Abstract
Insufficient evidence is available to reliably compare the roles of bone alkaline phosphatase (BAP) and bone mineral density (BMD) in diabetes. This study aimed to compare associations between BAP and BMD in adults with and without diabetes to elucidate fracture risk in diabetes.
Data were extracted from the National Health and Nutrition Examination Survey (NHANES), 2001–2004, including 4197 adults aged 20 to 49 years, 143 with diabetes (DM group), and 4054 without (non-DM group). Main outcome measure was BMD and regression analyses were performed to identify serum BAP and other covariates associated with total BMD.
BMD decreased significantly in DM patients when BAP was increased. In the non-DM group, all BMD results were significantly decreased when BAP was increased. Factors associated with total BMD varied with DM status. Lifestyle measures such as smoking and physical activity were also associated with BMD in the non-DM group.
BAP and BMD are inversely associated in DM and non-DM patients. BAP is significantly associated with BMD after controlling for other variables, suggesting that BAP may interact with other factors altering bone metabolism in DM patients.
Keywords: bone alkaline phosphatase, bone mineral density, diabetes, glycohemoglobin
1. Introduction
Throughout life, bone continuously remodels itself through bone resorption and replacement. Peak bone mass is reached in late adolescence and, in adulthood, bone health must be maintained to preserve as much bone mass as possible to help prepare for inevitable losses in later life. Although some bone loss may occur at the hip before age 50 years, most adult men and women maintain bone mass until middle age and beyond by avoiding falls and illnesses associated with bone loss and fractures.[1] Individuals with type 2 diabetes, however, exhibit increased fracture risk despite having adequate bone mass.[2] Researchers who evaluated BMD in diabetic and nondiabetic patients did not observe differences in BMD between the 2 groups but did find higher osteoporosis incidence in those with diabetes.[3] Recent meta-analyses and cohort studies confirm that bone turnover and skeletal integrity are affected negatively by diabetes, and that diabetes is associated with a higher risk of fracture.[4–7] However, BMD values vary among diabetes patients and can be increased, decreased, or remain normal.[2] Although the pathogenesis of altered bone metabolism in diabetic patients is known to be multifactorial, the mechanism is not entirely clear.[4]
Bone metabolism in diabetes is influenced by many factors, including depressed osteoblast activity and decreased numbers of osteoclasts (“sweet bones”) as a result of abnormal insulin secretion and/or insulin action.[4] Insulin-like growth factor and other osteoclastogenic cytokines are also implicated.[8] Serum alkaline phosphatase (ALP) is associated with vascular calcification,[9] while bone-specific alkaline phosphatase, or BAP, is a marker of bone formation and bone turnover and is used in the evaluation of skeletal status.[10] Nevertheless, both serum ALP and BAP appear to be associated with diabetes and metabolic syndrome (MetS) even though the mechanism and the common influencing factors are not fully understood.[9] Other studies of BAP have had conflicting results. In a study of patients with axial spondyloarthritis, Kang et al[11] found that elevated serum ALP correlated with low BMD and greater structural damage. In contrast, Lumachi et al[12] found no relationship between the bone formation marker BAP and bone density in elderly men, suggesting that BAP was not useful in monitoring bone integrity in this population.
Insufficient evidence is available to reliably compare the roles of BAP and BMD in diabetes and understand how they may interact. The authors hypothesized that comparing the associations and influencing factors between these 2 parameters in diabetic and nondiabetic patients might shed light on the increased fracture risk in diabetes. Data from a large nationally representative health survey of adults was used to test this hypothesis. The study purpose was to compare associations between BAP and BMD in adults with and without diabetes to elucidate fracture risk in diabetes.
2. Methods
2.1. Data source
The source of data for this study was the National Health and Nutrition Examination Survey (NHANES), a nationally representative survey conducted in the United States to provide current statistical data on the amount, distribution, and effects of illness in the United States. NHANES was first conducted in 1956, and has been a continuous field survey since 1999. All data for the present study were from NHANES, 2001–2004, conducted by the Centers for Disease Control and Prevention (CDC) and National Center for Health Statistics (NCHS).[13,14]
2.2. Ethical considerations
All NHANES data are de-identified and data analysis for research purposes does not require IRB approval or informed consent by participating subjects.
2.3. Study population
The sample for this study included participants aged 20 to 49 years who had been tested for BAP. Subjects were stratified into 2 groups: DM patients and non-DM patients. Diabetes was defined as a self-report of having been told by a doctor or healthcare professional that the subject had diabetes or high blood glucose. Those who responded “yes” were classified as having diagnosed diabetes. Individuals with invalid BAP values, unknown DM status, without BMD data or who had osteoporosis were excluded from the analysis. Finally, the data of 4197 subjects were included for analysis. Figure 1 depicts the subject selection process (Fig. 1).
Figure 1.
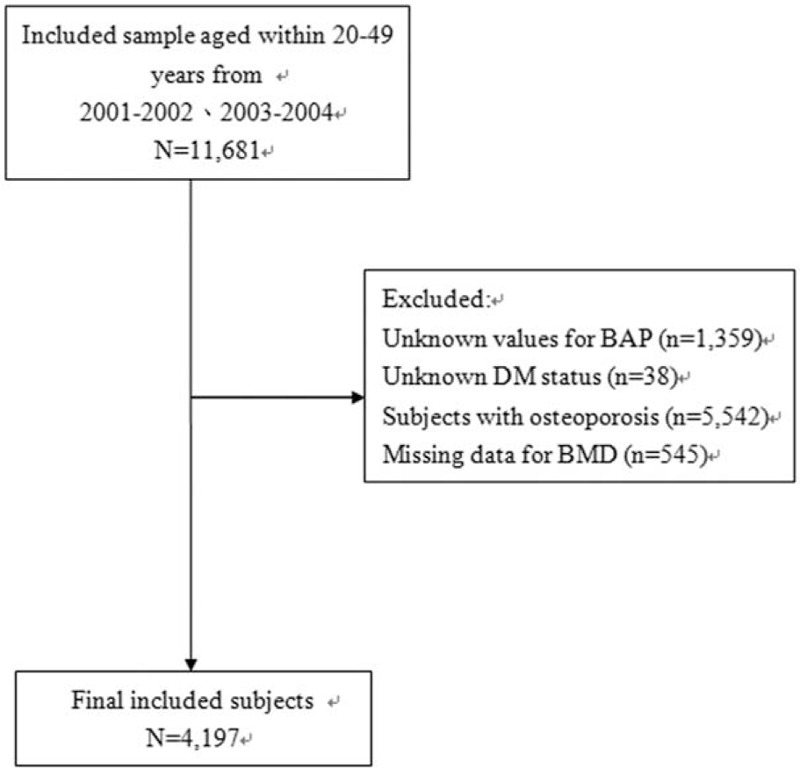
Flow chart of subject selection.
2.4. Main outcome measures
2.4.1. Bone mineral density (BMD)
BMD (g/cm2) was the primary outcome and was measured by total body dual-energy x-ray absorptiometry (DXA). In the NHANES 2001–2004, DXA scans were administered to a limited subset of eligible survey participants aged 8 years and older, excluding pregnant women, patients with self-reported history of radiographic contrast material (barium) use in the past 7 days or nuclear medicine studies in the past 3 days, and weight over 300 pounds or height over 6′5″. All subjects included in the present study received DXA total body and subregional scans. Whole body and subregional DXA scans were performed by certified radiology technologists using a Hologic QDR-4500A fan-beam densitometer (Hologic, Bedford, MA). Scans for all participants were reviewed and analyzed by the Department of Radiology, University of California, San Francisco, using standard radiologic techniques and study-specific protocols developed for the NHANES.[15] Analysis for each subject included total BMD and head, left arm, pelvis, lumbar spine, and trunk BMD.
2.4.2. Bone alkaline phosphatase (BAP)
Serum BAP was measured by Hybritech Tandem-MP Ostase ImmunoEnzymetric assay (Hybritech Inc., San Diego, CA) for NHANES 2001. For NHANES 2002–2004, the Beckman Access Ostase assay (Beckman Coulter, LaBrea, CA) was used to measure serum BAP.[16]
2.5. Variables
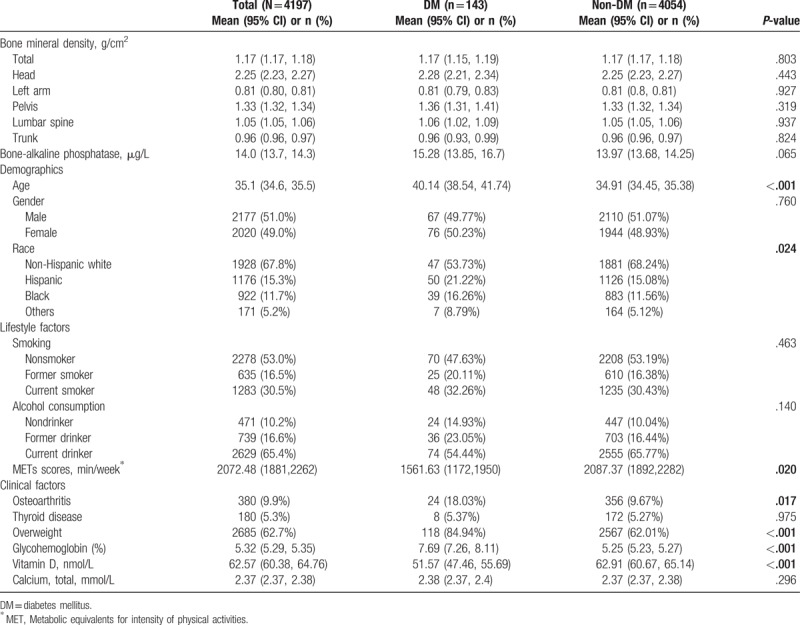
Variables measured and analyzed in this study included subjects’ demographic characteristics (age, gender, and race), lifestyle factors (smoking, alcohol consumption, and physical activity) and clinical characteristics (osteoarthritis, thyroid disease, overweight, glycated hemoglobin, serum 25-hydroxy vitamin D, and calcium concentration), as shown below Table 1.
Table 1.
Subject characteristics.
2.5.1. Tobacco smoking
Smoking was classified as never, former, or current smoker. Subjects who never had at least 100 cigarettes in their life were defined as nonsmokers. Those who had at least 100 cigarettes but did not smoke now were former smokers. Those who responded “yes” to the question: “Do you smoke now?” were defined as current smokers.
2.5.2. Alcohol consumption
Participants who, in their entire life (“In your entire life, have you had at least 12 drinks of any type of alcoholic beverage”?), had never had at least 12 drinks were defined as nondrinkers. Participants who had at least 12 drinks in their entire life, but had not consumed alcohol in the past 12 months (“In the past 12 months, how often did you drink any type of alcoholic beverage”?), were defined as former drinkers. Participants who consumed at least 12 drinks in their entire life and drank on at least 1 day in the past year were considered current drinkers, as previously described.[17]
2.5.3. Physical activity
A physical activity questionnaire was used to collect information on the frequency and duration of moderate- to vigorous-intensity physical activities subjects engaged in for transportation, household chores, and leisure-time recreation during the previous 30 days. The metabolic equivalents (MET-min/wk) was calculated for the intensity of physical activities, as previously described.[18]
2.5.4. Clinical characteristics
Osteoarthritis was defined as a self-report or having been told by a doctor or health professional that the individual had osteoarthritis. Thyroid disease was defined as a self-report or having been told by a doctor or health professional that the individual had thyroid problems. Patients who responded “yes” were classified as having diagnosed thyroid disease. Overweight status was dichotomized and defined as body mass index (BMI) >=25 kg/m2.
2.5.5. Glycosylated hemoglobin
Hemoglobin A1C levels, which reflect a person's average blood glucose level over the past 3 months, were measured by Primus Automated HPLC system Model CLC330 (Primus I, Primus Corp. Kansas City, MO) and converted to A1C levels.[16]
2.5.6. Serum 25-hydroxyvitamin D
Serum 25-hydroxyvitamin D (nmol/L) was measured at the National Center for Environmental Health, CDC, Atlanta, GA using the DiaSorin RIA kit (DiaSorin, Stillwater, MN) in NHANES 2001–2004, but were converted to equivalent 25(OH)D measurements using standardized liquid chromatography-tandem mass spectrometry (LC-MS/MS). The LC-MS/MS-equivalent data were used for the present study.[16]
2.5.7. Calcium levels
Calcium concentration in serum samples was measured on the Beckman Synchron LX20 system (Beckman-Coulter, LaBrea, CA) using indirect (or diluted) ion selective electrodes (ISE) methodology.[16]
2.6. Statistical analysis
Categorical data are presented as count and weighted percentages (%wt) and continuous data are presented as weighted means and 95% confidence interval (95% CI). Rao-Scott χ2-test and t-test estimated by the SAS procedure of Proc Survey were performed to evaluate differences in proportions and means, respectively, between subjects with and without diabetes. BMD was imputed by the SAS procedure of Proc Mianalyze that contains parameter estimates and associated covariance matrices for each imputation. Univariate and multivariate linear regression was used to analyze associations between total BMD and BAP stratified by DM status. All risk factors were included as covariates in the multivariate analysis. SAS survey analysis procedures (SAS Institute Inc., Cary, NC) were used for all statistical analyses.
3. Results
3.1. Characteristics of study subjects
Table 1 shows DM and non-DM subjects’ demographic, lifestyle and clinical characteristics. Among a total of 4197 subjects, 143 were DM patients and 4054 were non-DM; 2177 (51.0%wt) subjects were males; the majority of subjects were non-Hispanic whites (67.8%wt); and the mean age of the study population was 35.1 years (95% CI: 34.6–35.5 years). More than half of subjects had never smoked (53.0%wt) and most subjects consumed alcohol currently (65.4%wt). In addition, 380 subjects (9.9%wt) had arthritis, 180 (5.3%wt) had thyroid disease, and 2685 (62.7%wt) were overweight. The mean total BMD was 1.17 gm/cm2 (95% CI: 1.17–1.18), and subsite-specific BMD was 2.25 gm/cm2 (95% CI: 2.23–2.27) for head, 0.81 gm/cm2 (95% CI: 0.80–0.81) for left arm, 1.33 gm/cm2 (95% CI: 1.32–1.34) for pelvis, 1.05 gm/cm2 (95% CI: 1.05–1.06) for lumbar spine, and 0.96 gm/cm2 (95% CI: 0.96–0.97) for trunk. The mean BAP was 14.0 μg/L (95% CI: 13.7–14.3 μg/L).
No differences were found in BMD and BAP between DM and non-DM subjects. DM subjects were significantly older than non-DM subjects (40.14 years vs 34.91 years, P < .001). Mean glycohemoglobin was significantly higher in DM subjects than in non-DM subjects (7.69% vs 5.25%, P < .001). Mean Vitamin D levels were significantly higher in non-DM subjects compared to levels in DM subjects (62.91 nmol/L vs 51.57 nmol/L, P < .001), and metabolic equivalents of task (MET) scores were also significantly higher in non-DM subjects compared to the scores of DM subjects (2087.37 min/week vs 1561.63 min/week, P = .02). Significant differences were found in the proportions of race, osteoarthritis, and overweight status between the 2 groups (all P≤.024) (Table 1).
3.2. Associations between BMD and BAP in adults with and without diabetes
Table 2 shows the associations between BMD and BAP in the DM and non-DM groups. In the DM group, univariate analysis showed no significant associations between BAP and BMD, either total BMD or at specific subsites (all P > .05). After adjusting for other variables mentioned in Section 2, multivariate analysis showed that all BMD measures, except for head, decreased significantly when BAP was increased (total: β = −0.0042, P = .001; left arm: β = −0.0015, P = .046; pelvis: β = −0.0058, P = .002; lumbar spine: β = −0.0054, P = .003; trunk: β = −0.0048, P < .001) (Table 2).
Table 2.
Associations between bone mineral density and bone-alkaline phosphatase stratified by diabetic status.
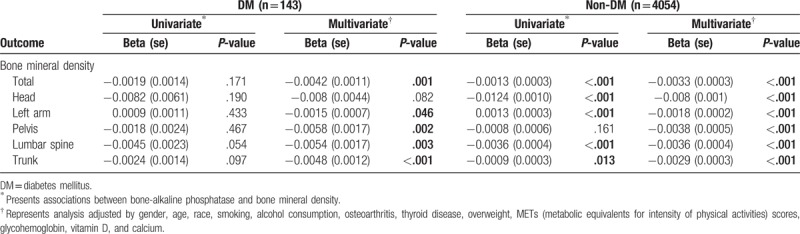
In the non-DM group, univariate analysis showed that BAP was significantly and independently associated with total BMD and head, left arm, lumbar spine, and trunk BMD (all P < .05). After adjusting for all covariates, multivariate analysis showed that all BMD results were significantly decreased when BAP was increased (total: β = −0.0033, P < .001; head: β = −0.008, P < .001; left arm: β = −0.0018, P < .001; pelvis: β = −0.0038, P < .001; lumbar spine: β = −0.0036, P < .001; trunk: β = −0.0029, P < .001) (Table 2).
3.3. Factors influencing total BMD in DM and non-DM subjects
Table 3 shows the factors influencing total BMD in adults with diabetes and those without. In the DM group, univariate analysis revealed that gender, race, calcium, and overweight were significantly associated with total BMD. After adjusting for the other covariates, multivariate analysis showed that BAP, calcium, gender, race, and overweight were significantly associated with total BMD. Total BMD was significantly decreased when BAP was increased (β=−0.0042, P = .001), significantly decreased when calcium was increased (β= −0.2995, P = .004); and significantly decreased in overweight subjects compared with those with normal weight (β= −0.0621, P = .008). Also, total BMD was significantly increased in males compared with females (β=0.0952, P < .001), and was significantly increased in Blacks compared with other races (β=0.085, P = .011) (Table 3).
Table 3.
Influencing factors for total bone mineral density stratified by diabetic status (DM, non-DM).
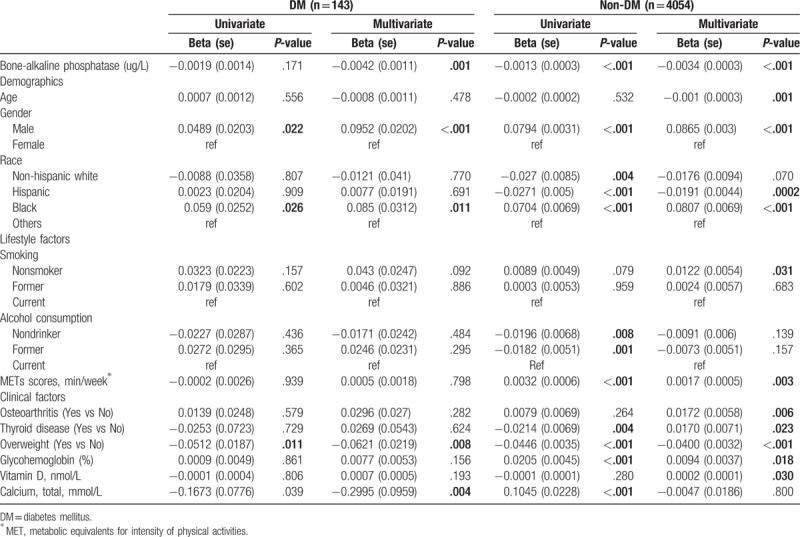
In the non-DM group, BAP, glycohemoglobin, calcium, gender, race, alcohol consumption, thyroid disease, overweight, and METs scores were significantly associated with total BMD in univariate analyses. After adjusting for the other covariates, multivariate analysis showed that BAP, age, glycohemoglobin, vitamin D, gender, race, smoking, osteoarthritis, thyroid disease, overweight, and METs scores were significantly associated with total BMD. The total BMD decreased significantly when BAP increased (β= −0.0034, P < .001); decreased significantly as age increased (β= −0.001, P = .001); was decreased significantly in overweight subjects compared with those of normal weight (β= −0.04, P < .001), increased significantly when glycohemoglobin increased (β=0.0094, P = .018); and increased significantly as vitamin D increased (β=0.0002, P = .03). Also, total BMD was significantly increased in males compared with females (β=0.0865, P < .001); was significantly decreased in Hispanics compared with other racial groups (β= −0.0191, P = .0002), but was increased in Blacks (β= 0.0807, P < .001). Total BMD was also significantly increased in subjects who never smoked compared with those who were smoking currently (β=0.0122, P = .031); was significantly increased in subjects with osteoarthritis compared with those without osteoarthritis (β=0.0172, P = .006); was significantly increased in subjects with thyroid disease compared with those without thyroid disease (β=0.017, P = .023); and was significantly increased when MetS scores increased (β= 0.0017, P = .003) (Table 3).
4. Discussion
BAP is the bone-specific isoform of the enzyme serum alkaline phosphatase. It is a glycoprotein found on the surface of osteoblasts and reflects the activity of these cells in bone metabolism.[19] As such, BAP has generally been considered a reliable indicator of bone metabolism. In bone remodeling, if the resorption rate is greater than the rate of bone formation, bone loss leads to metabolic bone disease (osteoporosis, Paget disease) and fractures.[19] However, although BAP is elevated in metabolic bone diseases, the numbers of osteoclasts are decreased in diabetic bone disease,[4] which might suggest that BAP would also be decreased in diabetic bone disease. Therefore, the question remains, is BAP associated with diabetic bone metabolism and increased risk of fracture?
To answer this question, the present study evaluated possible associations between BAP and BMD in adults with diabetes and without, finding that these 2 markers of bone metabolism have an inverse relationship in both populations—as one increases, the other is decreased, and vice versa. The absence of significant differences in BMD in diabetic and nondiabetic patients was also found by other authors who compared bone density between equal numbers of age-matched adults in both populations.[3] However, when associations between glucose metabolism, metabolic syndrome (MetS), and BAP were investigated, both glucose metabolism and MetS were significantly associated with serum BAP levels, which the authors suggest indicates BAP mediation of vascular calcification in diabetes and MetS, but may not indicate that BAP is a factor in bone metabolism.[9] In a study by Kang et al[11] increased serum ALP levels were associated with low BMD in patients with axial spondyloarthritis, which the authors attributed to inflammation; however, serum BAP levels were not associated with BMD, possibly indicating that BAP functions differently and plays an entirely different role than serum ALP in bone metabolism.
Not all studies have confirmed the value of BAP as a marker of bone formation, but this may be due to differences in study populations. When Lumachi et al[12] evaluated BAP along with other bone formation markers (osteocalcin, type I collagen and BMD) in elderly men with no history of fractures, they found no relationship at all between BAP and bone density in this population. In a similar comparison among elder Chinese women, Zhang et al[20] found a positive correlation between osteocalcin and BAP but a negative correlation with total and subregional BMD (at lumbar spine and total hip), suggesting that osteocalcin gene variants may not contribut to BMD in postmenopausal and elderly Chinese women. Those authors also found that BAP was a useful parameter for evaluating age-related changes in bone turnover. The present study also found that BAP and BMD were associated with age in diabetes and non-diabetes patients, and that total BMD decreased significantly as age increased and BAP increased; BMD was also significantly increased in males compared with females. Another report observed that higher bone turnover indicated by reduced serum levels of type I collagen correlated with increasing BMD in women aged over 40 years with surgical or medical menopause who were receiving hormone therapy, which was attributed to the continuous, rapid bone turnover rate in postmenopausal women.[21]
BAP is a marker of bone formation and may function differently in type 1 and type 2 diabetes. The fracture risk in type 1 diabetes is increased as a result of a decrease in BMD, and the impaired bone formation is a direct result of insulin deficiency and insulin-like growth factor; in type 2 diabetes, however, reduced bone quality attributed to obesity and insulin resistance increases risk for osteoporosis and fractures rather than BMD.[5] This is further supported by analysis of NHANES data showing that overweight is significantly associated with osteoporosis in older women.[22] In another analysis of NHANES data, differences in mean total and subregional BMD corresponded to fracture risk in study subjects within the same age ranges and gender.[23] In the Japanese population-based cohort study that assessed fracture risk in postmenopausal women with no diseases or medications affecting bone metabolism, BAP was found to predict vertebral fracture risk along with several other markers of bone turnover (osteocalcine, crosslinked telopeptide of type I collagen, and total and free deoxypyridinoline) independently of BMD. [24] The present study did not consider previous fractures and the results for BAP and BMD were only significantly associated in nondiabetes patients, but without confirmation of osteoporosis.
5. Limitations
This cross-sectional study has certain limitations, including that all data were examined retrospectively, limiting any attribution to causation, and all data except for physical examination were self-reported, which may result in response bias. In addition, the study population excluded institutionalized patients who may have lower bone density as a result of chronic illness, so results can only apply to the community population. Any indications of age patterns would require longitudinal data for confirmation even though trends may be reported reliably. Nevertheless, results of this study have the benefits of using a large nationally representative health survey of adults in a wide range of ages and 3 races.
6. Conclusions
BMD and BAP have an inverse relationship in both adults with diabetes and those without diabetes. Significant associations between BAP and BMD after controlling for other variables may suggest that BAP interacts with other factors to alter bone metabolism in DM patients. Further prospective study is needed to identify those factors and to again compare results with those of BMD. Results may help to further clarify the relationship between BAP and BMD in diabetes and to establish guidelines for the use of BAP and BMD in assessing fracture risk and preventive interventions.
Acknowledgments
The authors would like to thank Lili Deng for her valuable assistance.
Author contributions
Conceptualization: Hailing Chen.
Data curation: Hailing Chen, Jufen Li, Qian Wang.
Formal analysis: Hailing Chen, Jufen Li, Qian Wang.
Investigation: Hailing Chen, Qian Wang.
Project administration: Hailing Chen, Jufen Li.
Resources: Qian Wang.
Supervision: Hailing Chen.
Validation: Hailing Chen, Jufen Li, Qian Wang.
Writing – original draft: Hailing Chen, Jufen Li, Qian Wang.
Writing – review & editing: Hailing Chen, Jufen Li, Qian Wang.
Footnotes
Abbreviations: A1C = hemoglobin A1C, BAP = bone alkaline phosphatase, BMD = bone mineral density, DM = diabetes mellitus, DXA = dual-energy x-ray absorptiometry, MET = metabolic equivalents of task, MetS = metabolic syndrome.
Ethics: All NHANES data are de-identified and data analysis for research purposes does not require IRB approval or informed consent by participating subjects.
License to publish:
The authors have no funding and conflicts of interest to disclose.
References
- [1].Karsenty G. Themutual dependence between bone and gonads. J Endocrinol 2012;213:107–14. [DOI] [PubMed] [Google Scholar]
- [2].Dutta MK, Pakhetra R, Garg MK. Evaluation of bone mineral density in type 2 diabetes mellitus patients before and after treatment. Med J Armed Forces India 2012;68:48–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Asokan AG, Jaganathan J, Philiip R, et al. Evaluation of bone mineral density among type 2 diabetes mellitus patients in South Karnataka. J Nat Sci Biol Med 2017;8:94–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Al-Hariri M. Sweet bones: the pathogenesis of bone alteration in diabetes. J Diabetes Res 2016;2016:6969040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Jackuliak P, Payer J. Osteoporosis, fractures, and diabetes. Int J Endocrinol 2014;2014:820615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Tai V, Leung W, Grey A, et al. Calcium intake and bone mineral density: systematic review and meta-analysis. BMJ 2015;351:h4183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Perez-Diaz I, Sebastian-Barajas G, Hernandez-Flores ZG, et al. The impact of vitamin D levels on glycemic control and bone mineral density in postmenopausal women with type 2 diabetes. J Endocrinol Invest 2015;38:1365–72. [DOI] [PubMed] [Google Scholar]
- [8].Smith JK, Dykes R, Chi DS. The effect of long-term exercise on the production of osteoclastic and antiosteoclastogenic cytokines by peripheral blood mononuclear markers of bone metabolism. J Osteoporos 2016;2016:5925380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Cheung CL, Tan KC, Lam KS, et al. The relationship between glucose metaolism, metabolic syndrome, and bone-specific alkaline phosphatase: a structural equation modeling approach. J Clin Endocrinol Metab 2013;98:3856–63. [DOI] [PubMed] [Google Scholar]
- [10].Lim SM, Kim YN, Park KH, et al. Bone alkaline phosphatase as a surrogate marker of bone metastasis in gastric cancer patients. MC Cancer 2016;16:385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Kang KY, Hon YS, Park SH, et al. Increased serum alkaline phosphatase levels correlate with high disease activity and low bone mineral density in patients with axial spondyloarthritis. Semin Arthritis Rheum 2015;45:202–7. [DOI] [PubMed] [Google Scholar]
- [12].Lumachi F, Orlando R, Fallo F, et al. Relationship between bone formation markers bone alkaline phosphatase, osteocalcin and amino-terminal propeptide of type I collagen and bone mineral density in elderly men. Preliminary results. In Vivo 2012;26:1041–4. [PubMed] [Google Scholar]
- [13].About the National Health and Nutrition Examination Survey. 2/10/2014. 2017. Available at: http://www.cdc.gov/nchs/nhanes/about_nhanes.htm. Accessed April 10, 2017. [Google Scholar]
- [14].National Center for Health Statistics. Third National Health and Nutrition Examination Survey (NHANES III, 1988–1994): Multiply Imputed Data Set. CD-ROM, Series II, No. 7A. Documentation. Hyattsville, MD: Centers for Disease Control and Prevention, U.S. Department of Health and Human Services. 2001; Available at: http://www.cdc.gov/nchs/about/major/nhanes/datalink.htm. Accessed October 7, 2015. [Google Scholar]
- [15].Centers for Disease Control and Prevention National Center for Health Statistics. NHANES Analytic Guidelines. [cited 2015 October 7]. Available at: https:www.cdc.gov/nchs/data/nhanes/2003-2004/dxa/dxx_b.pdf. Accessed October 7, 2015. [Google Scholar]
- [16].Centers for Disease Control and Prevention. National Health and Nutrition Examination Survey (NHANES) Laboratory Procedures Manual. Hyattsville, MD: CDC, U.S. Department of Health and Human Services, 2004 [cited 2015 October 7]. Available at: https:www.cdc.gov/nchs/data/nhanes/2003-2004/labmethods/l11_c_met_bap.pdf. Accessed October 7, 2015. [Google Scholar]
- [17].Breslow RA, Guenther PM, Juan W, et al. Alcoholic beverage consumption, nutrient intakes, and diet quality in the US adult population, 1999–2006. J Am Diet Assoc 2010;110:551–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Ainsworth E, Haskell WL, Whitt MC, et al. Compendium of physical activities: an update of activity codes and MET intensities. Med Sci Sports Exerc 2000;32:S498–504. [DOI] [PubMed] [Google Scholar]
- [19].Bonnick S, De Villiers T, Odio A, et al. Effects of odanacatib on BMD and safety in the treatment of postmenopausal women previously treated with alendronate: a placebo-controlled trial. J Clin Endocrinol Metab 2013;98:4727–35. [DOI] [PubMed] [Google Scholar]
- [20].Zhang XY, He JW, Fu WZ, et al. Associations of serum osteocalcin and poly morphisms of the osteocalcin gene with bone mineral density in post-menopausal and elderly Chinese women. J Nutrigenet Nutrigenomics 2016;9:231–42. [DOI] [PubMed] [Google Scholar]
- [21].Srividhya NB, Singh N, Goel N, et al. Comparison of antiresorptive effect of hormone therapy and ibandonate in postmenopausal osteoporotic women by assessing type 1 collage C-telopeptide levels. Post Reprod Health 2015;21:48–55. [DOI] [PubMed] [Google Scholar]
- [22].Looker AC, Flegal KM, Melton LJ3rd. Impact of increased overweight on the projected prevalence of osteoporosis in older women. Osteoporos Int 2007;18:307–13. [DOI] [PubMed] [Google Scholar]
- [23].Looker AC, Melton LJ3rd, Harris T, et al. Age, gender, and race/ethnic differences in total body and subregional bone density. Osteoporos Int 2009;20:1141–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Tamaki J, Iki M, Kadowaki E, et al. JPOS Study Group. Biochemical markers for bone turnover predict risk of vertebral fractures in postmenopausal women over 10 years: the Japanese Population-based Osteoporosis (JPOS) Cohort Study. Osteoporos Int 2013;24:887–97. [DOI] [PubMed] [Google Scholar]


