Supplemental Digital Content is available in the text
Keywords: acute kidney injury, crystalloid, hydroxyethyl starch, surgery
Abstract
Background:
While hydroxyethyl starch (HES) solutions are not recommended any longer in critically ill patients, data on efficacy and safety during surgery are still limited.
Methods:
In a randomized controlled trial 63 patients were assigned to receive 10% HES (130/0.42), 6% HES (130/0.42), or crystalloid within a goal-directed hemodynamic algorithm during pancreatic surgery. The primary endpoints were intraoperative volume of HES and time until fully on oral diet.
Results:
The trial was terminated early upon recommendation of an independent data monitoring committee due to futility for efficacy at a planned interim analysis. The intraoperative volume of HES was not different between 10% and 6% HES group (2000 [1500; 2250] vs 2250 [1750; 3000] mL, P=.059). However, considering an inhomogeneity of patient's body weight between HES groups, there was a significant difference in intraoperative volume of HES between 10% and 6% group after adjusting for patient's body weight (24.0 [21.6; 28.3] vs 33.3 [28.2; 46.2] mL kg−1 BW, P = .002). Patients in the HES groups required less additional fluid after dose limit than those in the crystalloid group, resulting in lower intraoperative net balances. The time until fully on oral diet was not different between all study groups. Applying KDIGO oliguria criterion, patients receiving 10% HES had more AKI compared to patients receiving crystalloids (86.7 vs 45.0%, P = .010), whereas those receiving 6% HES and crystalloids did not differ (58.8 vs 45.0%, P = .253). Further explorative analyses using a gray-zone approach indicated that patients receiving 6% HES below 18.8 mL kg−1 will not experience AKI with near certainty.
Conclusions:
After adjusting for patient's body weight, patients receiving 6% HES required more volume of HES than patients receiving 10% HES. The relation of 140% represents very well the volume effect of a hyperoncotic 10% HES solution. Nonetheless, both HES solutions were similarly effective in reducing intraoperative fluid administration compared with crystalloid, but this did not result into differences in gastrointestinal outcomes. Patients receiving 10% HES showed an increased rate of AKI, whereas those receiving 6% HES and crystalloid did not differ. However, 6% HES should not be applied beyond 18 mL kg−1 during surgery.
1. Introduction
Major abdominal surgery is still associated with a substantial number of complications resulting in significant perioperative morbidity and mortality.[1]
In this context, perioperative fluid and volume therapy has been identified as a considerable determinant within clinical pathways in today's anesthesiological care aiming at enhancing recovery after surgery.[2,3] While a low dose continuous administration of crystalloid has been recommended for restoring and maintaining salt-water homeostasis, there is still an ongoing comprehensive debate with respect to colloid or crystalloid solutions for maintaining circulatory blood flow.[4] Due to the higher intravascular volume effects, the use of colloids could result in lower fluid demands and therefore might avoid fluid overload during major surgery. Fluid overload is supposed to be closely related to a delay in the return of normal bowel function and enhanced recovery from postoperative ileus (POI) is one of the most important factors to overall recovery of the patient during hospital stay.[5–7]
In 2013, the European Medicines Agency and the US Food and Drug Administration decided that hydroxyethyl starch (HES) solutions must no longer be used in critically ill patients due to increased risk for acute kidney injury (AKI) and mortality. Both statements were based on trials in critically ill patients where colloid administration was mainly guided without a goal-directed approach and was given for several days during ICU stay.[8,9] However, it is debatable if the deleterious effects of HES on renal function shown in critical ill patients are generally applicable to the short exposure that occurs during surgery. In the perioperative setting, available data from randomized controlled trials on efficacy and safety of HES are still sparse.
We hypothesized that a goal-directed therapy with 10% and 6% HES reduces fluid demands during surgery and is related to enhanced recovery from POI while 10% and 6% HES do not increase the risk for AKI compared with balanced crystalloid.
In this context, we compared 10% and 6% HES and crystalloid with respect to fluid administration and gastrointestinal outcomes, as well as evaluated as safety parameter AKI in high-risk patients undergoing major abdominal surgery.
2. Materials and methods
This study was a multicenter, randomized, double-blinded, parallel-group trial conducted in 3 tertiary care hospitals in Germany between June 2010 and July 2012. The study was registered internationally (European Union Drug Regulating Authorities Clinical Trials: EudraCT 2008-004175-22; ClinicalTrials.gov: NCT01117649) and approvals were attained from the national regulatory authority (Bundesinstitut für Arzneimittel und Medizinprodukte; BfArM No. 61-3910-4034969) and the ethics committee (Ethik-Kommission des Landes Berlin; No. ZS EK 11 026/09).
Eligible patients were adults, aged 18 years or older and aged 80 years or younger, scheduled for elective surgery of the pancreatic head due to primary pancreatic cancer or chronic pancreatitis at the University Hospital Charité, Campus Virchow-Klinikum Berlin, Germany; the Vivantes Humboldt Klinikum Berlin, Germany; and the University Hospital Bonn, Germany. Exclusion criteria included chronic heart failure defined as greater than class II according to the New York Heart Association (NYHA), American Society of Anesthesiologists (ASA) classification status greater than III, renal insufficiency (serum creatinine >1,5 mg dL−1 or >130 μmol L−1) or dependency on hemodialysis, impaired hepatic function (Quick-value <60% or liver cirrhosis Child–Pugh C), history of bleeding disorder or known bleeding diathesis, hematocrit ≤25%, aneurysm of the ascending and/or thoracic aorta, patients with any local esophageal disease, additional contraindications for application of study medication, pregnancy or lactation period, emergency surgery, simultaneous participation in another interventional clinical trial, and detained patients by judicial or enforceable order. Written informed consent was obtained from all patients.
2.1. Intervention, randomization and blinding
The patients were randomly assigned to receive either a hyperoncotic balanced 10% HES 130/0.42 solution (Tetraspan 10%, B. Braun, Melsungen, Germany), an isooncotic balanced 6% HES 130/0.42 solution (Tetraspan 6%, B. Braun) or a balanced crystalloid solution (Sterofundin ISO, B. Braun) within an outcome-based hemodynamic algorithm during surgery.[10,11]
The random list was generated by an independent study statistician with a block randomization to either treatment in a 1:1:1 ratio and handed out to the hospital's pharmacies for patient's allocation to the respective treatment and in sealed envelopes to the Principal Investigators for emergency unblinding. The random list considered the stratification for investigational center and ASA-class (ASA ≤2 and ASA 3). After receiving written informed consent from the patient, the study participation was faxed to the pharmacy in pseudonymised form with the random number (including center number, strata, and consecutive ascending number). The pharmacy provided the study medication in colored and transparent polybags and lines avoiding identification of the study fluids to maintain blinding while assuring that intravenous air administration could be prevented.
2.2. Clinical pathway and hemodynamic protocol
Patients were treated within an interdisciplinary clinical pathway defined by standard operating procedures at each hospital site (a brief summary is provided in Supplemental Digital Content—Methods). The hemodynamic management was performed according to a goal-directed hemodynamic algorithm guided by the esophageal Doppler monitor (EDM, CardioQ-ODMTM, Deltex Medical, Chichester, UK),[10,11] while the volume of study fluid for the fluid challenges was 250 mL (Supplemental Digital Content—Figure S1).
Briefly, after induction of anesthesia and establishing the hemodynamic monitoring an initial fluid challenge of 250 mL of intravenous study fluid was given over 5 minutes. If the EDM detected an increase of stroke volume (SVEDM) <10% no further fluid challenge was performed. If SVEDM increased ≥10%, additional fluid challenges with an intravenous bolus of 250 mL study fluid were given until no further increase of SVEDM ≥10% could be measured. After a period of 15 minutes or acute hemodynamic deterioration SVEDM was measured again and a decrease of >10% compared with SVEDM after the last fluid challenge re-indicated further fluid challenges.
Fluid challenges were performed during the entire course of surgery as indicated by the goal-directed hemodynamic algorithm and conducted with the randomized study medication. The maximum doses for 10% and 6% HES solutions were 30 and 50 mL kg−1 body weight (BW), respectively, corresponding to a maximum dose of 3 g kg−1 BW per day. After reaching the maximum dose, in the 10% HES group, the blinded treatment was continued with balanced crystalloid solution up to a dose of 50 mL kg−1 BW. Then an open-label balanced crystalloid solution was used for further fluid challenges within the goal-directed hemodynamic algorithm until the end of surgery. Regarding the 6% HES and crystalloid solution, similarly, at the maximum dose of 50 mL kg−1 BW, open-label balanced crystalloid solution was used if further fluid challenges were required within the goal-directed hemodynamic algorithm.
With respect to salt and water homeostasis, an intraoperative continuous and restrictive maintenance rate of 4 mL kg−1 BW h−1 of a balanced crystalloid solution was administered in each study group. Mean arterial pressure was maintained with bolus or continuous administration of norepinephrine and positive inotropic drugs were given if cardiac index dropped below 2.5 liter min−1 m−2, while stroke volume could not be raised further by volume administration.
2.3. Trial endpoints
The primary multiple ordered endpoints of the study were the intraoperative volume of HES [ml] administered within the goal-directed algorithm; and the time until fully on oral solid diet.
Secondary endpoints were intraoperative fluid characteristics and balances, time courses of hemodynamic variables during surgery, further gastrointestinal outcome measures, and acute kidney injury (AKI). AKI diagnosed by the definition of the Kidney Disease Improving Global Outcome (KDIGO) group[12] was performed as a post-hoc analysis. Regarding intraoperative fluid characteristics, the time to re-indication (TTRI) of a fluid challenge was recently introduced as a new approach to characterize the patient's individual fluid demands at different time points during surgery.[13] TTRI was calculated as the time frame from preload optimisation to re-indication of a fluid challenge within the used goal-directed algorithm. Due to the safety issues raised by the European Medicines Agency's (EMA) Pharmacovigilance Risk Assessment Committee (PRAC) and the U.S. Food and Drug Administration (FDA) with respect to HES solutions increasing risk for mortality and AKI in critical ill patients, comparisons with respect to the primary and secondary endpoints between 10% and 6% HES group were complemented by the statistical comparison with the balanced crystalloid group, which was initially planned for descriptive analysis only.
2.4. Statistical analysis
Assuming a type I error rate of 5% (two-sided), 76 patients per group, including a drop-out rate of 10%, were calculated to detect with 80% power a difference of 250 mL of administered HES solution within the goal-directed algorithm between 10% and 6% HES group. After including 60 patients into the pilot phase an interim analysis was performed. The sample size was reassessed without unblinding using the pooled standard deviation[14,15] of administered HES solution and revealed that 753 patients were required per group. An independent data monitoring committee (IDMC) recommended stopping the trial for futility after 63 patients were enrolled into the study. Analyses were performed according to an a priori statistical analysis plan. Due to the safety issues raised by the European Medicines Agency's (EMA) Pharmacovigilance Risk Assessment Committee (PRAC) and the U.S. Food and Drug Administration (FDA) with respect to detrimental effects of HES, the analysis was performed based on the per protocol population with respect to all endpoints.
Because of limited sample sizes and deviations from normal distribution of the observations, data were expressed as median (25%, 75% quartiles), or frequencies (%), respectively. Differences between study groups with respect to continuous data were tested using the exact Mann–Whitney U test for independent groups, while frequencies were tested by Fisher's exact test. Differences between study groups with respect to endpoints based on time-to-event data were tested using log-rank tests and presented as Kaplan–Meier curves with respect to cumulative events. Differences between study groups in hemodynamic variables with respect to time were analyzed using nonparametric analysis for longitudinal data in a two-factorial design (1st factor: groups, 2nd factor: time).[16] The following hypotheses were tested with these analyses: overall group differences over time [Group], pairwise group differences over time [Group pairwise], and interactions between group differences and time.
For further explorative analyses to investigate the association of administered HES dose and volume of crystalloid with AKI, a gray-zone approach[17] was performed and integrated into a boxplot presentation. In former studies, a receiver operating characteristic (ROC) curve with calculation of a single cut-off has been used to evaluate if a variable of interest (most common a biomarker) might be able to predict a binary outcome measure. However, most variables of interest do not perfectly discriminate between patients with and without the status of the binary outcome measure (i.e., AKI and No AKI in our case), so that the use of the variable of interest in daily practice does not allow certainty in the determination of the outcome measure. Therefore, the “gray-zone” approach has recently been introduced to avoid the binary constraint of a “black-or-white” decision of the ROC curve with calculation of a single cut-off that often does not fit the reality in daily practice.[17] The gray zone was defined as 95% CI of the mean value of the best cut-off determined according to the Youden index within a ROC curve and conducted for a 1,000 samples bootstrapped from the study population. This approach avoids a single cut-off that dichotomizes the population and provides 2 cut-offs that constitute the borders of the gray zone: patients with HES doses or volume of crystalloid below the lower cut-off of the gray zone will not experience AKI with near certainty, whereas patients with HES doses or volume of crystalloid above the upper cut-off will develop AKI with near certainty. In patients who fall into the gray zone, further clinical assessment is required as the HES dose or volume of crystalloid cannot be used to allow certainty in the determination of AKI. Consequently, this approach allows clinical decision making on HES and crystalloid administration during major surgery with respect to AKI.
A two-tailed P-value of .05 was considered statistically significant. All tests have to be understood in the area of exploratory data analysis. Therefore, no adjustments for multiple testing have been made. All numerical calculations were performed with the R project for Statistical Computing, Version 3.2.2 (R-packages used: foreign, gplots, nparLD, survival, pROC).
3. Results
The trial was stopped for futility after a total of 63 patients were enrolled between June 2010 and July 2012 (Fig. 1); 20 patients were allocated to the 10% HES group, 22 patients to the 6% HES group, and 21 patients to the crystalloid group. After randomization 2 patients were excluded before receiving study medication. In addition, 1 patient allocated to 10% HES group was incorrectly treated with 6% HES and in another 7 patients the intervention was discontinued, in most cases due to inoperability or changes in surgical procedures. In 1 patient, the study case report forms were lost during the postoperative course. Finally, a total of 15 patients in the 10% HES group, 17 patients in the 6% HES group and 20 patients in the crystalloid group were eligible for statistical analysis of primary and secondary endpoints. There were no protocol deviations reported with respect to stroke volume optimization loop within the goal-directed algorithm.
Figure 1.
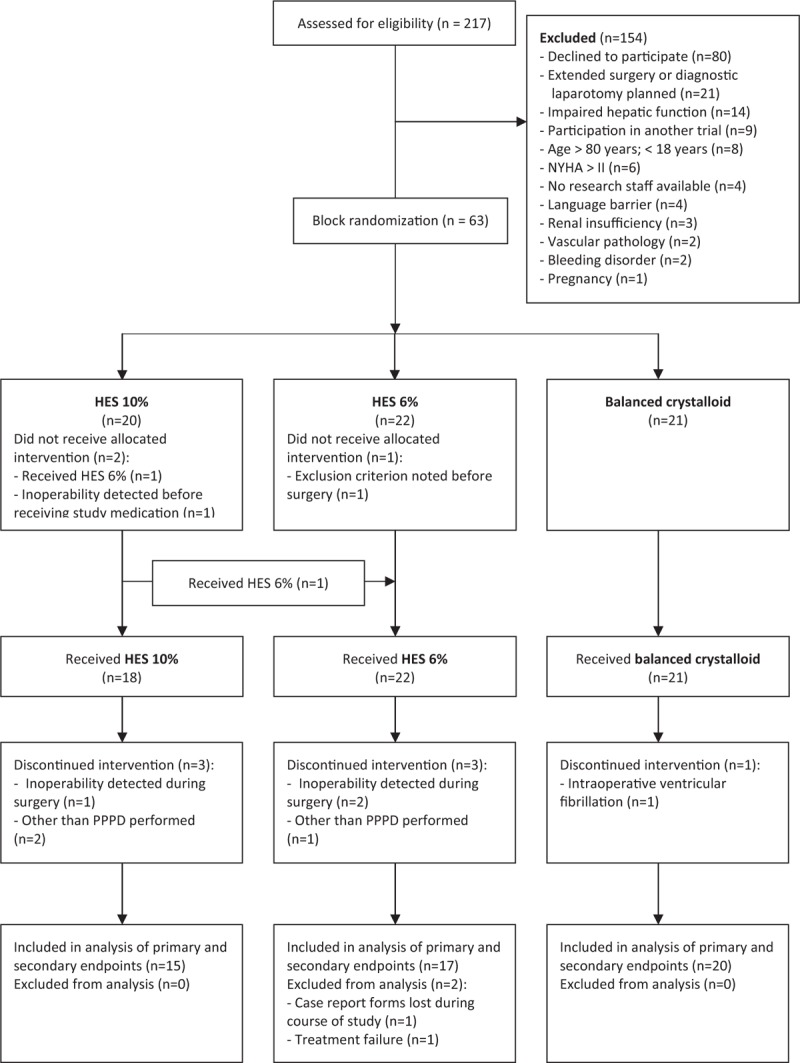
CONSORT flow diagram of the study.
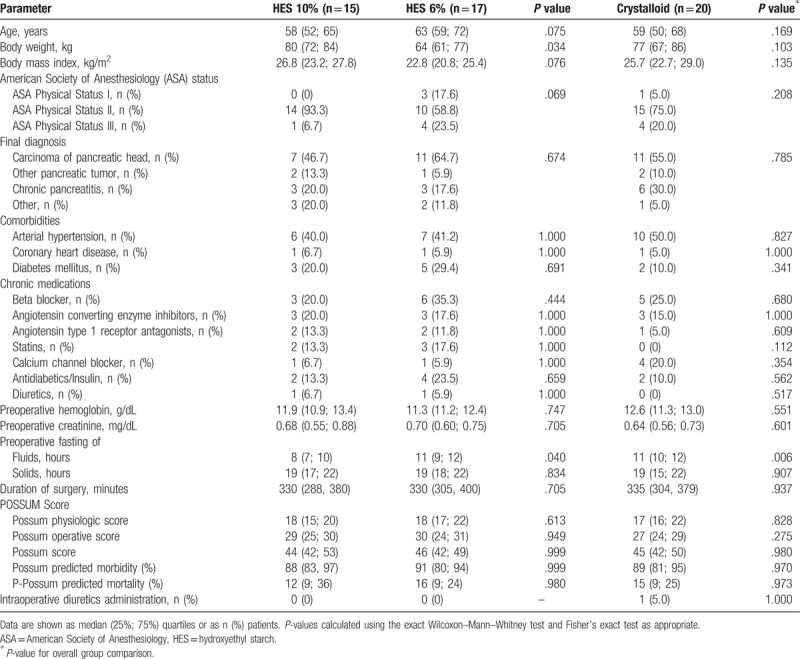
There were no differences in baseline characteristics between study groups, except for a lower body weight (BW) in the 6% HES group and a shorter period of preoperative fasting of fluids in the 10% HES group (Table 1). All patients received open surgery.
Table 1.
Baseline patient characteristics.
The intraoperative volume of HES administered within the goal-directed algorithm was not different between 10% and 6% HES group (2000 [1500; 2250] vs 2250 [1750; 3000] ml, P = .059) (Table 2). However, considering the inhomogeneity of patient's body weight, there was a significant difference in intraoperative volume of HES between 10% and 6% group after adjusting for patient's body weight (24.0 [21.6; 28.3] vs 33.3 [28.2; 46.2] mL kg−1 BW, P = .002). While the 10% HES group received a higher dose of HES during surgery (200 [150; 225] vs 135 [105; 180] g, P = .030), there was no difference when adjusting for patient's body weight. The intraoperative volume of study medication was similar between 10% HES, 6% HES, and crystalloid group (2250 [2000; 2750] vs 2250 [1750; 3000] vs 2500 [2062; 3500] mL, P = .259). However, patients in the HES groups reached the blinded dose limits of the study medication less frequently and required later and less frequently the administration of open-label fluid after dose limit compared to patients receiving crystalloid (Fig. 2, Supplemental Digital Content—Table S1). In this respect, the TTRI of a fluid challenge after preload optimization was shorter in the crystalloid group revealing increased fluid demands to maintain optimized stroke volume during the course of surgery. Baseline crystalloid infusion was similar across all study groups and met very well the requirements of a restrictive maintenance rate of 4 mL kg−1 h−1 as indicated within the goal-directed algorithm. In accordance with a similar blood loss among study groups, there was no difference regarding transfusion requirements and amount transfused of red packed cells or fresh frozen plasma across study groups. The cumulative net balance at the end of surgery was not different between patients in the 10% and 6% HES group (2989 [2365; 3869] vs 2532 [1936; 4599], P = .891), whereas patients in the crystalloid group revealed a higher cumulative net balance during surgery (4387 [3091; 6593], P = .014) (Table 2). Cumulative net balances on postoperative day 1 and 2 were similar between all groups (Supplemental Digital Content—Figure S2).
Table 2.
Intraoperative fluid administration, output, and cumulative net balance.
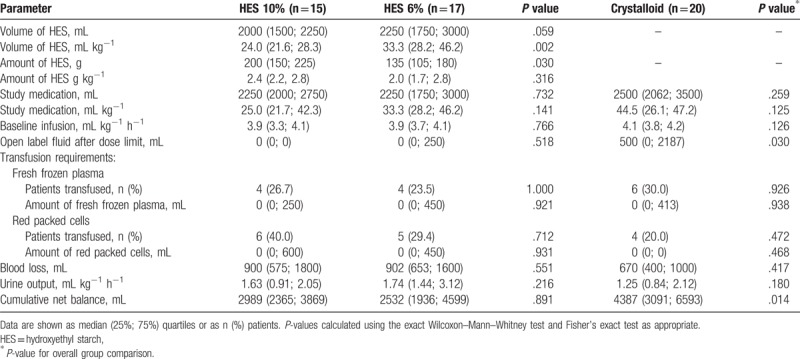
Figure 2.
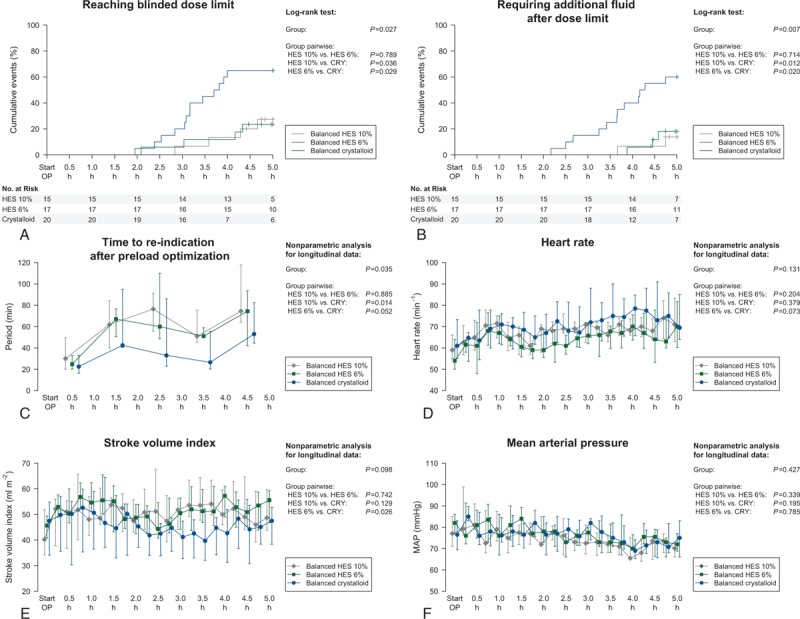
Kaplan–Meier curves for reaching blinded dose limit (A) and requiring additional fluid after dose limit (B) and intraoperative time courses of time to re-indication (C), heart rate (D), stroke volume (E), and mean arterial pressure (F). Data are shown as cumulative events with 95% confidence interval (A, B) and median and (25%; 75%) quartiles (C–F), respectively. Results of the log-rank test (A, B) and the nonparametric analysis (C–F) are indicated.
Analysis of intraoperative hemodynamic data showed no differences in heart rate, mean arterial, central venous pressure, or norepinephrine administration over time between all study groups. In contrast, while 10% and 6% HES group did not differ, patients receiving 6% HES had a significant higher stroke volume index (SVI) during the course of surgery as compared to crystalloid group (Fig. 2, Supplemental Digital Content—Figure S3). There were no interactions between group differences and time of the hemodynamic data, except for the pairwise comparison of SVI between 10% HES and crystalloid group (P < 0.001).
The time until fully on oral diet was not different between the 10% and 6% HES (P = .994) and crystalloid group (P = .924) (Supplemental Digital Content—Table S3, Fig. 3). There were no differences in further gastrointestinal outcomes related to the return of normal bowel function (Supplemental Digital Content—Table S3, Figure S4). An overall poor adherence to the most important determinants related to enhanced recovery from postoperative ileus was observed in all study groups (Supplemental Digital Content—Table S2).
Figure 3.
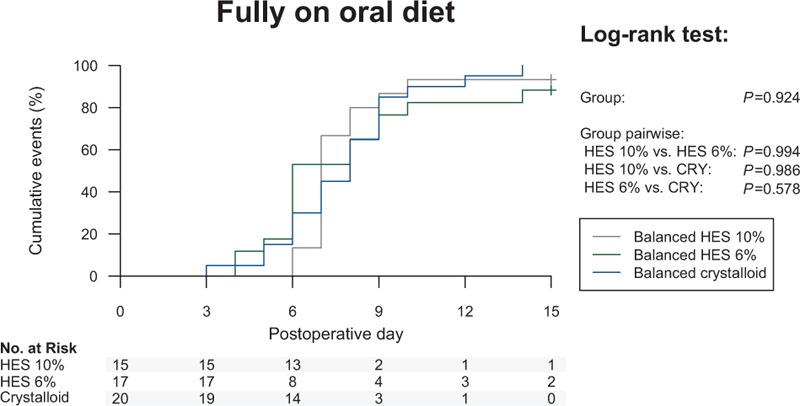
Kaplan–Meier curve for fully on oral diet. Results of the log-rank test are indicated.
Perioperative time courses of creatinine and diuresis showed no differences between the 10% HES, 6% HES and crystalloid group (Fig. 4). According to the KDIGO creatinine criterion, there were no differences between 10% HES, 6% HES, and crystalloid group during the postoperative course (10% HES vs 6% HES vs crystalloid: 46.7 vs 23.5 vs 20.0%, P = .207) (Fig. 4, Supplemental Digital Content—Table S3). Applying the KDIGO oliguria criterion, patients in the 10% HES group had more frequently AKI compared to patients in the crystalloid group (86.7 vs 45.0%, P = .010), even if adjusting the analysis for preventive diuretics administration during the ICU stay (86.7 vs 55.0%, P = .033). In contrast, there were no differences in occurrence of oliguria between 6% HES and crystalloid group in the unadjusted (58.8 vs 45.0%, P = .253) and diuretics adjusted analysis (58.8 vs 55.0%, P = .559). Combining the KDIGO creatinine and oliguria criterion, no differences were found between patients in the 6% HES and crystalloid group, whereas patients in the 10% HES group had a nonsignificant higher incidence of AKI during the postoperative course (64.7 vs 65.0 vs 86.7, P = .254).
Figure 4.
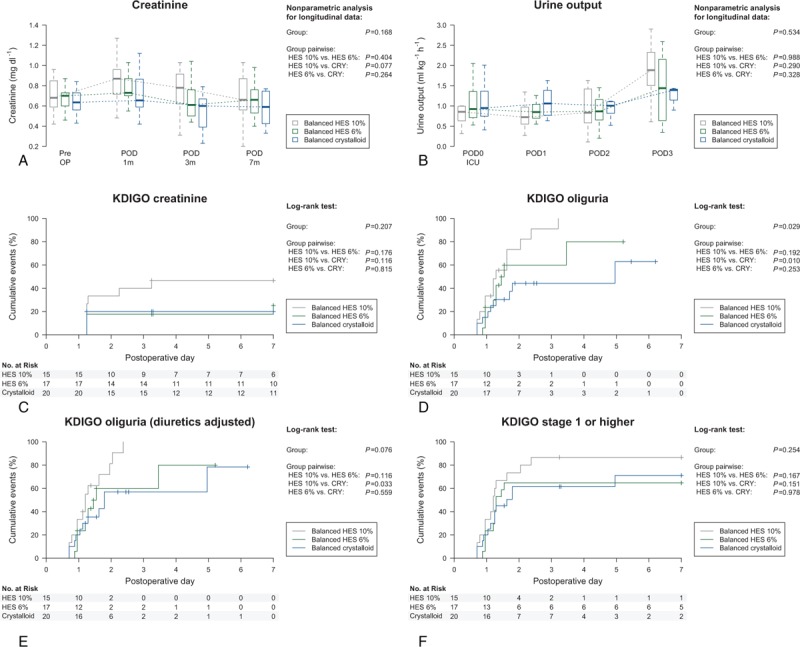
Perioperative time courses of creatinine (A) and urine output (B) and Kaplan–Meier curves for KDIGO outcomes (C–F). Data are shown as median and (25%; 75%) quartiles (A, B) and cumulative events with 95% confidence interval (C–F), respectively. Results of the nonparametric analysis (A, B) and the log-rank test (C–F) are indicated. KDIGO = Kidney Disease Improving Global Outcome.
Further explorative analysis among patients randomized to receive HES during surgery revealed that patients meeting the KDIGO AKI criteria received higher cumulative doses of HES (Fig. 5). The lower cut-offs of the gray zone calculated for all patients receiving HES, patients receiving 10% HES and patients receiving 6% HES were 103, 113, and 120 g as well as 1.3, 1.49, and 1.13 g kg−1, respectively. The upper cut-offs of the gray zone were 165, 125, and 165 g as well as 2.65, 2.17, and 2.7 g kg−1, respectively. The lower cut-offs of 10% HES corresponded to a volume of 1130 mL and 14.9 mL kg−1 and the upper cut-offs to a volume of 1250 mL and 21.7 mL kg−1, respectively. The lower cut-offs of 6% HES corresponded to a volume of 2000 mL and 18.8 mL kg−1 and the upper cut-offs to a volume of 2750 mL and 45.0 mL kg−1, respectively (Fig. 5). In contrast, there was no difference between patients with AKI and without AKI with respect to the administered volume of study medication and net balance in patients randomized to receive balanced crystalloid. Consequently, the volume of study medication and net balance in the crystalloid group were not found predictive for AKI and a gray-zone approach was not applied.
Figure 5.
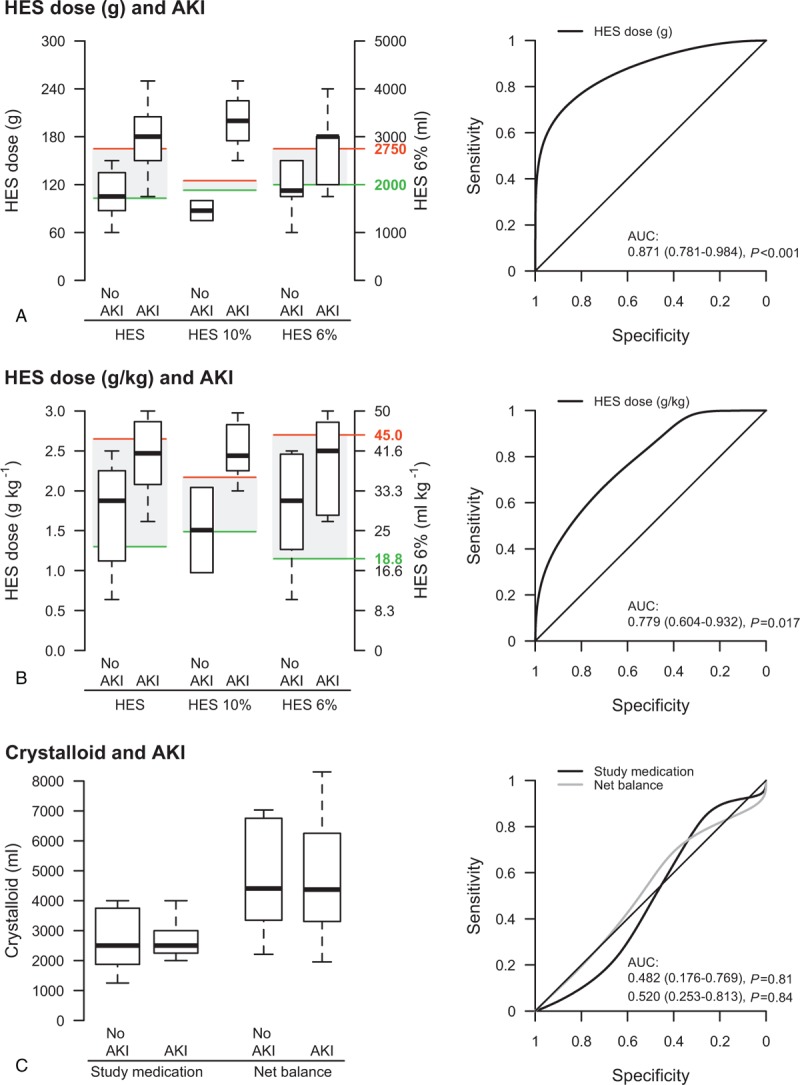
Comparison of patients with and without AKI with respect to administered HES dose in g (A) and g/kg (B) in patients randomized to receive 10% or 6% HES and with respect to volume of study medication and net balance in patients randomized to receive crystalloid (C). The boxplot presentation includes a gray-zone approach obtained by the 95% confidence interval of the best cut-off calculated from receiver operating characteristic (ROC) curve. The gray-zone approach provides 2 cut-offs: patients with HES doses below the lower cut-off of the gray zone (green line) will not experience AKI with near certainty, whereas patients with HES doses above the upper cut-off (red line) will develop AKI with near certainty. The gray-zone approach was not applied to the volume of crystalloid and net balance as there was no association found in ROC analysis. AKI = acute kidney injury, AUC = area under the receiver operating characteristic curve, HES = hydroxyethyl starch, ROC=receiver operating characteristic.
4. Discussion
The principal findings of the study are that the intraoperative volume of HES solution was similar between 10% and 6% HES group, but after adjusting for patient's body weight due to an inhomogeneity between study groups, the 10% HES group received a lower intraoperative volume of HES solution compared to the 6% HES group; that the intraoperative volume of study medication was similar between all study groups, but patients in the HES groups required less frequently additional fluid after dose limit resulting in a lower intraoperative net balance; that the time until fully on oral diet was not different between study groups, while there was an overall poor adherence to most determinants promoting enhanced recovery from postoperative ileus; and that AKI occurred equally frequent in 6% HES and crystalloid group, whereas patients receiving 10% HES had a significant higher incidence of AKI for the KDIGO oliguria criterion.
Fluid therapy has been identified as an important determinant aiming at enhancing recovery after surgery.[18] Our data indicate that after adjusting for patient's body weight due to an inhomogeneity between HES groups, patients in the 10% HES group received a lower intraoperative volume of HES solution compared to patients receiving 6% HES. In this context, the relation of 6% HES:10% HES of 33.3:24.0 mL/kg ≈ 140% represents very well the volume effect of a hyperoncotic 10% HES solution. Nonetheless, 10% and 6% HES were similarly effective in reducing intraoperative fluid administration compared with patients receiving crystalloid. In addition, patients receiving 6% HES had a better circulatory flow during the course of surgery measured by stroke volume index compared to patients receiving crystalloid. These findings are consistent with previous studies comparing 6% HES and crystalloid using a hemodynamic algorithm to guide fluid administration during surgery and very well reflect the prolonged intravascular volume effects of HES solutions that is not observed to the same extent after infusion of crystalloid solutions, at least not in this setting.[11,19,20] There was a similar trend of stroke volume in the 10% HES group, but the 10% HES group did not reach statistical significance. In this regard, the variability as indicated by the interquartile range was larger at several time points than in the 6% HES group, which might due to the lower sample size in this group.
In major abdominal surgery, fluid overload is closely related to a delay in the return of normal bowel function and enhanced recovery from postoperative ileus (POI) is one of the most important factors to overall recovery of the patient during hospital stay.[5–7] In this context, animal data suggest that fluid excess leading to intestinal edema formation causes stretch of the intestinal wall leading to a decreased intestinal contractile activity and therefore promoting postoperative ileus and delaying the return of normal bowel function.[6,21] Patients in the crystalloid group had increased fluid demands to maintain stroke volume within the goal-directed hemodynamic algorithm, which resulted in almost 2 L higher cumulative net balance at the end of surgery compared to patients receiving 10% and 6% HES, whereas net fluid balances on postoperative day 1 and 2 were similar between all groups. The lower intraoperative net balance in patients receiving HES did not translate into differences in any gastrointestinal outcomes related to POI compared with patients receiving crystalloid. Gastrointestinal endpoints as outcome measures in studies comparing different intravenous solutions in patients undergoing surgery are very challenging, as gastrointestinal endpoints are assumed to be multifactorial affected and can only be adequately compared between study groups if the most important determinants contributing to POI are sufficiently controlled.[22–24] However, several important perioperative items promoting enhanced recovery from POI were not sufficiently controlled within our study, which might explain the lack of differences in gastrointestinal endpoints between crystalloid and HES groups despite substantial differences in intraoperative fluid balance. When planning this study, particular guidelines for perioperative care in pancreatic surgery were not yet available,[24] but should be addressed in further research when comparing different interventions on patient's outcome.
It is debatable if the deleterious effects of HES on kidney function shown in critical ill patients[8,9] are generally applicable to surgical patients who receive HES predominantly during the intraoperative period. Recently, a large retrospective analysis found an association of 6% HES and renal failure, but the study had severe limitations disregarding the molecular weight and degree of substitution as well as lacking the adjustment for the most important determinants of AKI during the perioperative course.[25] Prospectively obtained detailed and high quality data addressing this issue are still sparse and recent meta-analyses are based on a small number of studies with heterogeneous AKI definitions.[26,27] In this context, our results indicate that AKI defined according to KDIGO criteria, which is the most recently published AKI classification,[12] occurred equally frequent in patients receiving 6% HES or crystalloid during surgery. These findings are well in line with 2 recent trials showing no harmful effects of 6% HES with respect to urinary and plasmatic markers of renal function in patients undergoing hip replacement and prostate surgery.[28,29] However, in contrast to 6% HES, our results also indicate that patients receiving 10% HES tended toward a higher incidence for the overall criteria of AKI, and showed a significant frequency rate for the KDIGO oliguria criterion. The latter is the only criterion sensitive enough in the perioperative context since creatinine increases after kidney injury require at least 4 hours up to 27 hours before they can be used to define the damage.[30]
Although our data suggest that 6% HES administered within a goal-directed algorithm might be safe in patients undergoing surgery, further explorative analyses carefully indicate that patients developing AKI received a higher cumulative dose of HES molecules. These results imply that there might be a dose-dependent effect of HES solutions. In 2013, following the EMA statement, the dose limits of 10% and 6% HES have been already lowered from 30 to 18 mL kg−1 and 50 to 30 mL kg−1, respectively. However, in our study population, using a gray-zone approach, a volume of 10% HES up to 14.9 mL kg−1 and of 6% HES up to 18.8 mL kg−1 was calculated as a volume the patient will not experience AKI with near certainty. Although conclusions from this post-hoc analysis have to be drawn carefully due the small number of patients included, these findings indicate that a “safe” volume might be lower than the current dose limits for 10% and 6% HES. The pathophysiology of HES-induced AKI is not fully understood. A recent systematic review on accumulation of HES in human and animal tissues showed that HES molecules are taken up in different human tissues with a dose-dependent storage.[31] Degraded HES molecules pass the renal glomerular filtration barrier and renal proximal tubular cells take up a certain proportion by pinocytosis.[32] The pinocytic vacuoles fuse with lysosomes forming vacuoles that appear as swelling of the renal proximal tubular cells under the light microscope. This histological morphological pattern referred to as osmotic nephrosis-like lesions (OL) has been the most frequently proposed mechanism for AKI after HES administration.[32,33] In many studies, OL were found in renal tissues after administration of older HES generations.[31] Data on OL after administration of newer HES solutions such as of 6% HES 130/0.4 are still limited. A recent investigation in 3 patients who underwent laparoscopic nephrectomy due to renal cancer and who received only low volumes of 6% HES 130/0.4 up to 1000 mL found no histological evidence for OL.[34] Animal data regarding this issue are inconsistent with one study showing no OL in a pediatric animal model after infusion of 6% HES 130/0.4,[35] while another study indicated a higher number of OL in an isolated renal perfusion model in pigs.[36] In the absence of any evidence that a hyperoncotic effect of colloids induces kidney injury,[37] the dose-dependent relation of HES with OL could be a mechanism that might explain the dose dependent effect of HES with respect to AKI observed in our study. In contrast, there was no association of administered volume of study medication or net balance with AKI in patients randomized to receive balanced crystalloid. Furthermore, increased renal interstitial proliferation and macrophage infiltration have been identified as potential pathophysiological mechanisms of HES induced AKI. However, these histological changes were predominantly observed after administration of 10% HES 200/0.5, whereas there were no differences between 6% HES 130/0.4 and crystalloid.[36]
This trial has limitations and strengths. The early termination of the study due to futility resulted in not sufficient power for testing the secondary and primary endpoints. Nevertheless, the results should be of clinical interest, but have to be taken with care. In addition, the second primary endpoint was difficult to interpret since evidence-based clinical pathways were not adequately adhered. Furthermore, the protocol-based intervention was only performed during surgery, but during the ICU stay the hemodynamic management including volume and fluid administration was conducted at discretion of the treating intensivist. In this regard, other determinants of medical care might have changed secondary endpoints with special regard to AKI after surgery. In contrast, this is the first study providing highly detailed data of 10% HES and 6% HES administration on renal safety in patients undergoing major abdominal surgery.
In conclusion, in a randomized controlled trial in patients undergoing pancreatic surgery, patients receiving 6% HES required more volume of HES than patients receiving 10% HES after adjusting for patient's body weight due to inhomogeneity between HES groups. The relation of 140% represents very well the volume effect of a hyperoncotic 10% HES solution. Nonetheless, 10% and 6% HES were similarly effective in reducing intraoperative fluid administration compared with patients receiving crystalloid. Considering that several important perioperative items related to enhanced recovery from POI were not sufficiently controlled, the lower intraoperative cumulative net balance in the 10% and 6% HES groups cannot be used to translate into in differences in any gastrointestinal outcomes related to postoperative ileus compared with patients receiving crystalloid. While there was no difference between 6% HES and crystalloid, patients receiving 10% HES had a higher incidence of AKI during the postoperative course. Although our data suggest that 6% HES might be safe, we recommend that it should be used with caution during surgery and not applied beyond 18.8 mL kg−1 during surgery.
Acknowledgments
The authors would like to thank Ulrike Wittkowski, MD, Steffen Herz, MD, Torsten Henze, MD, Alexander Pawlak, MD, and Maik Helfer for their help with the study.
Author contributions
Study concept, design of the study: CvH, CS. Biometrical planning: K-DW. Acquisition of data: AS, JW, HS, AF, CvH, AF. Interpretation of data: OH, JW, AF, CS. Statistical analysis: K-DW, OH, AF, CS. Drafting of the manuscript: OH, JW, AF, CS. Critical revision of the manuscript for important intellectual content: All authors. Final revision of manuscript: CS. Obtained funding: CS. Study supervision: CvH, CS.
Data curation: Julia Werner, Oliver Hunsicker, Anja Schneider, Henryk Stein, Adrian Freitag, Aarne Feldheiser.
Formal analysis: Julia Werner, Oliver Hunsicker, Aarne Feldheiser, Klaus-Dieter Wernecke, Claudia Doris Spies.
Investigation: Julia Werner, Anja Schneider, Henryk Stein, Christian von Heymann, Adrian Freitag.
Validation: Julia Werner, Oliver Hunsicker, Aarne Feldheiser, Klaus-Dieter Wernecke.
Writing –original draft: Julia Werner, Oliver Hunsicker, Aarne Feldheiser, Claudia Doris Spies.
Writing – review & editing: Julia Werner, Oliver Hunsicker, Anja Schneider, Henryk Stein, Christian von Heymann, Adrian Freitag, Aarne Feldheiser, Klaus-Dieter Wernecke, Claudia Doris Spies.
Visualization: Oliver Hunsicker, Aarne Feldheiser, Klaus-Dieter Wernecke.
Conceptualization: Christian von Heymann, Klaus-Dieter Wernecke, Claudia Doris Spies.
Methodology: Christian von Heymann, Claudia Doris Spies.
Supervision: Christian von Heymann, Claudia Doris Spies.
Funding acquisition: Claudia Doris Spies.
Project administration: Claudia Doris Spies.
Resources: Claudia Doris Spies.
Supplementary Material
Footnotes
Abbreviations: AKI = acute kidney injury, ASA = American Society of Anesthesiology, AUC = area under the curve, BW = body weight, CI = confidence interval, CRY = crystalloid, CVP = central venous pressure, EDM = esophageal Doppler monitor, EMA = European Medicines Agency, FDA = U.S. Food and Drug Administration, FFP = fresh frozen plasma, FTc = corrected flow time, GDT = goal-directed therapy, HES = hydroxyethyl starch, HR = heart rate, IDMC = independent data monitoring committee, KDIGO = Kidney Disease Improving Global Outcome, MAP = mean arterial pressure, NYHA = New York Heart Association, OL = osmotic nephrosis-like lesions, POD = postoperative day, POI = postoperative ileus, POSSUM = Physiologic and Operative Severity Score for the enumeration of Mortality and Morbidity, PRAC = Pharmacovigilance Risk Assessment Committee, ROC = receiver operating characteristic curves, SVI = stroke volume index, TTRI = time to re-indication.
JW and OH both contributed equally to this study.
Trial Registration: clinicaltrials.gov Identifier: NCT01117649
Funding: This research was a sponsor-initiated study (SIT) by B. Braun. The implementation of the EDM technology in the department was supported by Deltex Medical by an unrestricted grant unrelated to this study. The funders had no input into or control over data collection, our analysis, and our interpretation of data.
Declaration of interest: CvH, AF, and CS report grants from B. Braun Melsungen AG during conduct of the study. Financial activities outside the submitted work are available to the Editorial Office. All conflicts of interests are declared. The ICMJE Form for Disclosure of Potential Conflicts of Interest and the Conflict of interest form are available to the Editorial Office.
Ethical approval and registration: Ethical approval was given by the national regulatory authority (Bundesinstitut für Arzneimittel und Medizinprodukte; BfArM No. 61-3910-4034969) and the ethics committee (Ethik-Kommission des Landes Berlin; No. ZS EK 11026/09).
The study was internationally subscribed: ClinicalTrials.gov: NCT01117649; European Union Drug Regulating Authorities Clinical Trials: EudraCT 2008-004175-22.
Supplemental Digital Content is available for this article.
References
- [1].Pearse RM, Moreno RP, Bauer P, et al. Mortality after surgery in Europe: a 7 day cohort study. Lancet 2012;380:1059–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Lassen K, Soop M, Nygren J, et al. Consensus review of optimal perioperative care in colorectal surgery: Enhanced Recovery After Surgery (ERAS) Group recommendations. Arch Surg 2009;144:961–9. [DOI] [PubMed] [Google Scholar]
- [3].Feldheiser A, Aziz O, Baldini G, et al. Enhanced Recovery After Surgery (ERAS) for gastrointestinal surgery, part 2: consensus statement for anaesthesia practice. Acta Anaesthesiol Scand 2015;60:289–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Feldheiser A, Aziz O, Baldini G, et al. Enhanced Recovery After Surgery (ERAS) for gastrointestinal surgery, part 2: consensus statement for anaesthesia practice. Acta Anaesthesiol Scand 2016;60:289–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Lobo DN, Bostock KA, Neal KR, et al. Effect of salt and water balance on recovery of gastrointestinal function after elective colonic resection: a randomised controlled trial. Lancet 2002;359:1812–8. [DOI] [PubMed] [Google Scholar]
- [6].Chowdhury AH, Lobo DN. Fluids and gastrointestinal function. Curr Opin Clin Nutr Metab Care 2011;14:469–76. [DOI] [PubMed] [Google Scholar]
- [7].Nisanevich V, Felsenstein I, Almogy G, et al. Effect of intraoperative fluid management on outcome after intraabdominal surgery. Anesthesiology 2005;103:25–32. [DOI] [PubMed] [Google Scholar]
- [8].Myburgh JA, Finfer S, Bellomo R, et al. Hydroxyethyl starch or saline for fluid resuscitation in intensive care. N Engl J Med 2012;367:1901–11. [DOI] [PubMed] [Google Scholar]
- [9].Perner A, Haase N, Guttormsen AB, et al. Hydroxyethyl starch 130/0.42 versus Ringer's acetate in severe sepsis. N Engl J Med 2012;367:124–34. [DOI] [PubMed] [Google Scholar]
- [10].Feldheiser A, Conroy P, Bonomo T, et al. Development and feasibility study of an algorithm for intraoperative goaldirected haemodynamic management in noncardiac surgery. J Int Med Res 2012;40:1227–41. [DOI] [PubMed] [Google Scholar]
- [11].Feldheiser A, Pavlova V, Bonomo T, et al. Balanced crystalloid compared with balanced colloid solution using a goal-directed haemodynamic algorithm. Brit J Anaesth 2013;110:231–40. [DOI] [PubMed] [Google Scholar]
- [12].Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group. KDIGO Clinical Practice Guideline for Acute Kidney Injury. Kidney Int Suppl 2012;2:1–38. [Google Scholar]
- [13].Hunsicker O, Fotopoulou C, Pietzner K, et al. Hemodynamic consequences of malignant ascites in epithelial ovarian cancer surgery∗: a prospective substudy of a randomized controlled trial. Medicine 2015;94:e2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Kieser M, Friede T. Simple procedures for blinded sample size adjustment that do not affect the type I error rate. Stat Med 2003;22:3571–81. [DOI] [PubMed] [Google Scholar]
- [15].Kieser M, Friede T. Re-calculating the sample size in internal pilot study designs with control of the type I error rate. Stat Med 2000;19:901–11. [DOI] [PubMed] [Google Scholar]
- [16].John Wiley & Sons, Brunner E, Domhof S, Langer F. Nonparametric Analysis of Longitudinal Data in Factorial Experiments. 2002. [Google Scholar]
- [17].Cannesson M, Le Manach Y, Hofer CK, et al. Assessing the diagnostic accuracy of pulse pressure variations for the prediction of fluid responsiveness: a “gray zone” approach. Anesthesiology 2011;115:231–41. [DOI] [PubMed] [Google Scholar]
- [18].Scott MJ, Baldini G, Fearon KC, et al. Enhanced recovery after surgery (ERAS) for gastrointestinal surgery, part 1: pathophysiological considerations. Acta Anaesthesiol Scand 2015;59:1212–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Yates DR, Davies SJ, Milner HE, et al. Crystalloid or colloid for goal-directed fluid therapy in colorectal surgery. Brit J Anaesth 2014;112:281–9. [DOI] [PubMed] [Google Scholar]
- [20].Trof RJ, Sukul SP, Twisk JW, et al. Greater cardiac response of colloid than saline fluid loading in septic and non-septic critically ill patients with clinical hypovolaemia. Intensive Care Med 2010;36:697–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Uray KS, Shah SK, Radhakrishnan RS, et al. Sodium hydrogen exchanger as a mediator of hydrostatic edema-induced intestinal contractile dysfunction. Surgery 2011;149:114–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Hunsicker O, Scott MJ, Miller TE, et al. Gastrointestinal morbidity as primary outcome measure in studies comparing crystalloid and colloid within a goal-directed therapy. Brit J Anaesth 2015;115:128–9. [DOI] [PubMed] [Google Scholar]
- [23].Kehlet H. Postoperative ileus—an update on preventive techniques. Nat Clin Pract Gastroenterol Hepatol 2008;5:552–8. [DOI] [PubMed] [Google Scholar]
- [24].Lassen K, Coolsen MM, Slim K, et al. Guidelines for perioperative care for pancreaticoduodenectomy: Enhanced Recovery After Surgery (ERAS(R)) Society recommendations. World J Surg 2013;37:240–58. [DOI] [PubMed] [Google Scholar]
- [25].Opperer M, Poeran J, Rasul R, et al. Use of perioperative hydroxyethyl starch 6% and albumin 5% in elective joint arthroplasty and association with adverse outcomes: a retrospective population based analysis. BMJ 2015;350:h1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Raiman M, Mitchell CG, Biccard BM, et al. Comparison of hydroxyethyl starch colloids with crystalloids for surgical patients: a systematic review and meta-analysis. Eur J Anaesthesiol 2016;33:42–8. [DOI] [PubMed] [Google Scholar]
- [27].Gillies MA, Habicher M, Jhanji S, et al. Incidence of postoperative death and acute kidney injury associated with i.v. 6% hydroxyethyl starch use: systematic review and meta-analysis. Brit J Anaesth 2014;112:25–34. [DOI] [PubMed] [Google Scholar]
- [28].Kancir AS, Johansen JK, Ekeloef NP, et al. The effect of 6% hydroxyethyl starch 130/0.4 on renal function, arterial blood pressure, and vasoactive hormones during radical prostatectomy: a randomized controlled trial. Anesth Analg 2015;120:608–18. [DOI] [PubMed] [Google Scholar]
- [29].Kancir AS, Pleckaitiene L, Hansen TB, et al. Lack of nephrotoxicity by 6% hydroxyethyl starch 130/0.4 during hip arthroplasty: a randomized controlled trial. Anesthesiology 2014;121:948–58. [DOI] [PubMed] [Google Scholar]
- [30].Waikar SS, Bonventre JV. Creatinine kinetics and the definition of acute kidney injury. J Am Soc Nephrol 2009;20:672–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Wiedermann CJ, Joannidis M. Accumulation of hydroxyethyl starch in human and animal tissues: a systematic review. Intensive Care Med 2014;40:160–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Dickenmann M, Oettl T, Mihatsch MJ. Osmotic nephrosis: acute kidney injury with accumulation of proximal tubular lysosomes due to administration of exogenous solutes. Am J Kidney Dis 2008;51:491–503. [DOI] [PubMed] [Google Scholar]
- [33].Schortgen F, Brochard L. Colloid-induced kidney injury: experimental evidence may help to understand mechanisms. Crit Care 2009;13:130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Pinholt Kancir AS, Pleckaitis M, Ekelof NP, et al. Absence of osmotic nephrosis in renal tissue removed during laparoscopic nephrectomy after infusion of 6% hydroxyethyl starch 130/0.4. APMIS 2015;123:993–5. [DOI] [PubMed] [Google Scholar]
- [35].Witt L, Glage S, Lichtinghagen R, et al. Impact of high doses of 6% hydroxyethyl starch 130/0.42 and 4% gelatin on renal function in a pediatric animal model. Paediatr Anaesth 2016;26:259–65. [DOI] [PubMed] [Google Scholar]
- [36].Huter L, Simon TP, Weinmann L, et al. Hydroxyethylstarch impairs renal function and induces interstitial proliferation, macrophage infiltration and tubular damage in an isolated renal perfusion model. Crit Care 2009;13:R23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Wiedermann CJ, Dunzendorfer S, Gaioni LU, et al. Hyperoncotic colloids and acute kidney injury: a meta-analysis of randomized trials. Crit Care 2010;14:R191. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


