Abstract
Background:
Local advanced cervical cancer (LACC) is a considerable health crisis for women, and neoadjuvant chemotherapy (NACT) followed by radical surgery has been a suggested therapy method. However, the correlation between the tumor treatment response to NACT and the prognosis of LACC remains controversial.
Methods:
A comprehensive meta-analysis was performed to precisely assess the prognostic role of the clinical response and pathological response to NACT for LACC. The included studies were identified using PubMed and Web of Science up to July 2017. Hazard ratios (HR) and corresponding 95% confidence intervals (95% CI) for overall survival (OS) and disease-free survival (DFS) were determined using Review Manager (version 5.3) and Stata (version 12).
Results:
A total of 13 publications of 4727 cases were included. The treatment clinical response rate ranged from 58.49% to 86.54%, and the pathological response rate was 7.5% to 78.81%. Our combined results suggested that a clinical response was favorable for OS (HR=3.36, 95% CI: 2.41–4.69) and DFS (HR=2.36, 95% CI: 1.82–3.06). Further, a pathological response predicts favorable OS (HR=5.45, 95% CI: 3.42–8.70) and DFS (HR=3.61, 95% CI: 2.0–6.52).
Conclusion:
The response to NACT, including the clinical and pathological response, was associated with a favorable prognosis for patients with LACC. However, the predictive value of this factor in clinical practice warrants further in-depth research.
Keywords: cervical cancer, clinical response, neoadjuvant chemotherapy, pathological response, prognosis
1. Introduction
Cervical cancer is a considerable health crisis for women; it is the fourth most common cancer worldwide and the fourth leading cause of cancer death.[1,2] Surgery, radiation, and chemotherapy were recommended as standard treatment approaches by the National Comprehensive Cancer Network (NCCN) and the International Federation of Gynecology and Obstetrics (FIGO) according to the disease stage at the time of diagnosis. For patients with FIGO stage Ia1 to IIa1 cervical cancer, surgery including radical hysterectomy and pelvic lymph node dissection was the preferred therapy method.[3] However, for disease of FIGO stage IIb and above, namely advanced stage cervical cancer, hysterectomy is not usually performed based on the newest guidelines.[4]
In some countries and clinical studies, selected cases of local advanced cervical cancer (LACC, stages Ib2, IIa2, and IIb) have been treated with neoadjuvant chemotherapy (NACT) following radical surgery.[5–7] Data from clinical studies suggested that NACT followed by surgery did not improve survival compared with surgery alone for LACC.[8] A previous meta-analysis conducted by a collaboration group that included 9 trials also indicated that NACT can result in downstaging, by not only decreasing tumor size, but also by controlling lymph node metastasis. However, NACT failed to improve the survival of patients with LACC.[5] These clinical data meant that the guidelines do not recommend the use of NACT for LACC.
Previous studies found that the NACT treatment response has been adopted as an explicit predictive marker for the long-term prognosis of several cancers, such as breast cancer.[9,10] Other studies also implied that achievement of a therapy response was an independent prognostic parameter for LACC treated with NACT. A study conducted by Buda et al retrospectively analyzed 446 patients with stages Ib2–IVa disease who were treated with NACT followed by radical surgery. Results suggested that patients with an optimal pathologic response had better overall survival (OS) (hazard ratio (HR) = 4.65, 95% confidence interval (95% CI): 2.75–7.85, P < .0001), implying that the NACT response is a predictive marker for long-term survival.[11] Another study from China of 853 patients with LACC (stage Ib2–IIb) who received NACT suggested that a clinical response was a predictive marker of a favorable prognosis (HR = 1.83, 95% CI: 1.18–2.85, P = .007).[12] However, the data and conclusions regarding the predictive value of the NACT response all came from sporadic and small studies. This was the case for both pathologic and clinical responses.
Thus, the purpose of this meta-analysis was to analyze the published data of NACT for LACC to determine the tumor treatment response, including the pathologic and clinical response. We aimed to determine whether this could predict long-term outcomes in LACC.
2. Materials and methods
2.1. Literature search
Eligible articles for this comprehensive meta-analysis were identified using the electronic databases of PubMed and Web of Science up to July 2017. Search terms including “cervical cancer;” “neoadjuvant chemotherapy or preoperative chemotherapy;” and “prognosis or survival” were researched in the title, abstract, or keywords of published articles. The references of the eligible publications were intensively reviewed to identify additional possible articles for inclusion.
2.2. Eligibility criteria
The eligible studies in this analysis were also required to meet all of the following criteria: NACT had to be administered for cervical cancer and followed by radical surgery; detailed survival data had to be reported for clinical or pathologic responders; the full text of original studies had to be written in English; and published articles must containing sufficient data to calculate the HR and 95% CI.
2.3. Qualitative assessment and data extraction
The quality of the included studies was evaluated by 2 investigators based on the Newcastle–Ottawa Scale (NOS).[13] Further, 2 other authors independently evaluated and extracted the data in a standardized manner. Any disagreements were resolved by discussion with another investigator. The following relevant data from each included article were extracted: first author, study country, publication year, total number of included patients, duration of follow-up, primary FIGO stage, percentages of patients achieving clinical and pathologic responses, NACT regimen, and survival data.
In order to avoid bias, the NACT clinical response was assessed based on the Response Evaluation Criteria in Solid Tumors (RECIST) criteria.[14] The clinical responses were classified as complete resolution (CR) and a partial response (PR). Meanwhile, the pathological response was determined by pathological analysis post-NACT and surgery. Pathological responders were defined as a pathological CR (pCR) and a PR, and a pathological PR was defined as cervical cancer residual disease with less than 3 mm stromal invasion.
2.4. Statistical analysis
The HR and 95% CI were utilized to assess the association between patients with LACC who achieved a clinical and pathologic response from NACT and long-term survival (OS and disease-free survival, DFS). A fixed effect model (Mantel–Haenszel method) was used when there was minimal heterogeneity among the eligible studies. When significant heterogeneity existed, the random effect model (DerSimonian–Laird method) was used. The I2 test was applied for the interstudy heterogeneity of the HRs. Potential publication bias was evaluated using the funnel plot with Begg and Egger tests. Review Manager (version 5.3, Cochrane Collaboration, Oxford) and Stata (version 12; Stata Corporation, College Station, TX) were used for statistical analyses.
3. Results
3.1. Characteristics of eligible studies
A total of 759 related publications were identified from the PubMed and Web of Science databases and their bibliographies of relevant articles. After titles and abstracts were reviewed, the full texts of the remaining 39 potential studies were reviewed. Twenty-six articles were excluded because they did not meet the selection criteria. Ultimately, 13 studies were included for the final meta-analysis[11,12,15–25] (Fig. 1).
Figure 1.
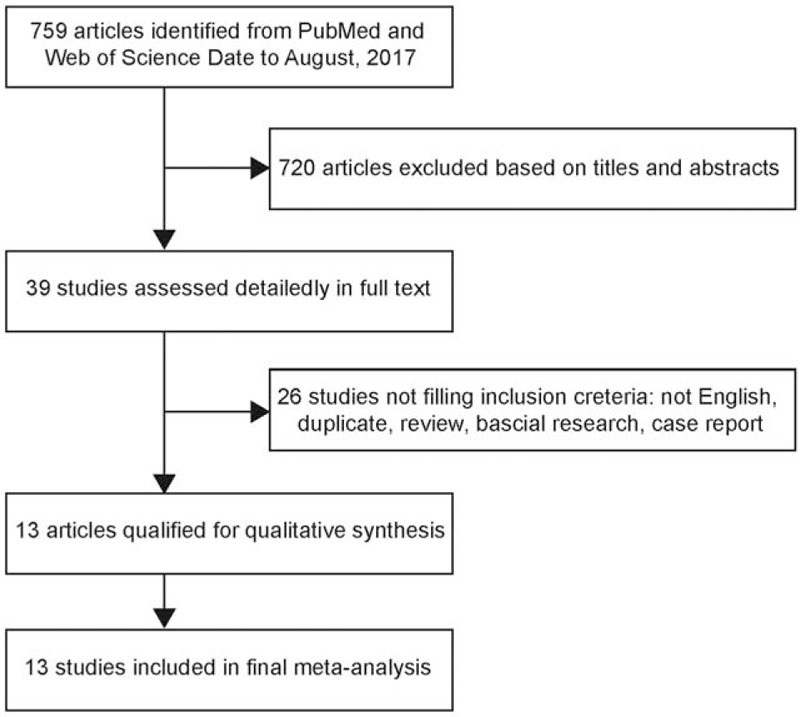
Flow diagram of study identification.
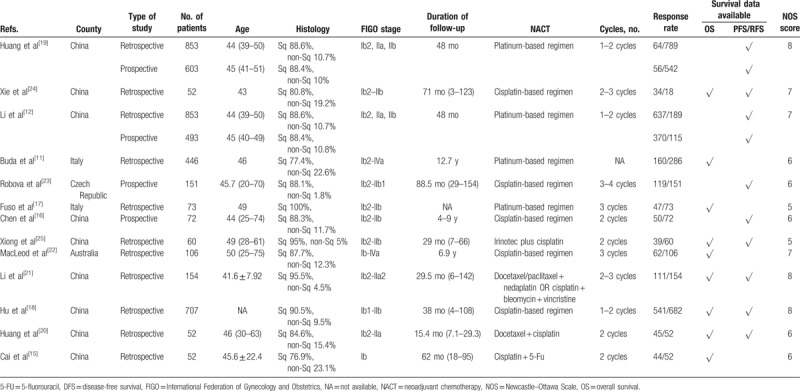
The baseline main traits of all included studies are listed in Table 1. In total, 4727 cases were included from 13 publications that provided the clinical and pathological response data from NACT. The number of patients in the eligible studies varied from 52 to 853. Most of the articles involved patients with FIGO stage Ib and IIb cervical cancer. According to the original studies, the pathological response rate after NACT was 7.5% to 78.81%, and the clinical response rate was 58.49% to 86.54%. The most used NACT regimen consisted of platinum and/or taxane-based chemotherapy.
Table 1.
Characteristics of the eligible studies.
3.2. Treatment response and cervical cancer OS
Published data of the relationship between pathologic response and OS were acquired from 2 of the 13 eligible studies and included 519 patients. The combined analysis showed that NACT pathologic responders could achieve favorable OS (pooled HR = 5.45, 95% CI = 3.42–8.70; P < .00001). A heterogeneity test was performed, and did not detect heterogeneity (I2 = 4%, P = .31). A fixed effect model was adopted (Fig. 2).
Figure 2.
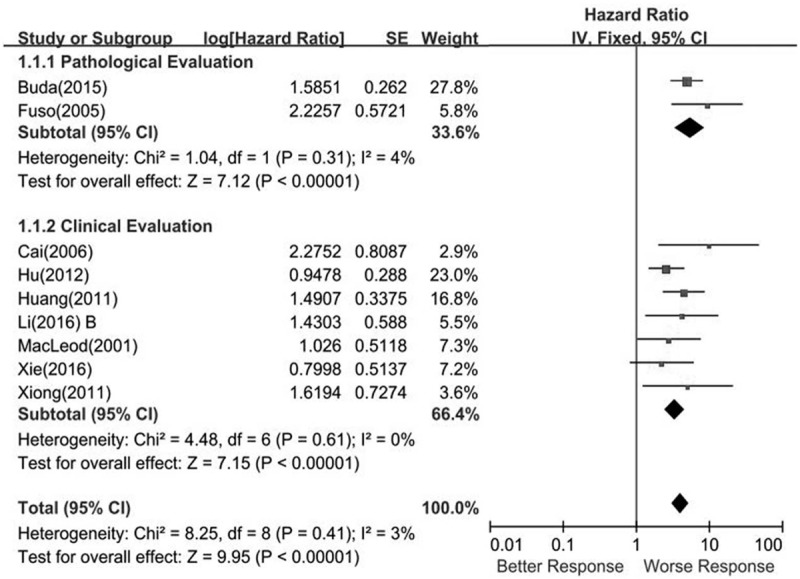
Forest plots showing the responsiveness of neoadjuvant chemotherapy for local advanced cervical cancer overall survival.
One thousand two-hundred fifty-five patients from 7 included studies provided the association between a clinical response and the OS data. Heterogeneity testing revealed I2 = 3%, showing that no heterogeneity existed (P = .41). In the fixed effects model, the summary HR was 3.36 (95% CI, 2.41–4.69; P < .00001) (Fig. 2).
3.3. Treatment response and cervical cancer DFS
Three studies including 1607 patients provided the association between pathologic response from NACT and DFS. Heterogeneity testing revealed I2 = 0% and P for heterogeneity .63. The HR was 3.61 (95% CI = 2.0–6.52; P < .00001), which showed that a better pathologic response was associated with favorable DFS (Fig. 3).
Figure 3.
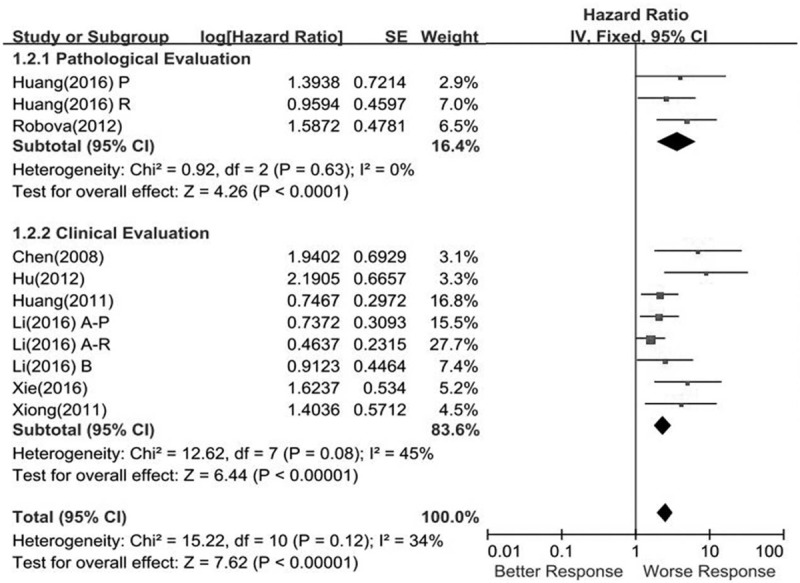
Meta-analysis of the correction between responsiveness of neoadjuvant chemotherapy on disease-free survival of local advanced cervical cancer.
Eight eligible publications showed the association between the clinical response and DFS data. Finally, 2443 patients were included in the pooled analysis. NACT responders showed an association with favorable DFS (pooled HR = 2.36, 95% CI = 1.82–3.06, P < .00001), and the results did not show heterogeneity (I2 = 45%, P = .08) (Fig. 3).
3.4. Sensitivity analysis and publication bias
Sensitivity analyses were conducted to estimate heterogeneity in this meta-analysis, and the analysis results showed no potential heterogeneity (data not shown). Publication bias was estimated by visual symmetry of funnel plots and the Egger test; the symmetric funnel plots indicated that no significant publication bias existed in this study (Fig. 4).
Figure 4.
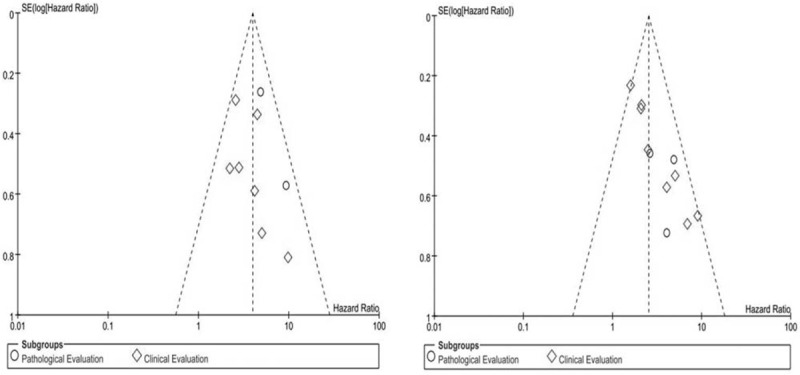
Funnel plot for the evaluation of potential publication bias in prognosis predict value of responsiveness of neoadjuvant chemotherapy for overall survival (A) and disease-free survival (B).
4. Discussion
Nowadays, the canonical therapy for cervical cancer is performed according to the 2009 FIGO staging system. Surgery or radiotherapy (RT) are optimized therapy methods for early stage cervical cancer.[4] In contrast, the appropriate therapy for LACC (stages Ib2, IIa2, and Ib) remains uncertain.[26] Major therapy options include radical surgery with or without postoperative RT; NACT as an alternative treatment method for LACC has also been applied for several years.[27] NACT helps to downstage tumors, increase their resectability, and potentially make young patients eligible for fertility-sparing surgery.[28] Despite the benefits, NACT could also conceal the primary pathologic features of the disease, such as vascular invasion and the number of involved lymph nodes.[12,29] A previous randomized controlled trial carried out by the Japan Clinical Oncology Group indicated that patients with stage Ib2, IIa2, or IIb cervical cancer who receive NACT before radical surgery are less likely to require postsurgery radiation therapy; however, it did not improve OS.[30] To date, the lack of large-scale randomized controlled studies and evidence-based medical evidence to support NACT means that NACT is not recommended in the NCCN guidelines for patients with LACC.
The NACT treatment response has been adopted as an explicit surrogate predictive marker of prognosis in many cancers, such as local advanced breast cancer (LABC). In contrast, for LACC, the prognosis predictive value of the NACT response has not been fully investigated. In this study, a pooled analysis about the prognostic predictive role of the NACT response in LACC (including the pathological and clinical response) was performed using 13 previously published studies containing 4727 cases. The treatment clinical response and the pathological response rates ranged from 58.49% to 86.54% and 7.5% to 78.81%, respectively; the treatment response indicated that LACC was sensitive to chemotherapy.
The treatment decisions for cervical cancer postsurgery were mostly dependent on postoperative pathological examination; however, for LACC post-NACT followed by radical surgery, it was hard to identify an alternative therapy according to the post-NACT pathological diagnosis. Therefore, it was necessary to identify prognostic indicators from NACT. Our combined results suggested that a clinical response to NACT was favorable for OS and DFS. Further, the pathological response to NACT predicts favorable OS and DFS. Hence, the NACT response could be a marker to predict survival and a marker for postsurgery treatment could be tailored to individual patients (Fig. 5).
Figure 5.
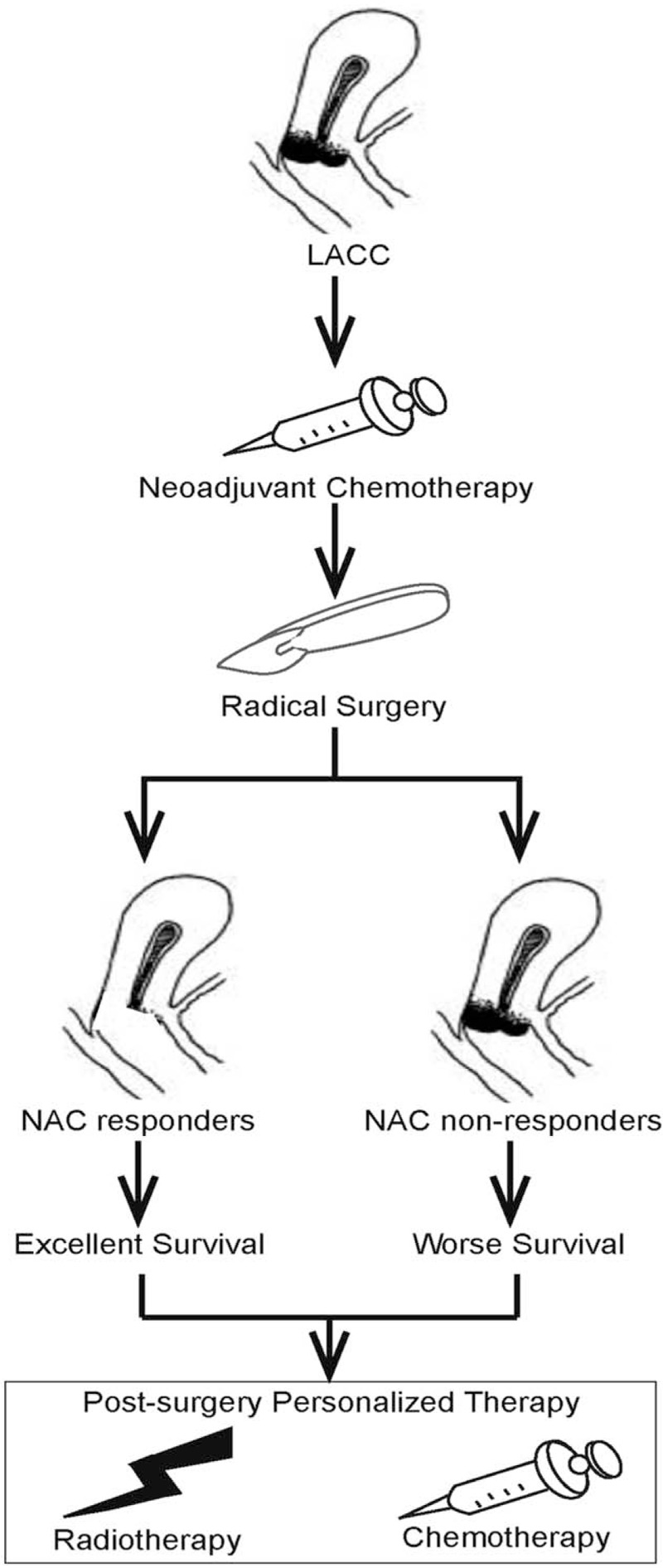
Overview of the neoadjuvant chemotherapy response could be tailored to individual patients for postsurgery treatment.
This study was properly designed using statistical analysis based on previous published studies, and no heterogeneity was observed when pooling the data. However, there were still some limitations. First, the majority of the included articles were retrospective. Second, 9 out of 13 studies were performed in China, which may have resulted in selection bias. Finally, great differences existed among the included studies. The chemotherapy regimens might be the main reason for response diversity; in NACT for LACC, a platinum-based regimen was used widely. Chemotherapeutic drugs, like irinotecan, docetaxel, nedaplatin, bleomycin, vincristine, and 5-fluorouracil (5-FU) were also used.
In conclusion, this meta-analysis demonstrated that the responsiveness to NACT, including the clinical and pathological response, was associated with a favorable prognosis for patients with LACC. Furthermore, better designed studies are required to clarify the prognostic predictive value of the NACT response in patients with LACC. This will also allow us to establish selective criteria for patients who would obtain the greatest survival benefit from NACT.
Acknowledgment
We wanted to acknowledge members of our research group for helpful insights.
Author contributions
Data curation: Yunshan Zhu, Xiao Zhang.
Writing—original draft: Yunshan Zhu.
Methodology: Jianhua Yang.
Writing—review and editing: Jianhua Yang, Songying Zhang.
Formal analysis: Danxia Chen.
Project administration: Songying Zhang.
Footnotes
Abbreviations: FU = 5-fluorouracil, 95% CI = 95% confidence intervals, CR = complete resolution, DFS = disease-free survival, FIGO = International Federation of Gynecology and Obstetrics, HR = hazard ratios, LACC = local advanced cervical cancer, NACT = neoadjuvant chemotherapy, NCCN = National Comprehensive Cancer Network, NOS = Newcastle–Ottawa Scale, OS = overall survival, pCR = pathological complete resolution, PR = partial response, RECIST = Response Evaluation Criteria in Solid Tumors, RT = radiotherapy.
Funding: This work was supported by the Key Research and Development Program of Zhejiang Province (2017C03022).
Ethical approval and patient consent: This study does not require ethical approval and patient consent because the study was a systematic review of previous studies and does not involve patients.
The authors have no conflicts of interest to disclose.
References
- [1].Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin 2017;67:7–30. [DOI] [PubMed] [Google Scholar]
- [2].Small W, Bacon MA, Bajaj A, et al. Cervical cancer: a global health crisis. Cancer 2017;123:2404–12. [DOI] [PubMed] [Google Scholar]
- [3].Brucker SY, Ulrich UA. Surgical treatment of early-stage cervical cancer. Oncol Res Treat 2016;39:508–14. [DOI] [PubMed] [Google Scholar]
- [4].Koh WJ, Greer BE, Abu-Rustum NR, et al. Cervical cancer, Version 2.2015. J Natl Compr Canc Netw 2015;13:395–404. [DOI] [PubMed] [Google Scholar]
- [5].Neoadjuvant Chemotherapy for Cervical Cancer Meta-Analysis Collaboration (NACCCMA) Collaboration. Neoadjuvant chemotherapy for locally advanced cervical cancer. Eur J Cancer 2003;39:2470–86. [DOI] [PubMed] [Google Scholar]
- [6].Lee J, Kim TH, Kim GE, et al. Neoadjuvant chemotherapy followed by surgery has no therapeutic advantages over concurrent chemoradiotherapy in International Federation of Gynecology and Obstetrics stage IB-IIB cervical cancer. J Gynecol Oncol 2016;27:e52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Gadducci A, Teti G, Barsotti C, et al. Clinicopathological variables predictive of clinical outcome in patients with FIGO stage Ib2-IIb cervical cancer treated with cisplatin-based neoadjuvant chemotherapy followed by radical hysterectomy. Anticancer Res 2010;30:201–8. [PubMed] [Google Scholar]
- [8].Ye Q, Yuan H-X, Chen H-L. Responsiveness of neoadjuvant chemotherapy before surgery predicts favorable prognosis for cervical cancer patients: a meta-analysis. J Cancer Res Clin Oncol 2013;139:1887–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Kong X, Moran MS, Zhang N, et al. Meta-analysis confirms achieving pathological complete response after neoadjuvant chemotherapy predicts favourable prognosis for breast cancer patients. Eur J Cancer 2011;47:2084–90. [DOI] [PubMed] [Google Scholar]
- [10].Petrelli F, Coinu A, Cabiddu M, et al. Correlation of pathologic complete response with survival after neoadjuvant chemotherapy in bladder cancer treated with cystectomy: a meta-analysis. Eur Urol 2014;65:350–7. [DOI] [PubMed] [Google Scholar]
- [11].Buda A, Lissoni AA, Floriani I, et al. Long-term clinical benefits of neoadjuvant chemotherapy in women with locally advanced cervical cancer. Int J Gynecol Cancer 2015;25:1468–75. [DOI] [PubMed] [Google Scholar]
- [12].Li X, Huang K, Zhang Q, et al. Early response to neoadjuvant chemotherapy can help predict long-term survival in patients with cervical cancer. Oncotarget 2016;7:87485–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13]. Wells GA Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses [webpage on the Internet] Ottawa. ON: Ottawa Hospital Research Institute. 2011 [Accessed February 5, 2013]. Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. [Google Scholar]
- [14].Tsuchida Y, Therasse P. Response evaluation criteria in solid tumors (RECIST): new guidelines. Med Pediatr Oncol 2001;37:1–3. [DOI] [PubMed] [Google Scholar]
- [15].Cai H-B, Chen H-Z, Yin H-H. Randomized study of preoperative chemotherapy versus primary surgery for stage IB cervical cancer. J Obstet Gynaecol Res 2006;32:315–23. [DOI] [PubMed] [Google Scholar]
- [16].Chen H, Liang C, Zhang L, et al. Clinical efficacy of modified preoperative neoadjuvant chemotherapy in the treatment of locally advanced (stage IB2 to IIB) cervical cancer: a randomized study. Gynecol Oncol 2008;110:308–15. [DOI] [PubMed] [Google Scholar]
- [17].Fuso L, Mazzola S, Marocco F, et al. Pretreatment serum hemoglobin level as a predictive factor of response to neoadjuvant chemotherapy in patients with locally advanced squamous cervical carcinoma: a preliminary report. Gynecol Oncol 2005;99:S187–91. [DOI] [PubMed] [Google Scholar]
- [18].Hu T, Li S, Chen Y, et al. Matched-case comparison of neoadjuvant chemotherapy in patients with FIGO stage IB1-IIB cervical cancer to establish selection criteria. Eur J Cancer 2012;48:2353–60. [DOI] [PubMed] [Google Scholar]
- [19].Huang K, Sun H, Chen Z, et al. Optimal pathological response indicated better long-term outcome among patients with stage IB2 to IIB cervical cancer submitted to neoadjuvant chemotherapy. Sci Rep 2016;6:28278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Huang X, Lan C, Huang H, et al. Neoadjuvant docetaxel combined with cisplatin and followed by radical surgery for the treatment of locally advanced (stage IB2–IIB) cervical cancer: preliminary results of a single-institution experience. Expert Opin Pharmacother 2011;12:165–73. [DOI] [PubMed] [Google Scholar]
- [21].Li R, Lu S-t, Si J-g, et al. Prognostic value of responsiveness of neoadjuvant chemotherapy before surgery for patients with stage IB2/IIA2 cervical cancer. Gynecol Oncol 2013;128:524–9. [DOI] [PubMed] [Google Scholar]
- [22].MacLeod C, O’Donnell A, Tattersall MH, et al. Locally advanced cervix cancer: chemotherapy prior to definitive surgery or radiotherapy. A single institutional experience. Australas Radiol 2001;45:491–5. [DOI] [PubMed] [Google Scholar]
- [23].Robova H, Rob L, Halaska MJ, et al. High-dose density neoadjuvant chemotherapy in bulky IB cervical cancer. Gynecol Oncol 2013;128:49–53. [DOI] [PubMed] [Google Scholar]
- [24].Xie Q, Liang J, Rao Q, et al. Aldehyde dehydrogenase 1 expression predicts chemoresistance and poor clinical outcomes in patients with locally advanced cervical cancer treated with neoadjuvant chemotherapy prior to radical hysterectomy. Ann Surg Oncol 2015;23:163–70. [DOI] [PubMed] [Google Scholar]
- [25].Ying X, Li-zhi L, Li-ping C, et al. Clinical effects of irinotecan hydrochloride in combination with cisplatin as neoadjuvant chemotherapy in locally advanced cervical cancer. Gynecol Oncol 2011;123:99–104. [DOI] [PubMed] [Google Scholar]
- [26].Marchetti C, De Felice F, Pinto AD, et al. Survival nomograms after curative neoadjuvant chemotherapy and radical surgery for stage IB2-IIIB cervical cancer. Cancer Res Treat 2017;DOI: 10.4143/crt.2017.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Lapresa M, Parma G, Portuesi R, et al. Neoadjuvant chemotherapy in cervical cancer: an update. Expert Rev Anticancer Ther 2015;15:1171–81. [DOI] [PubMed] [Google Scholar]
- [28].Robova H, Rob L, Halaska MJ, et al. Review of neoadjuvant chemotherapy and trachelectomy: which cervical cancer patients would be suitable for neoadjuvant chemotherapy followed by fertility-sparing surgery? Curr Oncol Rep 2015;17:446. [DOI] [PubMed] [Google Scholar]
- [29].Kim HS, Sardi JE, Katsumata N, et al. Efficacy of neoadjuvant chemotherapy in patients with FIGO stage IB1 to IIA cervical cancer: an international collaborative meta-analysis. Eur J Surg Oncol 2013;39:115–24. [DOI] [PubMed] [Google Scholar]
- [30].Katsumata N, Yoshikawa H, Kobayashi H, et al. Phase III randomised controlled trial of neoadjuvant chemotherapy plus radical surgery vs radical surgery alone for stages IB2, IIA2, and IIB cervical cancer: a Japan Clinical Oncology Group trial (JCOG 0102). Br J Cancer 2013;108:1957–63. [DOI] [PMC free article] [PubMed] [Google Scholar]


