Abstract
Introduction:
Azathioprine (AZA) is widely used as an immunosuppressive agent, and its efficacy has been recommended by many clinical studies. However, leukopenia, the most common toxicity, still restricts its clinical applications. Recent studies found that NUDT15 R139C polymorphism is strongly associated with AZA-induced leukopenia in Koreans. However, the follow-up studies available are all limited to inflammatory bowel disease (IBD). Here, we report a case of a Chinese patient with Sjögren syndrome (SS) with wild-type TPMT∗3C who was diagnosed with AZA-induced severe toxicity due to NUDT15 mutation based on clinical and laboratory characteristics.
Case presentation:
A 22-year-old Chinese woman with SS developed severe leukopenia after AZA administration for 21 days. Detection of 6-thioguanine nucleotides (6-TGN) showed that the erythrocyte concentration had beyond the monitoring range, indicating that severe leukopenia might be caused by AZA. Furthermore, gene sequencing showed that NUDT15 R139C (poor metabolizer) homozygosity might explain this adverse event. Based on the evidence, AZA administration was immediately stopped and supportive treatments provided, and the patient eventually recovered.
Conclusion:
In this report, we first provide detailed clinical and laboratory characteristics of AZA-induced leukopenia in a patient with SS with a mutant NUDT15 R139C genotype (TT allele) and normal TPMT activity. This case indicates that NUDT15 R139C and TPMT∗3C genotypes, and more importantly, 6-TGN levels, should be routinely monitored for those administered with AZA to predict and prevent AZA-induced toxicity.
Keywords: azathioprine, Chinese, NUDT15 R139C, severe leukopenia, Sjögren syndrome
1. Introduction
Azathioprine (AZA) is a thiopurine prodrug commonly used as an immunosuppressive agents in the treatment of autoimmune diseases, such as inflammatory bowel disease (IBD), Sjögren syndrome (SS), and other autoimmune diseases.[1] AZA is first nonenzymatically broken down to 6-mercaptopurine (6-MP), which is then converted to the predominant active metabolites 6-thioguanine nucleotides (6-TGN) by a key enzyme, thiopurine S-methyltransferase (TPMT).[2] Myelosuppression is the main toxicity induced by AZA, which presents as leukopenia, anemia, thrombocytopenia, and pancytopenia.[3] The leukopenia of AZA has been reported to be correlated with the erythrocyte level of 6-TGN.[4–5] In addition, the association between AZA-induced leukopenia and TPMT mutation is well established in Western patients, and a TPMT gene test before AZA exposure is recommended by the US Food and Drug Administration (FDA).[6] However, an increasing number of studies have found that the frequency of TPMT variation is approximately 0.9% in Chinese patients,[7] which is considerably lower than that found in European populations, but the incidence of AZA-induced leukopenia in the Chinese patient population is still high (27%–41.3%).[8] A recent study suggested that NUDT15 R139C (rs116855232), a gene that mediates the hydrolysis of some nucleoside diphosphate derivatives, was strongly associated with AZA-induced leukopenia in Koreans. Additionally, Zhu et al[9] reported an association between NUDT15 R139C and early leukopenia in Chinese patients with IBD.
SS is a long-term multisystem autoimmune disease that mainly affects the exocrine glands, with dryness of the main mucosal surfaces as the key symptom, and other symptoms including dry skin, chronic cough, and joint pains.[10] A regimen of corticosteroids combined with immunosuppressive drugs (e.g., cyclophosphamide, tacrolimus and AZA) was used to treat SS, and the guidelines recommend biologic therapy for SS patients with internal organ or systemic involvement.[11] This case reports a Chinese patient with SS with normal TPMT activity who developed AZA-induced severe leukopenia due to NUDT15 R139C variation.
2. Case presentation
A 22-year-old Chinese female patient was diagnosed with SS based on the American College of Rheumatology Classification Criteria (2012)[12] on January 27, 2015. Patient characteristics are listed in Table 1. She took 5 mg prednisone and 2 mg tacrolimus (FK506) every other day to treat SS before she revisited her physician on October 26, 2016. At that time, her proteinuria was still relatively high, indicating her disease was not controlled well by the previous medication. Given that the patient was in the childbearing period, the physician prescribed 50 mg/day AZA instead of FK506 because her genotype TPMT∗3C (rs1142345) was wild type (normal metabolizer). Unfortunately, severe leukopenia occurred after taking AZA for 21 days. All drugs were immediately stopped, and she was admitted to the rheumatology department on November 19, 2016. During her hospitalization, recombinant human granulocyte colony-stimulating factor (30 μg/day), red blood cells (2 U/day), and other supportive treatments were administered. Eventually, the patient recovered on day 10 and physician chose the biologic therapy as follow-up treatment. The results of her blood test during this period are shown in Table 2. Informed consent was obtained from the patient for publication of the case report.
Table 1.
Characteristics of the patient.
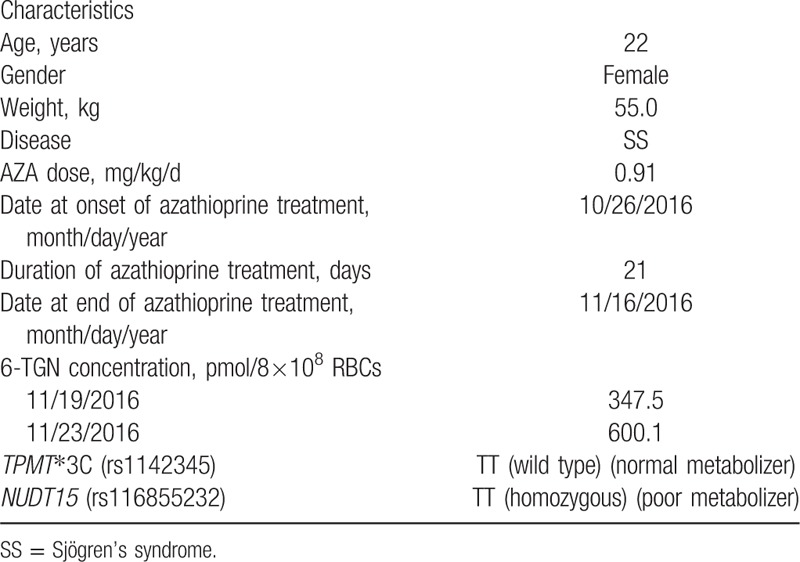
Table 2.
Routine blood counts during hospitalisation.
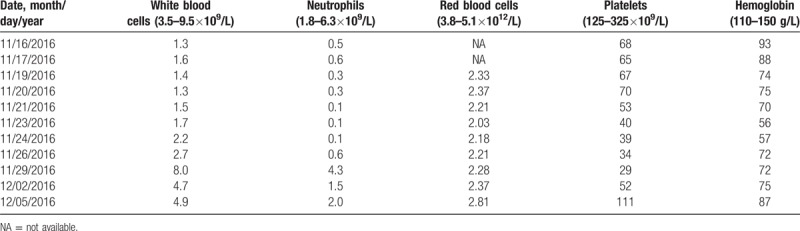
Bone marrow aspiration showed hypoplasia of granulocyte RBC and megakaryocytic series, indicating acute arrest of hemopoiesis. We suspected that severe leukopenia may be induced by AZA. On November 19, 2 mL of venous blood samples (EDTA anticoagulation) were obtained, and the 6-TGN concentration (347.5 pmol/8 × 108 RBCs) in the erythrocytes was measured using high performance liquid chromatography as previously described.[13] When 6-TGN was measured again on November 23, it was up to 600.1 pmol/8 × 108 RBCs. Furthermore, genotyping for NUDT15 R139C and TPMT∗3C was performed using Custom TaqManSNP genotyping assays in accordance with manufacturer's information. The NUDT15 R139C sequencing indicated that the patient was homozygous (TT), which is a poor metabolizer genotype of this enzyme (Fig. 1). Combined with the patient's clinical and laboratory characteristics, we were convinced that AZA contributed to her severe leukopenia.
Figure 1.
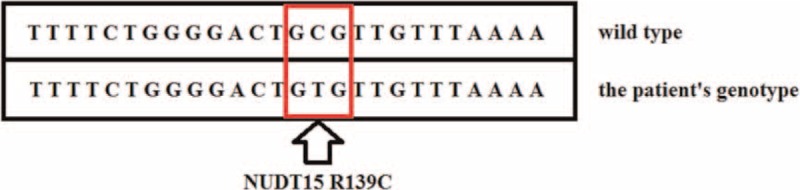
The patient's genotype of NUDT15 is homozygous (TT allele).
3. Literature review and discussion
To the best of our knowledge, one report has been published on primary biliary cirrhosis and autoimmune hepatitis in a patient with the NUDT15 R139C variant who developed severe leukopenia following AZA treatment.[14] In this report, we first provide detailed clinical and laboratory characteristics of AZA-induced leukopenia in a patient with SS with mutant (TT allele) NUDT15 R139C genotype and normal TPMT activity. Compared with TPMT∗3C, NUDT15 R139C maybe a promising biomarker that can be used to predict AZA-induced myelotoxicity.
TPMT plays a key role in AZA metabolism. Mutations in the enzyme cause abnormal metabolism and lead to accumulation of excess 6-TGN,[15] which can increase the risk of AZA-induced adverse reactions, particularly leukopenia.[16,17] Paradoxically, AZA-induced leukopenia is more common in Asians, and the mutant frequency is lower than the 10% prevalence of TPMT∗3C reported in the European population.[18] Single nucleotide polymorphisms (SNPs) of not only TPMT∗3C, but also NUDT15 R139C could account for thiopurine-induced toxicity in a variety of diseases.
A literature review showed that NUDT15 R139C gene was initially found in 2014 to have a strong association with AZA-induced leukopenia (odds ratio = 35.6; P = 4.8810–94).[19] Of 978, Korean patients with IBD, 346 (35.4%) developed leukopenia at a median AZA dose of 1.70 mg/kg/day, and all patients (14/14) homozygous for the NUDT15 R139C variant developed early leukopenia (occurrence of leukopenia before 8 weeks). In addition, 25.6% (45/176) and 50% (88/176) of patients who were heterozygous for the NUDT15 R139C variant developed early and late leukopenia (occurrence of leukopenia after 8 weeks), respectively. The association of NUDT15 R139C with AZA-induced leukopenia has subsequently been demonstrated by other IBD studies.[20–23] Besides, other studies in children with acute lymphoblastic leukemia (ALL) have recognized that the NUDT15 R139C variant increased the risk of developing thiopurine-induced toxicity (P < .00001) and identified a variant in NUDT15 R139C gene to be associated with intolerance of thiopurine dose.[17,24–26] Suzuki et al[27] found that NUDT15 R139C genotyping could be beneficial in estimating the tolerated dose of 6-MP for Japanese patients with childhood ALL, particularly during the preschool age (younger than 7 years) (P = .04). Furthermore, the variant frequency of NUDT15 R139C showed ethnic variability: 9.8% in East Asians, 4.1% in Hispanics, 0.2% in Europeans, and 0.0% in Africans.[24] Therefore, the NUDT15 R139C variant is common in East Asians and Hispanics and rare in Europeans and Africans.
NUDT15 is a member of the nudix hydrolase enzyme family, which consists mainly of pyrophosphohydrolases that act on nucleoside diphosphates linked to other moieties, X.[28] Moriyama et al[25] suggested that NUDT15 may prevent the incorporation of TGTP and TdGTP into the deoxyribonucleic acid by dephosphorylating the thiopurine-active metabolites TGTP and TdGTP and thus negatively affecting the desired cytotoxic effects of thiopurine in vivo. In vitro studies showed a higher percentage of apoptosis and necrosis in cells transfected with the NUDT15 R139C construct compared with cells with the wild type construct.[19] However, the specific role of NUDT15 R139C in toxicity induced by thiopurine remains unclear.
Additionally, increasing number of studies suggested that the 6-TGN concentration is correlated with clinical responses and adverse drug reactions. 6-TGN blood concentrations not only help physicians determine the patient's compliance to medication, but can also be used to timely detect AZA-induced adverse effects. Studies have recommended that the reasonable 6-TGN concentration monitoring range is approximately 235 to 450 pmol/8 × 108 RBCs for patients with IBD.[29] The patient's 6-TGN concentration was 347.5 pmol/8 × 108 RBCs when she stopped taking AZA for 3 days. However, after AZA was stopped for 7 days, the level increased up to 600.1 pmol/8 × 108 RBCs. The steady-state concentration of AZA is usually achieved after 6 to 8 weeks. The patient had been administered with AZA for 3 weeks; hence, the 6-TGN concentration was still accumulating at that time. Monitoring 6-TGN routinely is important and essential in detecting AZA-induced toxicity in time. However, we found that 6-TGN concentration in autoimmune diseases (such as systemic lupus erythematosus, SS, and others) is significantly lower than the monitoring range in IBD based on our studies (unpublished). The appropriate 6-TGN range for patients with autoimmune disease needs to be established in future. In contrast, Moriyama et al. suggested that NUDT15 may dephosphorylate the AZA-active metabolites TGTP and TdGTP rather than 6-TGN.[25] The new therapeutic monitoring method that detects the metabolites TGTP and TdGTP may have better clinical value than the one that detects 6-TGN.
4. Conclusion
We report a case of a Chinese patient with SS with normal metabolizer TPMT who developed AZA-induced severe toxicity due to NUDT15 R139C homozygosity (TT allele). Previous reports along with this study support the hypothesis that NUDT15 R139C variant is a factor for thiopurine toxicity. NUDT15 R139C and TPMT∗3C genotypes are recommended to be tested. Moreover, 6-TGN level should be monitored routinely to predict and prevent AZA-induced hematotoxicity.
Author contributions
Conceptualization: Xiang Fei.
Data curation: Xiang Fei.
Formal analysis: Xiang Fei, Qing Shu.
Funding acquisition: Weihong Ge.
Investigation: Xiang Fei.
Methodology: Xiang Fei.
Project administration: Xiang Fei, Bingzhu Hua, Shiying Wang, Zhiyong Chen.
Software: Xiang Fei.
Supervision: Yun Fang.
Validation: Xiang Fei.
Visualization: Xiang Fei.
Writing – original draft: Xiang Fei.
Writing – review & editing: Xiang Fei.
Footnotes
Abbreviations: 6-MP = 6-mercaptopurine, 6-TGDP = 6-thioguanine-diphosphate, 6-TGN = 6-thioguanine nucleotides, 6-TGTP = 6-thioguanine-triphopshate, AZA = azathioprine, FDA = the US Food and Drug Administration, FK506 = tacrolimus, IBD = inflammatory bowel disease, SNPs = single nucleotide polymorphisms, SS = Sjögren's syndrome, TPMT = thiopurine S-methyltransferase.
Ethical approval: This study was conducted in accordance with the ethical guidelines and approved by the research ethics committee of Drum Tower Hospital affiliated Nanjing University Medical School, Nanjing, China (2017-101-01). All methods were carried out in accordance with the approved guidelines.
The authors have no conflicts of interest to disclose.
References
- [1].Okon LG, Werth VP. Cutaneous lupus erythematosus: diagnosis and treatment. Best Pract Res Clin Rheumatol 2013;27:391–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Moon W, Loftus EV. Review article: Recent advances in pharmacogenetics and pharmacokinetics for safe and effective thiopurine therapy in inflammatory bowel disease. Aliment Pharmacol Ther 2016;43:863–83. [DOI] [PubMed] [Google Scholar]
- [3].Anstey A, Lear JT. Azathioprine: clinical pharmacology and current indications in autoimmune disorders. BioDrugs 1998;9:33–47. [DOI] [PubMed] [Google Scholar]
- [4].Cuffari C, Theoret Y, Latour S, et al. 6-Mercaptopurine metabolism in Crohn's disease: correlation with efficacy and toxicity. Gut 1996;39:401–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Dubinsky MC, Lamothe S, Yang HY, et al. Pharmacogenomics and metabolite measurement for 6-mercaptopurine therapy in inflammatory bowel disease. Gastroenterology 2000;118:705–13. [DOI] [PubMed] [Google Scholar]
- [6].Black AJ, McLeod HL, Capell HA, et al. Thiopurine methyl transferase genotype predicts therapy-limiting severe toxicity from azathioprine. Ann Intern Med 1998;129:716–8. [DOI] [PubMed] [Google Scholar]
- [7].Lee YJ, Hwang EH, Park JH, et al. NUDT15 variant is the most common variant associated with thiopurine-induced early leukopenia and alopecia in Korean pediatric patients with Crohn's disease. Eur J Gastroenterol Hepatol 2016;28:475–8. [DOI] [PubMed] [Google Scholar]
- [8].Connell WR, Kamm MA, Ritchie JK, et al. 5Bone marrow toxicity caused by azathioprine in inflammatory bowel disease: 27 years of experience. Gut 1993;34:1081–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Zhu X, Wang XD, Chao K, et al. NUDT15 R139C genotype is a determinant of thiopurines-induced leukopenia in Chinese patients with Crohn's disease. J Crohn's Colitis 2016;10:S475. [DOI] [PubMed] [Google Scholar]
- [10].Ramos-Casals M, Brito-Zeron P, Siso-Almirall A, et al. Primary Sjogren syndrome. BMJ 2012;344:e3821–13821. [DOI] [PubMed] [Google Scholar]
- [11].Ramos-Casals M, Tzioufas AG, Stone JH, et al. Treatment of primary Sjögren syndrome: a systematic review. JAMA 2010;304:452–60. [DOI] [PubMed] [Google Scholar]
- [12].Shiboski SC, Shiboski CH. Criteria for Sjögren syndrome: a data-driven, expert consensus approach in the Sjögren international collaborative clinical alliance cohort. Arthritis Care Res 2012;64:475–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Benson LA, Olson H, Gorman MP. Evaluation and treatment of autoimmune neurologic disorders in the pediatric intensive care unit. Semin Pediatr Neurol 2014;21:284–90. [DOI] [PubMed] [Google Scholar]
- [14].Ailing Z, Jing Y, Jingli L, et al. Further evidence that a variant of the gene NUDT15 may be an important predictor of azathioprine-induced toxicity in Chinese subjects: a case report. J Clin Pharm Ther 2016;41:572–4. [DOI] [PubMed] [Google Scholar]
- [15].Chouchana L, Narjoz C, Roche D, et al. Interindividual variability in TPMT enzyme activity: 10 years of experience with thiopurine pharmacogenetics and therapeutic drug monitoring. Pharmacogenomics 2014;15:745–57. [DOI] [PubMed] [Google Scholar]
- [16].Gearry RB, Barclay ML, Burt MJ, et al. Thiopurine drug adverse effects in a population of New Zealand patients with inflammatory bowel disease. Pharmacoepidemiol Drug Saf 2004;13:563–7. [DOI] [PubMed] [Google Scholar]
- [17].Tanaka Y, Kato M, Hasegawa D, et al. Susceptibility to 6-MP toxicity conferred by a NUDT15 variant in Japanese children with acute lymphoblastic leukaemia. Br J Haematol 2015;171:109–15. [DOI] [PubMed] [Google Scholar]
- [18].Collie-Duguid ES, Pritchard SC, Powrie RH, et al. The frequency and distribution of thiopurine methyltransferase alleles in Caucasian and Asian populations. Pharmacogenetics 1999;9:37–42. [DOI] [PubMed] [Google Scholar]
- [19].Yang S-K, Hong M, Baek J, et al. A common missense variant in NUDT15 confers susceptibility to thiopurine-induced leukopenia. Nat Genet 2014;46:1017–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Kakuta Y, Naito T, Onodera M, et al. NUDT15 R139C causes thiopurine-induced early severe hair loss and leukopenia in Japanese patients with IBD. Pharmacogenomics J 2015;16:1–6. [DOI] [PubMed] [Google Scholar]
- [21].Asada A, Nishida A, Shioya M, et al. NUDT15 R139C-related thiopurine leukocytopenia is mediated by 6-thioguanine nucleotide-independent mechanism in Japanese patients with inflammatory bowel disease. J Gastroenterol 2016;51:22–9. [DOI] [PubMed] [Google Scholar]
- [22].Shah SAV, Paradkar M, Desai D, et al. Nudt15 C415t variant as a predictor for thiopurine induced toxicity in Indian patients. J Gastroenterol Hepatol 2016;32:620–4. [DOI] [PubMed] [Google Scholar]
- [23].Zhu X, Wang XD, Chao K, et al. NUDT15 polymorphisms are better than thiopurine S-methyltransferase as predictor of risk for thiopurine-induced leukopenia in Chinese patients with Crohn's disease. Aliment Pharmacol Ther 2016;44:967–75. [DOI] [PubMed] [Google Scholar]
- [24].Yang JJ, Landier W, Yang W, et al. Inherited NUDT15 variant is a genetic determinant of mercaptopurine intolerance in children with acute lymphoblastic leukemia. J Clin Oncol 2015;33:1235–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Moriyama T, Nishii R, Perez-Andreu V, et al. NUDT15 polymorphisms alter thiopurine metabolism and hematopoietic toxicity. Nat Genet 2016;1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Zgheib NK, Akika R, Mahfoz R, et al. NUDT15 and TPMT genetic polymorphisms are related to 6-mercaptopurine intolerance in children treated for acute lymphoblastic leukemia at the Children's Cancer Center of Lebanon. Pediatr Blood Cancer 2017;64:146–50. [DOI] [PubMed] [Google Scholar]
- [27].Suzuki H, Fukushima H, Suzuki R, et al. Genotyping NUDT15 can predict the dose reduction of 6-MP for children with acute lymphoblastic leukemia especially at a preschool age. J Hum Genet 2016;61:797–801. [DOI] [PubMed] [Google Scholar]
- [28].Bessman MJ, Frick DN, O’Handley SF. The MutT proteins or ‘Nudix’ hydrolases, a family of versatile, widely distributed, ‘housecleaning’ enzymes. J Biol Chem 1996;271:25059–62. [DOI] [PubMed] [Google Scholar]
- [29].Osterman MT, Kundu R, Lichtenstein GR, et al. Association of 6-thioguanine nucleotide levels and inflammatory bowel disease activity: a meta-analysis. Gastroenterology 2006;130:1047–53. [DOI] [PubMed] [Google Scholar]


