Abstract
Increasingly, for a variety of indications, patients have their genomes sequenced and actionable results returned. A subset of returned results is pharmacogenomic (PGx) variants involved in the metabolism or action of medications. Although the impact of these variants on health is well‐documented, little research exists on how to communicate these findings to patients and clinicians. We conducted semistructured interviews with end users to understand how best to communicate PGx results. Overall, patients and clinicians had similar opinions regarding report content, delivery, and application. Unique concerns specific to each stakeholder group were also expressed. Patients wanted an easy‐to‐understand individualized report that clinicians utilized to guide their care. Clinicians wanted reports that were easy‐to‐use, actionable, and integrated into their workflow. Implementation of these reports in a clinical setting will allow for broader user feedback and iterative improvement.
Study Highlights
WHAT IS THE CURRENT KNOWLEDGE ON THE TOPIC?
✓ Pharmacogenomic (PGx) testing is becoming more readily available. Little information exists about what should be contained within a PGx report that would be used by patients and clinicians.
WHAT QUESTION DID THIS STUDY ADDRESS?
✓ The study aimed to understand information patients and clinicians would want to receive regarding a PGx report. This was used to inform the development of patient‐facing and clinical‐facing PGx reports using user‐centered design principles.
WHAT DOES THIS STUDY ADD TO OUR KNOWLEDGE?
✓ This study addresses what information is needed when developing a PGx report incorporating perspectives from patients and clinicians, simultaneously.
HOW THIS MIGHT CHANGE DRUG CLINICAL PHARMACOLOGY OR TRANSLATIONAL SCIENCE
✓ Patients are the only common factor in the United States healthcare delivery system. By putting this information in the hands of the patients, we can encourage the sharing of these results so that the PGx information is used in any healthcare setting they encounter, not just the system that performed the testing.
Despite the initial promise of pharmacogenomics (PGx), translation into clinical care and improved outcomes has been slower than expected.1 This has been attributed to practical application, knowledge gaps, uncertainty about the clinical utility of results, and concern about incidental findings.2, 3, 4, 5 Qualitative studies of clinicians’ perspectives report that they lack the requisite knowledge to appropriately order and return PGx results, have concerns regarding cost implications, and health insurance being denied for their patients.2, 3, 4, 6, 7, 8, 9, 10 Pharmacists have expressed positive feelings toward this information because of its potential to prevent adverse events and improve medication dosing.6 Studies examining the views of patients found they share similar positive views11, 12, 13, 14 and concerns, including a lack of PGx knowledge and concern about the cost implications of such results.2, 12, 13 These studies, however, focused on attitudes toward PGx testing and not how these results, when available, should be communicated.
These barriers hinder the implementation of PGx into routine clinical care. Multifaceted approaches to address these concerns are needed, particularly in providing PGx information that addresses clinician and patient concerns. Creating a patient‐centered results report could ameliorate some of these concerns and facilitate communication between the patient and clinician. Current laboratory reports are often complex, difficult to understand, contain minimal context for clinical implementation, and are targeted toward communicating the result to a clinician, rather than to both the clinician and the patient. We extended the use of a previously described patient‐facing and clinician‐facing results report15, 16 by adapting the report for the return of PGx results. This study investigated the perspectives of patients and clinicians regarding communication of PGx results for the development of a patient‐facing PGx report.
METHODS
Setting
Geisinger is an integrated rural health delivery system that serves central, south central, and northeast Pennsylvania and southern New Jersey. Geisinger's MyCode Community Health Initiative (MyCode) is a major resource for research that combines information obtained from DNA and other biospecimens with health information from the electronic health record (EHR) and other sources intended to improve the prevention, diagnosis, and treatment of disease.17
Study population
Adult MyCode patients and Geisinger clinicians were invited to participate in the study. Patients were identified using a random convenience sample and had to be willing to drive to the Henry Hood Research Center in Danville, PA. None of the patients had received a genetic test result from the MyCode initiative. Clinicians were defined as primary care providers (PCPs), specialists, and pharmacists who have referred patients to MyCode, participated in a MyCode genomic medicine workgroup, or in the MyCode oversight committee and were identified using purposive sampling. Pharmacists will have a significant role in the use of PGx information, as they are increasingly recognized as members of the care team and are often viewed as the medication experts,18 therefore, inclusion of their perspective is essential to optimize the reporting of this information. Additional clinicians were identified through snowball sampling. The clinicians had to be willing to meet in‐person for the interviews.
Procedures
We conducted in‐person semistructured interviews with patients and clinicians (Supplementary Materials S1). One‐on‐one in‐person interviews were conducted by a trained study interviewer with a study participant. The interviews lasted no longer than an hour and all participants received a $25 gift card for their time. Interviewers (A.K.R., L.K.J., J.L.W., M.R.G., and A.F.) were trained by an experienced qualitative researcher (A.K.R.). Each study participant was assigned a study number for privacy. Interviews were audio recorded with acknowledgment and verbal consent of the participants. Each participant was asked to complete a demographic survey (Supplementary Material S2).
Following a user‐centered design approach,19, 20 and based on information from our prior work,15, 16 mock reports were created and presented as part of the interview to patients and clinicians. For purposes of testing, a reading level was not chosen. The patient input informed the order and content of the report, which can then be written at an accepted reading level (e.g., fifth grade). Initially two gene‐drug reports were drafted (Figure 1): (1) SLCO1B1 for simvastatin and (2) TPMT for azathioprine, mercaptopurine, and thioguanine, individually. All versions of the SLCO1B1/simvastatin reports contained the same six sections but the order of the sections was varied to elicit end‐user preference: drug first, gene first, or condition first (Supplementary Material S3). For example, the gene first report started with the section entitled information about the genetic test result, whereas the drug first report started with information about this medication. The TPMT/azathioprine, mercaptopurine, and thioguanine reports were drafted to represent the information as if the participant was taking or not taking the identified medication (Supplementary Material S3). Changes were made to the report based on feedback and presented to the next interviewees in an iterative process until the report elicited no additional suggestions for improvement, which was interpreted as meeting the needs of patients and clinicians being interviewed (Figure 2).
Figure 1.
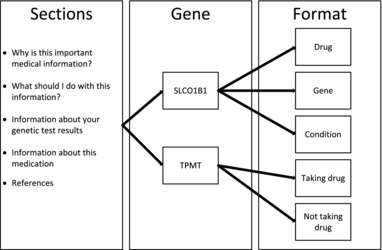
Report sections of mock concept sheets to patient and clinician by gene and presentation of report.
Figure 2.
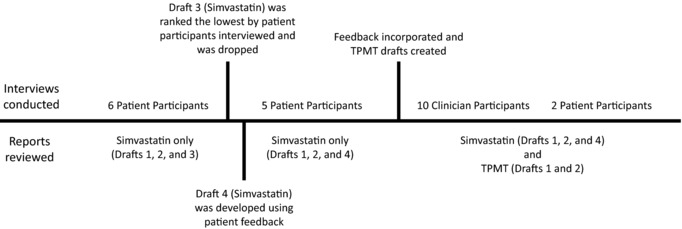
Description of iterative process.
This study was approved by the Geisinger Institutional Review Board.
Data analysis
We conducted an inductive analysis, and the themes were determined as they emerged from the data.21 Recordings were transcribed verbatim using the hospital transcription service. One reviewer confirmed the accuracy and completeness of transcripts (L.K.J.). A codebook was developed by two investigators (A.L.F. and L.K.J.) based on individual interview summaries, content of the interview guide, and divided into coding domains. Themes relevant to each domain were determined through review of transcripts by two coders (A.L.F. and L.K.J.) through consensus review with the coding team (A.L.F., L.K.J., A.K.R., J.L.W., M.R.G., and R.A.P.). Two coders, one trainee, and one experienced qualitative researcher coded each transcript and results were reviewed and discrepant codes resolved by consensus with the coding team. Thematic saturation was met in both populations interviewed when no new ideas were discussed concluding the interviews.22 Atlas.ti software version 7.5.16 was used to facilitate analysis and to compare themes across groups (Berlin, Germany).
RESULTS
Study participants included 13 MyCode participants and 10 clinicians. MyCode participants were 62% women (8/13), 71% (5/7) were between 55 and 64 years old, and all were white (reflective of the MyCode population, which is >95% white). Half (4/8) reported at least a college level education. Four (4/7) had an annual income of <$75,000. Demographic information was incomplete for five individuals. Five participants reported taking simvastatin, the medication used in one of the mock reports. Although unselected, two participants had experienced simvastatin‐related muscle pain. Participating clinicians were three PCPs, three specialists, and four pharmacists. Comments about report content and concerns were similar among the different clinician types; therefore, all clinicians were analyzed as a unit. The clinician participants were 50% men and had, on average, 12.6 years in practice (range 1–28; 9/10). Only two individuals had ordered PGx testing previously and three had received some type of genetic test result for their patients.
Common themes emerged for both patients and clinicians around report content, communication of the result, and application of content (Table 1). However, certain preferences, understandings, and opinions of utility varied between patients and clinicians.
Table 1.
Summary of key findings
| Summary of results | ||
|---|---|---|
| Themes | Patients | Clinicians |
| Report content |
|
|
| Communication of the result | ||
| Report format for PGx results |
|
|
| Expectations |
|
|
| Responsibility |
|
|
| Application of content | ||
| Perceived value |
|
|
| Unintended consequences |
|
|
EHR, electronic health record; PGx, pharmacogenomics.
Report content
Prior to reviewing the mock report, patients hypothesized that a PGx report should be both personalized and actionable. Specifically, patients requested to know “[why] you might not react well” (patient participant #16), and “what makes me react poorly to that drug” (patient participant #26), as well as “what was different about it” (patient participant #6), and “what could be done about it” (patient participant #6). Some patients requested to know everything about the result, “I'd like to know as much as I can” (patient participant #6), however, after reviewing the mock report, they thought that some sections of the report had too much information. Most patients reported that the mock report helped them understand what the results mean for them, including information about the medication(s) impacted, drug interactions, and the relevant indication for the medication. Participants noted that there was too much medical jargon (e.g., poor, intermediate, or normal metabolizer, and gene) and preferred a report written in lay language.
I don't think you need to get into all of the medical terms, 'cause a lot of the people wouldn't understand that. If you get too wordy, I think they just start tuning things out. I think you should just stick to the basics, you know, the highlights (patient participant #39).
Clinician preferences for report language were similar, including a patient‐facing report that is personalized, actionable, and easy‐to‐read without unnecessary medical jargon.
I think there needs to be more patient‐friendly information, slightly longer and more detailed for the patients to be able to look at, digest, go back to (PCP participant #104).
Although clinicians agreed overall that patients should receive the reviewed patient‐facing report, they also expressed a strong preference for a separate clinician‐specific report. The requested the report should be short, but with adequate detail for medical follow‐up.
If it's more than one page, we're not going to read it (PCP participant #104).
So I like, as a provider, to get some more details on it. As much detail as possible for me would… be ideal (Pharmacist participant #109).
According to the clinicians, a report should include data relevant to the care of their patient, how the result changes the care of their patient, and what they needed to do with this information. Clinicians suggested adding sections on alternative medications, dose reduction strategies, and important medical parameters to monitor.
So the only other thing to add would be from a physician standpoint, if there would be any testing that needs to be done routinely (PCP participant #104).
I think a little bit more of a detailed report on dosing, how high they may need to go with the dose, things of that nature& (Pharmacist participant #105).
Clinicians also requested a report section dedicated to how to talk to patients about the result and that covered information such as: what does the result mean, what to expect, does it affect the patient now and how urgent or severe is the problem, how to talk to the family, and how this affects insurance.
Here's what it is, here's why you're getting it, and then here's what we need to do now, or what you should consider doing. Because it's all patient's choice really. Giving them all the information that you can, helps them make better decisions (pharmacist participant #109).
Finally, clinicians also requested active links to additional resources to educate themselves on the genetic change and its implications for patient care, a function supported by the prior version of genomic test reports.15, 16
COMMUNICATION OF THE RESULTS
Report format for PGx results
Patients discussed multiple avenues for how they would prefer to receive a PGx result report, with one third of patients preferring a paper report copy mailed to them, whereas another third would prefer access to the report electronically through their EHR patient portal (MyGeisinger), and another third wanted both. Patients wanted to be able to access the report in the future and almost all of them would file this report away with other important documents.
Clinicians uniformly preferred the PGx results “live[s] in [EHR]” and facilitate patient care through an alert that “…pops up when a medication like this is going to be prescribed” (PCP participant #104). Clinicians had various ideas about where in the EHR PGx results would best fit in their workflow; 8 of the 10 clinicians interviewed suggested a separate tab or place for PGx results, whereas one other clinician specifically requested that it would be useful if presented with the patient's allergy information. Regardless, clinicians were adamant that the information be available at the point‐of‐care so that it could inform decision making. It was important for results to be integrated into the EHR in such a way that it encouraged appropriate prescribing and discouraged practices that could lead to medication errors or medication safety concerns.
So where it lives in the medical record I think is more important (PCP participant #104).
Patients and clinicians struggled to determine whether PGx results should be returned to the patient only when they are actionable (e.g., patient is on the drug or given a prescription for the drug) or as soon as the result is available. Two patients discussed when they would want to learn about their PGx result such that one wanted it “as soon as possible” (patient participant #6) and the other “would probably only want to know [the result] if the situation came up if I was actually on the medication” (patient participant #24). For the other patients, the timing was dependent on the meaning of the result to their current medical care. Clinicians agreed that more urgent results should be discussed promptly, whereas less urgent ones can wait. Regardless, clinicians discussed the importance of receiving this report before the patient so that they could be prepared for any questions about the result the patient might have.
I would want to be able to like see what the report says and either change the patient's therapy or not depending on what the result was (Pharmacist participant #111).
Expectations
Patients emphatically voiced an expectation that the healthcare system would use the result for their care—“There ought to be a way to get it on my list of medications somehow indicating that I wasn't taking it, but I shouldn't take it” (patient participant #35), store it in their medical record—“I guess it gets associated in my medical record and everything. I think this information ought to be in there” (patient participant #60), and “ensure that all relevant parties were notified of the result—I mean, doesn't the doctor know?” (patient participant #28) and could easily access it when needed.
Responsibility
Patients voiced uncertainty regarding who to discuss the result with and whether they are responsible to contact their clinician or the clinician should contact them. Individual patients had specific views regarding who should be responsible for communicating this information and this was dependent both on personal comfort with the desired clinician (e.g., their PCP) and the perceived relevance of the result to the clinician given their expertise (e.g., their cardiologist).
I probably would want to hear it from the person who was the closest to the information. You know, the person who may be studying the results more so than my primary care doctor (patient participant #60).
Clinicians supported the idea that the healthcare system had the responsibility to ensure that PGx results are communicated and that clinically appropriate actions are taken. Those who ordered the medication should be responsible for communicating this result and altering the treatment plan. Often, this opinion was influenced by the clinician's comfort level—“…I don't think I'd feel comfortable discussing all of them. I feel comfortable discussing the ones that pertain to my care of the patient” (Specialist participant #106).
Simvastatin is bread and butter of primary care. I feel like we deal with it more than any other specialty, so for this drug, I think primary care should take charge. … but if it's some drug that we don't prescribe that often, then definitely the [clinician] that prescribes it the most (PCP participant #112).
APPLICATION OF CONTENT
Perceived value
Patients strongly valued learning about PGx results and how the report would prepare them for conversations with their clinicians as well as their family members. For example, one patient said “Yeah, I need to share it. That's the important thing, I think, to really share it” (patient participant #15).
Patients discussed that these reports would be very important if the individual was currently taking the medicine affected by the genetic variant and believed this information would affect their decisions to remain on the medicine. Patients expressed that even if they were not currently taking the medication in question, these reports would be valuable due to uncertainty as to whether they may need the medication in the future.
…I would be scared to be on that drug. Of course, you see all the stuff on TV and they recommend these drugs to you, and then at the bottom of the screen, if you read it, it tells you what side effects you can have, and I would want to know what side effects I'm going to have, so that if I start to take this drug and I don't feel well, I would want to know what they are. You know, why are my arms starting to hurt or what? Why am I more tired than I have been in the past? I would want to know those things because then I would know it is kind of related to the drug… because when I start taking something, I don't feel right, I kind of start to blame it on the drug. So I want to know that (patient participant #16).
Well I'd probably read over it and then make a decision of whether or not I was going to stay on it or not (patient participant #30).
Although most patients were responding to the report information hypothetically, because they had never been prescribed the medication in the report or had never experienced an adverse drug reaction, the applicability of this report was especially valued for two individuals who had experienced the side effects described in the SLCO1B1/simvastatin report. Even though the mock report was not indicative of either patient's actual genetic variant, both described how this report would have been beneficial to receive prior to being prescribed simvastatin. Both emphasized knowing this information would have been invaluable because they would have been prescribed a different medication initially and would not have had to experience statin‐related side effects.
That's right, if I would have had this, we could have saved a lot of grief and money, and you know, gone right to the top (patient participant #29).
Well, it sounds accurate in terms of what's in there based on my knowledge of some of the issues that I just discussed with you in terms of muscle pain, so it does… I can relate to that. I know I was taken off of [simvastatin] and given… I'm sure it's pravastatin. Because of my muscle pain (patient participant #28).
The remainder of patients was asked to imagine receiving this report for a medication that they had already been taking. They asked that a healthcare professional review the result with them and decide whether they should continue taking the medication. Clinicians thought the patients could be educated by reading the report by themselves.
Unintended consequences
In contrast with patients who anticipated wholly positive outcomes from receiving the PGx report, half of the clinicians interviewed were concerned that the report might cause patients to become upset, panicked, or confused. In addition, four clinicians anticipated a substantial burden on themselves and their clinic from concerned patients contacting them via EHR patient portal messages (MyGeisinger) or telephone calls.
You know what's going to happen is they are either going to call or have a MyGeisinger message… (PCP participant #104).
So, I can see patients panicking, and the first thing they do is call their physician who (a) if they weren't informed, is going to get very upset, and (b) is also going to get upset if they get the phone call and have to deal with it (Specialist participant #108).
DISCUSSION
As PGx testing becomes integrated into clinical care, it is important to understand what information patients and clinicians desire and need to inform decision making. Previous studies described below have examined patient and clinician attitudes toward requesting or receiving PGx results. We conducted semistructured interviews with patients and clinicians to understand how to communicate this information with patients and clinicians. We found that in developing a PGx report it is important to consider not only the content of the report, but how that information will be communicated and applied in clinical care. This study builds upon prior work at Geisinger focusing on creating reports around the return of clinically actionable variants related to diseases (as opposed to medications).15, 16 Our study aimed to apply these principle findings to PGx results, incorporating guidance on themes requested in patient‐facing PGx reports.
Patients in our study reported feeling more knowledgeable about the medication, condition, and genetic result after reading the sample report; they reported that this report would allow them to be more engaged in the conversation with their clinician. However, both patients and clinicians agreed that this information needs to be written so that patients can understand. Clinicians described this information as important to clinical care and emphasized that it must be communicated in a way that is both usable and actionable. These reports could then be used to facilitate conversations between patients and clinicians about PGx results and aid the decision‐making process on appropriate medication selection. Recognizing that medicine is multidisciplinary, clinicians indicated that although the result reports should be communicated to the ordering prescriber, this information should reside within the EHR so that it was available to other clinicians involved in that patient's care.
Our findings are consistent with other studies that have described patients’ perceptions of positive benefits and usability of PGx testing.11, 12, 13, 14 Long‐term access to results was identified as an important need by this study as well as previous studies.23 The current approach of reporting and storing PGx results in the EHR does not easily translate to establishing long‐term sharing and patient access to PGx results. In addition, there was no consensus among patients about the optimal mechanism to access reports. Our study examined patient perceptions of a patient‐facing PGx report and found that PGx reports provide a suitable form to distribute PGx information, increase patient understanding, and facilitate patient and clinician communication. Having a hard copy of the report, or access through an electronic patient portal, means that the information can be available for the patient wherever they are receiving care.
Our study aligns with previous reports that clinicians have an overall positive view towards PGx.11, 14, 24 Others have found that clinicians feel that PGx data can inform treatment decision making by identifying patients likely to benefit or be harmed through the use of certain medications and, therefore, can be used to individualize therapy.11, 24 A previous study found that clinicians were concerned about their knowledge regarding the management of these variants and how and by whom these results would be returned and managed.14 We had similar opinions as other studies, in which clinicians voiced concerns over increased messaging and increased clinic burden.25, 26 Providing clear, relevant, and actionable PGx reports to patients and clinicians may provide a suitable solution to overcome some of the concerns expressed by patients and clinicians, but requires additional investigation and report optimization to determine the best format and delivery mechanism for the return of PGx reports.
Patients and clinicians were interviewed until no new ideas about these hypothetical reports emerged, indicating that we reached thematic saturation in this sample. Opinions expressed in these interviews may not be representative for all ideas requiring additional testing around timing of receipt of result reports for patients and development of a short but detailed report for clinicians. Patients were recruited solely from those consented to the MyCode initiative, which may not represent the views of those who chose not to participate. Our population represents a rural, white demographic, which could limit the generalizability of these findings to other populations. However, the themes identified about the perception of the use of the information reflect those found in prior studies. Finally, the applicability of our results may be limited by the fact that most of our participants were not taking the medications highlighted in our example reports. It is unclear whether the views expressed by our participants reflect those that might be expressed by those actively taking these medications.
Further investigation must be done to determine how to create a clinician‐facing report that is short but retains adequate detail for clinical decision making. Future research should be conducted to explore whether clinician type affects views of PGx result reports. Geisinger plans to incorporate these result reports into clinical care of our patients so that every patient receiving a PGx result will receive this type of report. This will initially be done in a pilot project of 2,500 patients receiving PGx results through the Electronic Medical Records in Genomics (eMERGE) project.
Our study provides the foundation for a framework for optimal design and delivery of PGx reports previously unstudied. This information can be used by health systems to develop PGx reports that are useful, usable, and desirable for both patients and clinicians.
Conflict of Interest
The authors declared no competing interests for this work.
Supporting information
Supplementary Materials 1. Interview Guide
Supplementary Materials 2. Demographic Questionnaires
Supplementary Materials 3. Mock Reports
Source of Funding
Funded through Patient Centered Outcomes Research Institute (PCORI Contract Number: CD‐1304‐6987. Project title: Enhancing Genomic Laboratory Reports to Enhance Communication and Empower Patients.
Author Contributions
L.K.J., A.K.R., M.R.G., J.L.W., A.L.F., R.A.P., E.A.W., and M.S.W. wrote the manuscript. L.K.J., A.K.R., J.L.W., E.A.W., and M.S.W. designed the research. L.K.J., A.K.R., M.R.G., J.L.W., and A.L.F. performed the research. L.K.J., A.K.R., M.R.G., J.L.W., A.L.F., R.A.P., and M.S.W. analyzed the data.
References
- 1. Moaddeb, J. & Haga, S.B. Pharmacogenetic testing: current evidence of clinical utility. Ther. Adv. Drug Saf. 4, 155–169 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Patel, H.N. , Ursan, I.D. , Zueger, P.M. , Cavallari, L.H. & Pickard, A.S. Stakeholder views on pharmacogenomic testing. Pharmacotherapy 34, 151–165 (2014). [DOI] [PubMed] [Google Scholar]
- 3. Johnson, J.A. Pharmacogenetics in clinical practice: how far have we come and where are we going? Pharmacogenomics 14, 835–843 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Haga, S.B. & Burke, W. Pharmacogenetic testing: not as simple as it seems. Genet. Med. 10, 391–395 (2008). [DOI] [PubMed] [Google Scholar]
- 5. Mills, R. , Voora, D. , Peyser, B. & Haga, S.B. Delivering pharmacogenetic testing in a primary care setting. Pharmgenomics Pers. Med. 6, 105–112 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tuteja, S. , Haynes, K. , Zayac, C. , Sprague, J.E. , Bernhardt, B. & Pyeritz, R. Community pharmacists’ attitudes towards clinical utility and ethical implications of pharmacogenetic testing. Per. Med. 10, 793–800 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. McCullough, K.B. et al Assessment of the pharmacogenomics educational needs of pharmacists. Am. J. Pharm. Educ. 75, 51 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Roederer, M.W. , Van Riper, M. , Valgus, J. , Knafl, G. & McLeod, H. Knowledge, attitudes and education of pharmacists regarding pharmacogenetic testing. Per. Med. 9, 19–27 (2012). [DOI] [PubMed] [Google Scholar]
- 9. Sansgiry, S.S. & Kulkarni, A.S. The human genome project: assessing confidence in knowledge and training requirements for community pharmacists. Am. J. Pharm. Educ. 67, 39 (2003). [Google Scholar]
- 10. de Denus, S. et al An evaluation of pharmacists’ expectations towards pharmacogenomics. Pharmacogenomics 14, 165–175 (2013). [DOI] [PubMed] [Google Scholar]
- 11. Rogausch, A. , Prause, D. , Schallenberg, A. , Brockmöller, J. & Himmel, W. Patients’ and physicians’ perspectives on pharmacogenetic testing. Pharmacogenomics 7, 49–59 (2006). [DOI] [PubMed] [Google Scholar]
- 12. Haga, S.B. , O'Daniel, J.M. , Tindall, G.M. , Lipkus, I.R. & Agans, R. Survey of U.S. public attitudes towards pharmacogenetic testing. Pharmacogenomics J. 12, 197–204 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Haga, S.B. , Tindall, G. & O'Daniel, J.M. Public perspectives about pharmacogenetic testing and managing ancillary findings. Gen. Test. Mol. Biomarkers 16, 193–197 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fargher, E.A. et al Patients’ and healthcare professionals’ views on pharmacogenetic testing and its future delivery in the NHS. Pharmacogenomics 8, 1511–1519 (2007). [DOI] [PubMed] [Google Scholar]
- 15. Stuckey, H. et al Enhancing genomic laboratory reports from the patients' view: a qualitative analysis. Am. J. Med. Genet. A 167A, 2238–2243 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Williams, J.L. et al Enhancing genomic laboratory reports: a qualitative analysis of provider review. Am. J. Med. Genet. A 170A, 1134–1141 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Carey, D.J. et al The Geisinger MyCode community health initiative: an electronic health record‐linked biobank for precision medicine research. Genet. Med. 18, 906–913 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chisholm‐Burns, M.A. et al US pharmacists' effect as team members on patient care: systematic review and meta‐analyses. Med. Care. 48, 923–933 (2010). [DOI] [PubMed] [Google Scholar]
- 19. Johnson, C.M. , Johnson, T.R. & Zhang, J. A user‐centered framework for redesigning health care interfaces. J. Biomed. Inform. 38, 75–87 (2005). [DOI] [PubMed] [Google Scholar]
- 20. Hartzler, A. et al Stakeholder engagement: a key component of integrating genomic information into electronic health records. Genet. Med. 15, 792–801 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Patton, M.Q. , ed. Qualitative evaluation and research methods. 3rd edn (Sage Publishing, Thousand Oaks, CA, 1990). [Google Scholar]
- 22. Strauss, A. & Corbin, J. Basics of qualitative research techniques. (Sage Publishing, Thousand Oaks, CA, 1998). [Google Scholar]
- 23. Haga, S.B. , Kawamoto, K. , Agans, R. & Ginsburg, G.S. Consideration of patient preferences and challenges in storage and access of pharmacogenetic test results. Genet. Med. 13, 887–890 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zachary, W. III & Armstrong, E.P. Health care professionals' perceptions of the role of pharmacogenomic data. J. Manag. Care Pharm. 8, 278–284 (2002). [DOI] [PubMed] [Google Scholar]
- 25. Delbanco, T. et al Inviting patients to read their doctors' notes: a quasi‐experimental study and a look ahead. Ann. Intern. Med. 157, 461–470 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Walker, J. et al Inviting patients to read their doctors' notes: patients and doctors look ahead: patient and physician surveys. Ann. Intern. Med. 155, 811–819 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Materials 1. Interview Guide
Supplementary Materials 2. Demographic Questionnaires
Supplementary Materials 3. Mock Reports


