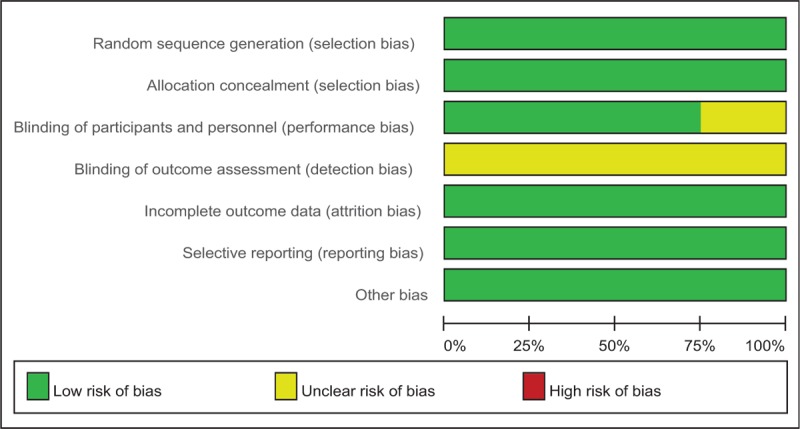
The methodological quality assessment was summarized in Tables 2 and 3. Randomized sequence generation was implemented adequately, in all studies which reported the random sequence generation. Allocation concealment was implemented adequately, in all of the included studies. Three RCTs[16–18] reported blinding of the participants. However, no study showed blinding of outcome assessors. Overall, all RCTs were considered to be high quality.
Table 2.
Risk of bias summary: review authors’ judgements about each risk of bias item for each included study.

Table 3.
Risk of bias graph: review authors’ judgements about each risk of bias item presented as percentages across all included studies.
3.3. Meta-analysis of clinical outcomes
3.3.1. BMD in Gruen zone 1
The results of BMD in Gruen zone 1 showed a statistically, significant difference between the risedronate group, and the placebo group (WMD = 0.163, 95% CI: 0.104 – 0.223, P < .001; Fig. 3). The heterogeneity among these included studies was small, and a fixed-effect model was adopted (χ2 = 1.70, df = 3, I2 = 0%, P = .637).
Figure 3.
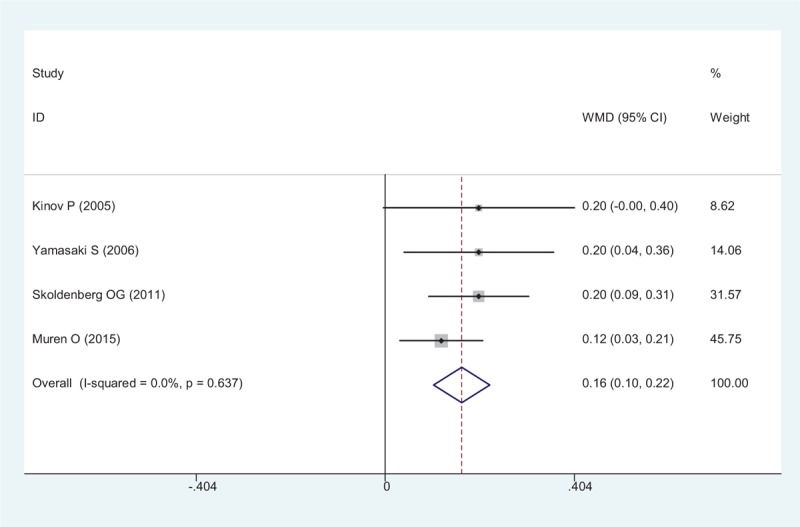
Forest plot showing the meta-analysis of the BMD in Gruen zone 1. BMD = bone mineral density.
3.3.2. BMD in Gruen zone 2
BMD in Gruen zone 2 after THA were reported in 4 RCTs, a pooled result indicated that risedronate was associated with a significant reduction of periprosthetic BMD loss in Gruen zone 2 after THA (WMD = 0.120, 95% CI: 0.069 – 0.171, P < .001; Fig. 4).
Figure 4.
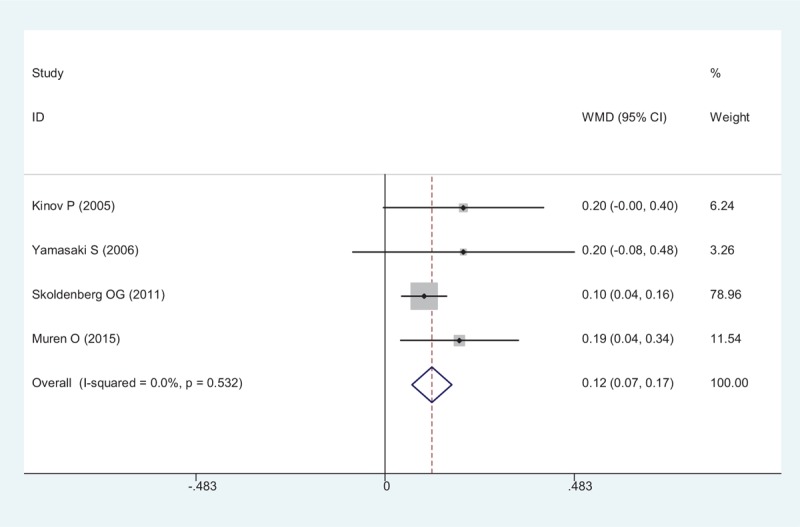
Forest plot showing the meta-analysis of the BMD in Gruen zone 2. BMD = bone mineral density.
3.3.3. BMD in Gruen zone 3
A total of 4 RCTs reported the BMD in Gruen zone 3 after THA. A fixed-effect model was adopted (χ2 = 0.46, df = 3, I2 = 0%, P = .928). The present meta-analysis indicated that there was no significant difference between group regarding the BMD in Gruen zone 3 (WMD = 0.038, 95% CI: − 0.076 – 0.151, P = .516; Fig. 5).
Figure 5.
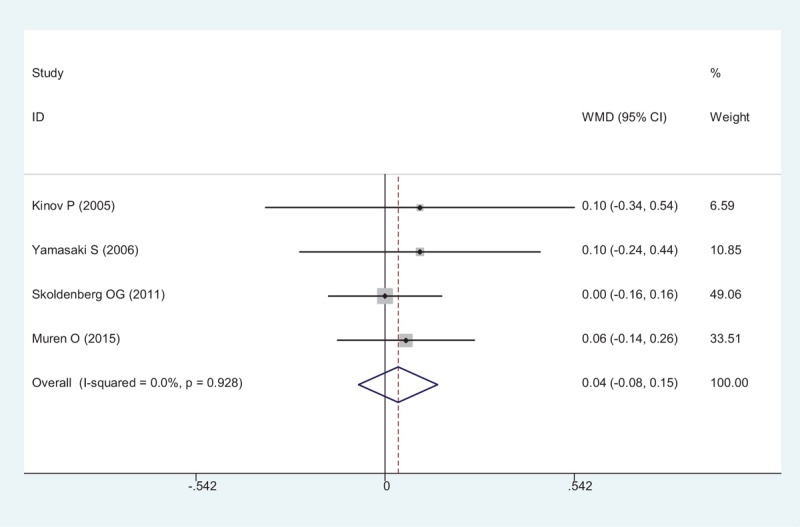
Forest plot showing the meta-analysis of the BMD in Gruen zone 3. BMD = bone mineral density.
3.3.4. BMD in Gruen zone 4
The results of BMD in Gruen zone 4 demonstrated no statistically, significant difference between the risedronate group, and the placebo group (WMD = 0.087, 95% CI: − 0.025 – 0.198, P = .127; Fig. 6). The heterogeneity among these included studies was small, and a fixed-effect model was adopted (χ2 = 4.55, df = 3, I2 = 34.1%, P = .208).
Figure 6.
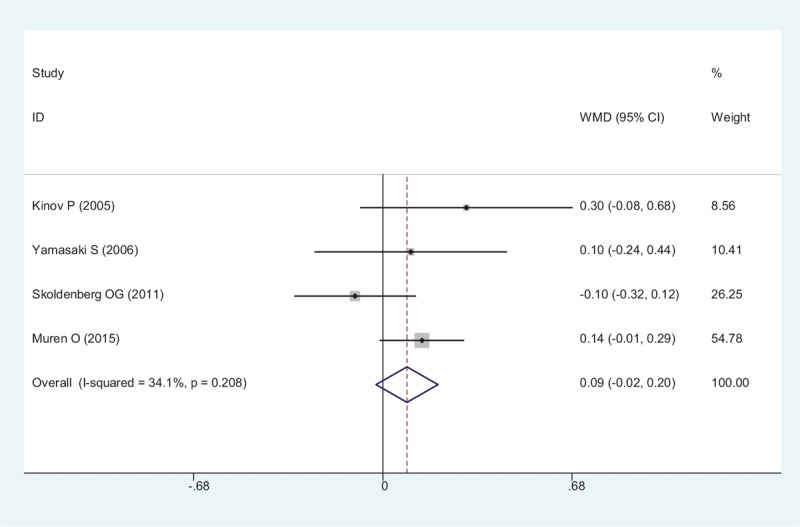
Forest plot showing the meta-analysis of the BMD in Gruen zone 4. BMD = bone mineral density.
3.3.5. BMD in Gruen zone 5
Four studies reported data of BMD in Gruen zone 5. Significant difference was observed between the oral risedronate and placebo groups (WMD = 0.103, 95% CI: 0.055 – 0.152, P < .001; Fig. 7). A fixed-effect model was used (χ2 = 3.61, df = 3, I2 = 16.9%, P = .307)
Figure 7.
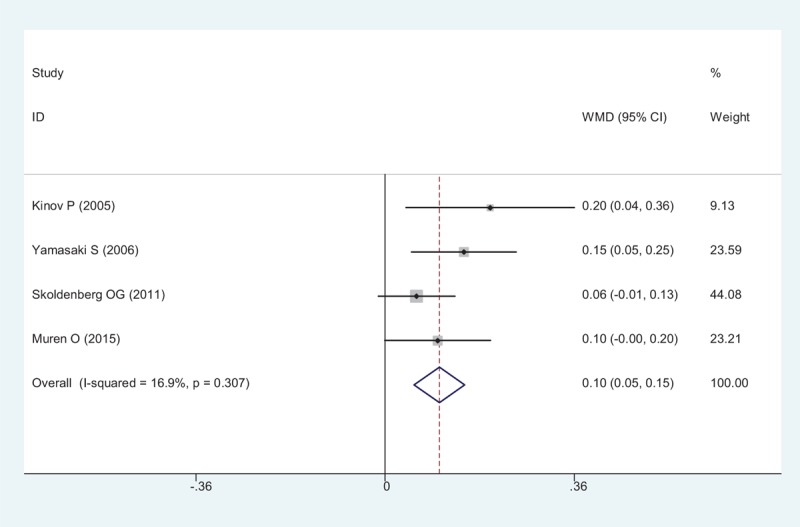
Forest plot showing the meta-analysis of the BMD in Gruen zone 5. BMD = bone mineral density.
3.3.6. BMD in Gruen zone 6
BMD in Gruen zone 6 after THA were provided in all RCTs, a pooled result indicated that risedronate was associated with a significantly, reduction of periprosthetic BMD loss after THA (WMD = 0.134, 95% CI: 0.043 – 0.226, P = .004; Fig. 8).
Figure 8.
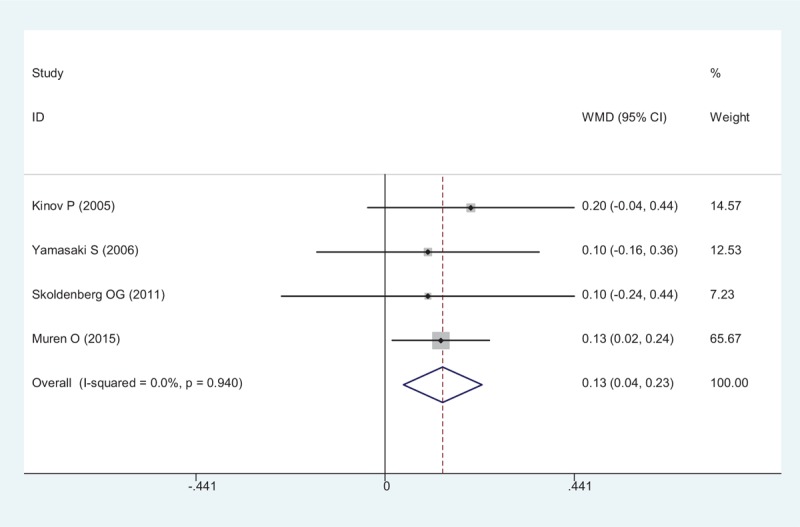
Forest plot showing the meta-analysis of the BMD in Gruen zone 6. BMD = Bone mineral density
3.3.7. BMD in Gruen zone 7
The results of BMD in Gruen zone 7 demonstrated a statistically, significant difference between the risedronate group, and the placebo group (WMD = 0.257, 95% CI: 0.127 – 0.386, P < .001; Fig. 9). A fixed-effect model was used (χ2 = 0.50, df = 3, I2 = 0%, P = .919).
Figure 9.
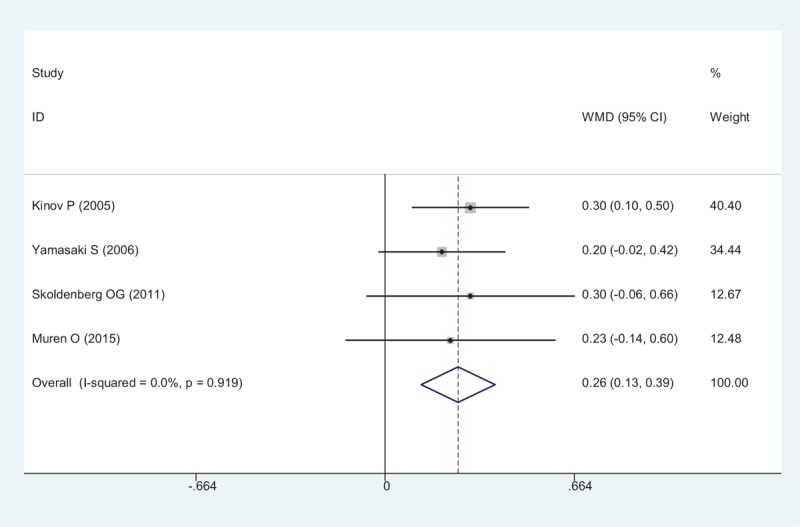
Forest plot showing the meta-analysis of the BMD in Gruen zone 7. BMD = Bone mineral density
3.4. Post-operative complications
All included RCTs showed post-operative complications including nausea and hip dislocation. The present meta-analysis indicated that there was no significant difference between groups (RD = -0.030, 95% CI: − 0.096 – 0.036, P = .367; Fig. 10).
Figure 10.
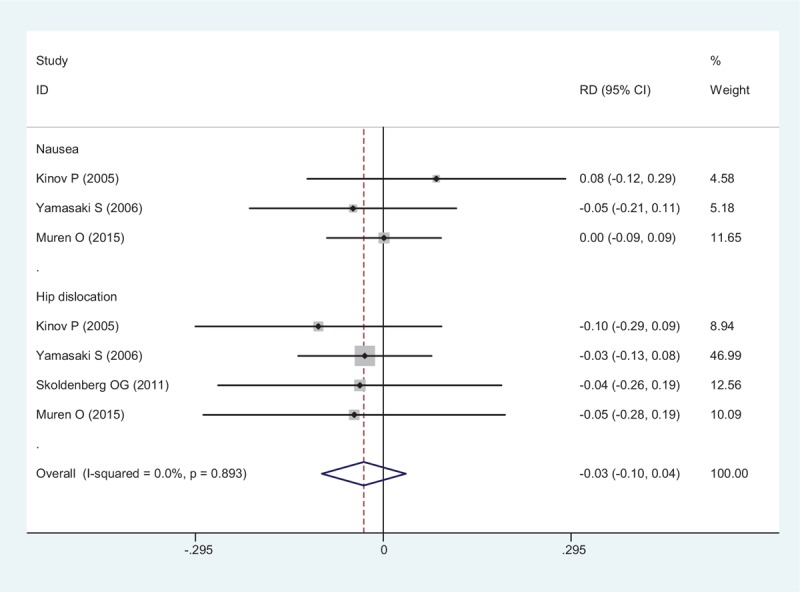
Forest plot showing the meta-analysis of the post-operative complications.
3.5. Hospitalization day
All RCTs showed the outcome of length of a hospital stay after THA. There was no significant heterogeneity between articles (χ2 = 0.20, df = 3, I2 = 0%, P = .978). Meta-analysis revealed that there was no significant difference between the groups regarding to length of a hospital stay (WMD = − 0.116, 95% CI: − 0.688 – 0.455, P = .690; Fig. 11).
Figure 11.
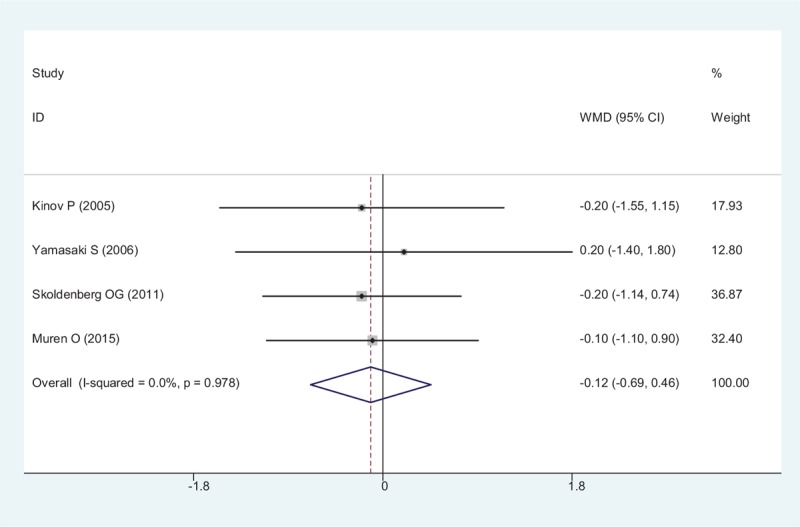
Forest plot showing the meta-analysis of the hospitalization day.
4. Discussion
In our meta-analyses, the primary outcomes were periprosthetic BMD in Gruen zone. The other outcomes were post-operative compactions and length of a hospital stay. To our knowledge, this was the first meta-analysis that compared the efficacy, and safety of oral risedronate with placebo in patients following primary THA. And the results of our meta-analysis that based on the available evidence from RCTs demonstrated that oral risedronate was associated with a significant reduction of periprosthetic BMD loss after THA. No increased post-operative complications were observed. Four RCTs were included in our study; we considered all of them to be at low risk of bias. Therefore, overall evidence quality for the meta-analysis was high.
Osteoarthritis is the most prevalent form of arthritis worldwide affecting nearly 52.5 million people, or 22.7% of the population in the US.[19] With the aging population, the incidence of hip osteoarthritis is increasing, and it becomes a serious social problem. THA is popular surgical treatment for end-stage osteoarthritis. However, bone resorption around a femoral stem after THA is a well-known phenomenon. Bisphosphonates are a class of drugs that can bind to the bone, and inhibit osteoclast activity by reducing bone resorption. Substantial articles have demonstrated that the application of bisphosphonates was associated with satisfactory outcomes for patients in THA.[20]
Bone remodeling occurred in the first 6 months after cementless THA which caused bone absorption and prosthetic loosening. Reducing stress-shielding has achieved by the use of bisphosphonate therapy which showed positive results in the early period. A vitro study on experimental animals showed that use of bisphosphonate inhibited wear-induced osteolysis. Bhandari et al[21] reported that bisphosphonates seemed to decrease early femoral periprosthetic bone resorption after primary cementless THA. Ericksen et al[22] showed that administration of bisphosphonates to patients with hip fracture after operation could increase hip BMD, and reduce the risk of subsequent clinical vertebral, nonvertebral, and hip fractures, and decrease mortality. Though potential benefit of bisphosphonates in THA, no consensus about effective therapeutic regimen has been reached due to the small sample size and short term follow up.
Risedronate is an orally administered bisphosphonate which is applied to inhibit bone resorption, and have been widely, used for treatment, and prevention of osteoporosis. Previous studies demonstrated that the anti-osteoporosis effect could last as long as 1 year after arthroplasty.[23] In our study, dual-energy X-ray device was used to recognize the periprosthetic Gruen zones automatically, and calculated the BMD at 6 months for patients with, or without oral risedronate after THA. Recently, the positive effect of oral risedronate in THA remains controversial. Skoldenberg et al[17] reported that risedronate taken once weekly for 6 months following THA was effective in reducing periprosthetic bone resorption around an uncemented femoral stem up. However, Muren et al[15] did not recommend the use of risedronate following THA for osteoarthritis of the hip. Thus, we performed the meta-analysis from RCTs, and the pooled results indicated that oral risedronate was associated with a significant reduction of BMD (Gruen 1, 2, 5, 6 and 7) after joint arthroplasty. We did not detect a significant difference in BMD in Gruen 3 and 4, although there was a higher average level in risedronate groups. Large sample size of RCTs were needed for further investigation. There remains debate regarding the course of risedronate treatment. Fundamental research showed that maximum bone resorption was identified in the first 6 months following THA. Arabmotlagh et al[24] reported that there was an improved bone quality with a course of 6 months compared that less than 6 months. Thus, experts recommended a more than half year course of diphosphonate treatment following THA. Risedronate 5 mg administration daily has the similar outcome compared to 35 mg given weekly, and once-weekly risedronate would be more popular among patients.[25,26] Due to the limited of the included RCTs, we did not perform a subgroup analysis, more RCTs were required.
With the widely used of bisphosphonates, the post-operative adverse effects were major concern. In our study, all adverse events were mild, and were managed easily, with supportive care. There was no significant difference between groups. Additional follow up was needed to investigate potential severe adverse events.
Some limitations of this study should be noted. Firstly, the number of included studies was small, and most of the studies included few patients, the cumulative sample size was also small, which could not provide sufficient evidence for our conclusions. Secondly, significant statistical heterogeneity still existed among the included trials, which may be explained by the clinical diversity among trials. Thirdly, several included studies had methodologic flaw, which might lead a high risk of bias for the results of the included studies. Fourth, some important outcomes were not assessed, such as range of motion and pain. More large-sample, multi-center, high-quality, RCTs are needed to verify the results of this meta-analysis.
5. Conclusion
Oral risedronate might reduce the periprosthetic bone resorption after cementless THA. Additionally, no severe adverse effects were observed. High-quality RCTs with large sample size were still required.
Author contributions
Weidong Wang designs the meta-analysis. Liang Ren collects the data and finishes the manuscript. They have read and approved the final manuscript.
Data curation: Weidong Wang.
Writing - original draft: Liang Ren.
Footnotes
Abbreviations: BMD = Bone mineral density, RCT = Randomized controlled trials, THA = Total hip arthroplasty.
The authors declare that there is no competing interests.
Funding: None.
References
- [1].Kawamura H, Mishima H, Sugaya H, et al. The 21- to 27-year results of the Harris-Galante cementless total hip arthroplasty. J Orthop Sci 2016;21:342–7. [DOI] [PubMed] [Google Scholar]
- [2].Tran P, Zhang BX, Lade JA, et al. Periprosthetic bone remodeling after novel short-stem neck-sparing total hip arthroplasty. J Arthroplasty 2016;31:2530–5. [DOI] [PubMed] [Google Scholar]
- [3].Yan SG, Li D, Yin S, et al. Periprosthetic bone remodeling of short cementless femoral stems in primary total hip arthroplasty: a systematic review and meta-analysis of randomized-controlled trials. Medicine (Baltimore) 2017;96:e8806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Janssen L, Wijnands KAP, Janssen D, et al. Do stem design and surgical approach influence early aseptic loosening in cementless THA? Clin OrthopRelat Res 2018;doi: 10.1007/s11999.0000000000000208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Bugbee WD, Culpepper WJ, 2nd, Engh CA, Jr, et al. Long-term clinical consequences of stress-shielding after total hip arthroplasty without cement. J Bone Joint Surg Am 1997;79:1007–12. [DOI] [PubMed] [Google Scholar]
- [6].Allen CS, Yeung JH, Vandermeer B, et al. Bisphosphonates for steroid-induced osteoporosis. Cochrane Database Syst Rev 2016;10:CD001347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Alwahhabi BK, Alsuwaine BA. Long-term use of bisphosphonates in osteoporosis. Saudi Med J 2017;38:604–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Cankaya D, Tabak Y, Ozturk AM, et al. Perioperative alendronate, risedronate, calcitonin and indomethacin treatment alters femoral stem fixation and periprosthetic bone mineral density in ovariectomized rats. J Orthop Sci 2015;20:728–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Gao J, Gao C, Li H, et al. Effect of zoledronic acid on reducing femoral bone mineral density loss following total hip arthroplasty: a meta-analysis from randomized controlled trails. Int J Surg 2017;47:116–26. [DOI] [PubMed] [Google Scholar]
- [10].Roll KT. Risedronate: a new oral bisphosphonate. Clin Ther 2002;24:835–6. [DOI] [PubMed] [Google Scholar]
- [11].Ohara M, Imanishi Y, Nagata Y, et al. Clinical efficacy of oral risedronate therapy in Japanese patients with Paget's disease of bone. J Bone Miner Metab 2015;33:584–90. [DOI] [PubMed] [Google Scholar]
- [12].Arabi A, Fuleihan Gel H. How effective is risedronate in preventing bone loss in patients on high-dose steroids? Nat Clin Pract Endocrinol Metab 2008;4:540–1. [DOI] [PubMed] [Google Scholar]
- [13].Saari TM, Digas G, Karrholm JN. Risedronate does not enhance fixation or BMD in revision cups: randomised study with three years follow-up. Hip Int 2014;24:49–55. [DOI] [PubMed] [Google Scholar]
- [14].Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA Statement. PLoS Med 2009;6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Muren O, Akbarian E, Salemyr M, et al. No effect of risedronate on femoral periprosthetic bone loss following total hip arthroplasty. A 4-year follow-up of 61 patients in a double-blind, randomized placebo-controlled trial. Acta Orthop 2015;86:569–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Kinov P, Tivchev P, Doukova P, et al. Effect of risedronate on bone metabolism after total hip arthroplasty: a prospective randomised study. Acta Orthop Belg 2006;72:44–50. [PubMed] [Google Scholar]
- [17].Skoldenberg OG, Salemyr MO, Boden HS, et al. The effect of weekly risedronate on periprosthetic bone resorption following total hip arthroplasty: a randomized, double-blind, placebo-controlled trial. J Bone Joint Surg Am 2011;93:1857–64. [DOI] [PubMed] [Google Scholar]
- [18].Yamasaki S, Masuhara K, Yamaguchi K, et al. Risedronate reduces postoperative bone resorption after cementless total hip arthroplasty. Osteoporos Int 2007;18:1009–15. [DOI] [PubMed] [Google Scholar]
- [19].Barbour KE, Boring M, Helmick CG, et al. Prevalence of Severe Joint Pain Among Adults with Doctor-Diagnosed Arthritis - United States, 2002-2014. MMWR Morb Mortal Wkly Rep 2016;65:1052–6. [DOI] [PubMed] [Google Scholar]
- [20].Thillemann TM, Pedersen AB, Mehnert F, et al. Postoperative use of bisphosphonates and risk of revision after primary total hip arthroplasty: a nationwide population-based study. Bone 2010;46:946–51. [DOI] [PubMed] [Google Scholar]
- [21].Bhandari M, Bajammal S, Guyatt GH, et al. Effect of bisphosphonates on periprosthetic bone mineral density after total joint arthroplasty. A meta-analysis. J Bone Joint Surg Am 2005;87:293–301. [DOI] [PubMed] [Google Scholar]
- [22].Eriksen EF, Lyles KW, Colon-Emeric CS, et al. Antifracture efficacy and reduction of mortality in relation to timing of the first dose of zoledronic acid after hip fracture. J Bone Miner Res 2009;24:1308–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Ha KY, Park KS, Kim SI, et al. Does bisphosphonate-based anti-osteoporosis medication affect osteoporotic spinal fracture healing? Osteoporos Int 2016;27:483–8. [DOI] [PubMed] [Google Scholar]
- [24].Arabmotlagh M, Rittmeister M, Hennigs T. Alendronate prevents femoral periprosthetic bone loss following total hip arthroplasty: prospective randomized double-blind study. J Orthop Res 2006;24:1336–41. [DOI] [PubMed] [Google Scholar]
- [25].Ilter E, Karalok H, Tufekci EC, et al. Efficacy and acceptability of risedronate 5 mg daily compared with 35 mg once weekly for the treatment of postmenopausal osteoporosis. Climacteric 2006;9:129–34. [DOI] [PubMed] [Google Scholar]
- [26].Brown JP, Kendler DL, McClung MR, et al. The efficacy and tolerability of risedronate once a week for the treatment of postmenopausal osteoporosis. Calcif Tissue Int 2002;71:103–11. [DOI] [PubMed] [Google Scholar]


