Abstract
Introduction:
Systemic Bartonella spp. infections are being increasingly reported in association with complex medical presentations. Individuals with frequent arthropod exposures or animal contact appear to be at risk for acquiring long standing infections with Bartonella spp.
Case report:
This case report describes infections with Bartonella koehlerae and Bartonella henselae in a female veterinarian whose symptoms were predominantly rheumatologic in nature. Infection was confirmed by serology, polymerase chain reaction (PCR), enrichment blood culture, and DNA sequencing of amplified B koehlerae and B henselae DNA. Long-term medical management with antibiotics was required to achieve elimination of these infections and was accompanied by resolution of the patient's symptoms. Interestingly, the patient experienced substantial improvement in the acquired joint hypermobility mimicking Ehlers–Danlos Syndrome (EDS) type III.
Conclusion:
To facilitate early and directed medical interventions, systemic bartonellosis should potentially be considered as a differential diagnosis in patients with incalcitrant rheumatological symptoms and frequent arthropod exposures or extensive animal contact.
Keywords: Bartonella henselae, Bartonella koehlerae, bartonellosis, breast cyst, EDS Ehlers–Danlos, joint laxity, serology
1. Introduction
In addition to historically important Bartonella bacilliformis (Carrion's disease) and Bartonella quintana, (Trench Fever), the genus Bartonella now comprises numerous (38 named and candidatus species) emerging, zoonotic pathogens. Veterinarians and others with extensive arthropod and animal exposures appear to be at occupational risk for acquiring Bartonella infections, including but not limited to Bartonella henselae and Bartonella koehlerae.[1–3] Improved, sensitive diagnostic modalities continue to enhance recognition of an increasingly diverse spectrum of disease manifestations associated with bartonellosis. In this case report, we document B koehlerae and B henselae infection using serological and molecular diagnostic methods in a previously healthy veterinarian who developed extensive tenosynovitis, snapping elbow, and acquired joint hypermobility mimicking Ehlers–Danlos Syndrome type III (EDS type III). Clinical and microbiological results emphasize the diagnostic and therapeutic challenges associated with the patient's medical management.
2. Case report
In 2010, a 31-year-old female veterinarian with a progressive, 2-year history of rheumatologic and orthopedic symptoms elected to enter a Bartonella research study (North Carolina State University Institutional Review Board Protocol #1960, Detection of Bartonella Species in the Blood of Healthy and Sick People). Recent evaluations by rheumatologists and EDS experts at Harvard and Johns Hopkins Hospitals reported that the patient met criteria for EDS, hypermobility type III. Genetic testing was not performed (as there is no genetic lesion known presently to be associated with EDS type III).
MRI studies had documented extensor digitorum and extensor carpi tendinosis, extensor carpi radialis tenosynovitis, a radial head bone cyst, and mild degenerative joint disease involving the cervical spine. Rheumatologic screening tests for systemic lupus erythematosus, anticardiolipin antibodies, and rheumatoid factor were negative. Erythrocyte sedimentation rate, vitamin D levels, thyroid stimulating hormone, and liver function tests were within reference ranges. Infectious disease testing for Parvovirus B, Borrelia burgdorferi, B henselae, B quintana, and Ehrlichia chaffeensis antibodies were negative. An echocardiogram was normal. The patient was treated with various NSAIDS, topical analgesics physical therapy and splints without substantial benefit. Other treatments had included daily cetirizine, daily supplements (fiber, B12, vitamin D, a multivitamin, vitamin C, glucosamine, creatinine, coenzyme Q10, flax oil, fish oil), daily topical tazarotene cream and benzoyl peroxide, clindamycin, intermittent calcium carbonate antacids, and omeprazole.
Dating from childhood, the woman had an extensive history of exposure to various companion animals (cats, dogs, birds, and reptiles), production animals (cattle, goats, poultry, swine, and sheep) and wildlife (rescue and rehabilitation activities) and their associated arthropod or insect vectors that could have served as sources for Bartonella species transmission. Prior medical history included axillary lymphadenopathy from cat scratch disease (CSD) at 12 years of age, a tibial sesamoid bone fracture at 21 years of age and plantar fasciitis at 29 years of age (running injury). At the time of study entry, the veterinarian was no longer able to perform daily living or employment activities. Symptoms reported on the study questionnaire included generalized muscle/joint pain, muscle weakness, headaches, tingling, and fatigue. In 2009, the previously healed sesamoid bone re-fractured and had not re-healed. Newly noted joint hypermobility had progressively worsened (Beighton score 7/9), and the woman experienced multiple joint subluxations daily. Breast cysts, meeting criteria for benign classification, were previously diagnosed.
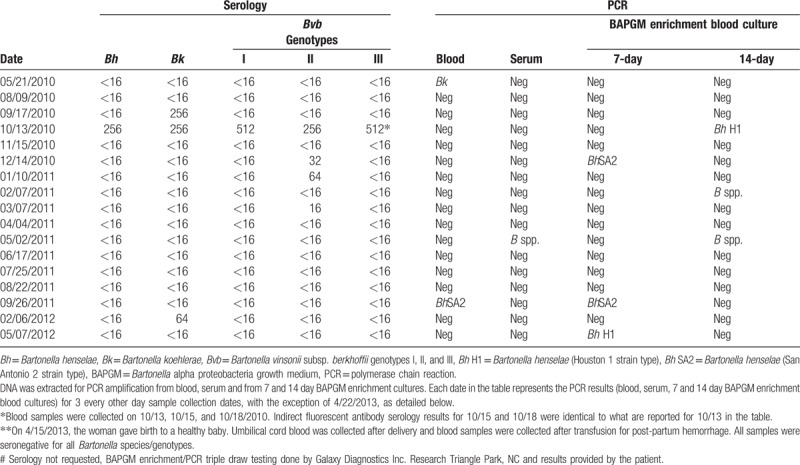
In May 2010, 3 blood sample sets were drawn over a 7-day period. Blood was collected aseptically into ethylenediaminetetraacetic acid (EDTA)-anticoagulated and serum separator tubes (SST) for shipment to the Intracellular Pathogens Research Laboratory (IPRL), Comparative Medicine Institute, College of Veterinary Medicine, North Carolina State University for Bartonella testing. Throughout treatment, the patient was sequentially followed by indirect fluorescent antibody testing (IFA) using a panel of Bartonella species antigens and BAPGM (Bartonella alpha proteobacteria growth medium) enrichment blood culture/PCR, as previously described.[1–3] Amplicon identity was confirmed by DNA sequencing in a commercial laboratory (GENEWIZ Inc. 7030 Kit Creek Rd Suite 120, Research Triangle Park, NC 27709.). Three blood sample sets were submitted (triple draw) at each testing time point throughout this study to enhance Bartonella spp. detection by BAPGM enrichment PCR (ePCR).[4] A breast cyst was aspirated for Bartonella PCR testing. Bacterial species and genotype were defined by examining similarities to other sequences deposited in the GenBank database using the basic local alignment search tool (BLAST; version 2.0). DNA extraction, PCR, and uninoculated BAPGM culture controls remained negative throughout the course of this study
At study entry, B koehlerae DNA was amplified and sequenced (219/221 bp similarity GenBank B koehlerae AF312490) from blood, with substantially less sequence similarity to B henselae (Houston I strain NC005956, 170/221 bp). BAPGM enrichment blood culture/PCR results were negative. By IFA testing, her serum was not reactive to any of 5 Bartonella spp. antigens, including B koehlerae. Initial and subsequent serological and molecular microbiological findings are provided in Table 1.
Table 1.
Bartonella species serology and PCR results from blood, serum and BAPGM enrichment blood culture for a veterinarian with B koehlerae and B henselae bacteremia, joint laxity, and articular pannus formation.
On presentation to the primary author (BRM) in June 2010, the patient was wearing bilateral wrist and elbow braces. She had cervical lymph node enlargement, extremity edema, ligamentous laxity, tenosynovitis, shoulder and elbow subluxations, and elbow joint crepitus. Immediately prior to this examination, she was being considered for surgical interventions. In view of the joint crepitance and “popping” reproduced with each articulation, her findings of joint subluxation were consistent with mechanisms of meniscal dislocation, articular plica or pannus. In the context of EDS, skin elasticity was normal.
Based on the positive B koehlerae PCR and because veterinarians are occupationally at-risk for acquiring B koehlerae infections,[1–3] she was treated with azithromycin, rifampin, and minocycline. Four weeks after starting antibiotics, joint pain was decreased. By August 2010, joint hypermobility had resolved (Beighton score 0/9) and the sesamoid bone had united. Retesting in September 2010 confirmed B koehlerae-specific seroconversion with no cross-reactivity to the other Bartonella spp. antigens. Three weeks after sustaining fleabites and while continuing on antibiotic therapy, she seroconverted to all 5 Bartonella spp./genotype antigens, and a 14-day BAPGM enrichment blood culture contained 2 B henselae strains (388/389 bp similarity GenBank B henselae NC005956, and 387/389 bp similarity GenBank B henselae CAL1 AF369527). By November 2010, she had seroreverted (nonseroreactive to all test antigens), but BAPGM ePCR blood cultures were negative. In December 2010, the patient remained seronegative, despite PCR amplification of B henselae DNA (406/406 bp similarity GenBank B henselae San Antonio2 AF369529) from a 14-day BAPGM enrichment blood culture. During the same time frame, 2 other B henselae strains (347/347 bp similarity GenBank B henselae NC_005956 and B henselae CAL-1 (346/347 bp similarity GenBank B henselae AF369527) were PCR amplified and sequenced from a breast cyst aspirate.
To assess progress of joints during treatment, a musculoskeletal ultrasound with power Doppler in December 2010 indicated bilateral (R>L) epicondylitis and a normal flow pattern with no evidence for synovitis. Based upon sequential serological testing, Bartonella spp. IFA antibodies were not detected throughout 2011, whereas Bartonella PCR was positive in February (14-day enrichment blood culture) and May (blood and 14-day enrichment blood culture); however, efforts to sequence these 3 amplicons were not successful, presumably due to the low quantity of amplified Bartonella DNA. In September 2011, B henselae (407/407 bp similarity GenBank B henselae AF369529) DNA was again PCR amplified and successfully sequenced from both blood and 7-day BAPGM enrichment blood culture. In 2012, with the exception of PCR amplification of B henselae from a May 7-day enrichment blood culture, all serology and BAPGM enrichment blood culture/PCR results were negative. Due to persistent bacteremia during antibiotic therapy, her regimen was changed to clindamycin and rifampin.
In August 2012, antibiotic therapy (clindamycin and rifampin) was discontinued for pregnancy. The woman subsequently delivered a healthy baby. At parturition, serology and BAPGM enrichment blood culture of umbilical cord blood and the mother's blood were negative. A repeat musculoskeletal ultrasound with power Doppler in May 2014 indicated bilateral elbow extensor tenosynovitis and right antero-lateral elbow meniscus subluxation, with a widened joint space during articulation, thereby implicating (but not directly visualizing) an intra-articular mass lesion, such as a plica. Retesting of the mother's blood in December 2016, serology, and BAPGM/ePCR were negative and she has not experienced recurrence of rheumatologic symptoms as of February 2018.
3. Discussion
An expanding spectrum of disease manifestations are being associated with the genus Bartonella.[5] In this patient, clinical, microbiological and therapeutic results suggest that Bartonella spp. may play a role in the pathogenesis of joint hypermobility. In addition to reporting fatigue, muscle pain and joint pain, the veterinarian in this case report experienced a progressive increase in joint laxity resulting in a diagnosis at 2 major medical centers of hypermobile Ehlers–Danlos syndrome (EDS type III). Recent research supports a potential role for mast cell activation and dysregulation in a subset of nongenetically mediated EDS patients with joint hypermobility syndrome.[6] In addition to the lung and gastrointestinal tract, mast cells are prevalent in cutaneous tissues throughout the body.[6] In the context of a plausible pathogenesis, a long-standing Bartonella spp. infection, accompanied by chronic mast cell activation could potentially contribute to ongoing damage to connective tissues; thereby resulting in clinical findings indicative of EDS.
Medical management decisions for this patient were based upon sequential and simultaneous Bartonella spp. serology, PCR, and enrichment blood culture results. Currently, there is minimal data upon which to base diagnostic testing recommendations or effective treatment modalities for patients with chronic rheumatological manifestations, which have been reported in a subset of patients after diagnosis of B henselae-induced CSD[7] and in nonimmunocompromised patients that were confirmed bacteremic by PCR/enrichment blood culture testing.[8,9] Similar to our patient, joint and muscle pain are among complaints reported by Bartonella bacteremic animal health workers.[1–3] Recently, B henselae was documented by immunohistochemistry and BAPGM enrichment culture in a surgically excised femoral head and the synovium from a veterinarian undergoing hip replacement due to osteoarthritis.[9] The published medical literature regarding B koehlerae is sparse, in part due to limitations in documenting infection with this species using currently available serology, PCR, or enrichment blood culture approaches. In a previously published case series that included 4 veterinarians infected with B koehlerae, fatigue, joint pain, and muscle pain were frequently reported rheumatological symptoms.[1] Due to extensive animal contact and arthropod exposures, veterinary workers may represent a sentinel study population to better characterize the clinical spectrum and medical importance of the genus Bartonella.
When, how, and how often this veterinarian became infected with each Bartonella spp. and strain type (amplified from blood, enrichment cultures, and breast cyst) is unknown. Persistent and potentially relapsing Bartonella bacteremia is a well-documented phenomenon in reservoir hosts and can occur in healthy[10] or chronically ill humans.[1–2] Therefore, it is possible B koehlerae transmission occurred in 1990 in association with CSD axillary lymphadenopathy. Interestingly in the context of this patient's medical history, previous studies have reported CSD cases presenting as solitary masses in the breast; infection with B quintana mimicking inflammatory breast cancer in a 50-year-old woman; and B henselae isolation in pleural fluid cell cultures derived from 2 patients with metastatic inflammatory breast cancer (IBC) (Fernandez SV, L Aburto, RG Maggi, EB Breitschwerdt, M Critofanilli: Bartonella henselae infection detected in patients with inflammatory breast cancer. Thirty-fifth Annual CTRC-AACR San Antonio Breast Cancer Symposium, San Antonio, December 2012). In the patient in this study, B henselae DNA was amplified and sequenced from the biopsy of a breast cyst that was reportedly static for 6 years prior to study entry. The cyst may have been one source for recurrent bacteremia.
Based upon sequential serology and BAPGM enrichment blood culture findings, it is also possible that this veterinarian became infected with one or more B henselae strains from fleabites. If correct, flea transmission of B henselae to humans may occur independent of a cat scratch[11] and neither concurrent antibiotic therapy nor B. koehlerae antibodies prevented transmission or development of B henselae bacteremia. Experimentally, cats and dogs infected with one B henselae strain type do not develop protective immunity when challenged with a different Bartonella spp. or B henselae strain type.[12,13] Alternatively, infection with B koehlerae and multiple B henselae strains may have occurred prior to the fleabite episode. Fluctuations in antibody levels may have been caused by immune complex formation and immuno-precipitation, due to changes in antibody-antigen stoichiometry occurring during treatment and immune recovery.[14]
Consistent with previous studies[1–3] in which a subset of patients with persistent bacteremia were not IFA seroreactive to a panel of Bartonella spp. antigens, this patient was not initially B koehlerae or B henselae IFA seroreactive. However, within 4 weeks of antibiotic administration, the woman seroconverted to B koehlerae, the species previously amplified and sequenced from her blood. Dogs that were naturally infected with B koehlerae and subsequently experimentally infected with either B henselae or B vinsonii subsp. berkhoffii were consistently seroreactive to only B koehlerae (prior to challenge) or only to B koehlerae and the challenge Bartonella species post-challenge.[13] Similarly, a veterinarian seroconverted specifically to B vinsonii subsp. berkhoffii genotypes I and III after putative needle stick transmission of these 2 genotypes from an infected dog.[15] Although data upon which to base any conclusion is limited, both dogs and humans appear to mount a Bartonella species-specific humoral antibody response, at least during the early stages of infection.
To enhance Bartonella spp. detection by BAPGM ePCR, 3 blood sample sets were submitted (triple draw) at each testing time point throughout this study.[4] This approach allowed us to confirm identical IFA serology results in 3 independently collected sera at each testing time interval between May and December 2010 (data not shown), thereby supporting both the seroconversion and seroreversion patterns reported in Table 1. The data from this patient and previously published studies[1–4] emphasize diagnostic limitations of IFA serology and suggest that enrichment blood culture and PCR should be used in conjunction with pre- and post-treatment Bartonella spp. serology when attempting to confirm bacteremic infection with a Bartonella spp. and during follow-up patient assessments.
As the veterinarian in this study made every effort to avoid possible re-infection, repeated documentation of B henselae DNA over 2-year period most likely reflected several possible modes of antibiotic treatment failures, such as limited antibiotic efficacy with intermittent breakthrough bacteremia, poor tissue penetration, or intermittent release from tissues such as cysts. Therapeutic elimination of Bartonella bacteria has been difficult to achieve in a subset of patients. We have previously described veterinarians who failed protracted courses of one or more antibiotics. Because isolation of Bartonella spp. from persistently bacteremic patients remains insensitive, in vitro antibiotic susceptibility testing cannot usually be attained and is, therefore, not clinically available to determine resistance patterns. Research is needed to determine the extent to which Bartonella spp. may colonize collagen and/or bone and if persistent infection and inflammation can contribute to the pathogenesis of hypermobility (EDS) that may include progressive joint pain, tendinosis, and meniscal instability.
4. Conclusion
Patients with rheumatologic symptoms occurring in conjunction with other constitutional symptoms should have a thorough medical history interview that considers risk factors for acquiring Bartonella spp. infections. In addition, individuals with frequent arthropod exposures and extensive animal contact with rheumatic manifestations including joint symptoms should consider occupational screening to facilitate early medical interventions.^^
Acknowledgements
We would like to thank the veterinarian described in this report for her study participation and review of manuscript content.
Author contributions
Conceptualization: Robert Mozayeni.
Data curation: Edward Breitschwerdt, Ricardo Maggi, Julie Bradley.
Formal analysis: Ricardo Maggi.
Investigation: Robert Mozayeni, Ricardo Maggi, Julie Bradley.
Methodology: Edward Breitschwerdt, Ricardo Maggi, Julie Bradley.
Project administration: Edward Breitschwerdt.
Supervision: Edward Breitschwerdt.
Validation: Ricardo Maggi, Julie Bradley.
Visualization: Robert Mozayeni.
Writing – original draft: Edward Breitschwerdt.
Writing – review & editing: Edward Breitschwerdt, Robert Mozayeni, Ricardo Maggi, Julie Bradley.
Footnotes
Abbreviations: B. = Bartonella, BAPGM = Bartonella alpha proteobacteria growth medium, bp = base pairs, BRM = B. Robert Mozayeni, Bvb = Bartonella vinsonii subsp. berkhoffii, C. felis = Ctenocephalides felis, CSD = cat scratch disease, DNA = deoxyribonucleic acid, EDS = Ehlers–Danlos syndrome type III, EDTA = ethylenediaminetetraacetic acid, ePCR = enrichment polymerase chain reaction, IBC = inflammatory breast cancer, IFA = indirect fluorescent antibody, IPRL = Intracellular Pathogens Research Laboratory, IRB = Institutional Review Board, MRI = magnetic resonance imaging, PCR = polymerase chain reaction, spp = species, subsp = subspecies.
Compliance with ethical standards: The microbiological data described in this case report was generated at North Carolina State University College of Veterinary Medicine in conjunction with IRB approval (Institutional Review Board Protocol #1960, Detection of Bartonella Species in the Blood of Healthy and Sick People).
Patient Consent Statement: The study subject has signed an Informed Consent Form that is on file in our laboratory. She has read and approved the publication of the case report.
Conflicts of interest: In conjunction with Dr Sushama Sontakke and North Carolina State University, EBB, DVM holds US Patent no. 7,115,385; Media and Methods for cultivation of microorganisms, which was issued on October 3, 2006. He is a co-founder, shareholder and Chief Scientific Officer for Galaxy Diagnostics, a company that provides advanced diagnostic testing for the detection of Bartonella species infections. BRM, MD is Chief Medical Officer and Dr RM is the Chief Technical Officer for Galaxy Diagnostics. The remaining author has no competing interests. This research was supported in part by unrestricted donations to the Foundation for the Study of Inflammatory Diseases, North Bethesda, MD, and the NCSU-CVM Foundation for Bartonella/Vector Borne Diseases Research, Raleigh, NC.
The authors have no conflicts of interest to disclose.
References
- [1].Breitschwerdt EB, Maggi RG, Mozayeni BR, et al. Isolation or PCR amplification of Bartonella koehlerae from human blood. Parasit Vectors 2010;3:e76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Maggi RG, Mascarelli PE, Pultorak EL, et al. Bartonella spp. bacteremia in high-risk immunocompetent patients. Diagn Microbiol Infect Dis 2011;71:430–7. [DOI] [PubMed] [Google Scholar]
- [3].Lantos PM, Maggi RG, Ferguson B, Varkey JPLP, et al. Detection of Bartonella species in the blood of veterinarians and veterinary technicians: a newly recognized occupational hazard. Vector Borne Zoonotic Dis 2015;14:563–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Pultorak EL, Maggi RG, Mascarelli PE, et al. Serial testing from a 3-day collection period by use of the Bartonella alphaproteobacteria growth medium platform may enhance the sensitivity of Bartonella species detection in bacteremic human patients. J Clin Microbiol 2013;51:1673–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Pulliainen AT, Dehio C. Persistence of Bartonella spp. stealth pathogens: from subclinical infections to vasoproliferative tumor formation. FEMS Microbiol Rev 2012;36:563–99. [DOI] [PubMed] [Google Scholar]
- [6].Seneviratne SL, Maitland A, Afrin L. Mast cell disorders in Ehlers–Danlos syndrome. Am J Med Genet Part C Semin Med Genet 2017;9999C:1–1. [DOI] [PubMed] [Google Scholar]
- [7].Giladi M, Maman E, Paran D, et al. Cat-scratch disease-associated arthropathy. Arthritis Rheum 2005;52:3611–7. [DOI] [PubMed] [Google Scholar]
- [8].Maggi RG, Mozayeni BR, Pultorak EL, et al. Bartonella spp. bacteremia and rheumatic symptoms in patients from Lyme disease-endemic region. Emerg Infect Dis 2012;18:783–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Ericson M, Balakrishnan N, Mozayeni BR, et al. DNA sequencing, and second harmonic generation (SHG) visualization of Bartonella henselae from a surgically excised human femoral head. Clin Rheumatol 2016;36:1669–75. [DOI] [PubMed] [Google Scholar]
- [10].Pitassi LH, de Paiva Diniz PP, Scorpio DG, et al. Bartonella spp. bacteremia in blood donors from Campinas. Brazil PLoS Negl Trop Dis 2015;9:e0003467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Bouhsira E, Franc M, Boulouis HJ, et al. Assessment of persistence of Bartonella henselae in Ctenocephalides felis. Appl Environ Microbiol 2013;79:7439–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Yamamoto K, Chomel BB, Kasten RW, et al. Homologous protection but lack of heterologous-protection by various species and types of Bartonella in specific pathogen-free cats. Vet Immunol Immunopathol 1998;65:191–204. [DOI] [PubMed] [Google Scholar]
- [13].Balakrishnan N, Cherry NA, Linder KE, et al. Maggi RG Breitschwerdt EB. 2013. Experimental infection of dogs with Bartonella henselae and Bartonella vinsonii subsp. berkhoffii. Vet Immunol Immunopath 2013;156:153–8. [DOI] [PubMed] [Google Scholar]
- [14].Kaufman DL, Kogelnik AM, Mozayeni RB, et al. Neurological and immunological dysfunction in two patients with Bartonella henselae bacteremia. Clin Case Rep 2017;5:931–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Oliveira AM, Maggi RG, Woods CW, et al. Putative needle stick transmission of Bartonella vinsonii subsp. berkhoffii to a veterinarian. J Vet Intern Med 2010;24:1229–32. [DOI] [PubMed] [Google Scholar]


