Abstract
Data on the association of dietary patterns with non-alcoholic fatty liver disease (NAFLD) among adolescents are scarce. Hence, the purpose of this study was to ascertain the influence of dietary patterns and key foods on NAFLD among adolescents in Shandong, China. Data were extracted from Linyi Nutrition and Health study during 2015 to 2016. This cross-sectional study population comprised 1639 participants aged between 16 and 23 years. Dietary intake was assessed by the use of a semiquantitative food frequency questionnaire (FFQ), containing 85 food items. NAFLD diagnosis was defined as individuals whose ultrasound examination disclosed hepatic steatosis at any stage, in the absence of excess intake of alcoholic beverages. The odds ratio (OR) and 95% confidence interval (CI) were estimated for each quartile of the dietary pattern adherence scores using logistic regression analysis. Of 1639 participants, 221 (13.5%) were classified as having NAFLD. Three major dietary patterns were derived from factor analysis: traditional Chinese, Western, and high-energy dietary patterns. There were significant differences in the intake of whole grains, tuber, and vegetable across quartiles of the traditional Chinese and Western pattern (P < .05). Besides, compared with adolescents in the lowest quartile, those in the highest quartile for whole grains intake had a lower OR for NAFLD (OR = 0.72; 95%CI: 0.61–0.98; P < .05), and for red meat and soft drink consumption had greater OR for NAFLD (OR = 1.34; 95% CI: 1.06–1.72; OR = 1.69; 95% CI: 1.34–2.56; respectively, P < .05). After adjustment for several potential confounders, participants in the highest quartile of the traditional Chinese pattern scores had lower OR for NAFLD (OR = 0.726; 95% CI: 0.383–0.960, P < .05) than did those in the lowest quartile, whereas those in the highest quartile of the Western pattern score had greater OR for NAFLD (OR = 1.197; 95% CI: 1.013–1.736, P < .01) than did those in the lowest quartile. No statistically significant association was found between the high-energy pattern and the risk of NAFLD.
Our findings demonstrated that the traditional Chinese dietary pattern was associated with a lower risk, whereas the Western dietary pattern was associated with a higher risk of NAFLD.
Keywords: adolescent, china, dietary patterns, non-alcoholic fatty liver disease
1. Introduction
Non-alcoholic fatty liver disease (NAFLD) includes a wide spectrum of liver diseases, from simple steatosis to non-alcoholic steatohepatitis (NASH), which can progress to cirrhosis and hepatocellular carcinoma.[1] In China, NAFLD has been recognized as a serious health problem, and the incidence of NAFLD is increasing year by year.[2] In the United States and Asia, NAFLD has also attracted increasing attention given its high prevalence, estimated at 24% to 30%.[3] Presently, NAFLD is considered to be a hepatic component of metabolic syndrome, and elements of metabolic syndrome, such as obesity, insulin resistance and dyslipidemia, all of which are important risk factors for NAFLD.[4,5]
During the last several decades, diet has been considered as an important pathogenic factor of NAFLD. There are a number of studies reporting the associations between diet and NAFLD risk, concentrating on the effects of individual foods or nutrients.[6–9] However, nutrients or foods are not consumed in isolation but in numerous different combinations.[10] To address this problem, dietary pattern analysis has emerged as an alternative and complementary approach to reporting the association of diet with the risk of chronic diseases, and it encompasses complex relations and synergistic effects of nutrients and foods consider the complexity of overall diet and facilitate nutritional recommendations.[11]
Nowadays, rapid economic growth, globalization, and urbanization are all leading to a dramatic shift in the traditional dietary patterns to Western patterns in China. Consequently, the nutritional transition is fuelling the epidemic of chronic diseases.[12] Herein, it is important to investigate the associations between dietary patterns and the risk of noncommunicable chronic disease among Chinese, particularly in adolescents, who are easy to suffer from these chronic diseases.[13] To date, only 1 study has been performed to elucidate the association of dietary patterns with the risk of NAFLD in Australian adolescents.[7] Therefore, the purpose of this study was to corroborate the associations between dietary patterns and its major components and NAFLD in Chinese adolescents.
2. Subjects and methods
2.1. Study population
The subjects were students recruited in the dietetic course at 6 universities and/or colleges in the city of Linyi, Shandong Province in China from September 2015. A total of 1875 subjects (aged between 16 and 23 years), were invited to attend the new entrance health examination at the Medical Center for Physical Examination, Linyi People's Hospital. Meanwhile, these study participants were interviewed face-to face by a trained staff with written questionnaire. For this analysis, 129 participants were excluded based on the following reasons: had missing or incomplete dietary information in their questionnaires (n = 47); self-reported a history of usage of drugs or drinking (n = 65); had a history of hereditary diseases (n = 17). We further excluded 107 participants who did not provide information on dietary intake or had more than 8 items blank on the food frequency questionnaire (FFQ). After all these exclusions, we considered 1639 participants remained for the analysis. Approval for the present study was obtained from the Ethics Committee of Linyi People's Hospital, China, and all participants provided written informed consent.
2.2. Assessment of dietary intake
Dietary data were collected using a semiquantitative FFQ including 85 food items (27 food groups) based on the 2012 China National Nutrition and Health Survey. Study subjects were asked about their frequency of consumption of each food group during the previous 12 months and the estimated portion size, using local weight units (1 Liang = 50 g) or natural units (cups). For instance, the frequency response for food consumption included 9 categories, as follows: never, less than once/month, 1 to 3 times/month, 1 to 2 times/week, 3 to 4 times/week, 5 to 6 times/week, 1 time/day, 2 times/day, and 3 times/day. Consequently, individual food items were converted into an average daily consumption.
2.3. Assessment of biomarker
Blood samples were obtained after 12 hours of fasting overnight. After blood was collected, samples were allowed to clot at room temperature for 1 to 3 hours and serum was separated. Subsequently, a separation of serum was via centrifugation for 15 minutes at 3000 rpm. Then samples were analyzed in the Medical Center for Physical Examination, Zhejiang Hospital for fasting glucose, total cholesterol, triglycerides, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, serum uric acid, alanine aminotransferase and asparagine aminotransferase using a Hitachi 7600 automatic analyzer (Hitachi, Tokyo, Japan).
2.4. Blood pressure measurement
After participants rested for 10 minutes in the sitting position, blood pressure was measured by using a standard mercury sphygmomanometer. A trained nurse measured the blood pressure 3 times in seated participants, and thereafter the means of 3 measurements of systolic blood pressure and diastolic blood pressure were calculated and considered for analysis.
2.5. Definition of terms (ascertainment)
Hypertension was defined as a systolic pressure of ≥140 mmHg and/or a diastolic pressure of ≥90 mmHg. Obesity was defined by body mass index (BMI) ≥28 kg/m2 and abdominal adiposity was defined as: male: waist circumference (WC) ≥85 cm; female: WC ≥80 cm.[10] NAFLD was defined as the presence of moderate-severe hepatic steatosis (by B-ultrasonic examination), the absence of excessive alcohol use (>20 g/day in men and 10 g/day in women), no use of steatogenic medications within the last 6 months, no exposure to hepatotoxins, and no history of bariatric surgery.[14]
2.6. Statistical analyses
The Kaiser-Meyer-Olkin measure of sampling adequacy and the Bartlett test of sphericity were used to evaluate the adequacy of correlation matrices with the data. Factor analysis (principal component) was used to assess the dietary patterns based on the frequency of consumption of 27 food groups in the FFQ. The factors identified were rotated using an orthogonal transformation (varimax rotation) to achieve uncorrelated factors and greater interpretability. In determining the number of factors to retain, we applied eigen value (>1) and a scree plot in the present study.[15] In view of the factor scores in 3 patterns overlapping, only food groups with a factor loading >|0.30| were considered to be important contributors to this pattern and included in the present study. Then, we only observed that 2 factors (snack food and carbonated beverages) were overlapped. Finally, labeling of dietary patterns was based on the interpretation of foods with high factor loadings for each dietary pattern.[16] Participant scores were categorized into quartiles separately for each dietary pattern. Thus, for each dietary pattern, quartile 4 was composed of persons whose diets conformed most closely to that particular pattern.
The characteristics of study participants were calculated across quartiles of each dietary pattern. Data were expressed as the mean values and standard deviation for continuous variables and as sum (proportions) for categorical variables. The χ2 tests and independent samples t test or Mann-Whitney U test were used to compared the characteristics of study participants, according to NAFLD diagnosis. Besides, the odds ratio (OR) and 95% confidence interval (CI) were assessed using multiple logistic regression to confirm the NAFLD risk depending on the quartile categories of each dietary pattern scores, with the Q1 (the lowest) category used as the reference. Furthermore, to further explore the association between the dietary pattern and NAFLD, intakes of the major food groups in each pattern were examined in relation to NAFLD risk.
All data were analyzed using the Statistical Package for Social Sciences (version 21.0, SPSS Inc, Chicago, IL), and P values <.05 were considered statistically significant.
3. Results
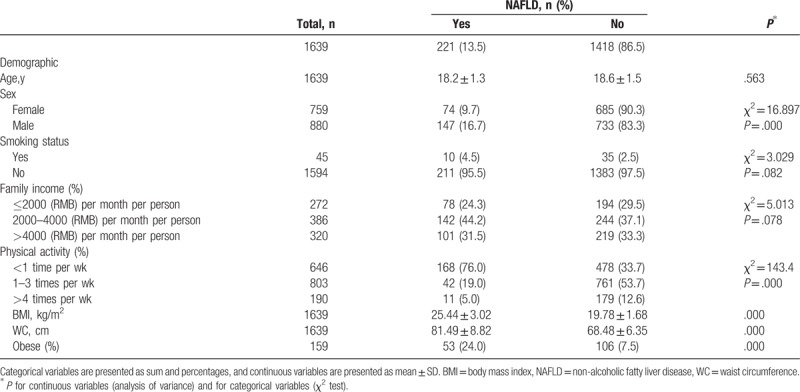
Total prevalence of NAFLD in the present study was 13.5%, with male was 9.0% and female was 4.5%. The demographic and clinical characteristics of subjects with and without NAFLD are shown in Table 1. There were significant differences between participants with and without NAFLD by sex and physical activity. In addition, participants with NAFLD had higher BMI, WC, and the prevalence of obesity than without NAFLD.
Table 1.
Demographic and lifestyle characteristics of participants and non-alcoholic fatty liver disease in Chinese adolescents.
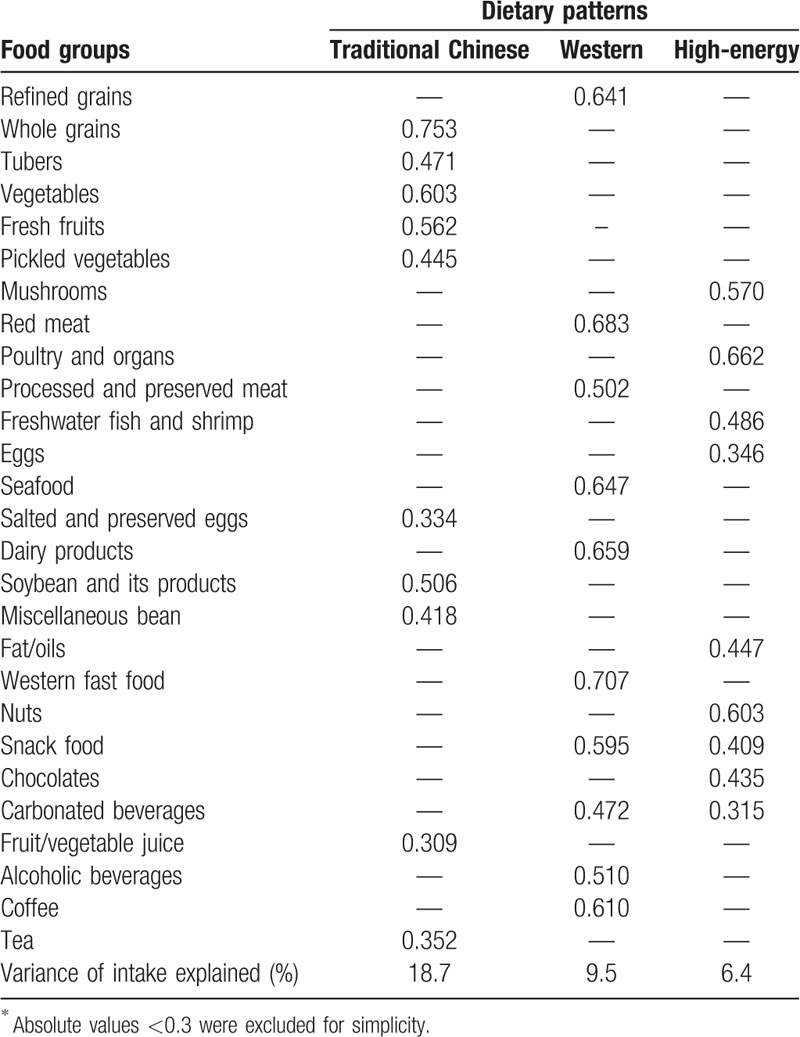
Three major dietary patterns were identified in this population which together accounted for 34.6% of the total variance in food items (Table 2). We labeled the first pattern “traditional Chinese,” as it was mainly characterized by high intake of whole grains, tubers, vegetables, fresh fruits, salted and preserved eggs, soybean and its products, miscellaneous bean, fruit/vegetable juice, and tea. The second pattern was named as “Western” dietary pattern, which is characterized by high intakes of refined grains, red meat, processed and preserved meat, seafood, dairy products, western fast food, snack food, carbonated beverages, alcoholic beverages, and coffee. The third pattern was named as “high-energy” dietary pattern, which is characterized to have high consumption of mushrooms, poultry and organs, freshwater fish and shrimp, eggs, fat/oils, nuts, snacks, chocolates, and carbonated beverages. The factor-loading matrixes for three dietary patterns were shown in Table 3.
Table 2.
The 27 food items in the FFQ with examples.
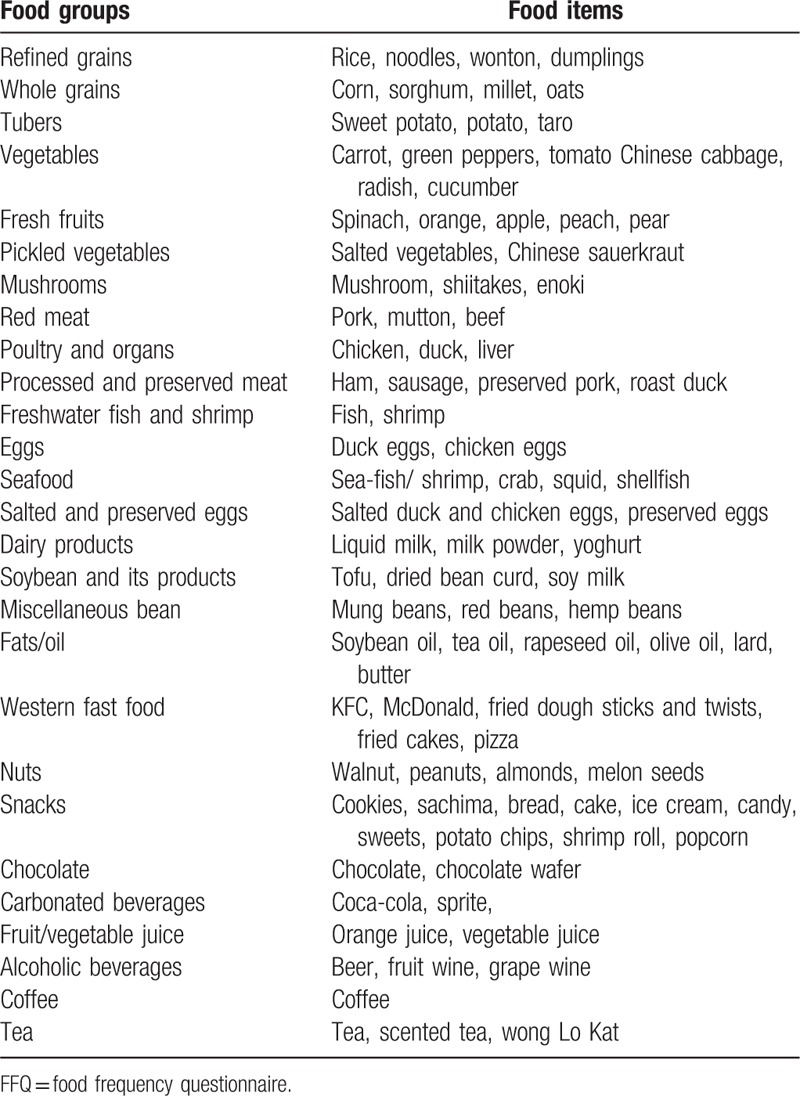
Table 3.
Roated factor loading matrix for the 3 dietary patterns among 1639 Chinese adolescents aged 16 to 23 years∗.
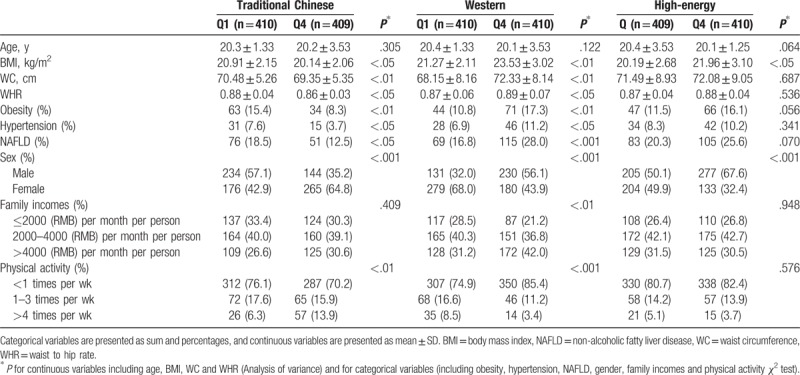
The characteristics of study participants across quartile categories of the dietary pattern scores were shown in Table 4. Compared with the lowest quartile of the “traditional Chinese” dietary pattern, those participants in the highest quartile were more likely to be female, and had a lower prevalence of obesity, hypertension, and NAFLD, lower BMI, WC, waist to hip ratio (WHR), higher physical activity level than those in the lowest quartile. Besides, subjects in the top quartile of the “Western” dietary pattern were more likely to be male, and had higher prevalence of obesity, hypertension, and NAFLD, higher BMI, WC, WHR, family income, and lower physical activity level than those in the lowest quartile. Similarly, subjects in the highest quartile of the “high-energy” dietary pattern were more likely to be male and had a higher BMI than those in the lowest quartile.
Table 4.
Characteristics of the study participants by quartile (Q) categories of dietary pattern scores in Linyin.
There were significant differences in the intake of whole grains, tuber, fresh fruits, and vegetable across quartiles of the traditional Chinese dietary pattern. Participants in the top quartile of the traditional Chinese dietary pattern had a lower intake of energy and fat than those in the lowest quartile. However, no significant difference was observed in the intake of refined grains, red meat, and soft drinks across quartiles of this pattern. In addition, we did not find the significant difference in the intake of fresh fruits and energy intake across quartiles of the Western dietary pattern. Meanwhile, there were also significant differences in consumption of refined grains, red meat, soft drinks across quartiles of the high-energy dietary pattern (Table 5).
Table 5.
Food and nutrient intakes across quartiles (Q) of the dietary patterns at baseline in Chinese adolescents.
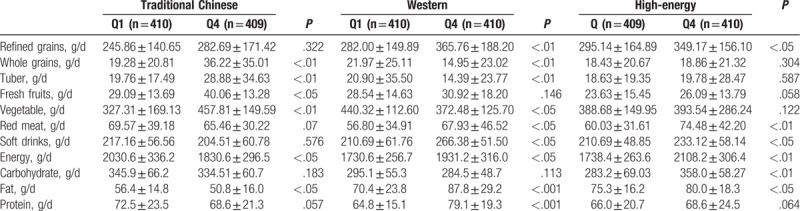
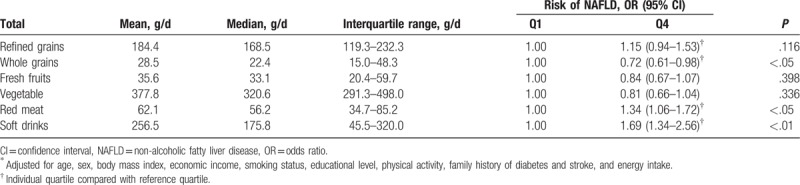
The associations between the components of the major dietary pattern and NAFLD risk were shown in Table 6. Compared with adolescents in the lowest quartile, those in the highest quartile for whole grains intake had a lower OR for NAFLD (OR = 72; 95% CI: 0.61–0.98; P < .05). Meanwhile, adolescents in the top quartile for red meat and soft drink consumption had greater OR for NAFLD (OR = 1.34; 95% CI: 1.06–1.72; OR = 1.69; 95% CI: 1.34–2.56; respectively, P < .05). However, we observed no significant association between refined grains, fresh fruits and vegetables and the risk of NAFLD.
Table 6.
Quartiles of components of the major dietary patterns (g/d) and risk of NAFLD in Chinese adolescents (n = 1639)∗.
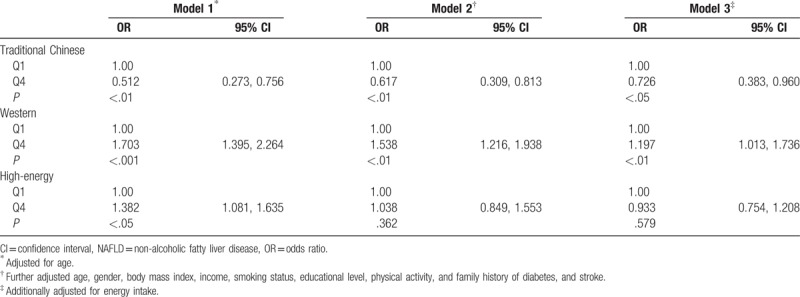
The relationship between dietary patterns and NAFLD risk by multivariate regression analysis is shown in Table 7. After control for potential confounders, participants in the highest quartile of the traditional Chinese pattern scores had lower OR for NAFLD (OR = 0.726; 95% CI: 0.383–0.960, P < .05) than did those in the lowest quartile, whereas those in the highest quartile of the Western pattern score had greater OR for NAFLD (OR = 1.197; 95% CI: 1.013–1.736, P < .01) than did those in the lowest quartile. However, no statistically significant association was found between the high-energy pattern and the risk of NAFLD in the present study.
Table 7.
Multivariable models adjusted for NAFLD across the quartile (Q) categories of the dietary patterns in Shandong Province, China.
4. Discussion
Limited epidemiological studies have reported the association of major dietary patterns with the risk of NAFLD in Chinese population. In the present study, 3 dietary patterns were identified: traditional Chinese, Western, and high-energy. We found that the traditional Chinese dietary pattern was associated with a decreased risk, whereas the Western dietary pattern was associated with an increased risk of NAFLD. Moreover, no association was observed between the high-energy dietary pattern and the risk of NAFLD. To the best of our knowledge, the present study is the first study to assess the associations between dietary patterns and NAFLD risk in Chinese adolescents.
In our analysis, the traditional Chinese dietary pattern was associated with a decreased risk of NAFLD. Our results were inconsistent with previous study,[17] reporting that the traditional Chinese pattern was not associated with the risk of NAFLD. The apparently protective effect of the traditional Chinese pattern on the prevention of NAFLD could be related to this pattern's healthy constituents (e.g., whole grains, vegetables, and fruits). Whole grains contain large amounts of dietary fiber, which has been shown to be positively associated with insulin resistance, an important risk factor for NAFLD.[18] In addition, antioxidant and phytonutrient-rich vegetables and fruits may contain active dietary components linking NAFLD and antioxidative constituents because the inverse association has been shown between vitamin C and E intake and NAFLD.[19] Furthermore, some foods in the traditional Chinese pattern have a low glycemic index (GI), which have been found to decrease total cholesterol levels, resulting in the decreased risk of NAFLD.[20] Likewise, in a Chinese population, Kong et al[21] also found that high low GI intake might decrease the risk of visceral obesity. The fundamental cause of obesity is the long-term imbalance in energy intake and expenditure (e.g., positive energy balance) leading to the increased body mass including the accumulation of subcutaneous and visceral fat. In previous a review, Finelli et al[22] have emphasized that visceral obesity is a main determinant of developing NAFLD. However, in this study, we also observed that participants in the highest quartile of the traditional Chinese pattern had a lower BMI and WC than those in the lowest quartile. Some epidemiological studies have also implicated visceral fat as a major risk factor for insulin resistance, type 2 diabetes mellitus, cardiovascular disease and metabolic syndrome,[23,24] some major risk factors for NAFLD. Finally, epidemiological and animal studies also indicated that drinking green tea was associated with a decreased risk of obesity and chronic disease, which is considered as a risk factor for NAFLD.[25,26]
The Western dietary pattern was characterized by high consumption of red meat, processed and preserved meat, fish and shrimp, seafood, dairy products, western fat food, snack food, carbonated beverages, alcoholic beverages, and coffee. In the present study, we found the positive association between the Western dietary pattern and NAFLD in Chinese adolescence. Our findings were agreement with previous studies, which showed that the “animal food” pattern was associated with an increased risk of NAFLD.[17] The detrimental effect of Western dietary pattern could be attributed to this pattern's unhealthy constituents (e.g., red and processed meat and carbonated beverages). First, the Western dietary pattern contains high amounts of saturated fat, trans-fatty acids, and cholesterol. A large number of studies have indicated that high intakes of fat may play an important role in hepatic steatosis.[27,28] Second, Oddy et al[7] showed that a higher intake of soft drink was associated with a greater risk of NAFLD. It is well-known that soft drinks contain large amounts of high-fructose corn syrup, which has been found to be positively associated with the risk of NAFLD.[29] In addition, it is also rich in caramel and aspartame that potentially increase insulin resistance and inflammation.[30] In this study, the subjects are predominately a group of Chinese teenagers aged 16 to 23 years, who consumed large amounts of carbonated beverages in per day. Third, the refined grains included in this pattern have a high GI. Animal studies indicated that high GI foods could cause increased hepatic fat storage.[31] Previous study also found that foods with high GI were associated with increased hepatic steatosis.[32] Last, fast foods in the Western dietary pattern have been reported to be positively associated with the risk of obesity, insulin resistance, and diabetes, all of which are the risk factors for NAFLD.[7,33]
In the present study, we found no significant association between high-energy pattern and NAFLD risk, although the prevalence of NAFLD for the highest quartile of this pattern was higher compared with the lowest quartile (10.2% vs. 8.3%). There are several possible explanations for this null association. On one hand, previous studies have reported a positive association between consumption of soft drinks and NAFLD.[29] Besides, some foods (e.g., nuts, snack food, and chocolate) in this pattern have been reported to increase the risk of obesity, an important risk factor for NAFLD.[34] On the other hand, it is known that freshwater fish and shrimp contain large amounts of unsaturated fatty acids, for example, omega-3 PUFA, which have been shown to be negatively associated with NAFLD.[35] In addition, the mushroom included in this pattern is low-fat and healthy food, and constituents of this food have been reported to be associated with a reduced risk of NAFLD.[36] Furthermore, the study subjects were a group of Chinese adolescents aged 16 to 23 years, who often participate physical exercise. However, physical activity as a form of energy expenditure has been considered as a standard clinical recommendation for obese people.[37] Previous researches have reported that higher physical activity level has a protective effect against long-term gain in weight.[38,39] As mentioned above, obesity is an important risk factor for NAFLD.[26] A recent systematic review and meta-analysis of physical activity and hypertension risk also concluded that the level of physical activity was significantly associated with the risk of hypertension,[40] major risk factor for NAFLD. Above all, Mouzaki et al[41] have reported that physical activity can decrease the risk of developing NAFLD. Finally, the null association could also be to because of reverse causality. These students with risk of NAFLD may be advised to change dietary habits during the process of entrance examination, such as limiting the intake of high-energy foods. Thus, these possibilities could not be excluded in the present study.
5. Strengths and limitations
Our study had several strengths and limitations. First, to our knowledge, this is the first study examining the influence of dietary patterns with NAFLD among Chinese adolescents. Second, dietary data were obtained from a validated semiquantitative FFQ by face-to-face interview. This method ensured the data on food intake we collected more accurate. Third, our findings are reliable because we have controlled for potential known confounders in our analysis. Nonetheless, several limitations of our study warrant mention. First, the data we used were cross-sectional; therefore, causal relations could not be determined. Our results need to be confirmed in prospective studies. Second, principal component analysis includes several subjective decisions in choosing the number of retained factors and method of rotation, labelling, and explaining the dietary patterns.[42] Third, the measurement errors in reporting diet using the FFQ affected our results by introducing random variation and lowering the significance of the associations. Fourth, it is hard to classify 1 individual into certain independent pattern group in performing factor analysis. Last, although potential confounding factors were adjusted for, the presence of unmeasured confounders is possible.
In conclusion, we found that the traditional Chinese pattern was associated with a decrease risk, whereas the Western dietary pattern was associated with an increased risk of NAFLD. Our findings may guide dietary interventions based on dietary pattern for the prevention of NAFLD. However, further studies are needed to clarify these findings.
Acknowledgments
The authors thank all participants from Department of Endocrinology, Linyi People's Hospital for their assistance and support. The authors also acknowledge the Medical Center for Physical Examination, Linyi People's Hospital, for their important contributions to collection of data in this study.
Author contributions
The authors’ responsibilities were as follows: Y.P. And S.-H. C contributed to the study design. Y.P. and X.-N.L performed the statistical analysis for the manuscript and drafted the paper. Q.-Y. S. and X.-N.L conducted research. All authors contributed to a critical review of the manuscript during the writing process. All authors approved the final version to be published.
Formal analysis: Yan Peng.
Writing – original draft: Yan Peng.
Data curation: Xiaonan Liu.
Methodology: Xiaonan Liu.
Writing – review & editing: Shuhong Chen.
Investigation: Qingyun Sun.
Footnotes
Abbreviations: ANOVA = analysis of variance, BMI = body mass index, CI = confidence interval, FFQ = food frequency questionnaire, NAFLD = non-alcoholic fatty liver disease, NASH = non-alcoholic steatohepatitis, OR = odds ratio, WC = waist circumference, WHR = waist to hip ratio.
The authors declare no conflicts of interest.
References
- [1].Smith BW, Adams LA. Non-alcoholic fatty liver disease. Crit Rev Clin Lab Sci 2011;48:97–113. [DOI] [PubMed] [Google Scholar]
- [2].Hou XH, Zhu YX, Lu HJ, et al. Non-alcoholic fatty liver disease's prevalence and impact on alanine aminotransferase associated with metabolic syndrome in the chinese. J Gastroenterol Hepatol 2011;26:722–30. [DOI] [PubMed] [Google Scholar]
- [3].Younossi ZM, Koenig AB, Abdelatif D, et al. Global epidemiology of nonalcoholic fatty liver disease-meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016;64:73–84. [DOI] [PubMed] [Google Scholar]
- [4].Zelber-Sagi S, Lotan R, Shlomai A, et al. Predictors for incidence and remission of NAFLD in the general population during a seven-year prospective follow-up. J Hepatol 2012;56:1145–51. [DOI] [PubMed] [Google Scholar]
- [5].Machado MV, Cortez-Pinto H. Management of fatty liver disease with the metabolic syndrome. Expert Rev Gastroenterol Hepatol 2014;8:487–500. [DOI] [PubMed] [Google Scholar]
- [6].Di Minno MN, Russolillo A, Lupoli R, et al. Omega-3 fatty acids for the treatment of non-alcoholic fatty liver disease. World J Gastroenterol 2012;18:5839–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Oddy WH, Herbison CE, Jacoby P, et al. The Western dietary pattern is prospectively associated with nonalcoholic fatty liver disease in adolescence. Am J Gastroenterol 2013;108:778–85. [DOI] [PubMed] [Google Scholar]
- [8].Adriano LS, Sampaio HA, Arruda SP, et al. Healthy dietary pattern is inversely associated with non-alcoholic fatty liver disease in elderly. Br J Nutr 2016;115:2189–95. [DOI] [PubMed] [Google Scholar]
- [9].Yang X, Deng F. Iron overload is associated with non-alcoholic fatty liver disease (NAFLD): results from The NHANES III survey. Eur J BioMed Res 2017;3:10–5. [Google Scholar]
- [10].Zheng PF, Shu L, Zhang XY, et al. Association between dietary patterns and the risk of hypertension among Chinese: a cross-sectional study. Nutrients 2016;8:239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Shim P, Choi D, Park Y. Association of blood fatty acid composition and dietary pattern with the risk of non-alcoholic fatty liver disease in patients who underwent cholecystectomy. Ann Nutr Metab 2017;70:303–11. [DOI] [PubMed] [Google Scholar]
- [12].Ganguli D, Das N, Saha I, et al. Major dietary patterns and their associations with cardiovascular risk factors among women in West Bengal, India. Br J Nutr 2011;105:1520–9. [DOI] [PubMed] [Google Scholar]
- [13].Mu M, Wang SF, Sheng J, et al. Dietary patterns are associated with body mass index and bone mineral density in Chinese freshmen. J Am Coll Nutr 2014;33:120–8. [DOI] [PubMed] [Google Scholar]
- [14].Nascimbeni F, Pais R, Bellentani S, et al. From NAFLD in clinical practice to answers from guidelines. J Hepatol 2013;59:859–71. [DOI] [PubMed] [Google Scholar]
- [15].Poggio R, Elorriaga N, Gutierrez L, et al. Associations between dietary patterns and serum lipids, apo and C-reactive protein in an adult population: evidence from a multi-city cohort in South America. Br J Nutr 2017;117:548–55. [DOI] [PubMed] [Google Scholar]
- [16].Newby PK, Tucker KL. Empirically derived eating patterns using factor or cluster analysis: a review. Nutr Rev 2004;62:177–203. [DOI] [PubMed] [Google Scholar]
- [17].Yang CQ, Shu L, Wang S, et al. Dietary patterns modulate the risk of non-alcoholic fatty liver disease in Chinese adults. Nutrients 2015;7:4778–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Musso G, Gambino R, De Michieli F, et al. Dietary habits and their relations to insulin resistance and postprandial lipemia in nonalcoholic steatohepatitis. Hepatology 2003;37:909–16. [DOI] [PubMed] [Google Scholar]
- [19].Harrison SA, Torgerson S, Hayashi P, et al. Vitamin E and vitamin C treatment improves fibrosis in patients with nonalcoholic steatohepatitis. Am J Gastroenterol 2003;98:2485–90. [DOI] [PubMed] [Google Scholar]
- [20].York LW, Puthalapattu S, Wu GY. Nonalcoholic fatty liver disease and low-carbohydrate diets. Annu Rev Nutr 2009;29:365–79. [DOI] [PubMed] [Google Scholar]
- [21].Kong AP, Choi KC, Chan RS, et al. A randomized controlled trial to investigate the impact of a low glycemic index (GI) diet on body mass index in obese adolescents. BMC Public Health 2014;14:180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Finelli C, Sommella L, Gioia S, et al. Should visceral fat be reduced to increase longevity. Ageing Res Rev 2013;12:996–1004. [DOI] [PubMed] [Google Scholar]
- [23].Carr DB, Utzschneider KM, Hull RL, et al. Intra-abdominal fat is a majordeterminant of the National Cholesterol Education Program Adult Treatment Panel III criteria for the metabolic syndrome. Diabetes 2004;53:2087–94. [DOI] [PubMed] [Google Scholar]
- [24].Foster MC, Hwang SJ, Massaro JM, et al. Association of subcutaneous and visceral adiposity with albuminuria: the Framingham Heart Study. Obesity (Silver Spring) 2011;19:1284–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Tan Y, Kim J, Cheng J, et al. Green tea polyphenols ameliorate non-alcoholic fatty liver disease through upregulating AMPK activation in high fat fed Zucker fatty rats. World J Gastroenterol 2017;23:3805–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Marventano S, Salomone F, Godos J, et al. Coffee and tea consumption in relation with non-alcoholic fatty liver and metabolic syndrome: A systematic review and meta-analysis of observational studies. Clin Nutr 2016;35:1269–81. [DOI] [PubMed] [Google Scholar]
- [27].Tetri LH, Basaranoglu M, Brunt EM, et al. Severe NAFLD with hepatic necroinfl ammatory changes in mice fed trans fats and a high-fructose corn syrup equivalent. Am J Physio Gastro Liver Physio 2008;295:G987–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Buettner R, Parhofer KG, Woenckhaus M, et al. Defining high-fat-diet rat models: metabolic and molecular effects of different fat types. J Mol Endocrinol 2006;36:485–501. [DOI] [PubMed] [Google Scholar]
- [29].Schulze MB, Manson JE, Ludwig DS, et al. Sugar-sweetened beverages,weight gain, and incidence of type 2 diabetes in young and middle-aged women. JAMA 2004;292:927–34. [DOI] [PubMed] [Google Scholar]
- [30].Nseir W, Nassar F, Assy N. Soft drinks consumption and nonalcoholic fatty liver disease. World J Gastroenterol 2010;16:2579–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Scribner KB, Pawlak DB, Ludwig DS. Hepatic steatosis and increased adiposity in mice consuming rapidly vs slowly absorbed carbohydrate. Obesity 2007;15:2190–9. [DOI] [PubMed] [Google Scholar]
- [32].Valtuena S, Pellegrini N, Ardig ÃD, et al. Dietary glycemic index and liver steatosis. Am J Clin Nutr 2006;84:136–42. [DOI] [PubMed] [Google Scholar]
- [33].Li M, Dibley MJ, Sibbritt DW, et al. Dietary habits and overweight/obesity in adolescents in Xi’ an City, China. Asia Pac J Clin Nutr 2010;19:76–82. [PubMed] [Google Scholar]
- [34].Pereira MA, Kartashov AI, Ebbeling CB, et al. Fast-food habits, weight gain, and insulin resistance (the cardia study): 15-year prospective analysis. Lancet 2005;365:36–42. [DOI] [PubMed] [Google Scholar]
- [35].Zelber-Sagi S, Nitzan-Kaluski D, Goldsmith R, et al. Long term nutritional intake and the risk for non-alcoholic fatty liver disease (NAFLD): a population based study. J Hepatol 2007;47:711–7. [DOI] [PubMed] [Google Scholar]
- [36].Arendt BM, Allard JP. Effect of atorvastatin, vitamin e and c on nonalcoholic fatty liver disease: Is the combination required? Am J Gastroenterol 2011;106:78–80. [DOI] [PubMed] [Google Scholar]
- [37].Weinsier RL, Hunter GR, Heini AF, et al. The etiology of obesity: relative contribution of metabolic factors, diet, and physical activity. Am J Med 1998;105:145–50. [DOI] [PubMed] [Google Scholar]
- [38].Hankinson AL, Daviglus ML, Bouchard C, et al. Maintaining a high physical activity level over 20 years and weight gain. JAMA 2010;304:2603–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Schulze MB, Fung TT, Manson JE, et al. Dietary patterns and changes in body weight in women. Obesity 2006;14:1444–53. [DOI] [PubMed] [Google Scholar]
- [40].Semlitsch T, Jeitler K, Hemkens LG, et al. Increasing physical activity for the treatment of hypertension: a systematic review and meta-analysis. Sports Med 2013;43:1009–23. [DOI] [PubMed] [Google Scholar]
- [41].Mouzaki M, Allard JP. The role of nutrients in the development, progression, and treatment of nonalcoholic fatty liver disease. J Clin Gastroenterol 2012;46:457–67. [DOI] [PubMed] [Google Scholar]
- [42].Li Y, He Y, Lai J, et al. Dietary patterns are associated with stroke in Chinese adults. J Nutr 2011;141:1834–9. [DOI] [PubMed] [Google Scholar]


