Abstract
Rationale:
Complete loss of splenic function increases infection and cardiovascular disease risks, so there is growing emphasis on spleen-preserving treatments, such as laproscopic partial splenectomy (LPS). However, LPS carries higher risk for hemorrhage. Sequential splenic embolization can obliterate the perilesional vascular bed while preserving flow through healthy tissue, substantially reducing risk of uncontrolled hemorrhage during LPS. Preoperative partial splenic embolization (PSE) may soften the spleen and reduce its size, which enhances space exposure for laparoscopic operation. Furthermore, immediate LPS guaranties these effects of PSE and prevents abscess, non-traumatic splenic rupture, post-embolization syndrome, and other complications. In light of these advantages, we conducted combined PSE and LPS for a case of hemangioma.
Patient concerns:
The patient presented with left abdominal discomfort of >1 year.
Diagnoses:
Ultrasound examination at the outpatient clinic identified a space-occupying lesion in the spleen. Contrast-enhanced computed tomography scan of the upper abdomen revealed a hypodense lesion, approximately 33 × 21 mm in size, located in the upper pole of the spleen, suggesting possible hemangioma.
Interventions:
The patient was treated by preoperative PSE followed by LPS.
Outcomes:
Treatment resulted in only mild intraoperative hemorrhage, fast postoperative recovery, and no recurrence during follow-up. And the postoperative histopathology confirmed splenic cavernous hemangioma.
Lessions:
Preoperative PSE combined with LPS is an effective therapy for elective patients that minimizes intraoperative hemorrhage during laparoscopic surgery, reduces surgical risk, and enhances surgical safety.
Keywords: laparoscopic partial splenectomy, partial splenic embolization, splenic hemangioma
1. Introduction
In 1991, Delaitre and Maignien[1] performed the first successful laparoscopic splenectomy, which has since become the standard approach for most splenectomy cases.[2] However, total splenectomy may lead to overwhelming post-splenectomy infection (OPSI), portal vein thrombosis, and other complications.[3,4] A previous study suggested that total splenectomy may also increase the risks of pulmonary hypertension, atherosclerosis, and coronary heart disease.[5] As understanding of splenic function increases, especially immunologic function, a growing number of surgeons are implementing spleen-preserving surgery.
Laparoscopic partial splenectomy (LPS), first performed by Poulin et al in 1995,[6] is widely regarded as an ideal minimally invasive spleen-preserving surgery. Nevertheless, LPS has been implemented mainly in a few large medical institutions because of surgical complexity and high-risk of intraoperative hemorrhage. Control of intraoperative hemorrhage, especially during splenic parenchyma transection, is pivotal for successful LPS. Herein, we report a case of splenic hemangioma successfully treated by combined preoperative partial splenic embolization (PSE) with subsequent LPS.
In this report, PSE was combined with LPS for spleen-preserving surgery. The report conforms to CARE guidelines. All study procedures were reviewed and approved by the Institutional Ethics Review Board of the Second Affiliated Hospital of Army Medical University and conducted according to the principles expressed in the Declaration of Helsinki. Informed consent was provided by both the patient and her family before operation, and informed consent for publication of the case has been signed by the patient.
2. Case report
A 36-year-old female was admitted to our hospital because of left abdominal aching discomfort for >1 year. Ultrasound examination at the outpatient clinic identified a space-occupying lesion in the spleen. She reported no personal medical history of trauma or abdominal surgery and no cancer history in her family. Physical examination revealed no positive signs. Contrast-enhanced computed tomography (CT) scan of the upper abdomen revealed a hypodense lesion, approximately 33 × 21 mm in size, located in the upper pole of the spleen, suggesting possible hemangioma (Fig. 1A and B). Laboratory examinations including serum tumor marker testing yielded normal results. Before surgery, physicians informed the patient of the surgical procedure and risks in detail. Written informed consent was obtained from the patient.
Figure 1.
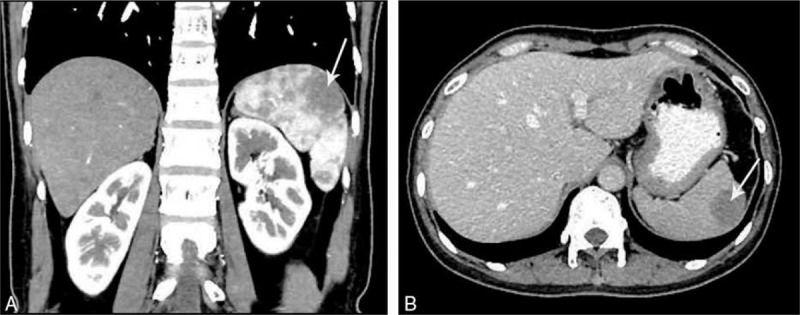
(A and B) Computed tomography scans showing a hypodense lesion located in the upper pole of the spleen.
The patient was kept in a supine position during LPS. She received femoral artery puncture using the Seldinger technique under topical anesthesia. Angiography of the celiac axis and splenic artery was performed using a 5F catheter (Cook Medical Inc., Bloomington, IN) to reveal the dissection status, stretching, branching pattern, and diameter of the splenic artery (Fig. 2A). After the insertion of a 3F microcatheter (Cook Medical Inc.), the elective catheter was inserted into the superior splenic lobar artery under wire-guided angiography (Fig. 2B). PSE was implemented in 2 steps. First, iodized oil (w/vol 48%, 10 mL; Luyin Pharmaceutical Co., Ltd, Shandong, China) and gelatin sponge particles (diameter 560−710 μm; Ailikang, Hangzhou, China) dissolved in iohexol solution (Beilu Pharmaceutical Co., Ltd, Beijing, China) were used successively for embolization of the splenic parenchyma. Subsequently, spring coil (MWCE; 5 mm; Cook Medical Inc.) was used for embolization of the superior splenic lobar artery. Following embolization, angiography demonstrated complete occlusion of the superior splenic lobar artery (Fig. 2C).
Figure 2.
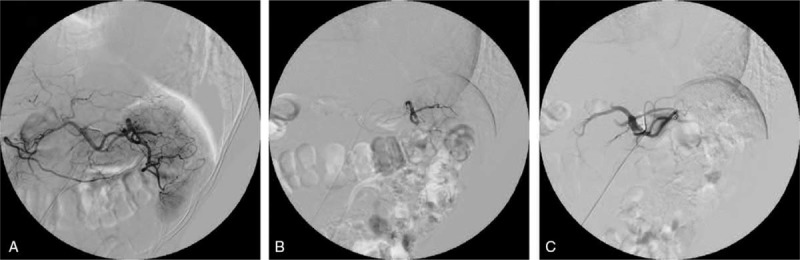
(A) Angiography of the celiac axis and splenic artery. (B) Angiography of the superior splenic lobar artery. (C) Angiography of the splenic artery after embolization, showing complete occlusion of the superior splenic lobar artery.
After embolization, the patient was immediately transferred to the operating room for LPS. Under general anesthesia, she was placed in a 30 degree right oblique position for 4-port LPS. A 10-mm trocar was inserted surrounding the umbilicus as the observation port, a 12-mm trocar at the left mid-clavicular line parallel to the umbilicus as the main operation port, and two 5-mm trocars beneath the xiphoid process and subcostal left anterior axillary line as the assisted operation ports. Abdominal exploration revealed ischemia and darkening of the spleen upper pole after embolization, with a well defined line of demarcation (Fig. 3A). The gastrocolic ligament was cut open using a harmonic scalpel (Ethicon Inc., Somerville, NJ), and the splenogastric ligament (including short gastric vessels) and lienophrenic ligament were successively transected. The splenic hilum was dissected carefully, and the branches of the superior splenic lobar blood vessels were separated, ligated, and cut. The splenic parenchyma approximately 1 cm adjacent to the splenic ischemic band was transected using a harmonic scalpel. Errhysis from the preserved splenic section was controlled by electrocoagulation (Fig. 3B and C). The resected partial spleen was placed into a plastic bag, extracted via the enlarged main operation port, and sent for pathological examination. An abdominal drainage tube was inserted into the splenic fossa and fixed via the subcostal puncture opening at the left anterior axillary line. Estimated intraoperative hemorrhage volume was ∼50 mL, and total operation time was ∼100 minutes. The abdominal drainage tube was removed on postoperative day 2. The patient recovered without complication and was discharged on postoperative day 5.
Figure 3.
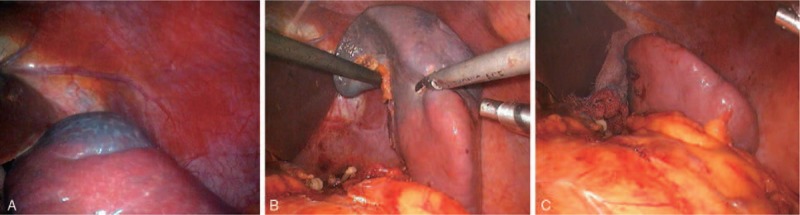
(A) Well-defined line of ischemic demarcation at the upper pole after embolization. (B) After partial mobilization of the splenic ligaments and dissection of the superior splenic lobar vessels, a harmonic scalpel was used to dissect the splenic parenchyma, preserving a 1-cm rim of devascularized splenic tissue. (C) The residual tissue beyond the splenic parenchyma.
On postoperative day 7, contrast-enhanced CT scan of the upper abdomen revealed good preservation of the inferior splenic lobar vessels (Fig. 4A–C). Postoperative pathological analyses revealed large vascular lacunae filled with blood within the resected partial spleen itself, consistent with splenic cavernous hemangioma (Fig. 5). During 6 months of follow-up, the patient presented no symptoms of discomfort, and ultrasound examination detected no signs of recurrence. The platelet count was maintained within the normal range.
Figure 4.
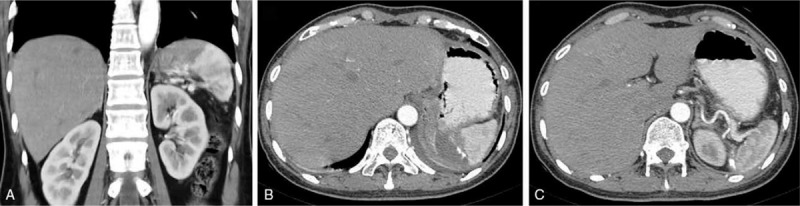
(A–C) Postoperative computed tomography scans acquired on postoperative day 7 showing preserved arterial and venous dynamics in the lower pole of the spleen.
Figure 5.
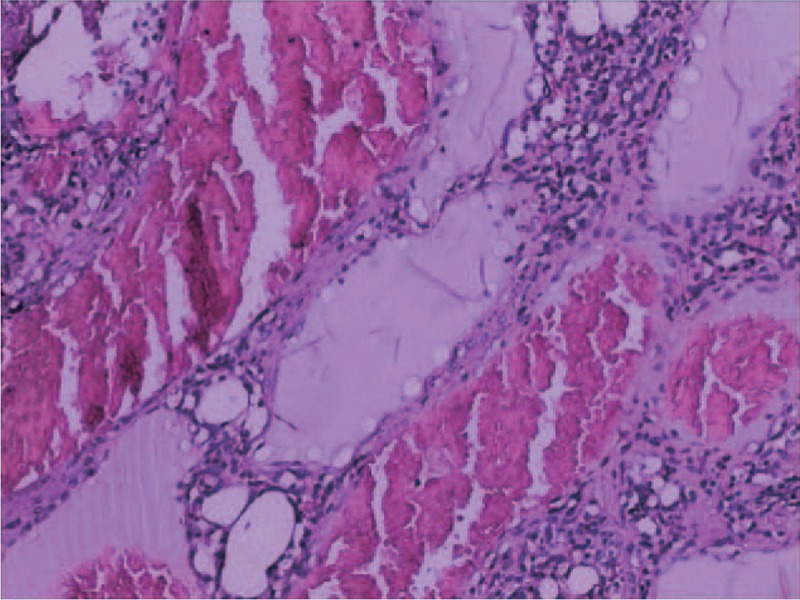
Postoperative pathological analyses revealed large vascular lacunae filled with blood within the resected partial spleen itself, consistent with splenic cavernous hemangioma (H&E staining, 100×).
3. Discussion
As the largest systemic peripheral lymphoid organ, the spleen is a critical component of the immune–nervous–endocrine secretion system. The spleen is involved in many vital processes, including immunity, blood storage, blood filtration, and hematopoiesis. Thus, total splenectomy can cause a variety of postoperative complication, of which POSI is the most severe, with an incidence of approximately 4.4% and a mortality rate for established OPSI cases as high as 50% to 80%.[7] In parallel with the growing appreciation of splenic function and postoperative complications after total splenectomy, more surgeons are choosing spleen-preserving surgery for elective patients.
The segmental blood supply of the spleen provides the anatomical basis for partial splenectomy. The branch artery at each segment can be dissociated from surrounding spleen, and artery occlusion or ligation performed according to the extent of target resection. After occlusion or ligation of the splenic artery branch, a well-defined line of demarcation reflecting underlying tissue ischemia is observed on the splenic surface, and regular partial splenectomy can then be performed based on this demarcation line.[8] However, previous investigations have demonstrated that the splenic lobar artery branch is proximal to the hilar surface of the spleen in approximately two-thirds of individuals,[9] which increases the difficulty of intraoperative dissection. Insufficient separation of splenic artery branches is likely to cause splenic hilar vascular injury and elevate the risk of hemorrhage.
PSE is a conservative spleen-preserving technique in which elective embolization of the partial splenic circulation is performed to cause local ischemia and infarction, which weakens splenic phagocytosis, thereby achieving surgical efficacy equivalent to partial splenectomy. Nevertheless, PSE may induce multiple postoperative complications, such as splenic abscess, non-traumatic rupture, and post-embolization syndrome. These complications not only affect the clinical efficacy of PSE, but may also lead to death.[10] Moreover, radical resection is still required for the treatment of space-occupying lesions of the spleen.
In this report, PSE in combination with LPS was adopted for spleen-preserving surgery according to the PSE characteristics, and relatively favorable clinical efficacy was obtained. This combined technique has the following advantages. First, preoperative PSE of the elective splenic lobar artery can reduce the risk of hemorrhage during splenic hilar dissection, separation, and ligation of splenic artery branches. It is particularly applicable for patients with splenomegaly, anatomical variation of splenic arteries, an artery branch adjacent to the hilar surface, and dissection difficulty. Second, PSE is conducted in 2 steps. Initially, splenic parenchyma and the splenic arterial end are embolized using celloidin sponges and lipiodol. Subsequently, the splenic artery branch is embolized using spring coils. This sequential technique allows for comprehensive splenic embolization, not only reducing the risk of uncontrolled massive hemorrhage, but also decreasing errhysis from the splenic transection surface. Third, through preoperative elective embolization, blood supply to the spleen can be partially blocked and the area of ischemia delineated to determine the resection area and guide the separation and ligation of splenic vascular branches before parenchyma transaction. This minimizes the hemorrhage volume during splenic parenchyma transection and reduces the surgical risk. Fourth, laparoscopic surgery is performed immediately after embolization to prevent splenic abscess, non-traumatic rupture of the spleen, post-embolization syndrome, and other complications.
However, PSE can still lead to complications such as ectopic embolism and pulmonary complications including pneumonia, pulmonary atelectasis, and pleural effusion. In addition, the 2 surgeries may cause additional mental and physical stress as well as increase medical expenses. Therefore, not all cases are appropriate for PSE before LPS. Nonetheless, this technique offers more options for LPS. Preoperative elective PSE is recommended for patients with a high risk of hemorrhage, such as those with megalosplenia, splenic artery variation, and anticipated difficulty in artery branch exposure and separation. Preoperative PSE in combination with LPS is capable of reducing intraoperative hemorrhage, decreasing surgical risk, and enhancing surgical safety and success rate.
4. Conclusion
Preoperative PSE combined with LPS is a safe, minimally invasive spleen-preserving surgery with substantial clinical benefits for elective patients.
Author contributions
Conceptualization: Lu zheng, Jing Li.
Data curation: Jing Li, Ke Wu.
Formal analysis: Ke Wu.
Funding acquisition: Lu zheng.
Methodology: Liang Wang.
Project administration: Liang Wang.
Resources: Weiwei Wang.
Software: Nan You, Weiwei Wang.
Supervision: Nan You.
Validation: Jing Li.
Visualization: Lu zheng, Jing Li.
Writing – original draft: Changlin Deng.
Writing – review & editing: Lu zheng.
Footnotes
Abbreviations: CT = Computed tomography, LPS = Laparoscopic partial splenectomy, OPSI = overwhelming post-splenectomy infection, PSE = partial splenic embolization.
LZ and CD contributed equally to this study and share first authorship.
This work was supported by a grant from National Natural Science Foundation of China (81372561), a grant from Clinical Scientific Research Project of the Second Affiliated Hospital of Third Military Medical University (2014YLC07), and a grant from New Clinical Technology of the Second Affiliated Hospital of Third Military Medical University (2016LCXJS048).
The authors report no conflicts of interest.
References
- [1].Delaitre B, Maignien B. Splenectomy by the laparoscopic approach. Report of a case. Presse Méd 1991;20:2263–12263. [PubMed] [Google Scholar]
- [2].Habermalz B, Sauerland S, Decker G, et al. Laparoscopic splenectomy: the clinical practice guidelines of the European Association for Endoscopic Surgery (EAES). Surg Endosc 2008;22:821–48. doi: 10.1007/s00464-007-9735-5. [DOI] [PubMed] [Google Scholar]
- [3].Bhandarkar DS, Katara AN, Mittal G, et al. Prevention and management of complications of laparoscopic splenectomy. Indian J Surg 2011;73:324–30. doi: 10.1007/s12262-011-0331-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Hassn AM, Al-Fallouji MA, Ouf TI, et al. Portal vein thrombosis following splenectomy. Br J Surg 2000;87:362–73. [DOI] [PubMed] [Google Scholar]
- [5].Balaphas A, Buchs NC, Meyer J, et al. Partial splenectomy in the era of minimally invasive surgery: the current laparoscopic and robotic experiences. Surg Endosc 2015;29:3618–27. [DOI] [PubMed] [Google Scholar]
- [6].Poulin EC, Thibault C, DesCôteaux JG, et al. Partial laparoscopic splenectomy for trauma: technique and case report. Surg Laparosc Endosc 1995;5:306–10. [PubMed] [Google Scholar]
- [7].Buesing KL, Tracy ET, Kiernan C, et al. Partial splenectomy for hereditary spherocytosis: a multi-institutional review. J Pediatr Surg 2011;46:178–83. [DOI] [PubMed] [Google Scholar]
- [8].Ignjatovic D, Stimec B, Zivanovic V. The basis for splenic segmental dearterialization: a post-mortem study. Surg Radiol Anat 2005;27:15–8. [DOI] [PubMed] [Google Scholar]
- [9].Liu DL, Xia S, Xu W, et al. Anatomy of vasculature of 850 spleen specimens and its application in partial splenectomy. Surgery 1996;119:27–33. [DOI] [PubMed] [Google Scholar]
- [10].Koconis KG, Singh H, Soares G. Partial splenic embolization in the treatment of patients with portal hypertension: a review of the English language literature. J Vasc Interv Radiol 2007;18:463–81. [DOI] [PubMed] [Google Scholar]


