Abstract
Background:
Health care providers commonly encounter blisters when treating burn patients. The question as to whether burn blisters should be drained or deroofed has long been debated. To our knowledge, there has been no controlled, randomized clinical trial to determine which treatment is the best management option.
Methods:
Between March 2016, and September 2016; 40 patients with burn blisters greater than 6-mm were enrolled in our study. Patients were randomized into 2 groups: aspiration group and deroofing group. The number of days to complete re-epithelialization was noted. Patient and Observer Scar Assessment Scale data were recorded from subjects and investigators at 4 time points. Pain during dressing changes was evaluated using a visual pain scale. Bacterial cultures were also obtained.
Results:
Average number of days to complete wound healing was 12 days in the aspiration group and 12.55 days in deroofing group. On the Patient and Observer Scar Assessment Scale, investigators found that the aspiration group scars demonstrated improvements in relief and thickness while subjects rated aspiration scars better in terms of pain. Patients with palm/sole blister in the deroofing group scored higher than aspiration group on the visual analogue pain score but it was also not statistically significant (2.66 vs 3.25). The overall incidence of colonization with microorganisms in each group was not significant (15% vs 40%).
Conclusion:
Neither aspiration nor deroofing is a superior treatment of burn blister. However, some objective indicators suggest that aspiration treatment might be more effective than deroofing treatment.
Keywords: aspiration, blister management, burn, deroofing
1. Introduction
Burn blisters are a common wound finding for health care providers treating burn patients in burn centers, emergency rooms, and other medical facilities. Burn injuries are unique in that they incorporate several zones of tissue damage due to difference in heat transfer. The formation of unblemished blisters at the exposed parts of the heat is characteristic of the superficial partial-thickness burn.[1,2] In the inflammatory phase, blisters can occur as a physiological response to burn damage if the epidermis is separated from the underlying dermis.[3] Deep partial-thickness blisters are either impaired or intact sensation and thick-walled and contain white skin, whereas superficial partial-thickness blisters are intact sensation and contain weeping skin and typically thin-walled.[4] The optimal treatment of burn blisters is controversial and few literature reports on which types of burns are more predisposed to blister formation.
Recommended procedures for burn blister management include deroofing, aspiration, and leaving blisters intact. Intact blisters form mechanical barriers to protect against invasion of microorganism and create a moist environment that helps re-epithelization.[5,6] Additionally, the blister fluid was found to have a wound healing effect favorable to fibroblasts and keratinocytes.[7–9] By contrast, vasoactive prostanoids that are capable of progressive damage by increasing vasoconstriction in microcirculation of zone of stasis are abundant in blister fluid.[10,11] Finally, blister fluid may impede fibrinolysis and inhibit opsonic activity against Pseudomonas aeruginosa, leading to progressive dermal necrosis.[12,13] Thus, questions about aspiration or deroofing have been discussed for many years as a treatment for burn blisters in different views by the medical literature and medical experts. The choice of treatment is usually based on the clinical impression, preferences, and expertise of the medical team involved in the care and management of the burn patient.
Herein, we report a randomized controlled trial that was done in our institution to assess the effectiveness and the advantages of different management options of burn blisters; aspiration and deroofing. The outcome assessment was divided into 4 categories: wound healing, functional and aesthetic outcome, patient comfort, and colonization. This is the first prospective study to evaluate the benefits of burn blister management.
2. Patients and methods
2.1. Study design and participants
After obtaining the appropriate approval from our Institutional Review Board, we conducted a parallel-group randomized controlled trial with concealed allocation to measure the effectiveness of aspiration versus deroofing as a management method for burn blisters in patients who visited the emergency room and outpatient clinic our plastic and reconstructive surgery facility. The aim of the study was clearly explained to the potential participants, and written informed consent was obtained from all registered patients before randomization at the emergency room or outpatient clinic. From March 2016 to September 2016, 40 patients with >6-mm burn blisters were included in the trial. The exclusion criteria were an age of <18 years, severe systemic disease or psychotic disorders, and a blister that had already ruptured at the time of the visit or after first aid treatment by another institution. Randomization was performed at the first hospital visit. Patients were classified into an aspiration group and a deroofing group. Randomization was performed by block randomization and the coin tossing method. Patients in each group were treated without being informed of whether they were undergoing aspiration or deroofing. Three board-certified plastic surgeons assessed the treatments while blinded to the patient and group information. The dressing of each group was matched with antibacterial ointment, anti-adhesive material, and gauze dressing.
2.2. Outcome assessment
2.2.1. Wound healing
We compared the number of days until complete re-epithelialization in each group. We measured the wound healing time according to the bodily location of the burn wound and blister size in patients who were managed nonoperatively. The criterion for re-epithelialization was the absence of oozing at the time of taking the dressings off. Re-epithelialization of the burn wounds were assessed at the bedside by an experienced plastic surgeon.
2.2.2. Functional and aesthetic outcome
The patient and observer scar assessment scale (POSAS) scores were obtained from the patients and investigators at 4 weeks, 3 months, 6 months, and 1 year. At the 12-month follow-up, the score for each item of the POSAS was compared between the 2 groups. The POSAS system was validated and defined by Truong et al and van de Kar et al in 2005.[14,15] The evaluation involves the use of a 10-point numerical rating scale, which is a valid and reliable scar assessment tool. The scale ranges from a score of 1 (normal skin) to 10 (worst scar imaginable).[14] The mean score for the patient and observer was calculated by averaging the scores for the separate items. The burn scars in each group were measured independently of each other by board-certified blinded plastic surgeons.
2.2.3. Patient comfort
The patients’ pain during dressing was measured on days 1, 3, 7, and 14 using a visual analog scale (VAS). Patients were asked to score their level of burn wound pain from 0 (no pain) to 5 (worst imaginable pain). The VAS score for all patients according to the body location and blister size was measured on hospital day 1. The patients’ pain tended to decrease rapidly from day 3; therefore, we decided that the comparison on day 1 was more objective and meaningful than the subsequent data. The VAS is an established measure of differences in pain.[16] We examined the pain score for the patients in each group to determine which procedure provided more comfort during dressing. All patients underwent standard surveillance and wound care.
2.2.4. Colonization
The bacterial culture results in each group were assessed according to the treatment method. At the first treatment in the aspiration group, the lumen of the blister was swabbed and a bacterial culture was performed. In the deroofing group, the blister was debridement and then the wound bed was swabbed. The swabbed fluid (aspiration group) and swab sample from the exposed burn (deroofing group) were analyzed according to standard bacteriological methods. On the days 3, 7, and 14 days, the wound beds of each group were swabbed.
2.2.5. Statistical analysis
The POSAS scores were quantitatively analyzed using an independent-samples t test to detect differences between the 2 groups. Categorical variables were analyzed using Fisher exact test. Data analyses were carried out using SPSS v.20.0 for Windows (IBM Corp., Armonk, NY). A P value of <.05 was considered statistically significant.
3. Results
From March 2016 to September 2016, 40 patients were evaluated (20 in the aspiration group and 20 in the deroofing group). The general patient characteristics and the causes and locations of the burns are summarized in Table 1. No statistically significant differences were found between the 2 groups. The flowchart of the trial is presented in Fig. 1.
Table 1.
Patient and injury characteristics.
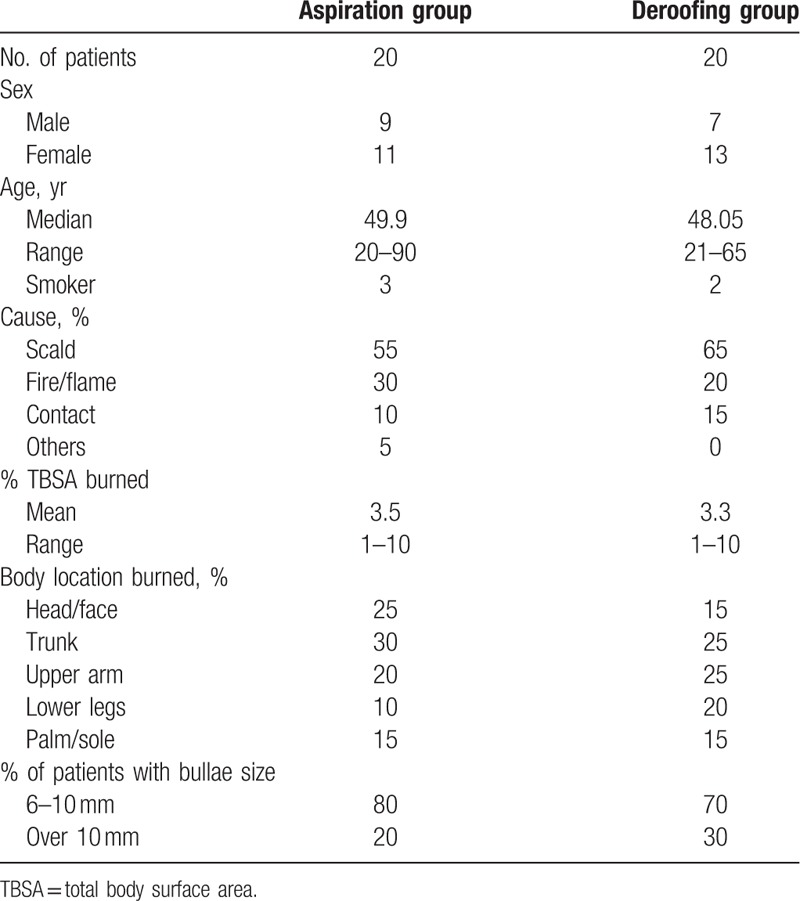
Figure 1.
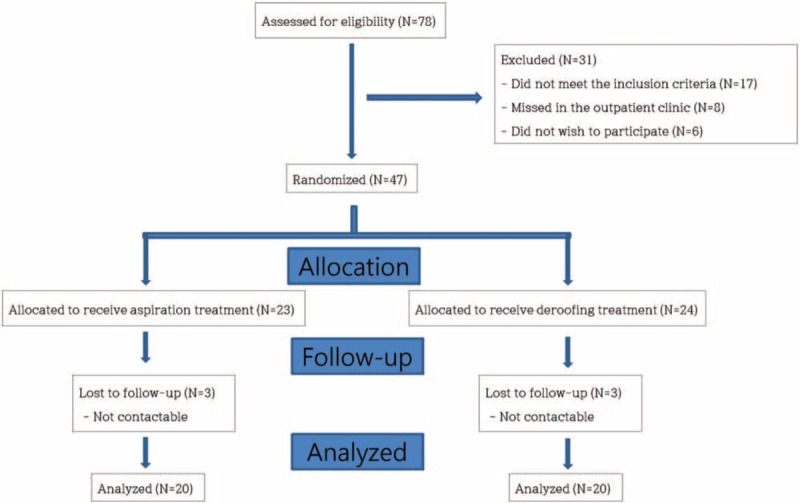
A flow chart of the patient enrollment process and the variables that were measured throughout the study.
The mean number of days to complete wound healing was 12.00 in the aspiration group and 12.55 in the deroofing group, and the difference was not statistically significant (P = .959) (Table 2). Patients with palm/sole burns had a longer recovery period than those with burns at other sites, but again the difference was not statistically significant (15.66 vs 10.66 days, respectively; P = .184).
Table 2.
Days to complete wound healing∗.
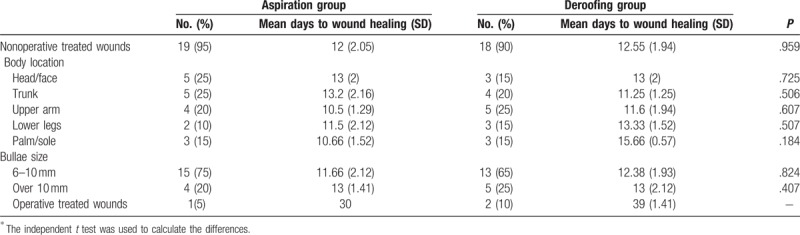
The wound did not heal adequately in 1 patient in the aspiration group and was treated by escharectomy. Two patients in the deroofing group were treated by skin grafting and escharectomy. The mean recovery period in the 2 groups was 30 and 39 days, respectively.
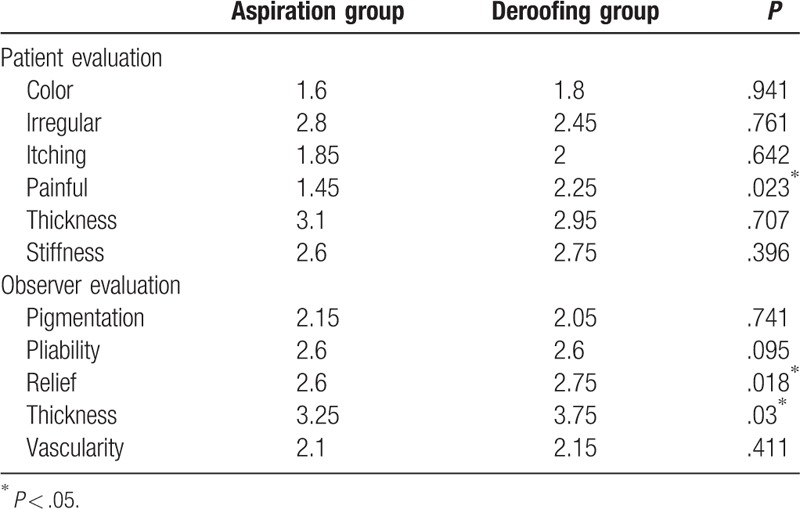
The POSAS scores are shown in Fig. 2. The mean POSAS score (i.e., the average score of 6 items) for both patients and observers slowly decreased after treatment. At different points in time, we were able to compare the differences between the 2 groups. Figure 2 demonstrates the opinions of the treating surgeons and patients. It is worth mentioning that the differences remained consistent over time. Additionally, the difference was that the patient score was higher than the observer score. Both patients and observers noted significant improvements at the first 3-month follow-up. In the aspiration group, a 1.53% reduction in the observer score was found from the 4-week to 3-month follow-up (13.0–12.8). Likewise, in the deroofing group, a 4.37% reduction in the observer score was found (13.7–13.1). The patient scores also decreased in both groups (2.20% in the aspiration group [13.6–13.3] and 1.32% in the deroofing group [15.1–14.9]). From 4 weeks to 12 months, however, the POSAS scores for both the investigators and patients declined monotonically for both groups. The observers found that at 12 months, the scars in the aspiration group demonstrated improvements in pain (P = .023) (Table 3). The patients rated the aspiration scars better with respect to pain relief (P = .018) and tissue thickness (P = .03).
Figure 2.

Overall POSAS Score over time. Overall scar score measured by observer (above) and patient (below) using the patient and observer scar assessment scale (POSAS) at all recorded time points.
Table 3.
Patient and observer scar assessment scale evaluation at 12 months.
No significant difference in the mean pain score on day 1 was observed between the deroofing group (2.56 out of 5.00) and aspiration group (2.31 out of 5.00) (P = .3) (Fig. 3). On day 3, these mean scores in the 2 groups were 1.30 and 1.13, respectively (P = .262). The mean VAS score in the deroofing group was higher on days 1 and 3, but the rate of decrease from day 1 to 3 was similar between the aspiration group (51.08%; from 2.31 to 1.13) and the deroofing group (49.00%; from 2.56 to 1.30). There was no significant difference in the mean VAS score on day 1 in either group according to burn location (head/face, P = .3; trunk, P = .44; upper arm, P = .69; and lower legs, P = .11) or blister size (6–10 mm, P = .16 and >10 mm, P = .16) (Table 4). Although patients with palm/sole burns in the deroofing group had higher scores than those in the aspiration group, there was no statistically significant difference (3.25 vs 2.66, respectively; P = .083). The pain was alleviated in most patients of both groups after 14 days.
Figure 3.
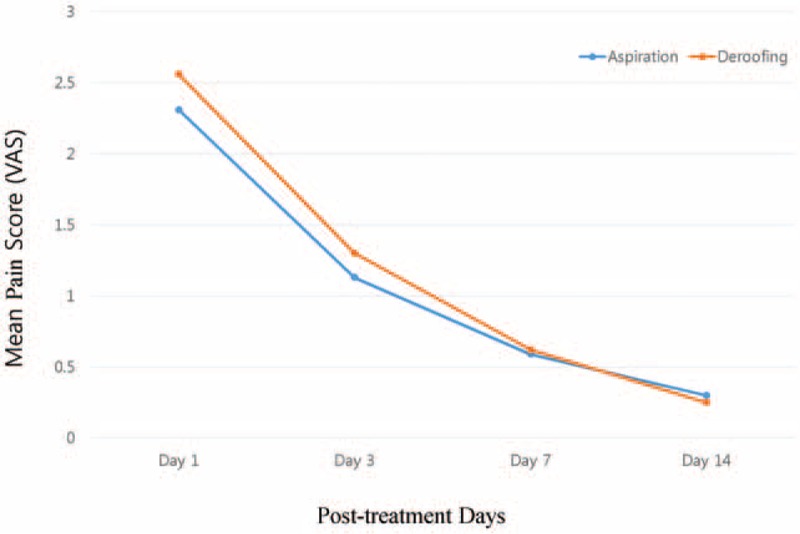
Mean visual analogue scale (VAS) scores for all participants in the aspiration and deroofing groups during 14 days of treatment period.
Table 4.
Mean visual analogue scale (VAS) scores for all participants according to body location and bullae size in the aspiration and deroofing groups in Day 1∗.
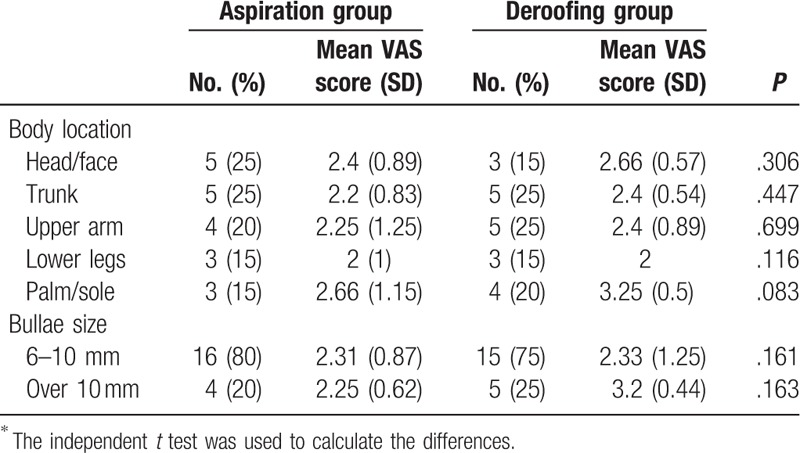
Table 5 denotes the incidence of bacterial colonization in both groups. The overall incidence of colonization with microorganisms was more likely to be lower in the aspirated blisters than in the exposed burn tissue on day 3, but the difference was not significant (15% vs 40%, respectively; P = .15). Colonization was most frequently observed on day 3 and persisted through day 7. On the day 14, however, the most of the wound colonization had resolved.
Table 5.
Effect of different treatments on bacterial colonization.

4. Discussion
This is the first prospective randomized controlled trial to investigate the efficacy of aspiration or deroofing as a method of burn blister management. Sargent[17] described the standards of superficial partial-thickness burns that could be used to devise an evidence-based policy and he discussed clinical applications and conflicting recommendations. Burn blister management remains a controversial subject, and no universal standard of practice has been established among people who deal with burn blisters, even among certified medical burn centers.[18,19] Although the usefulness of deroofing versus aspiration in the management of ideal blisters was evaluated by Swain et al,[20] their study had the disadvantages of a lack of randomization and blinding. The authors recommended aspiration rather than deroofing of blisters in most cases because the pain and exposed surfaces associated with deroofing may promote bacterial colonization. Another prospective, blinded, controlled trial of epidermal debridement and re-epithelialization was performed in swine.[21] In that study, the epidermal debridement group showed a higher infection rate and slower re-epithelialization. However, because pigs do not form burn blisters, the applicability of the findings to human burns is unclear.
In the present study, we divided the patients into 2 groups and measured the treatment efficacy in 4 different categories. Our study did not lead to strong evidence that either aspiration or deroofing was superior. However, some objective indicators support the superiority of aspiration.
Superficial partial-thickness burns usually recover within 5 to 7 days, but deep-thickness burns expect 10 to 21 days or more for re-epithelialization.[22] In our study, the mean number of days until healing was 12.00 in the aspiration group and 12.55 in the deroofing group, without statistical significance (P = .959). Additionally, there was no difference in the wound recovery time according to the burn site or blister size. Among patients with palm/sole burns, the wound healing time was longer in the deroofing group; however, the difference between the groups was not significant (P = .184). These results indicate that no treatment had a significant effect on the number of required treatment days. Although many factors are involved in wound healing and re-epithelialization, moisture is one of the most important factors. In moist versus dry conditions, the healing process may be accelerated by as much as 50%.[23–26] In the current study, appropriate dressing material was used for the burn wounds of each group, and the moisture balance was maintained through appropriate wound care. Therefore, we conclude that the lack of a significant difference in the wound healing time between the 2 groups occurred because proper moisture was maintained in this study.
The POSAS score was measured at 4 weeks, 3 months, 6 months, and 12 months in the 2 groups of patients. We measured the improvement in the POSAS score in both groups over time. We suggest that scar maturation and remodeling occurred mostly between the first 4 weeks and 3 months because the greatest decrease was measured in this period. Furthermore, the remodeling phase may take up to ≥1 year after the injury, as shown in this study.
We found that the patient score was higher than the observer score in this study. This finding seems to indicate that the patient is more sensitive, serious, and subjective than the observer when assessing the same scar.
The investigator assessments of pain relief and tissue thickness were significant in favor of aspiration at the 12-month follow-up (P = .018 and P = .03, respectively). Similarly, the patient-related item for pain was also highly significant in favor of aspiration (P = .023). This suggests that pain relief may be affected by the treatment method and that aspiration could be more effective than deroofing for improvement of the pliability and thickness of the scar.
By covering the underlying cutaneous nerves with an intact epidermis, it can provide a natural way to relieve pain.[17] However, the difference in the VAS score between the debrided and intact epidermis groups was not significant throughout the treatment period in our study. Based on this report and our results, we suggest that regardless of the treatment used, patient comfort can be achieved with the use of proper dressing materials that adhere to the wound site until healing occurs because these materials provide protection to the underlying cutaneous nerves by acting as a temporary epidermis.
The VAS score most frequently decreased from days 1 to 3, and few patients had severe pain after day 3. This result indicates that careful and proper dressing and wound management during the first 3 days of burn management is important for pain relief.
We also measured the VAS score according to the bodily location of the burn and blister size on day 1 between the 2 groups, but no significant differences were found. However, patients with palm/sole burns in the deroofing group had higher scores than those in the aspiration group. Previous reports have indicated that blisters on the palm/sole of the thick-walled may be left intact because of high level of patient discomfort associated with a debridement and because they are less likely to become infected.[27,28] Therefore, based on the information available in the literature and our results, it is more advantageous in terms of patient's pain to aspirate and note remove palm/sole blisters.
Many burn injuries involve various burn depths. Blisters typically exhibit a more superficial partial-thickness wound but occasionally mid-dermal injury requiring excision or skin graft can occur. The accurate depth of wound identification can be achieved by a deroofing of the blisters that allow direct visualization of the wound bed.[27,29] Correct identification of the wound depth is thus imperative because it is correlated with wound infection risk and leads to additional treatment decisions. Only 3 patients in the present study received surgical treatment. Therefore, it is difficult for us to support literature stating that deroofing facilitates wound bed identification. We suggest that if appropriate wound management is performed, the wound depth can be sufficiently judged even in an aspirated blister.
In some patients, we found colonization on day 3 despite a thorough aseptic dressing. We believe that whether the wound was clean or not at the time of injury is more important than the treatment method. The frequency of colonization in the deroofing group was high, but the difference between the 2 groups was not statistically significant. Therefore, proper wound management could prevent wound conversion, and this could in turn reduce the infection risk because burn wounds that maintain tissue viability and intact vascular supply are less susceptible to invasion of microorganism.
We would like to address that there are points of discussion relevant to the setup of the current study. The inclusion of a selected population and the intraindividual comparison may have introduced bias. We are aware of potential confounding factors that were not adjusted for within the data collection, including smoking status, underlying disease (e.g., hypertension, diabetes mellitus, etc.), the patient compliance with the study protocol.
However, our population is a reflection of a broad spectrum of burn scar and blisters seen in daily clinical practice. The randomized and blinded attribute of the study means that the likelihood of bias is minimal, though differences cannot be ruled out. A limitation of current study was the relatively small sample size. For this reason, these findings cannot be generalized to the broader community based on this study alone and further larger studies are required to confirm these results.
5. Conclusion
The main strengths of this study are its prospective, randomized controlled design and this was the first trial to evaluate the effectiveness of aspiration versus deroofing as a method of burn blister management. This study provides high-level evidence supporting the lack of overall superiority of burn blister management techniques but shows that aspiration may be more effective in terms of pain relief and scar thickness.
Author contributions
Conceptualization: Hyung-Suk Ro, M. Diya Sasbbagh, Si-Gyun Roh, Suk Choo Chang.
Data curation: Hyung-Suk Ro, Nae-Ho Lee.
Investigation: Hyung-Suk Ro.
Project administration: Hyung-Suk Ro, Nae-Ho Lee.
Software: Hyung-Suk Ro, Si-Gyun Roh.
Visualization: Hyung-Suk Ro, Jin Yong Shin.
Writing – original draft: Hyung-Suk Ro, Si-Gyun Roh, Suk Choo Chang.
Funding acquisition: Jin Yong Shin, Suk Choo Chang.
Resources: Jin Yong Shin.
Supervision: Jin Yong Shin, Nae-Ho Lee.
Validation: M. Diya Sasbbagh, Suk Choo Chang, Nae-Ho Lee.
Formal analysis: Si-Gyun Roh.
Writing – review & editing: Si-Gyun Roh, Suk Choo Chang.
Footnotes
Abbreviations: POSAS = patient and observer scar assessment scale, VAS = visual analogue scale.
Financial disclosure: This paper was supported by Fund of Biomedical Research Institute, Chonbuk National University Hospital.
The authors report no conflicts of interest.
References
- [1].Arturson G. Mechanism of injury. Principles and Practice of Burns Management. New York: Churchill Livingstone; 1996:61–82. [Google Scholar]
- [2].Richard R, Johnson RM. Managing superficial burn wounds. Adv Skin Wound Care 2002;15:246–7. [DOI] [PubMed] [Google Scholar]
- [3].DuKamp A. Deroofing minor burn blisters–what is the evidence? Accid Emerg Nurs 2001;9:217–21. [DOI] [PubMed] [Google Scholar]
- [4].Wardrope J, Edhouse JA. The Management of Wounds and Burns, Vol 20. USA: Oxford University Press; 1999. [Google Scholar]
- [5].Winter GD. Formation of the scab and the rate of epithelization of superficial wounds in the skin of the young domestic pig. Nature 1962;193:293–4. [DOI] [PubMed] [Google Scholar]
- [6].Winter GD. Effect of air exposure and occlusion on experimental human skin wounds. Nature 1963;200:378–9. [DOI] [PubMed] [Google Scholar]
- [7].Chen WJ, Rogers AA, Lydon MJ. Characterization of biologic properties of wound fluid collected during early stages of wound healing. J Invest Dermatol 1992;99:559–64. [DOI] [PubMed] [Google Scholar]
- [8].Wilson Y, Goberdhan N, Dawson R, et al. Investigation of the presence and role of calmodulin and other mitogens in human burn blister fluid. J Burn Care Rehabil 1994;15:303–14. [DOI] [PubMed] [Google Scholar]
- [9].Kupper T, Deitch E, Baker C, et al. The human burn wound as a primary source of interleukin-1 activity. Surgery 1986;100:409–15. [PubMed] [Google Scholar]
- [10].Heggers JP, Loy GL, Robson MC, et al. Histological demonstration of prostaglandins and thromboxanes in burned tissue. J Surg Res 1980;28:110–7. [DOI] [PubMed] [Google Scholar]
- [11].Del Beccaro EJ, Heggers JP, Robson MC. Preventing the prostaglandin effect on dermal ischemia in the burn wound. Surg Forum 1978;29:603–5. [PubMed] [Google Scholar]
- [12].Deitch EA, Dobke M, Baxter CR. Failure of local immunity: a potential cause of burn wound sepsis. Arch Surg 1985;120:78–84. [DOI] [PubMed] [Google Scholar]
- [13].Rockwell W, Ehrlich H. Fibrinolysis inhibition in human burn blister fluid. J Burn Care Rehabil 1989;11:1–6. [DOI] [PubMed] [Google Scholar]
- [14].van de Kar AL, Corion LU, Smeulders MJ, et al. Reliable and feasible evaluation of linear scars by the Patient and Observer Scar Assessment Scale. Plast Reconstr Surg 2005;116:514–22. [DOI] [PubMed] [Google Scholar]
- [15].Truong PT, Lee JC, Soer B, et al. Reliability and validity testing of the Patient and Observer Scar Assessment Scale in evaluating linear scars after breast cancer surgery. Plast Reconstr Surg 2007;119:487–94. [DOI] [PubMed] [Google Scholar]
- [16].Farrar JT, Portenoy RK, Berlin JA, et al. Defining the clinically important difference in pain outcome measures. Pain 2000;88:287–94. [DOI] [PubMed] [Google Scholar]
- [17].Sargent RL. Management of blisters in the partial-thickness burn: an integrative research review. J Burn Care Res 2006;27:66–81. [DOI] [PubMed] [Google Scholar]
- [18].Fakhry SM, Alexander J, Smith D, et al. Regional and institutional variation in burn care. J Burn Care Res 1995;16:85–90. [DOI] [PubMed] [Google Scholar]
- [19].Hill MG, Bowen CC. The treatment of minor burns in rural Alabama emergency departments. J Emerg Nurs 1996;22:570–6. [DOI] [PubMed] [Google Scholar]
- [20].Swain AH, Azadian BS, Wakeley CJ, et al. Management of blisters in minor burns. Br Med J (Clin Res Ed) 1987;295:181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Singer AJ, Thode HC, McClain SA. The effects of epidermal debridement of partial-thickness burns on infection and reepithelialization in swine. Acad Emerg Med 2000;7:114–9. [DOI] [PubMed] [Google Scholar]
- [22].Kagan RJ, Warden GD. Management of the burn wound. Clin Dermatol 1994;12:47–56. [DOI] [PubMed] [Google Scholar]
- [23].Ayello EA, Dowsett C, Schultz GS, et al. TIME heals all wounds. Nursing 2004;34:36–41. [DOI] [PubMed] [Google Scholar]
- [24].Smith DJ, Thomson PD, Garner WL, et al. Burn wounds: infection and healing. Am J Surg 1994;167:S46–8. [DOI] [PubMed] [Google Scholar]
- [25].Collier M. Wound bed preparation: theory to practice. Nurs Stand 2003;17:45–52. [DOI] [PubMed] [Google Scholar]
- [26].Geronemus RG, Robins P. The effect of two new dressings on epidermal wound healing. J Dermatol Surg Oncol 1982;8:850–2. [DOI] [PubMed] [Google Scholar]
- [27].Johnson RM, Richard R. Partial-thickness burns: identification and management. Adv Skin Wound Care 2003;16:178–87. [DOI] [PubMed] [Google Scholar]
- [28].Honari S. Topical therapies and antimicrobials in the management of burn wounds. Crit Care Nurs Clin North Am 2004;16:1–1. [DOI] [PubMed] [Google Scholar]
- [29].Merz J, Schrand C, Mertens D, et al. Wound care of the pediatric burn patient. AACN Clin Issues 2003;14:429–41. [DOI] [PubMed] [Google Scholar]


