Abstract
Introduction:
Recent progress in medical technology has resulted in improved surgical outcomes of pars plana vitrectomy (PPV); with microincision systems, the incidence of procedure-related complications during surgery has been reduced. However, unpredictable visual field defects after PPV remain an unresolved issue. A few reports have shown that damage to the retinal neurofibers owing to dry-up during air/fluid exchange or retinal neurotoxicity of the dye used to visualize the internal limiting membrane (ILM), as well as unintentional removal of retinal neurofibers during ILM peeling, are responsible for such visual field disorders. In this report, we present a case of extensive visual field defect due to optic neuropathy exhibiting vertical hemianopsia after PPV.
Case Summary:
A 50-year-old woman underwent PPV and cataract surgery for a macular hole and mild cataract under retrobulbar anesthesia with 3.5 mL of xylocaine. At the time of opening an infusion cannula for PPV, the intraocular lens was herniating, with an acute increase in pressure from the posterior eyeball; thus, intraocular pressure configuration level had to be decreased from the default level, whereas the other procedures including 20% SF6 injection were performed without any modification. The macular hole was closed postoperatively. However, the patient experienced nasal hemianopsia, which turned out to be optic neuropathy, as assessed via electric physiological examinations. The pattern of the visual field defect was not typical for glaucoma or anterior ischemic optic neuropathy. Her optic nerve head was pale at the temporal side soon after the surgery, and her blood pressure was low, suggesting that there may have been a congestion of the optic nerve feeder vessels because of the relatively high pressure in the orbit. The space occupancy with xylocaine and extensively stretched and plumped out eye ball with infusion during PPV may have pressed the surrounding tissue of the optic nerve and the feeder vessels.
Conclusion:
PPV is safe for most patients; however, individual variations in local and/or systemic conditions may cause complications. Future studies to optimize the surgical condition for each individual patient may be warranted.
Keywords: complication, macular hole, optic neuropathy, pars plana vitrectomy, visual field defect
1. Introduction
Recent progress in medical technology has resulted in reduced incidence of procedure-related complications during pars plana vitrectomy (PPV) with micro-incision systems. However, the presence of unpredictable visual field defects after PPV remains an unresolved issue. Post-PPV visual field defects have been attributed to the retinal neurofiber damage caused by intravitreal procedures. The use of air-moisturizing systems to prevent retinal drying,[1] and the use of a less neurotoxic reagent, such as brilliant blue G, to visualize the internal limiting membrane (ILM),[2] have been introduced as standard methods to prevent retinal damage, whereas unplanned mechanical neurofiber removal related to ILM peeling has not been completely avoided.[3] Thus, the appropriateness of ILM peeling is now under discussion. In the present report, we present a case of drastic visual field loss and vertical hemianopsia after macular hole surgery, which was caused by optic neuropathy, rather than by retinal damage related to ILM peeling.
2. Case presentation
In October 2015, a 50-year-old woman presented with impaired central vision in her left eye and was diagnosed with a macular hole (Fig. 1A) and mild cataract at the Vitreo-Retina Division Clinic of the Department of Ophthalmology, Keio University Hospital. The best-corrected visual acuity (BCVA) in her left eye was 0.5 in decimal (0.30 in logMAR), the intraocular pressure (IOP) was 15 mmHg, and the axial length was 23.6 mm. On February 1, 2016, she underwent cataract surgery with intraocular lens (IOL) implantation followed by PPV, after retrobulbar anesthesia with 3.5 mL of xylocaine injected from the inferior temporal margin of the orbit. At the time of opening an infusion cannula for PPV, the IOL was herniating, with an acute increase in pressure from the posterior eyeball. The default IOP configuration for PPV was set to 30 mmHg. After manual decrease of the IOP configuration level, the IOL position recovered, and PPV with ILM peeling using brilliant blue G and 20% SF6 injection was performed without any modification from the general procedure.
Figure 1.
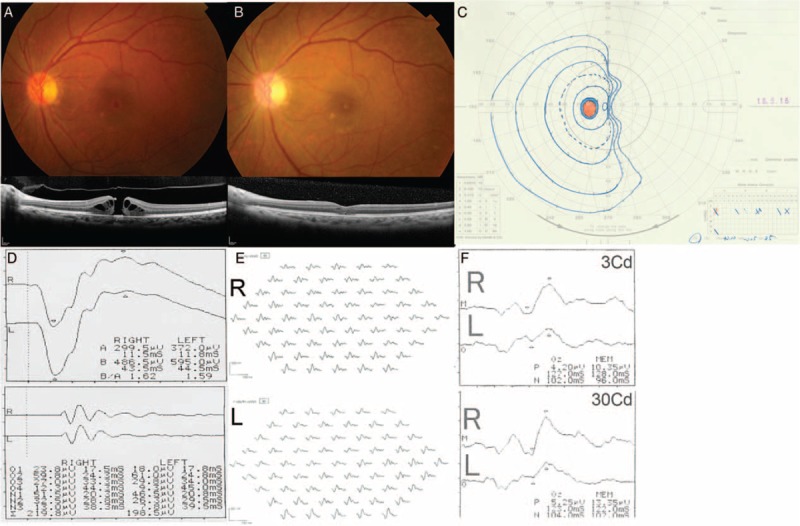
Ocular findings before and after the pars plana vitrectomy procedure. Preoperative fundus photography and optical coherence tomography performed on October 29, 2015 showed a macular hole in the patient's left eye (A). The macular hole was closed by February 22, 2016 (B). Goldman perimetry performed on May 16, 2016 (C) showed a nasal vertical visual field defect in her left eye. At this time, there were no right-left differences in the full-field (D) and multifocal (E) electroretinograms. However, the flash visual evoked potential was impaired in her left eye (F).
The macular hole was closed by February 22, 2016 (Fig. 1B). Her BCVA recovered to 0.8 (0.10) on March 7, 2016. The postoperative IOP remained constant between 15 and 16 mmHg. However, she felt something strange in her visual field at this time, although she could not describe the symptoms clearly. By April 14, 2016 she noticed postoperative nasal visual field loss. There was temporal pallor of the optic nerve head. On May 16, 2016 a visual field examination confirmed nasal hemianopia in her left eye only (Fig. 1C). There were no particular cranial and orbital magnetic resonance imaging findings (data not shown) or full-field and multifocal electroretinogram findings (Fig. 1D and E). Conversely, flash visual evoked potential clearly showed low response in her left eye (Fig. 1F), indicating optic neuropathy. Her BCVA recovered to 1.2 (−0.079) and the macular hole remained closed, whereas her hemianopsia was unchanged at the latest follow-up visit (August 2017; data not shown). She continued taking a topical steroid and non-steroidal anti-inflammatory drug for the first 3 months after PPV. No other adverse events were noted during the follow-up period.
3. Discussion
Based on the clinical observations, this case of postoperative, unilateral nasal vertical hemianopsia was caused by an optic nerve disorder of the temporal side. Because the symptoms and pale optic nerve head appeared early postoperatively, the damage most likely occurred intraoperatively. The temporal orbital connective tissue should have been distended by xylocaine injected for local anesthesia, and one side of the optic nerve and/or its feeding vessels may have been extensively compressed. Additionally, relatively high ocular pressure set as the IOP configuration for PPV may have stretched the eye ball extensively to plump the eye ball out, and reduced the space and relatively increased the pressure in the orbit. Therefore, feeder vessels of the optic nerve at one side may have had a transient circulatory failure. Alternatively, increased IOP at the beginning of PPV may have also contributed to the optic nerve damage. Because the oscillatory potential of the full-field electroretinogram showed no abnormalities, the retinal inner function was intact, and the retinal circulation was not disturbed. However, circulatory failure may have occurred in the feeder vessels of the optic nerve level, potentially causing optic neuropathy.
Optic neuropathy post-PPV for retinal detachment may be caused by reduced ocular circulation in patients with low blood pressure.[3] The estimation of ocular perfusion pressure uses the systemic blood pressure value, and our patient had low blood pressure (100/60 mmHg), indicating a risk for local circulatory disorders; the compression of the orbital space by the anesthetic and the ocular expansion with an extremely high IOP caused by using default ocular infusion conditions for PPV may have easily caused circulation-related complications in her orbit. This finding differed from that of our previous report of a patient with high blood pressure (176/107 mmHg) who exhibited horizontal visual field defects and was diagnosed with anterior ischemic optic neuropathy post-PPV.[4]
When clinicians from various fields encounter cases of vertical hemianopsia, they will first examine the patient for intracranial diseases, but not for ocular diseases. However, this case highlights the possibility of occurrence of unilateral vertical hemianopsia as a complication related to eye surgery.
4. Conclusions
PPV is safe for most patients; however, individual variations in local and/or systemic conditions may cause complications. Future studies to optimize the surgical condition on a case-by-case basis may be warranted.
Acknowledgements
The authors thank their colleagues in the Vitreo-Retina Division Clinic of the Department of Ophthalmology, Keio University Hospital (Tokyo, Japan) and the paramedical staff in the department for assisting us.
Author contributions
Conceptualization: Yoko Ozawa.
Data curation: Hirohiko Kawashima, Yoko Ozawa.
Methodology: Yoko Ozawa.
Supervisio: Norihiro Nagai, Hajime Shinoda, Kazuo Tsubota.
Validation: Yoko Ozawa.
Footnotes
Abbreviations: BCVA = best-corrected visual acuity, ILM = internal limiting membrane, IOL = intraocular lens, IOP = intraocular pressure, PPV = pars plana vitrectomy.
H.K. and Y.O. have contributed equally to this study.
This study followed the tenets of the Declaration of Helsinki, and was approved by the Ethics Committee of the Keio University School of Medicine (approval number; 20100003). Written informed consent was obtained from the patient.
The authors report no conflict of interest.
References
- [1].Yan H, Dhurjon L, Chow DR, et al. Visual field defect after pars plana vitrectomy. Ophthalmology 1998;105:1612–6. [DOI] [PubMed] [Google Scholar]
- [2].Baba T, Hagiwara A, Sato E, et al. Comparison of vitrectomy with brilliant blue G or indocyanine green on retinal microstructure and function of eyes with macular hole. Ophthalmology 2012;119:2609–15. [DOI] [PubMed] [Google Scholar]
- [3].Bansal AS, Hsu J, Garg SJ, et al. Optic neuropathy after vitrectomy for retinal detachment: clinical features and analysis of risk factors. Ophthalmology 2012;119:2364–70. [DOI] [PubMed] [Google Scholar]
- [4].Uchida A, Shinoda K, Matsumoto CS, et al. Acute visual field defect following vitrectomy determined to originate from optic nerve by electrophysiological tests. Case Rep Ophthalmol 2012;3:396–405. [DOI] [PMC free article] [PubMed] [Google Scholar]


