Abstract
Video-assisted minilaparotomy surgery (VAMS) is a hybrid of open and laparoscopic surgical techniques, so has advantages of both approaches. Here, we examined the learning curve for this procedure.
We retrospectively evaluated 50 consecutive patients who underwent VAMS donor nephrectomy performed by a single surgeon (YEY) between March 2015 and March 2016. The learning curve was evaluated using the cumulative sum (CUSUM) method. Measures of surgical performance included total operation time, warm ischemic time, and estimated blood loss.
The mean patient age, body mass index, and body surface area were 43.5 years, 23.8 kg/m2, and 1.7 m2, respectively. The mean operation time and warm ischemic time were 160.0 minutes and 124.4 seconds. The learning curve of total operation time was best modeled as a second-order polynomial with equation CUSUMOT (minutes) = –0.3802 × case number2 + 20.315 × case number – 41.333 (R2 = 0.7707). The curve included 3 unique phases: phase 1 (the initial 17 cases), which is the initial learning curve; phase 2 (the middle 23 cases), expert competence, and phase 3 (the subsequent cases), mastery. In terms of warm ischemic time and estimated blood loss, the initial learning was achieved after 16 cases and after 9 to 10 cases, one could achieve competency.
The VAMS donor nephrectomy learning curve is shorter than for laparoscopic or robotic hand-assisted donor nephrectomy. Surgeons can become familiar with the procedure and perform it without complications after approximately 16 to 17 operations.
Keywords: cumulative sum (CUSUM), donor nephrectomy, learning curve, video-assisted minilaparotomy surgery (VAMS)
1. Introduction
Surgical instrumentation and technologic innovations have tremendously improved surgical proficiency. However, surgeons will fall behind if they fail to learn new techniques. Investigating the learning curve is useful for assessing how surgeons acquire novel operative techniques,[1] and assessing healthcare quality using statistical process-control methods is becoming more commonplace.[2] The cumulative sum (CUSUM) technique was originally developed to monitor industrial sector performance and quality but has been adopted in the medical field to analyze surgical technique learning curves.[3,4]
Laparoscopic donor nephrectomy (LDN) was developed to meet the increasing demand for renal transplants and has become the preferred organ recovery method for living donors since it has advantages of less postoperative pain, decreased length of hospital stay with rapid recovery, faster return to work, and enhanced cosmesis.[5–8] Living donor transplants provide better graft function and survival than deceased donor kidney transplants.[9] Hand-assisted laparoscopic donor nephrectomy (HAL) is considered an important step in LDN since it is easier to learn, more rapid to perform, and is associated with less bleeding and fewer intestinal complications than full laparoscopy.[10]
Video-assisted minilaparotomy surgery (VAMS) is a hybrid of laparoscopic and open surgical techniques that does not require pneumoperitoneum or gas insufflation. This makes it particularly suitable for extracting an intact solid organ through a small incision such as that required for living donor nephrectomy.[11] We have reported the efficacy, efficiency, and favorable surgical outcomes by VAMS donor nephrectomy.[12–14] However, although several studies have reported the learning curves of LDN, HAL, and robotic hand-assisted donor nephrectomy (RHADN),[15–18] the learning curve of VAMS donor nephrectomy has not been described. In the present work, we estimated the learning curve for VAMS donor nephrectomy using CUSUM methodology.
2. Patients and methods
Medical records of patients treated at Severance Hospital in Seoul, South Korea were retrospectively retrieved after the study was approved by the Institutional Review Board of the Yonsei University Health System (project no: 4-2017-0457). A single experienced urologist (YEY) performed 50 consecutive VAMS donor nephrectomy surgeries between March 2015 and March 2016.
The VAMS technique was used in all donor nephrectomy surgeries with the patient in the semilateral position. A piercing abdominal wall elevator was used to secure the retroperitoneal space for the operative area. The surgical techniques were described previously.[11–13,19] The time from the first incision to the final closure was defined as the operation time. Demographic data including patient age, sex, height, weight, body mass index (BMI), body surface area (BSA), American Society of Anesthesiologists (ASA) score, and history of hypertension and diabetes mellitus were retrospectively retrieved. Intraoperative parameters including operation time, warm ischemic time (WIT), and estimated blood loss (EBL) were analyzed, as well as the hospital length of stay (LOS). Laboratory test results of preoperative, immediate postoperative, and 1 day after the operation are included. The measure of surgical performance was composed of 3 distinct categories, operation time, WIT, and EBL.
2.1. Cumulative sum analysis
The CUSUM technique was used for quantitative assessment of the learning curve; it calculates the running total of differences between the individual data points and the mean of all data points and can therefore be performed recursively.[1]
The 50 cases were ordered chronologically, from the earliest to the latest surgery dates. The operation time of each case is defined as xi, and the mean operation time of all cases is μ.
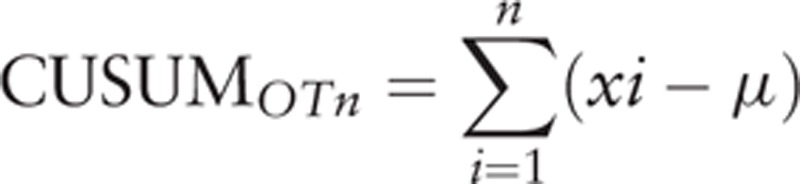 |
The CUSUMOT1 of the first case was the difference between the operation time for the first case and the μOT. The CUSUMOT2 of the second case was the previous case's CUSUMOT\ added to the difference between the operation time for the second case and μOT. This recursive process continued until we calculated the CUSUMOT for the last case. Similarly, additional parameters, WIT and EBL, were evaluated using CUSUM method.
2.2. Statistical analysis
The results are reported as mean (standard deviation) for continuous variables and as percentage values for categorical variables. To compare phases 1, 2, and 3, analysis of variance (ANOVA) was used for continuous variables, and chi-square or Fisher exact tests were carried out for categorical variables. SPSS software version 23.0 (IBM Corp., Armonk, NY) was used for the statistical analyses. All statistical tests were two-tailed, and a P-value <.05 was considered statistically significant.
3. Results
Patient characteristics are listed in Table 1. The study population included 27 (54.0%) men and 23 (46.0%) women with a mean age, BMI, and BSA of 43.5 ± 12.1 years, 23.8 ± 2.6 kg/m2, and 1.7 ± 0.2 m2, respectively. The median ASA score was 1, accounting for 70% of the study population. Six (12.0%) patients had hypertension, but none had diabetes mellitus. Most kidney donations were performed on the left kidney (45 cases, 90%). The mean operation time and WIT were 160.0 ± 29.5 minutes and 124.4 ± 14.9 seconds, respectively. The mean EBL was 66.5 ± 68.0 cm3.
Table 1.
Patient characteristics.
Figure 1 shows the operation times plotted in chronological case order, and the CUSUMOT learning curve was best modeled as a second-order polynomial with equation CUSUMOT in minutes equal to –0.3802 × case number2 + 20.315 × case number – 41.333, which had a high R2 value of 0.7707. The CUSUMOT learning curve consisted of 3 unique phases: phase 1 (the initial 17 cases), phase 2 (the middle 23 cases), and phase 3 (the final 10 cases). Comparisons between the 3 phases identified by CUSUMOT analysis are presented in Tables 2 and 3. There were no significant differences in demographic characteristics among the 3 phases. Operation time was significantly decreased in phase 3 (P < .001) compared with phase 1; however, the decrease was not significant from phase 1 to phase 2 (P = .115). The WIT and EBL of phases 2 and 3 were shorter and smaller than those of phase 1, but the differences were not significant.
Figure 1.
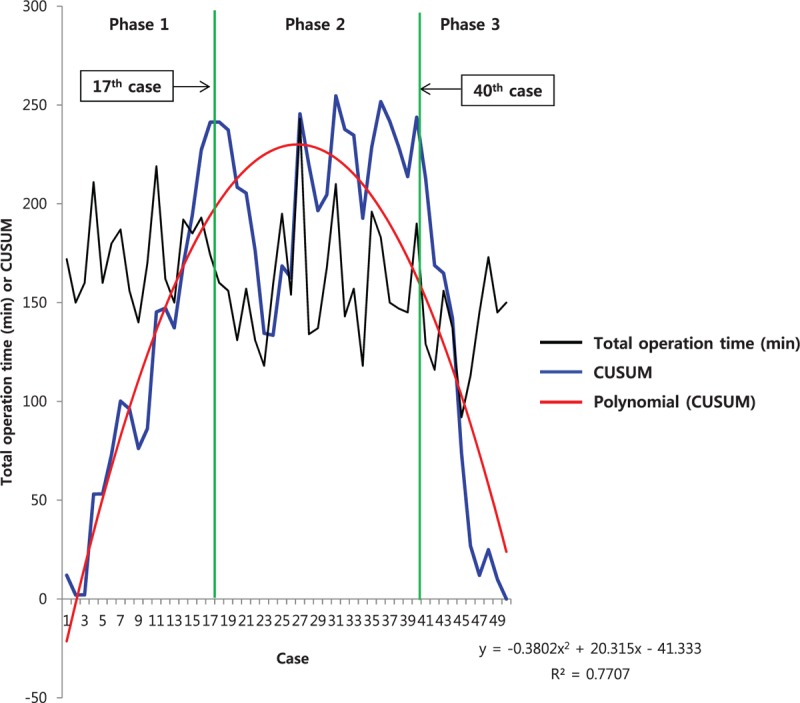
Total operation time (black line) and cumulative sum (CUSUM)OT (blue line) plotted against case number. The red line represents the best fit for the plot using a second-order polynomial with equation CUSUMOT = –0.3802 × case number2 + 20.315 × case number – 41.333 (R2 = 0.7707), corresponding to 3 distinct phases of the total operation time.
Table 2.
Interphase comparisons of patient characteristics and other parameters∗.
Table 3.
Pre- and postoperative laboratory measurements, mean (SD).
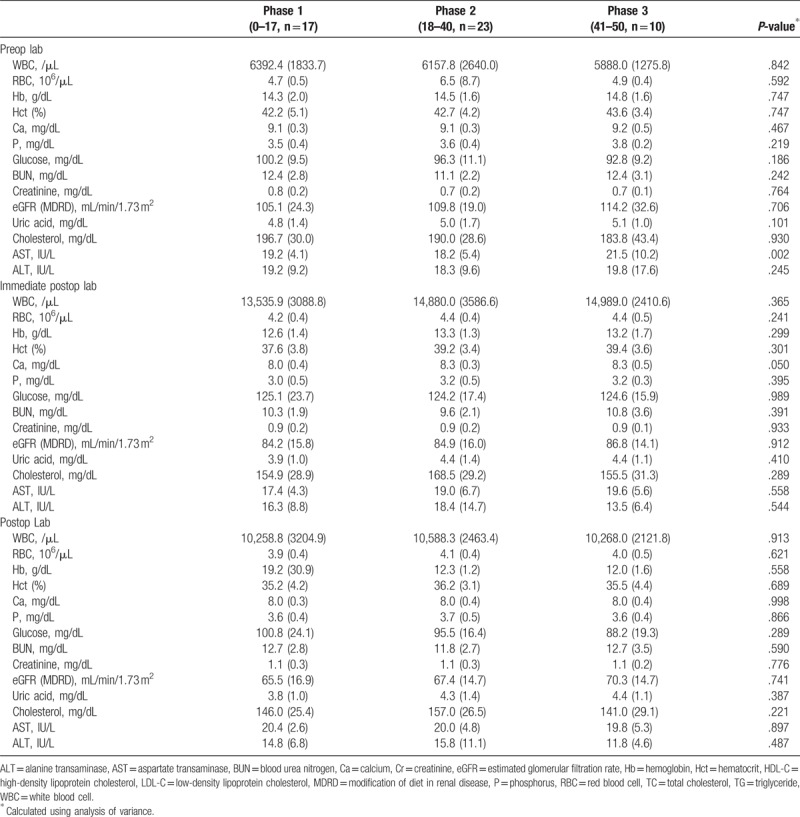
Figures 2 and 3 show the CUSUM learning curves of WIT and EBL, respectively. Both of the curves consisted of 3 unique phase with initial 16 cases of phase 1, and additional 9 cases (WIT) and 10 cases (EBL) in phase 2.
Figure 2.
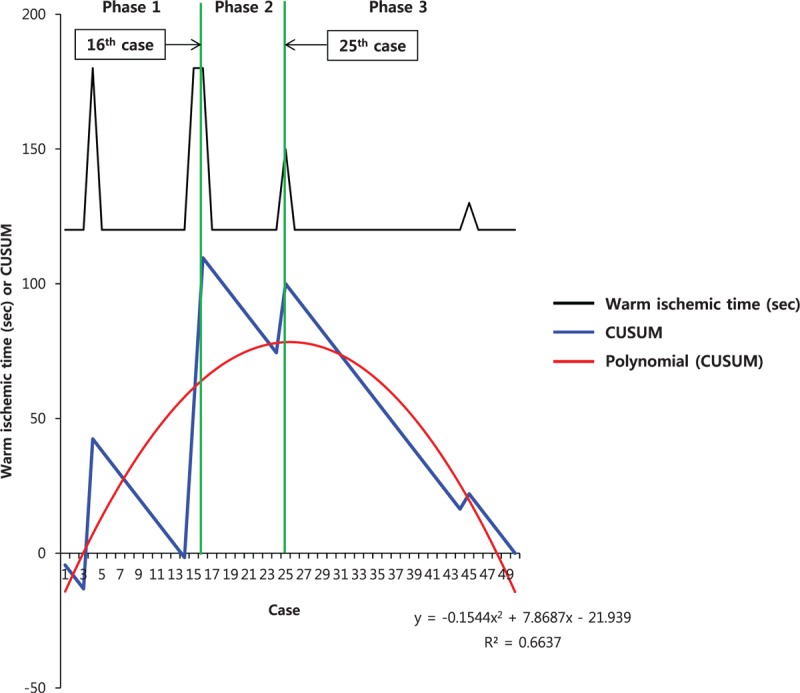
Warm ischemic time (black line) and cumulative sum (CUSUM)OT (blue line) plotted against case number. The red line represents the best fit for the plot using a second-order polynomial with equation CUSUMWIT = –0.1544 × case number2 + 7.8687 × case number – 21.939 (R2 = 0.6637), corresponding to 3 distinct phases of the warm ischemic time.
Figure 3.
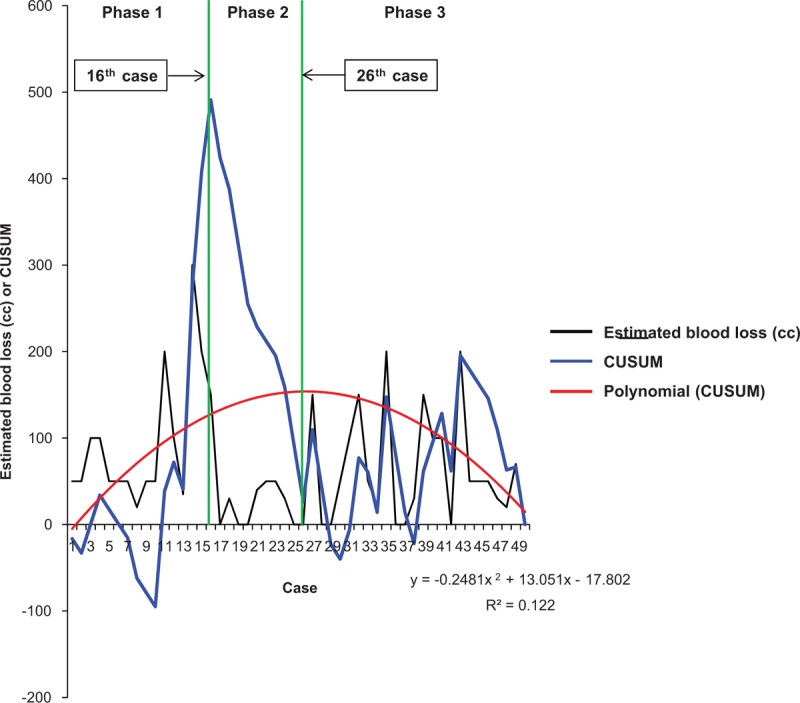
Estimated blood loss (black line) and cumulative sum (CUSUM)OT (blue line) plotted against case number. The red line represents the best fit for the plot using a second-order polynomial with equation CUSUMEBL = –0.2481 × case number2 + 13.051 × case number – 17.802 (R2 = 0.122), corresponding to 3 distinct phases of the estimated blood loss.
4. Discussion
This is the first investigation into the learning curve for performing VAMS donor nephrectomies in living donors. There is increasing demand for living donor renal transplants due to their superior graft survival. Many surgical techniques including LDN, RHADN, HAL have been developed to improve outcomes of both the donor and the recovered kidney. Our institution has used the VAMS technique for donor nephrectomy since 1991 based on benefits of the laparoscopic approach including shorter hospital stays, pain duration, and recovery periods, as well as those of the open approach such as no need for careful handling of the kidney vessels.
Ratner et al[20] performed the first clinically successful LDN at John Hopkins University. LDN has many advantages of minimal invasive surgery; however, it requires extensive vascular dissection, careful handling of the kidney and vessels, and rapid specimen extraction to minimize WIT.[16] Several studies noted that a certain level of experience is required to decrease technical complications, and Su et al[21] reported that there was no significant decrease in the mean operative time, EBL, or WIT even after 381 cases. However, there was a significant decrease in donor complications after the first 285 cases. Leventhal et al[22] determined that complication rates significantly decreased after a surgeon performed 100 out of 500 donor nephrectomies. Similar results have been reported in other studies.[23,24] Notably, 1 study stated that the learning curve for RHADN was 74 cases.[18] Compared with other techniques, VAMS donor nephrectomy requires a shorter learning period before clinical complications decrease.
For the comparison of WIT and EBL in LDN, HAL, and RHADN with VAMS donor nephrectomy, VAMS donor nephrectomy showed decreased in WIT and EBL compared with LDN, HAL, and RHADN, except for the WIT of RHADN according to the literature. The WIT and EBL for the 381 cases of LDN reported as 4.9 ± 3.4 minutes and 334 ± 690.3 cm3, respectively.[21] Another study of 382 cases of LDN with single renal artery showed WIT and EBL of 2.6 ± 0.6 minutes and 127 ± 118 cm3.[22] Other study of LDN of 738 cases also showed similar results of WIT and EBL of 169 ± 90.8 seconds and 128 ± 194 cm3.[23] For HAL, the WIT and EBL were 3.6 ± 1.5 minutes and 128 ± 70 cm3 for 31 cases.[25] WIT and EBL of RHADN with signle renal artery was 98 ± 20 seconds and 72 ± 173 cm3.[18]
This study evaluated a single surgeon's operative competency based on operation time and divided the cohort into phases corresponding with the surgeon's learning curve. The CUSUM technique was previously used in pediatric cardiac surgery[26] and is still used to monitor cardiac surgeon performance and patient outcomes.[27] In terms of operation time, our CUSUM analysis showed that phase 1 (17 cases), a surgeon with no experience in donor nephrectomy could complete the initial learning phase. After additional 23 cases, one could achieve expert competency. In terms of WIT and EBL, initial learning phase could be achieved in 16 cases which are similar to CUSUM analysis in operation time. However, about 9 to 10 cases were required to achieve expert competency in terms of WIT and EBL which are shorter than those required for operation time. The time required to achieve competency of VAMS donor nephrectomy which are represented by WIT and EBL is shorter than those required to minimize operation time. After achieving competency of procedure in VAMS donor nephrectomy, than surgeon could reduce the operation time.
High BMI, previous operation history, previous recurrent pyelonephritis history, congenital anomaly such as horseshoe kidney and duplication of ureter, complicated anomaly of renal vessels, and familiarity with the surgical devices would affect the surgical performance in initial phase of any surgery associated with donor nephrectomy. However, we have not performed donor nephrectomy in patients with previous operation history, previous recurrent pyelonephritis history, and congenital anomaly. According to our experiences, high BMI, complicated anomaly of renal vessels, and familiarity with the surgical devices have affected the surgical performance at phase 1. However, surgeon became familiar with the VAMS devices after phase 1 with 17 cases but BMI and complicated renal vessels still affected the performance at phase 2. After phase 3, surgeon was competent without any factors affecting the performance.
There are several possible reasons why VAMS donor nephrectomy has a shorter learning curve than other techniques. First, VAMS is a hybrid of open and laparoscopic surgeries. Both laparoscopic and robot-assisted approaches employ needle drivers that many find difficult to use. However, VAMS does not require laparoscopic equipment handling. Second, the use of telescope with a magnified view and an internal light source provides clear, direct surgical observation. Third, VAMS employs an extraperitoneal approach, which has no bowel injury with low morbidity. The surgeon can freely perform the operation without fear of bowel injury, and there is no need to consider adhesiolysis, thus shortening the learning curve. Fourth, VAMS is easily converted to open surgery in the event of a vascular accident.[19,28]
There are several limitations of this study. First, we only analyzed cases for a single surgeon, and future investigations including outcomes for multiple surgeons in different centers are needed to verify our results. Second, although there were no differences in patient characteristics among the different phases in our study, selection bias could have affected the learning curve.
5. Conclusion
To our knowledge, this is the first analysis with CUSUM identifying 3 unique learning curve phases for VAMS donor nephrectomy. The surgeon completes the initial learning phase of VAMS donor nephrectomy after 16 to 17 cases, which is comparably shorter than other techniques. In terms of operation time, after 40 cases, and in terms of WIT and EBL, after 9 to 10 cases, a surgeon can effectively perform VAMS donor nephrectomy with optimized WIT, total operation time, and low EBL.
Author contributions
Conceptualization: Jee Soo Park, Woong Kyu Han.
Data curation: Jee Soo Park.
Formal analysis: Jee Soo Park.
Investigation: Jee Soo Park, Young Eun Yoon.
Methodology: Jee Soo Park, Joonchae Na.
Project administration: Woong Kyu Han.
Resources: Jee Soo Park, Young Eun Yoon, Min Gee Yoon.
Supervision: Young Eun Yoon, Woong Kyu Han.
Validation: Jee Soo Park, Hyun Kyu Ahn, Young Eun Yoon.
Visualization: Jee Soo Park.
Writing—original draft: Jee Soo Park.
Writing—review and editing: Jee Soo Park, Hyung Ho Lee, Woong Kyu Han.
Footnotes
Abbreviations: ANOVA = analysis of variance, ASA = American Society of Anesthesiologists, BMI = body mass index, BSA = body surface area, CUSUM = cumulative sum, EBL = estimated blood loss, HAL = hand-assisted laparoscopic donor nephrectomy, LDN = laparoscopic donor nephrectomy, LOS = length of stay, RHADN = robotic hand-assisted donor nephrectomy, VAMS = video-assisted minilaparotomy surgery, WIT = warm ischemic time.
Compliance with ethical standards:
Ethical approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent: Informed consent was not required for the purposes of this study as it was based upon retrospective anonymous patient data and did not involve patient intervention or the use of human tissue samples.
All of the authors declare that they have no conflicts of interest to declare.
References
- [1].Bokhari MB, Patel CB, Ramos-Valadez DI, et al. Learning curve for robotic-assisted laparoscopic colorectal surgery. Surg Endosc 2011;25:855–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Suñol R, Vallejo P, Thompson A, et al. Impact of quality strategies on hospital outputs. Qual Saf Health Care 2009;18:i62–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].De Saintonge DC, Vere D. Why don’t doctors use cusums? Lancet 1974;303:120–1. [DOI] [PubMed] [Google Scholar]
- [4].Wohl H. The cusum plot: its utility in the analysis of clinical data. N Engl J Med 1977;296:1044–5. [DOI] [PubMed] [Google Scholar]
- [5].Brown SL, Biehl TR, Rawlins MC, et al. Laparoscopic live donor nephrectomy: a comparison with the conventional open approach. J Urol 2001;165:766–9. [PubMed] [Google Scholar]
- [6].Flowers JL, Jacobs S, Cho E, et al. Comparison of open and laparoscopic live donor nephrectomy. Ann Surg 1997;226:483–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Merlin TL, Scott DF, Rao MM, et al. The safety and efficacy of laparoscopic live donor nephrectomy: a systematic review. Transplantation 2000;70:1659–66. [DOI] [PubMed] [Google Scholar]
- [8].Troppmann C, Ormond DB, Perez RV. Laparoscopic (vs. open) live donor nephrectomy: a UNOS database analysis of early graft function and survival. Am J Transplant 2003;3:1295–301. [DOI] [PubMed] [Google Scholar]
- [9].Cho Y, Cecka J, Gjertson D, et al. The UNOS Scientific Renal Transplant Registry: multistep regression models on kidney graft survival. Clin Transpl 1990;397–405. [PubMed] [Google Scholar]
- [10].Gill IS. Hand-assisted laparoscopy: con. Urology 2001;58:313–7. [DOI] [PubMed] [Google Scholar]
- [11].Han WK, Lee HY, Jeon HG, et al. Quality of life comparison between open and retroperitoneal video-assisted minilaparotomy surgery for kidney donors. Transpl Proc 2010;42:1479–83. [DOI] [PubMed] [Google Scholar]
- [12].Lee YS, Jeon HG, Lee SR, et al. The feasibility of solo-surgeon living donor nephrectomy: initial experience using video-assisted minilaparotomy surgery. Surg Endosc 2010;24:2755–9. [DOI] [PubMed] [Google Scholar]
- [13].Choi KH, Yang SC, Lee SR, et al. Standardized video-assisted retroperitoneal minilaparotomy surgery for 615 living donor nephrectomies. Transpl Int 2011;24:973–83. [DOI] [PubMed] [Google Scholar]
- [14].Kim SI, Rha KH, Lee JH, et al. Favorable outcomes among recipients of living-donor nephrectomy using video-assisted minilaparotomy. Transplantation 2004;77:1725–8. [DOI] [PubMed] [Google Scholar]
- [15].Dalla VR, Mazzoni MP, Capocasale E, et al. Laparoscopic donor nephrectomy: short learning curve. Transpl Proc 2006;38:1001–2. [DOI] [PubMed] [Google Scholar]
- [16].Martin GL, Guise AI, Bernie JE, et al. Laparoscopic donor nephrectomy: effects of learning curve on surgical outcomes. Transplant Proc 2007;39:27–9. [DOI] [PubMed] [Google Scholar]
- [17].Wadström J, Biglarnia A, Gjertsen H, et al. Introducing hand-assisted retroperitoneoscopic live donor nephrectomy: learning curves and development based on 413 consecutive cases in four centers. Transplantation 2011;91:462–9. [DOI] [PubMed] [Google Scholar]
- [18].Horgan S, Galvani C, Gorodner M, et al. Effect of robotic assistance on the “learning curve” for laparoscopic hand-assisted donor nephrectomy. Surg Endosc 2007;21:1512–7. [DOI] [PubMed] [Google Scholar]
- [19].Yang SC, Lee DH, Rha KH, et al. Retroperitoneoscopic living donor nephrectomy: two cases. Transplant Proc 1994;26:2409. [PubMed] [Google Scholar]
- [20].Ratner LE, Ciseck LJ, MooRE RG, et al. Laparoscopic live donor nephrectomy. Transplantation 1995;60:1047–9. [PubMed] [Google Scholar]
- [21].Su LM, Ratner LE, Montgomery RA, et al. Laparoscopic live donor nephrectomy: trends in donor and recipient morbidity following 381 consecutive cases. Ann Surg 2004;240:358–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Leventhal JR, Kocak B, Salvalaggio PR, et al. Laparoscopic donor nephrectomy 1997 to 2003: lessons learned with 500 cases at a single institution. Surgery 2004;136:881–90. [DOI] [PubMed] [Google Scholar]
- [23].Jacobs SC, Cho E, Foster C, et al. Laparoscopic donor nephrectomy: the University of Maryland 6-year experience. J Urol 2004;171:47–51. [DOI] [PubMed] [Google Scholar]
- [24].Melcher ML, Carter JT, Posselt A, et al. More than 500 consecutive laparoscopic donor nephrectomies without conversion or repeated surgery. Arch Surg 2005;140:835–40. [DOI] [PubMed] [Google Scholar]
- [25].Buell JF, Abreu SC, Hanaway MJ, et al. Right donor nephrectomy: a comparison of hand-assisted transperitoneal and retroperitoneal laparoscopic approaches. Transplantation 2004;77:521–5. [DOI] [PubMed] [Google Scholar]
- [26].Leval MR, François K, Bull C, et al. Analysis of a cluster of surgical failures: application to a series of neonatal arterial switch operations. J Thorac Cardiovasc Surg 1994;107:914–23. discussion 923-4. [PubMed] [Google Scholar]
- [27].Grunkemeier GL, Jin R, Wu Y. Cumulative sum curves and their prediction limits. The Ann Thorac Surg 2009;87:361–4. [DOI] [PubMed] [Google Scholar]
- [28].Byun YJ, Yang SC. Laparoscopy-assisted urologic surgery through minilaparotomy. Yonsei Med J 1999;40:596–9. [DOI] [PubMed] [Google Scholar]


