Supplemental Digital Content is available in the text
Keywords: coronary artery disease, meta-analysis, obstructive sleep apnea, percutaneous coronary intervention
Abstract
The relationship between obstructive sleep apnea (OSA) and adverse cardiovascular outcomes in patients undergoing percutaneous coronary intervention (PCI) remains unclear. We performed a systematic review and meta-analysis to assess the impact of OSA on subsequent cardiovascular events after PCI.
We searched the PubMed, EMBASE, and Cochrane library from their inceptions to August 5, 2017. We included cohort studies that described the association between OSA (based on apnea-hypopnea index) and cardiovascular outcomes after PCI with stenting. The primary endpoint was major adverse cardiovascular event (MACE), including all-cause or cardiovascular death, myocardial infarction, stroke, repeat revascularization, or heart failure. Outcomes data were pooled using random effects models and heterogeneity was assessed with the I2 statistic.
We identified 9 studies with 2755 participants. The prevalence of OSA in patients treated with PCI ranged from 35.3% to 61.8%. OSA was associated with increased risk of MACE after PCI (pooled risk ratio [RR] 1.96, 95% confidence interval [CI]: 1.36–2.81, P < .001, I2 = 54%). Between-study heterogeneity was partially explained by sample size (2 studies with ≤100 participants; RR 9.12, 95% CI: 2.69–31.00, I2 = 0% vs 7 studies with >100 participants; RR 1.64, 95% CI: 1.23–2.18, I2 = 35%). Moreover, the presence of OSA significantly increased the incidence of all-cause death (4 studies), cardiovascular death (4 studies), and repeat revascularization (7 studies) in patients undergoing PCI.
Patients with OSA are at greater risk of subsequent cardiovascular events after PCI. Whether treatment of OSA prevents such events warrants further investigation.
1. Introduction
Obstructive sleep apnea (OSA) is an increasingly common chronic disorder in adults.[1] Compared to the general population, OSA occurs more often in patients with coronary artery disease (CAD), with a reported prevalence of 38% to 65%.[2] Emerging evidence indicates OSA initiates and exacerbates coronary atherosclerosis[3,4] and is associated with higher risk of subsequent cardiovascular events in patients with established CAD.[5,6]
Percutaneous coronary intervention (PCI) is nowadays part of standard therapy in patients with symptomatic CAD.[7] However, the long-term cardiovascular outcomes after PCI remain suboptimal despite optimal medical therapy to manage traditional risk factors.[8] In the past decade, multiple observational studies,[5,6,9–15] including the recently published multicenter sleep and stent study,[15] have examined whether presence of OSA significantly increased risk of recurrent cardiovascular events in patients treated with PCI, but the results are inconsistent and the interpretation of these results remains disputable given variability in sample size, follow-up duration, and adjustment for potential confounders (coexisting clinical variables) across studies. Hence, we conducted a systematic review and meta-analysis of cohort studies to assess the impact of OSA on subsequent cardiovascular outcomes after PCI.
2. Methods
2.1. Search strategies
This meta-analysis was conducted in accordance to the Meta-analysis of Observational Studies in Epidemiology (MOOSE) guidelines.[16] We systematically searched the PubMed, EMBASE, and Cochrane library from their inceptions to August 5, 2017 for studies describing the association between OSA and cardiovascular outcomes after PCI. We used Medical Subject Heading terms “Sleep Apnea Syndromes,” “Percutaneous Coronary Intervention,” “Myocardial Ischemia,” and related text words including sleep apnea, stent, and coronary disease, without language restrictions. Reference lists of all identified publications and relevant review articles were further checked manually for potential citations. An example search strategy is presented in Supplemental Table 1.
2.2. Study selection and eligibility criteria
Two authors (XW and JYF, both cardiologists) assessed the eligibility of articles by initially screening the titles and abstracts. Articles were considered for inclusion if they reported original data on clinical outcomes in patients with OSA and CAD and/or receiving PCI. Full-text articles were subsequently reviewed in duplicate. We only selected cohort studies (retrospective cohort or prospective cohort) that recruited patients undergoing PCI with stenting, with a comparison between untreated OSA versus controls (no OSA or with varying degrees of OSA severity). In case of studies comparing treated OSA with untreated OSA and to those without OSA, only untreated OSA and no OSA (control) cohorts were included in this study. The diagnosis of OSA was based on apnea-hypopnea index (AHI) obtained by standardized polysomnography or portable diagnostic devices. Included studies had to have a longitudinal follow-up duration of >6 months. The primary outcome of interest for inclusion was major adverse cardiovascular events (MACEs). The following exclusion criteria were used: no PCI or stent information; OSA diagnosis was not based on AHI; studies that did not specify exclusion of patients with predominantly central sleep apnea; studies comparing treated OSA versus untreated OSA; and cardiovascular outcomes not reported or no long-term follow-up. Any disagreement was resolved by consensus through referral to a 3rd reviewer (SPN). An ethical approval was not necessary since meta-analysis was based on secondary data.
2.3. Data extraction and validity assessment
Data extraction was performed by 2 independent reviewers (XW and JYF) using a prespecified standardized form, and verified by a senior author (SPN). All discrepancies were resolved by consensus. We also contacted authors for missing data. The following study information were recorded: study design, location, date, sample size, demographic characteristics, procedural characteristics, methods and timing of OSA assessment, duration and completeness of follow-up, cardiovascular outcomes, and adjustments of potential confounding factors. The definitions of exposure (OSA) and control groups were based on AHI cut-points in each study.
The quality of included studies was evaluated independently by 2 authors (XW and JYF) using the Newcastle-Ottawa Scale for cohort studies.[17] A quality score was calculated according to a maximum of 1 star for each item upon selection (4 items: representativeness of the exposed cohort, selection of the nonexposed cohort, ascertainment of exposure, and demonstration that outcome was not present at study start), comparability (2 items: controls for the most important factor and any additional factor), and outcome (3 items: assessment, duration, and adequacy of follow-up) categories.
The primary endpoint was MACE, defined as a composite of all-cause or cardiovascular death, myocardial infarction (MI), stroke, repeat revascularization, or heart failure. Secondary endpoints included all-cause death, cardiovascular death, MI, stroke, and repeat revascularization. Definitions of events were in accordance to guidelines during each study period, although the timing of definitions varies among studies. Repeat revascularization was generally defined as unplanned or target vessel revascularization. If these data were not recorded, data on any repeat revascularization were used. Endpoints were assessed at the longest follow-up. The quality of each outcome was assessed using the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) system.[18]
2.4. Data synthesis and analysis
We used risk ratio (RR) as the effect estimate of OSA on the risk of cardiovascular events after PCI. In general, we collected multivariable-adjusted hazard ratio (HR) or RR from original studies. HR was directly considered as RR. In case of unreported HR or RR of the outcomes of interest, we calculated unadjusted RR using crude values. We pooled RR with 95% confidence interval (CI) for MACE and significant secondary outcomes by random effects model (DerSimonian-Laird), which incorporates between-study heterogeneity. The Cochran Q test (at a significance level of P < .10) and the I2 statistic were used to examine statistical heterogeneity across studies. For primary outcome, we performed prespecified sensitivity analysis by excluding retrospective cohort, studies with unadjusted data, or low-to-moderate quality studies, respectively. Post hoc sensitivity analysis was conducted based on drug-eluting stent (DES) use, AHI definition, sample size, or follow-up duration. Publication bias was assessed by funnel plots, Egger test, and trim-and-fill analysis. All analyses were performed with Cochrane Review Manager software (version 5.3) and Stata version 11.2 (StataCorp LP, College Station, TX). Unless otherwise indicated, a 2-sided P value < .05 was considered statistically significant.
3. Results
3.1. Study selection and characteristics
Our search yielded 4653 citations. By first screen, 26 studies were retained for further review. We subsequently excluded 17 studies, of which 7 studies included patients with CAD but no PCI or stent information was specified, and 4 studies did not report cardiovascular events or long-term (>6 months) follow-up. Other reasons for study exclusion are presented in Fig. 1.
Figure 1.
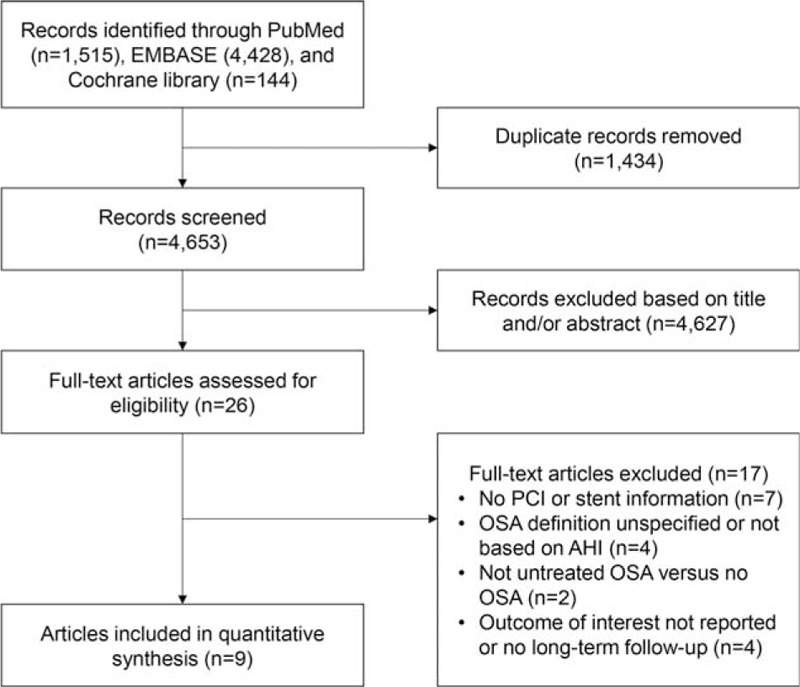
Flow chart of the study selection process for meta-analysis. OSA = obstructive sleep apnea, PCI = percutaneous coronary intervention.
Therefore, a total of 9 studies with 2755 participants were included in this meta-analysis.[5,6,9–15] The prevalence of OSA ranged from 35.3% to 61.8%. Characteristics of the studies are listed in Tables 1 and 2. Studies were published in the past 10 years. Eight studies were prospective cohorts,[5,6,9–12,14,15] and 1 study was retrospective cohort.[13] Seven studies enrolled mostly acute coronary syndrome (ACS) patients with PCI,[5,6,9,11,13–15] and 2 studies enrolled only MI patients undergoing primary PCI.[10,12] Five studies specified DES use in most patients,[9,11,13–15] whereas 4 studies used bare-metal stents,[5,6] endothelial progenitor cell capturing stents,[10] or did not indicate stent information.[12] OSA was assessed primarily by overnight polysomnography in 2 studies,[12,13] and by validated portable diagnostic devices in 7 studies.[5,6,9–11,14,15] Almost all sleep studies were done within 1 month after PCI or admission,[5,6,9–12,14,15] except 1 retrospective study done within 1 month before or after PCI.[13]
Table 1.
Study design, patients demographics, and procedural characteristics.
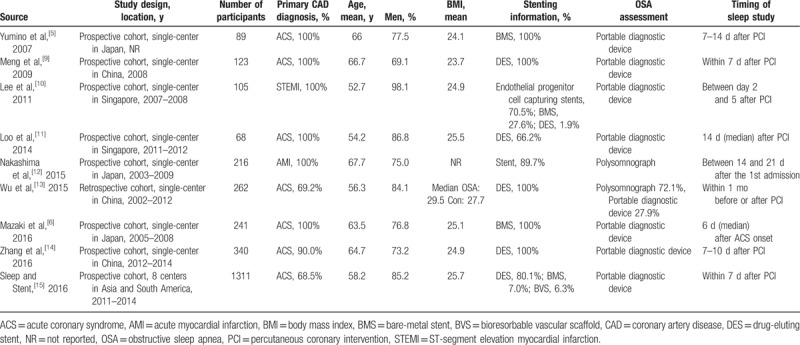
Table 2.
Study groups, outcomes, results, and risk of bias.
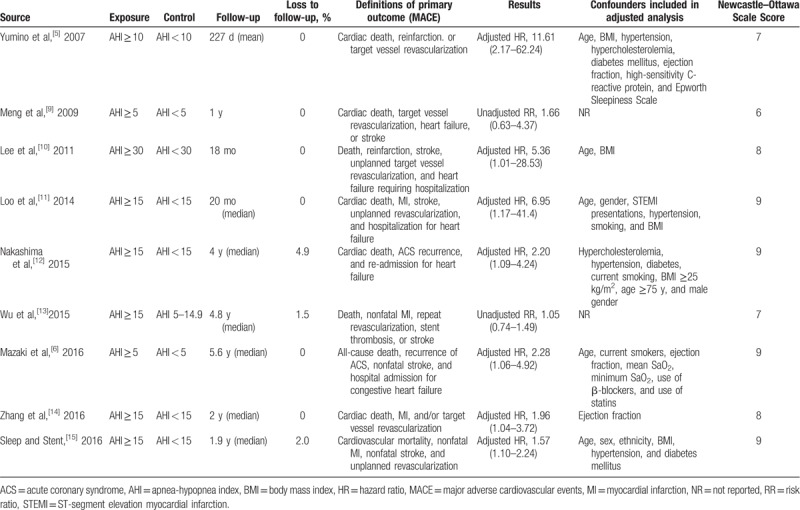
The definition of OSA was based on standardized assessment of AHI in all studies, with AHI≥15 as cut-off value in most studies.[11–15] Two studies compared treated OSA with untreated OSA and to those without OSA (control), and only untreated OSA and control cohorts were included in this meta-analysis.[12,13] The median follow-up duration was from 227 days to 5.6 years, and a mild proportion of patients were lost to follow-up (up to 4.9%). All studies had no treatments for OSA during this period. To be noted, most of the studies reported adjusted risk estimates for the primary endpoint, but some studies did not adjust for all or part of potential confounding factors, including age, sex, body mass index, hypercholesterolemia, hypertension, diabetes, and smoking, thus contributing to risk of bias. The quality scores of studies are shown in Supplemental Table 2.
3.2. OSA and MACE after PCI
Overall, OSA was significantly associated with increased risk of MACE after PCI (RR 1.96, 95% CI: 1.36–2.81, P < .001) (Fig. 2). There was evidence of statistical heterogeneity for the composite endpoint (Q statistic P = .02; I2 = 54%). Subgroup analysis according to sample size showed that the pooled RR of 2 studies with ≤100 patients (RR 9.12, 95% CI: 2.69–31.00, I2 = 0%) was greater than that of 7 studies with >100 patients (RR 1.64, 95% CI: 1.23–2.18, I2 = 35%), and the heterogeneity was attenuated in both subgroups (Supplemental Fig. 1). The increased risk of MACE remained significant in 7 studies with adjusted results (RR 2.30, 95% CI: 1.56–3.37, I2 = 36%), but was not significant in 2 studies without adjustment (RR 1.11, 95% CI: 0.80–1.54, I2 = 0%) (Supplemental Fig. 2). Noteworthy, the risk estimate of MACE without the large multicenter sleep and stent study (2.22) was consistent with the overall effect from all studies (1.96), but the 95% CI became narrower with the addition of the sleep and stent study (1.38–3.57 to 1.36–2.81) (Supplemental Fig. 3).
Figure 2.
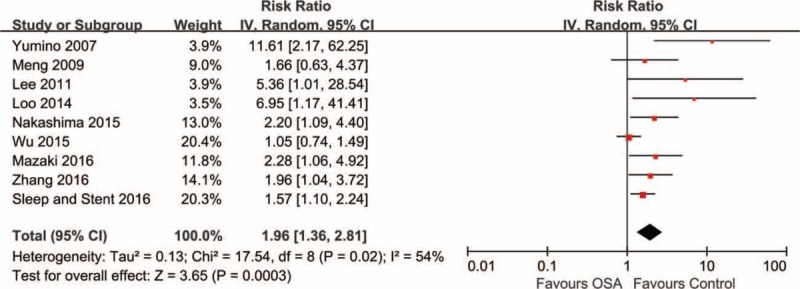
Risk estimates for MACE. Forest plot displays the risk ratio with 95% CI for MACE in patients with OSA compared to control. The diamond indicates the point estimate, and the left and right end of the line indicate the 95% CI. CI = confidence interval, IV = inverse variance, MACE = major adverse cardiovascular events, OSA = obstructive sleep apnea.
We further performed sensitivity analyses to explore the effect of variations in quality and potential sources of heterogeneity (Table 3). We excluded those studies with low-to-moderate quality (Newcastle-Ottawa Scale score < median value) and found no change in the significance of effect size and between-study heterogeneity reduced significantly (I2 from 54% to 0%). We also evaluated the effects of excluding studies with retrospective cohort, unadjusted values, a cutoff value of other than AHI ≥15, sample size ≤100, follow-up duration ≤1 year, as well as 4 studies that used other stent types or did not indicate stent information. All the analysis did not change the statistical significance or direction of effect for the primary endpoint, and showed slight decrease of heterogeneity. It should be noted that the quality of primary outcome was low based on GRADE system (Supplemental Table 3), so the result should be interpreted with caution.
Table 3.
Sensitivity analysis for MACE.
The funnel plot for the outcome of MACE was asymmetrical indicating possibility of publication bias, and it was suggested by Egger test (P = .002). When we used the trim-and-fill analysis to quantify the potential effect of small-study bias, addition of hypothetical missing studies reduced the pooled RR to 1.55 (95% CI: 1.07–2.24, P = .02) in random effects model but continued to show a significant association between OSA and MACE (Supplemental Figure 4).
3.3. OSA and individual cardiovascular events after PCI
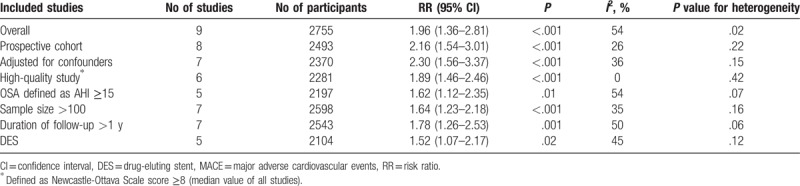
In studies reporting outcomes of death, OSA was found to significantly increase the risk of all-cause death (4 studies with 1919 participants; RR 1.70, 95% CI: 1.05–2.77, I2 = 0%) (Fig. 3A)[6,10,13,15] and cardiovascular death (4 studies with 1863 participants; RR 2.23, 95% CI: 1.08–4.59, I2 = 0%) (Fig. 3B)[5,9,14,15] in the pooled analysis, with no evidence of statistical heterogeneity.
Figure 3.
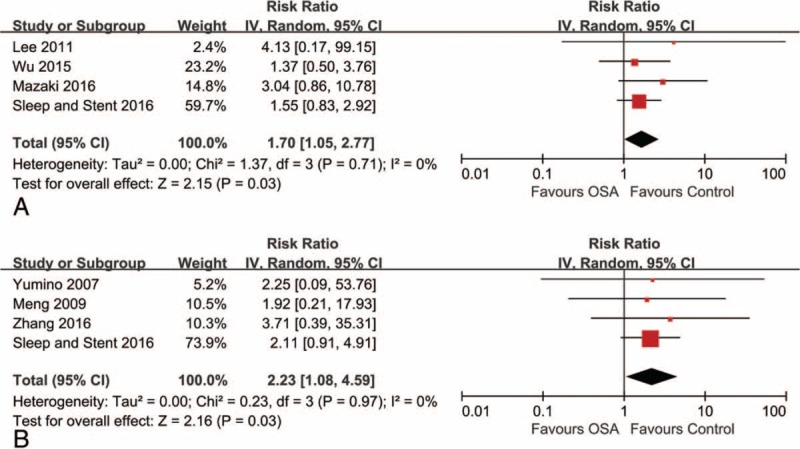
Risk estimates for all-cause death and cardiovascular death. Forest plot displays the risk ratio with 95% CI for all-cause death (A) and cardiovascular death (B) in patients with OSA compared to control. The diamond indicates the point estimate, and the left and right end of the line indicate the 95% CI. CI = confidence interval, IV = inverse variance, OSA = obstructive sleep apnea.
We also included 7 studies (2298 participants) with data on repeat revascularization and found higher risk in patients with OSA (RR 1.54, 95% CI: 1.17–2.02, I2 = 0%) (Fig. 4C), with no evidence of heterogeneity.[5,9–11,13–15] However, there were no significant difference for outcomes of MI and stroke (Fig. 4A and B). The quality of each individual outcome was shown in Supplemental Table 3.
Figure 4.
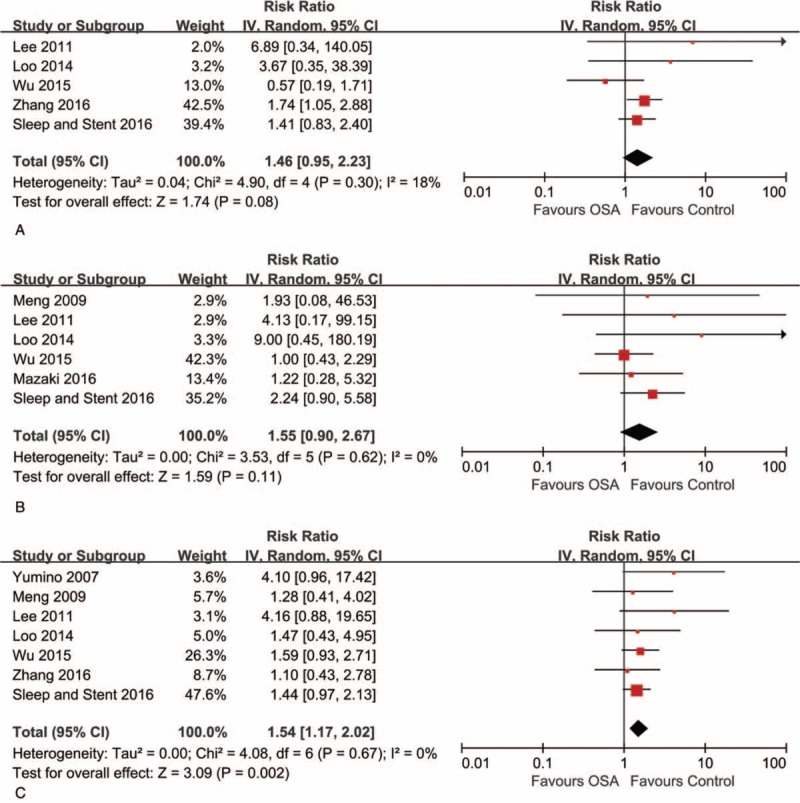
Risk estimates for myocardial infarction, stroke, and repeat revascularization. Forest plot displays the risk ratio with 95% CI for the outcomes of myocardial infarction (A), stroke (B), and repeat revascularization (C) in patients with OSA compared to control. The diamond indicates the point estimate, and the left and right end of the line indicate the 95% CI. CI = confidence interval, IV = inverse variance, OSA = obstructive sleep apnea.
4. Discussion
Our meta-analysis demonstrated that OSA was associated with a pooled 2-fold risk of recurrent cardiovascular events after PCI, which supports existing evidence including the multicenter sleep and stent study.[15] Between-study heterogeneity was partially explained by sample size and study quality based on Newcastle-Ottava Scale score or whether adjustment for confounders was performed. Our estimates remained robust to a variety of sensitivity analysis. Furthermore, the presence of OSA significantly increased the incidence of all-cause death, cardiovascular death, and repeat revascularization in patients undergoing PCI.
The correlation between OSA and risk of cardiovascular events in the general population has been well established in longitudinal observational studies[19] and meta-analysis,[20] whereas data on patients with known cardiovascular diseases were sparse. In 1 meta-analysis that enrolled patients with ischemic heart disease, OSA was an independent predictor of recurrent cardiac events.[21] However, the definitions of ischemic heart disease and outcome measures vary across studies, therefore precluding definitive conclusions.[21] In contrast, the present meta-analysis focused on a high-risk group of patients with established CAD (mostly ACS) and receiving PCI. Our findings suggested a higher risk of composite cardiovascular events following PCI in patients with OSA. In the meta-analysis by Zhao et al,[22] OSA can independently increase the risk of cardiovascular events in 5 studies, which was consistent with our results. Noteworthy, we did multiple sensitivity analysis by excluding studies with retrospective cohort, unadjusted values, sample size ≤100, and follow-up duration ≤1 year, and found no change in the statistical significance or direction of effect for the primary endpoint. In terms of mortality, our results were consistent with those that reported in the general population.[20] The insignificant association for the outcomes of stroke may be due to a small number of included studies or the relatively lower risk in patients with CAD compared to those with stroke.[21]
OSA-mediated intermittent hypoxia, that is triggered by repetitive episodes of apneas and hypopneas, is being recognized as the major factor contributing to cardiovascular impairment.[23] Recurrent cycles of hypoxemia with reoxygenation promote oxidative stress, sympathetic activation, and inflammatory responses, leading to endothelial dysfunction[23] and reduction of repair capacity,[24] which are responsible for the initiation and progression of atherosclerosis. By intravascular ultrasound assessment for symptomatic CAD, patients with OSA had a larger total atheroma volume than those without OSA, even after adjustment for traditional risk factors.[4] In patients with ACS, the presence of OSA was associated with higher rate of restenosis after PCI at 6 months.[5] Our analysis showed an increased risk of repeat revascularization after PCI in patient with OSA, which further supports current evidence. OSA may also act as a trigger for nocturnal myocardial ischemia.[25] In patients presenting with MI, this could exert a continuous effect and result in less myocardial salvage and impaired cardiac function, despite after successful PCI.[26] In addition, patients with OSA showed increased platelet activation and aggregation[27] and reduced fibrinolytic capacity.[28] All these findings predispose to thrombosis or even fatal cardiovascular events, which was verified in our analysis. Specifically, the etiology of OSA varies across patients, including general factors (male sex, age, and obesity), anatomic factors (such as small upper airway lumen), mechanical factors, poor upper airway muscle function, and low arousal threshold,[29,30] all of which would have a different impact on the subsequent cardiovascular risk. Further studies evaluating the association of specific etiology and individualized therapy with adverse events are needed.
Although OSA was found to be a significant predictor of recurrent cardiovascular events, it is prevalent that OSA is underdiagnosed by cardiologists and consequently undertreated.[31] Konecny et al[32] reviewed 798 patients who were hospitalized for acute MI and found only 12% of patients had documentation of diagnosed or suspected OSA. In contrast, more than two thirds had at least mild OSA evaluated by overnight polysomnography in a prospective cohort. In view of higher prevalence of OSA (up to 61.8% in our analysis) and subsequent cardiovascular risk, it is more reasonable and clinically significant to screen for OSA in patients with CAD and/or undergoing PCI than in the general population.
On the other hand, the low awareness of OSA may be explained by few randomized trials to assess the efficacy of continuous positive airway pressure (CPAP) as well as neutral results of current studies. In the multicenter Sleep Apnea Cardiovascular Endpoints trial that randomized 2717 participants, CPAP failed to reduce cardiovascular events in patients with moderate to severe OSA and established cardiovascular disease at a median follow-up of 3.7 years.[33] Another single-center Randomized Intervention With CPAP in Coronary Artery Disease and Sleep Apnea trial enrolled 224 patients with OSA and CAD who had undergone revascularization. The results showed no difference in a composite endpoint of repeat revascularization, MI, stroke, or cardiovascular death in patients with CPAP versus those without CPAP therapy. However, adjusted on-treatment analysis exhibited better outcomes among patients who were adherent to CPAP therapy (≥4 hours per night).[34] Although the beneficial effects of OSA intervention remain controversial, there is still a need for large-scale randomized trials to further explore the treatment effects of CPAP in a high-risk group with homogenous CAD populations (ACS, MI, or PCI, etc).
4.1. Study limitations
First, we observed significant statistical heterogeneity in the risk estimate for MACE. This may be partly explained by differences in sample size, definition of outcomes, and study quality according to Newcastle-Ottava Scale score or whether adjustment for confounders was performed. Second, there is evidence of publication bias for the primary endpoint. However, the effect size remains significant after trim-and-fill analysis. Third, the stent type may have an effect on the association of OSA with cardiovascular outcomes. Therefore, we excluded patients receiving bare-metal stent or other stent types and found no change in the significance of main outcome measure. However, most included studies were conducted in the era of 1st-generation DES. The impact of OSA on recurrent events in the 2nd-generation DES era requires further evaluation. Fourth, all included studies did not report the association of different categories of OSA with cardiovascular events after PCI. The relative risk based on the severity of OSA warrants further investigation. Fifth, results of secondary individual cardiovascular events could be underpowered due to a small number of included studies and variations in definitions of events. Sixth, this meta-analysis was not done based on patient level data. Seventh, because the included studies recruited primarily Asian patients, studies pertaining to other ethnicities are needed. Finally, the data from observational studies should be interpreted with caution. Although our analysis was predominantly based on adjusted values, the potential residual confounding remains a threat to the validity of results.
5. Conclusions
The present meta-analysis suggests that in patients undergoing PCI, the presence of OSA is associated with greater risk of recurrent MACE, all-cause death, cardiovascular death, and repeat revascularization. The results should be interpreted with caution given potential inconsistency of observational studies. Whether treatment of OSA prevents subsequent events after PCI warrants further investigation.
Author contributions
Data curation: Xiao Wang, Jing-Yao Fan, Shao-Ping Nie.
Funding acquisition: Xiao Wang.
Methodology: Xiao Wang, Jing-Yao Fan, Ying Zhang.
Validation: Xiao Wang, Jing-Yao Fan, Ying Zhang.
Writing – original draft: Xiao Wang, Jing-Yao Fan.
Writing – review & editing: Xiao Wang, Jing-Yao Fan, Ying Zhang, Shao-Ping Nie, Yong-Xiang Wei.
Conceptualization: Shao-Ping Nie.
Supervision: Yong-Xiang Wei.
Supplementary Material
Footnotes
Abbreviations: ACS = acute coronary syndrome, AHI = apnea-hypopnea index, CAD = coronary artery disease, CI = confidence interval, CPAP = continuous positive airway pressure, DES = drug-eluting stent, HR = hazard ratio, MACE = major adverse cardiovascular event, MI = myocardial infarction, OSA = obstructive sleep apnea, PCI = percutaneous coronary intervention, RR = risk ratio.
Funding/support: This study was funded by grants from International Science & Technology Cooperation Program of China (2015DFA30160), Beijing Municipal Science & Technology Commission (Z141100006014057) to Y-XW; grants from National Natural Science Foundation of China (81600209), Beijing Municipal Administration of Hospitals’ Youth Program (QML20160605), Beijing Municipal Administration of Hospitals Incubating Program (PX2016048), and Beijing Municipal Organization Department (2016000021469G194) to XW.
The authors have no conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
References
- [1].Peppard PE, Young T, Barnet JH, et al. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol 2013;177:1006–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Javaheri S, Barbe F, Campos-Rodriguez F, et al. Sleep apnea: types, mechanisms, and clinical cardiovascular consequences. J Am Coll Cardiol 2017;69:841–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Weinreich G, Wessendorf TE, Erdmann T, et al. Association of obstructive sleep apnoea with subclinical coronary atherosclerosis. Atherosclerosis 2013;231:191–7. [DOI] [PubMed] [Google Scholar]
- [4].Tan A, Hau W, Ho HH, et al. OSA and coronary plaque characteristics. Chest 2014;145:322–30. [DOI] [PubMed] [Google Scholar]
- [5].Yumino D, Tsurumi Y, Takagi A, et al. Impact of obstructive sleep apnea on clinical and angiographic outcomes following percutaneous coronary intervention in patients with acute coronary syndrome. Am J Cardiol 2007;99:26–30. [DOI] [PubMed] [Google Scholar]
- [6].Mazaki T, Kasai T, Yokoi H, et al. Impact of sleep-disordered breathing on long-term outcomes in patients with acute coronary syndrome who have undergone primary percutaneous coronary intervention. J Am Heart Assoc 2016;5:e003270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Levine GN, Bates ER, Blankenship JC, et al. 2011 ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. Circulation 2011;124:e574–651. [DOI] [PubMed] [Google Scholar]
- [8].Fokkema ML, James SK, Albertsson P, et al. Population trends in percutaneous coronary intervention: 20-year results from the SCAAR (Swedish Coronary Angiography and Angioplasty Registry). J Am Coll Cardiol 2013;61:1222–30. [DOI] [PubMed] [Google Scholar]
- [9].Meng S, Fang L, Wang CQ, et al. Impact of obstructive sleep apnoea on clinical characteristics and outcomes in patients with acute coronary syndrome following percutaneous coronary intervention. J Int Med Res 2009;37:1343–53. [DOI] [PubMed] [Google Scholar]
- [10].Lee CH, Khoo SM, Chan MY, et al. Severe obstructive sleep apnea and outcomes following myocardial infarction. J Clin Sleep Med 2011;7:616–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Loo G, Tan AY, Koo CY, et al. Prognostic implication of obstructive sleep apnea diagnosed by post-discharge sleep study in patients presenting with acute coronary syndrome. Sleep Med 2014;15:631–6. [DOI] [PubMed] [Google Scholar]
- [12].Nakashima H, Kurobe M, Minami K, et al. Effects of moderate-to-severe obstructive sleep apnea on the clinical manifestations of plaque vulnerability and the progression of coronary atherosclerosis in patients with acute coronary syndrome. Eur Heart J Acute Cardiovasc Care 2015;4:75–84. [DOI] [PubMed] [Google Scholar]
- [13].Wu X, Lv S, Yu X, et al. Treatment of OSA reduces the risk of repeat revascularization after percutaneous coronary intervention. Chest 2015;147:708–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Zhang JJ, Gao XF, Ge Z, et al. Obstructive sleep apnea affects the clinical outcomes of patients undergoing percutaneous coronary intervention. Patient Prefer Adherence 2016;10871–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Lee CH, Sethi R, Li R, et al. Obstructive sleep apnea and cardiovascular events after percutaneous coronary intervention. Circulation 2016;133:2008–17. [DOI] [PubMed] [Google Scholar]
- [16].Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000;283:2008–12. [DOI] [PubMed] [Google Scholar]
- [17].Wells G, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. [Accessed Sep 15, 2017]. [Google Scholar]
- [18].Atkins D, Best D, Briss PA, et al. Grading quality of evidence and strength of recommendations. BMJ 2004;328:1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Gottlieb DJ, Yenokyan G, Newman AB, et al. Prospective study of obstructive sleep apnea and incident coronary heart disease and heart failure: the sleep heart health study. Circulation 2010;122:352–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Loke YK, Brown JW, Kwok CS, et al. Association of obstructive sleep apnea with risk of serious cardiovascular events: a systematic review and meta-analysis. Circ Cardiovasc Qual Outcomes 2012;5:720–8. [DOI] [PubMed] [Google Scholar]
- [21].Xie W, Zheng F, Song X. Obstructive sleep apnea and serious adverse outcomes in patients with cardiovascular or cerebrovascular disease: a PRISMA-compliant systematic review and meta-analysis. Medicine (Baltimore) 2014;93:e336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Zhao Y, Yu BY, Liu Y, et al. Meta-analysis of the effect of obstructive sleep apnea on cardiovascular events after percutaneous coronary intervention. Am J Cardiol 2017;120:1026–30. [DOI] [PubMed] [Google Scholar]
- [23].Dewan NA, Nieto FJ, Somers VK. Intermittent hypoxemia and OSA: implications for comorbidities. Chest 2015;147:266–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Jelic S, Padeletti M, Kawut SM, et al. Inflammation, oxidative stress, and repair capacity of the vascular endothelium in obstructive sleep apnea. Circulation 2008;117:2270–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Kuniyoshi FH, Garcia-Touchard A, Gami AS, et al. Day-night variation of acute myocardial infarction in obstructive sleep apnea. J Am Coll Cardiol 2008;52:343–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Buchner S, Satzl A, Debl K, et al. Impact of sleep-disordered breathing on myocardial salvage and infarct size in patients with acute myocardial infarction. Eur Heart J 2014;35:192–9. [DOI] [PubMed] [Google Scholar]
- [27].Bokinsky G, Miller M, Ault K, et al. Spontaneous platelet activation and aggregation during obstructive sleep apnea and its response to therapy with nasal continuous positive airway pressure. A preliminary investigation. Chest 1995;108:625–30. [DOI] [PubMed] [Google Scholar]
- [28].Rangemark C, Hedner JA, Carlson JT, et al. Platelet function and fibrinolytic activity in hypertensive and normotensive sleep apnea patients. Sleep 1995;18:188–94. [DOI] [PubMed] [Google Scholar]
- [29].Jordan AS, McSharry DG, Malhotra A. Adult obstructive sleep apnoea. Lancet 2014;383:736–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Deegan PC, McNicholas WT. Pathophysiology of obstructive sleep apnoea. Eur Respir J 1995;8:1161–78. [DOI] [PubMed] [Google Scholar]
- [31].Costa LE, Uchoa CH, Harmon RR, et al. Potential underdiagnosis of obstructive sleep apnoea in the cardiology outpatient setting. Heart 2015;101:1288–92. [DOI] [PubMed] [Google Scholar]
- [32].Konecny T, Kuniyoshi FH, Orban M, et al. Under-diagnosis of sleep apnea in patients after acute myocardial infarction. J Am Coll Cardiol 2010;56:742–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].McEvoy RD, Antic NA, Heeley E, et al. CPAP for prevention of cardiovascular events in obstructive sleep apnea. N Engl J Med 2016;375:919–31. [DOI] [PubMed] [Google Scholar]
- [34].Peker Y, Glantz H, Eulenburg C, et al. Effect of positive airway pressure on cardiovascular outcomes in coronary artery disease patients with nonsleepy obstructive sleep apnea. The RICCADSA Randomized Controlled Trial. Am J Respir Crit Care Med 2016;194:613–20. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


