INTRODUCTION
Multiple myeloma (MM) is a malignant disease of plasma cells, with a 5‐year relative survival rate lower than 50%.1 In recent years, the introduction of proteasome inhibitors (PIs) and immunomodulatory drugs has improved treatment outcomes, leading to the standard of care consisting largely of immunomodulatory drug‐ and PI‐based regimens, or triplet therapy involving a combination of both.2 Nonetheless, MM remains largely incurable and there is an unmet need for additional therapies.
Immunotherapy is a rapidly emerging area in cancer treatment. Immuno‐oncology (I‐O) agents (such as the antisignaling lymphocytic activation molecule F7 (SLAMF7) monoclonal antibody elotuzumab) enhance the immune response against cancer cells, either by directly stimulating the activity of immune cells or by targeting specific cell surface tumor antigens.3
Until recently, use of immunotherapy in MM has faced two major hurdles. First, suitable targets on plasma cells have been elusive. Second, the immune system in patients with MM is significantly impaired (e.g., functional defects in T cells, B cells, natural killer (NK) cells, and dendritic cells),4 resulting in defective endogenous immune responses, particularly in adaptive immunity. Despite these limitations, recent clinical data suggest that immune effectors retain sufficient functionality in MM to mediate significant clinical benefit when I‐O agents targeted to appropriately expressed antigens are given.5
In addition to elotuzumab, the anti‐CD38 antibodies daratumumab, isatuximab, and MOR202, and the antikiller cell immunoglobulin‐like receptor (KIR) 2DL1/2/3 antibody lirilumab, are examples of immunostimulatory antibodies approved or in development for the treatment of MM.6, 7, 8, 9, 10 Daratumumab (in patients who have received at least three prior treatments) and elotuzumab (combined with lenalidomide and dexamethasone (Ld) in patients who have received one to three prior therapies) were approved in November 2015 by the US Food and Drug Administration (FDA) for MM treatment.7, 8 In May 2016, this combination of elotuzumab with Ld was approved for use in Europe in adult patients with MM who had received at least one prior therapy.11 Daratumumab received FDA approval in November 2016, for use in combination with Ld, or with bortezomib and dexamethasone, in patients who had received at least one prior therapy. In June 2017, it was approved for use, in combination with pomalidomide and dexamethasone, in patients who had received at least two prior therapies, including lenalidomide and a PI.12, 13, 14
The introduction of immunotherapies may lead to improvements in MM treatment outcomes, as agents such as elotuzumab have the potential to induce a long‐term immune response coupled with a durable clinical benefit,15 which reflects a mechanism of action and response kinetics that differ from that of conventional chemotherapeutic regimens.16
This review discusses the novel dual immunotherapeutic mechanism of action of elotuzumab and its associated clinical outcomes.
PATHOPHYSIOLOGY OF MM AND RELATIONSHIP WITH SLAMF7
SLAMF7 structure and expression
SLAMF7 is a member of the SLAM family of receptors, which are involved in cytotoxicity, humoral immunity, autoimmunity, cell survival, cell adhesion, and lymphocyte development.17 SLAMF7 is a cell surface transmembrane molecule (Figure 1).18, 19, 20 The extracellular region consists of two immunoglobulin (Ig) superfamily domains containing several N‐glycosylation sites. The hydrophobic transmembrane region is followed by a cytoplasmic region containing four tyrosine‐based motifs, two of which recruit signaling proteins.18, 19, 20 Using gene expression profiling or anti‐SLAMF7 antibody, SLAMF7 was found to be expressed in malignant hematopoietic cells, normal NK cells, CD8+ T cells, a subset of CD4+ T cells, plasmacytoid dendritic cells, B cells,18, 21 activated monocytes, and mature dendritic cells.21 Normal nonlymphoid tissues tested negative for SLAMF7 expression.21
Figure 1.
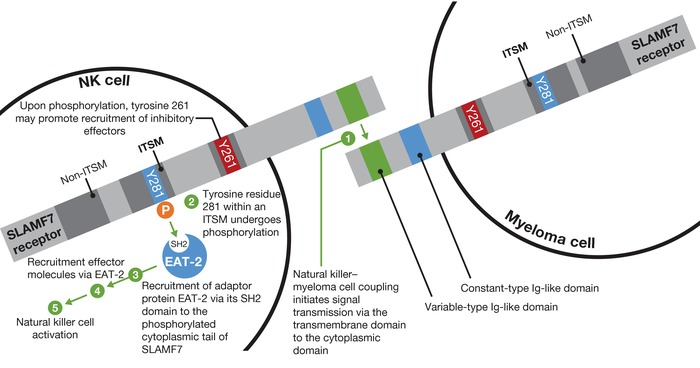
Signaling lymphocytic activation molecule F7 (SLAMF7) structure and signaling mechanism. SLAMF7 is a self‐ligand that recognizes and binds to SLAMF7 on other cells (e.g., myeloma cells can bind to other myeloma cells via SLAMF7 coupling). Natural killer (NK) cell activation after SLAMF7 coupling is dependent on Ewing's sarcoma‐associated transcript 2 (EAT‐2) signaling. Ig, immunoglobulin; ITSM, immunoreceptor tyrosine‐based switch motif; SH2, Src homology 2.
SLAMF7 is highly expressed in myeloma plasma cell samples and in plasma cells from patients with asymptomatic MM (smoldering MM and monoclonal gammopathy of undetermined significance).21 Expression is also maintained in patients who have received prior MM treatment—SLAMF7 gene expression was found to be comparable in previously untreated patients both before and after a single dose of bortezomib.22
ELOTUZUMAB: AN I‐O AGENT THAT DIRECTLY ACTIVATES NK CELLS AND INDUCES ANTIBODY‐DEPENDENT CELLULAR CYTOTOXICITY
Elotuzumab is a humanized, IgG1 anti‐SLAMF7 monoclonal antibody that elicits its effect via a dual mechanism of action: direct activation of NK cells and antibody‐dependent cellular cytotoxicity (ADCC; Figure 2, 23). Using a cytotoxicity assay that measures granzyme B secretion, coupling of elotuzumab to SLAMF7 on NK cells has been shown to cause granzyme B release targeted against myeloma cells,24 a process that is independent from CD16‐mediated ADCC.
Figure 2.
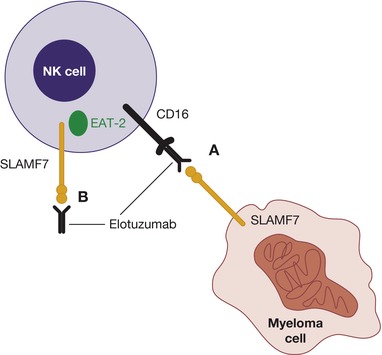
Elotuzumab mechanism of action. Elotuzumab primarily acts by activating natural killer (NK) cell–mediated killing of myeloma cells via antibody‐dependent cellular cytotoxicity (A). Elotuzumab also directly activates NK cells to kill myeloma cells (B). EAT‐2, Ewing's sarcoma‐associated transcript 2; SLAMF7, signaling lymphocytic activation molecule F7. Figure from Lonial, S. et al. Expert Opin. Biol. Ther. 16, 1291–1301 (2016). Reprinted with permission from Taylor & Francis © 2016.
Although NK cell activation and ADCC are the most researched mechanisms of action for elotuzumab, it is likely that elotuzumab exerts additional effects on the immune system via other SLAMF7‐positive cells (e.g., CD8+ T cells, monocytes, and dendritic cells).17, 18, 21, 25 For example, SLAMF7 expression on dendritic cells21 may trigger antitumor cellular immunity. Treatment with elotuzumab has been shown to reduce myeloma tumor burden in a xenograft model with defective NK cells in a CD16‐dependent manner, suggesting that mechanisms other than ADCC and direct activation of NK cells may mediate the antitumor effect of elotuzumab.26 Other hypotheses include the possibility that elotuzumab may inhibit plasmacytoid dendritic cells, which have been implicated in myeloma cell growth and survival.18, 21, 25 Taken together, further preclinical research is warranted to establish whether elotuzumab elicits an effect via mechanisms other than NK cell activation and ADCC.
ELOTUZUMAB MECHANISM OF ACTION DOWNSTREAM OF SLAMF7 SIGNALING
SLAMF7 is a homotypic receptor that recognizes and binds to SLAMF7 on other cells, thus allowing myeloma cells to adhere to other myeloma cells or to conjugate with NK cells. NK cells are involved in the immune defense against transformed cells,27 such as myeloma cells. This process is regulated by their expression of inhibitory or activating receptors. However, in patients with MM, changes in NK cell receptors may enable myeloma cells to evade immunosurveillance.28, 29, 30
Activation of NK cells following SLAMF7 coupling is dependent on the SLAM‐associated protein Ewing's sarcoma‐associated transcript 2 (EAT‐2), which binds to the cytoplasmic domain of SLAMF7 (Figure 1).18, 19, 20 EAT‐2 expression is high in NK cells freshly isolated from blood or cultured in interleukin‐2, and in NK cell lines,20 but it is not expressed by CD4+ T cells, B cells, dendritic cells, or myeloma cells; hence, these cells are not activated by SLAMF7–SLAMF7 coupling.19, 20
The importance of SLAMF7 in mouse NK cell activation has been demonstrated.31 In SLAMF7‐deficient mice, NK cells lacked all cell surface expression of SLAMF7 (confirmed by immunoblot analysis), whereas other SLAM family receptors and SLAM‐associated protein‐related adaptors were unchanged.31 These mice failed to show cytotoxicity towards target cells expressing SLAMF7. EAT‐2 involvement was established by examining the effects of the absence of EAT‐2 on the NK cell‐activating function of SLAMF7. Function was impaired in NK cells isolated from mice with inactivating mutations in EAT‐2.31 Taken together in the presence of EAT‐2, SLAMF7 coupling activates NK cells, but in its absence this activation is lost and NK cell function is inhibited.31
In ADCC, the Fab portion of elotuzumab binds to SLAMF7 on myeloma cells and the Fc portion binds to the Fc receptor CD16 (FcγRIII) on NK cells,31, 32 tagging myeloma cells for ADCC and myeloma cell death via the release of cytotoxic granules.24 ADCC occurs in a dose‐dependent manner against SLAMF7‐expressing cell lines, and in patients with newly diagnosed MM as well as in patients resistant to conventional therapies.33 Blocking the Fc receptor on NK cells with an anti‐CD16 antibody inhibits this action.21
ELOTUZUMAB CLINICAL OUTCOMES
Efficacy
In addition to the preclinical studies on elotuzumab and SLAMF7 described above, the efficacy of elotuzumab in combination with approved agents has been assessed in a number of clinical trials: in combination with Ld (ELd) in the ELOQUENT‐2 study (NCT01239797) and in combination with bortezomib and dexamethasone (EBd) in study 009 (NCT01478048).5, 15, 34, 35 Other ongoing elotuzumab clinical trials are using alternative combinations, such as pomalidomide and dexamethasone (EPd),36, 37 and there are immunotherapies currently in development—for example, elotuzumab in combination with the anti‐KIR antibody lirilumab.6
The phase III ELOQUENT‐2 study assessed ELd vs. Ld alone in patients with relapsed or refractory multiple myeloma (RRMM).5 In the primary analysis, ELd reduced the risk of disease progression or death by 30% vs. Ld (hazard ratio (HR) 0.70; 95% confidence interval (CI) 0.57, 0.85; P < 0.001), with an early separation between the Kaplan–Meier progression‐free survival (PFS) curves that was maintained over time (Figure 3).5 The observed early curve separation may be attributed to the conventional therapy backbone (Figure 4).38 Median PFS was 19.4 months with ELd vs. 14.9 months with Ld; PFS at 1 year was 68% in the ELd arm vs. 57% in the Ld arm; at 2 years, it was 41% vs. 27%, respectively.5 The overall response rate (ORR) was 79% in the ELd arm vs. 66% in the Ld arm (P < 0.001).5
Figure 3.
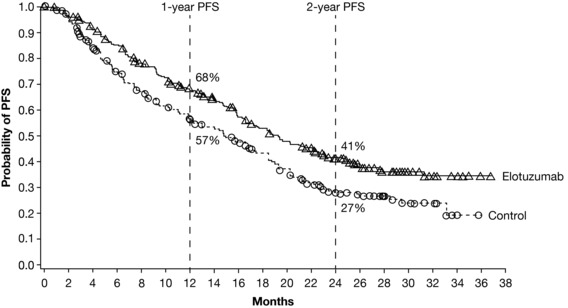
Kaplan–Meier curves showing progression‐free survival (PFS) in patients with relapsed or refractory multiple myeloma receiving elotuzumab plus lenalidomide and dexamethasone (“Elotuzumab”) or lenalidomide and dexamethasone (“Control”). Figure from Lonial, S. et al. Elotuzumab therapy for relapsed or refractory multiple myeloma. N. Engl. J. Med. 373, 621–631. ©2015 Massachusetts Medical Society. Reprinted with permission from Massachusetts Medical Society.
Figure 4.
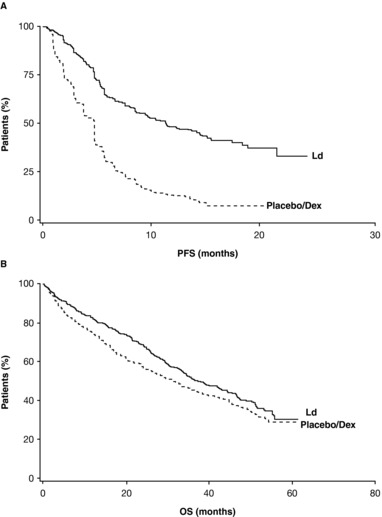
Kaplan–Meier curves showing (A) progression‐free survival (PFS) and (B) overall survival (OS) in patients with relapsed or refractory multiple myeloma receiving lenalidomide (L) and/or dexamethasone (d/Dex). Reprinted with permission from Macmillan Publishers: Dimopoulos, M.A. et al. Leukemia 23, 2147–2152 (2009), ©2009.
I‐O agents exhibit response kinetics defined by the building of a cellular immune response, followed by tumor regression. This is associated with durable clinical benefit that may persist after the therapy is discontinued,16, 39, 40, 41 leading to long‐term survival benefits. Thus, an immunotherapy‐specific approach with end points that reflect the increased durability of response is required for assessment of I‐O therapy outcomes. Such end points may include long‐term follow‐up, timepoint analyses of survival, and HRs over the study duration.16
Extended 3‐year follow‐up of ELOQUENT‐2 demonstrated that ELd reduced the risk of disease progression or death by 27% (HR 0.73; 95% CI 0.60, 0.89; P = 0.0014).15 The separation between the Kaplan–Meier curves for PFS in the primary analysis was maintained in the 3‐year follow‐up, showing long‐term durability of response with ELd vs. Ld alone.5, 15 The 3‐year PFS was 26% and 18% in the ELd vs. Ld arm, respectively, indicating a relative improvement in PFS of 44% at 3 years, while ORR was 79% with ELd and 66% with Ld (P = 0.0002).15 An interim overall survival (OS) analysis demonstrated a strong trend in favor of ELd (HR 0.77; 95% CI 0.61, 0.97; P = 0.0257), in which there was a clear separation in the tail end of the Kaplan–Meier curves (Figure 5).15 Median (95% CI) OS was 43.7 months (40.3, not estimable (NE)) in the ELd arm and 39.6 months (33.3, NE) in the Ld arm.15 The 4‐year PFS data support a sustained durable PFS benefit with ELd in comparison with Ld, showing a relative improvement in PFS of 50% (PFS rates of 21% vs. 14%) and a reduction in the risk of progression or death of 29% (HR 0.71; 95% CI 0.59, 0.86) consistent with prior follow‐up analysis.42 The OS trend in favor of ELd was also sustained at the 4‐year follow‐up (HR 0.78; 95% CI 0.63, 0.96; non‐prespecified OS analysis), with a median (95% CI) OS of 48 months (40.3, 54.4) and 40 months (33.3, 45.4) for ELd and Ld, respectively.42 Long‐term survival follow‐up is still ongoing.
Figure 5.
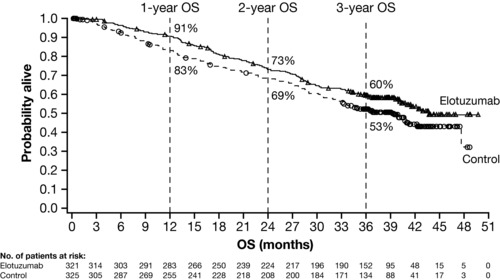
Kaplan–Meier curves showing overall survival (OS) in patients with relapsed or refractory multiple myeloma receiving elotuzumab plus lenalidomide and dexamethasone (“Elotuzumab”) or lenalidomide and dexamethasone (“Control”). Figure from Dimopoulos, M.A. et al. Br. J. Haematol. 178, 896–905 (2017). Reprinted with permission from John Wiley & Sons ©2017.
The extended 3‐year follow‐up of ELOQUENT‐2 also demonstrated that ELd reduces the risk of starting a subsequent line of therapy during follow‐up by 38% (HR 0.62; 95% CI 0.50, 0.77).15 Median (95% CI) time to next treatment (TTNT) was 33 months (26.2, 40.2) with ELd vs. 21 months (18.1, 23.2) with Ld, revealing that ELd‐treated patients had a median delay of 1 year in TTNT vs. Ld‐treated patients,15 which may be indicative of extended PFS and longer OS.
Study 009, a proof‐of‐concept, open‐label, phase II study, assessed EBd or bortezomib and dexamethasone (Bd) alone in patients with RRMM.35 In the primary analysis, the study met the primary end point of PFS: the HR was 0.72 (70% CI 0.59, 0.88; stratified log‐rank P = 0.09), representing a 28% reduction in the risk of disease progression or death.35 In an updated analysis performed 1 year after the primary analysis, ORR (95% CI) was 66% (55%, 77%) in the EBd arm and 63% (51%, 74%) in the Bd arm.35 Early OS results favored EBd, revealing an HR of 0.61 (70% CI 0.43, 0.85).35 Although these data suggest a survival benefit with EBd over Bd, with a tail‐end separation in the Kaplan–Meier curves35 as seen in ELOQUENT‐2,15 it is too early to draw firm conclusions and longer‐term follow‐up is ongoing.
Safety and tolerability
The safety and tolerability of elotuzumab has been shown to be consistent across clinical studies, which demonstrated minimal incremental toxicity with the addition of elotuzumab to established regimens. This may be due to the lack of SLAMF7 expression in normal tissue,21 limiting the toxicity associated with SLAMF7‐targeted therapy with elotuzumab.
The most common adverse events (AEs) of any grade in the ELOQUENT‐2 study were lymphocytopenia (ELd, 99%; Ld, 98%), anemia (ELd, 96%; Ld, 95%), and thrombocytopenia (ELd, 84%; Ld, 78%).5 Infusion reactions (IRs), which are commonly associated with antibody therapy and included pyrexia, chills, and hypertension, were reported in 10% of patients receiving ELd, most of which were Grade 1 or 2 in severity.5 Importantly, safety and tolerability data from the 3‐ and 4‐year extended follow‐up of ELOQUENT‐2 are consistent with the primary analysis.15, 42 In the 009 study, infection (67%), diarrhea (44%), and constipation (40%) were the most common AEs of any grade in the EBd arms, compared with infection (53%), peripheral neuropathy (36%), and diarrhea (33%) in the Bd arm. The rate of IRs in the 009 study was low; only 5% of patients experienced IRs, all of which occurred in the EBd arm and were Grade 1 or 2 in severity.35
Initial data from study 142 (NCT02612779), an ongoing, phase II, multicenter, single‐arm study of EPd in patients with RRMM, indicated that the combination is well tolerated, with a safety profile consistent with ELd.37 Efficacy data from this study will inform therapeutic decisions regarding EPd for patients who experience relapse after, or are refractory to, lenalidomide.
SUMMARY
The data described herein indicate that the I‐O agent elotuzumab, when combined with Ld or Bd, provides a durable and clinically meaningful benefit for patients with RRMM. The clinical efficacy data presented could result from elotuzumab inducing long‐term effects in the immune system, as has been shown with other immunotherapies. Measurable antitumor activity may take longer to appear with immunotherapies, as responses to such therapies may occur after apparent disease progression, and discontinuation of therapy may not always be appropriate.43 Taking these points into consideration, since a clinical response can take longer to become apparent with immunotherapies than with conventional regimens, caution should be exercised when deciding to terminate therapy with an I‐O agent. In the future, it will be important to establish how prior treatment regimens, which may affect immune system function, impact further treatment decisions (i.e., elotuzumab plus anti‐KIR agents or programmed death‐1 inhibitor combination therapies). It is evident that additional analyses such as TTNT should be considered when measuring response to treatment with I‐O agents, as current standards may not take into consideration delayed clinical benefits or the durability of response vs. the fast/deep responses seen with some regimens; these durable responses have been demonstrated with I‐O agents, including elotuzumab.
Conflict of Interest
D.R.: honoraria: Bristol‐Myers Squibb; research funding: Amgen, Bristol‐Myers Squibb, Celgene, Novartis, and Takeda. M.C.: no conflicts of interest to declare.
Supporting information
Supporting information
Supporting information
Supporting information
Supporting information
Author Contributions
Both D.R. and M.C. wrote the article, performed the research, and analyzed the data.
Funding
Professional medical writing and editorial assistance provided by Sarah Addison and Cristina Tomas at Caudex was funded by Bristol‐Myers Squibb.
References
- 1. Siegel, R. , Ma, J. , Zou, Z. & Jemal, A. Cancer statistics, 2014. CA Cancer J. Clin. 64, 9–29 (2014). [DOI] [PubMed] [Google Scholar]
- 2. National Comprehensive Cancer Network . NCCN Clinical Practice Guidelines in Oncology. Multiple Myeloma. Version 3. http://www.nccn.org/professionals/physician_gls/f_guidelines.asp (2016).
- 3. Lonial, S. , Kaufman, J. , Laubach, J. & Richardson, P. Elotuzumab: a novel anti‐CS1 monoclonal antibody for the treatment of multiple myeloma. Expert Opin. Biol. Ther. 13, 1731–1740 (2013). [DOI] [PubMed] [Google Scholar]
- 4. Pratt, G. , Goodyear, O. & Moss, P. Immunodeficiency and immunotherapy in multiple myeloma. Br. J. Haematol. 138, 563–579 (2007). [DOI] [PubMed] [Google Scholar]
- 5. Lonial, S. et al Elotuzumab therapy for relapsed or refractory multiple myeloma. N. Engl. J. Med. 373, 621–631 (2015). [DOI] [PubMed] [Google Scholar]
- 6. ClinicalTrials.gov . A Phase I open label study of the safety and tolerability of elotuzumab (BMS‐901608) administered in combination with either lirilumab (BMS‐986015) or urelumab (BMS‐663513) in subjects with multiple myeloma (study 028) (NCT02252263). https://clinicaltrials.gov/ct2/show/NCT02252263?term=02252263&rank=1 (2017).
- 7. Food and Drug Administration . FDA approves Darzalex for patients with previously treated multiple myeloma. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm472875.htm (2016).
- 8. Food and Drug Administration . FDA approves Empliciti, a new immune‐stimulating therapy to treat multiple myeloma. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm474684.htm (2016).
- 9. Raab, M.S. et al A phase I/IIa study of the human anti‐CD38 antibody MOR202 (MOR03087) in relapsed or refractory multiple myeloma (rrMM). J. Clin. Oncol. 33, 8574 (2015). [Google Scholar]
- 10. Vij, R. et al A phase Ib dose escalation trial of isatuximab (SAR650984, anti‐CD38 mAb) plus lenalidomide and dexamethasone (Len/Dex) in relapsed/refractory multiple myeloma (RRMM): interim results from two new dose cohorts. J. Clin. Oncol. 34, 8009 (2016). [Google Scholar]
- 11. European Medicines Agency . Elotuzumab: Summary of product characteristics. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/003967/WC500206673.pdf (2016).
- 12. Janssen Biotech . Darzalex® (daratumumab) prescribing information. https://www.janssenmd.com/pdf/darzalex/DARZALEX_PI.pdf (2017).
- 13. Bahlis, N.J. et al Daratumumab, lenalidomide, and dexamethasone (DRd) vs lenalidomide and dexamethasone (Rd) in relapsed or refractory multiple myeloma (RRMM): efficacy and safety update (POLLUX). J. Clin. Oncol. 35, 8025 (2017). [Google Scholar]
- 14. Lentzsch, S. et al Daratumumab, bortezomib and dexamethasone (DVd) vs bortezomib and dexamethasone (Vd) in relapsed or refractory multiple myeloma (RRMM): efficacy and safety update (CASTOR). J. Clin. Oncol. 35, 8036 (2017). [Google Scholar]
- 15. Dimopoulos, M.A. et al Elotuzumab plus lenalidomide/dexamethasone for relapsed or refractory multiple myeloma: ELOQUENT‐2 follow‐up and post‐hoc analyses on progression‐free survival and tumour growth. Br. J. Haematol. 178, 896–905 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hoering, A. , Durie, B. , Wang, H. & Crowley, J. End points and statistical considerations in immuno‐oncology trials: impact on multiple myeloma. Future Oncol. 13, 1181–1193 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cannons, J.L. , Tangye, S.G. & Schwartzberg, P.L. SLAM family receptors and SAP adaptors in immunity. Annu. Rev. Immunol. 29, 665–705 (2011). [DOI] [PubMed] [Google Scholar]
- 18. Bouchon, A. , Cella, M. , Grierson, H.L. , Cohen, J.I. & Colonna, M. Activation of NK cell‐mediated cytotoxicity by a SAP‐independent receptor of the CD2 family. J. Immunol. 167, 5517–5521 (2001). [DOI] [PubMed] [Google Scholar]
- 19. Guo, H. , Cruz‐Munoz, M.E. , Wu, N. , Robbins, M. & Veillette, A. Immune cell inhibition by SLAMF7 is mediated by a mechanism requiring Src kinases, CD45, and SHIP‐1 that is defective in multiple myeloma cells. Mol. Cell. Biol. 35, 41–51 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tassi, I. & Colonna, M. The cytotoxicity receptor CRACC (CS‐1) recruits EAT‐2 and activates the PI3K and phospholipase Cgamma signaling pathways in human NK cells. J. Immunol. 175, 7996–8002 (2005). [DOI] [PubMed] [Google Scholar]
- 21. Hsi, E.D. et al CS1, a potential new therapeutic antibody target for the treatment of multiple myeloma. Clin. Cancer Res. 14, 2775–2784 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. van Rhee, F. et al Combinatorial efficacy of anti‐CS1 monoclonal antibody elotuzumab (HuLuc63) and bortezomib against multiple myeloma. Mol. Cancer Ther. 8, 2616–2624 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lonial, S. , Kaufman, J. , Reece, D. , Mateos, M.V. , Laubach, J. & Richardson, P. Update on elotuzumab, a novel anti‐SLAMF7 monoclonal antibody for the treatment of multiple myeloma. Expert Opin. Biol. Ther. 16, 1291–1301 (2016). [DOI] [PubMed] [Google Scholar]
- 24. Collins, S.M. et al Elotuzumab directly enhances NK cell cytotoxicity against myeloma via CS1 ligation: evidence for augmented NK cell function complementing ADCC. Cancer Immunol. Immunother. 62, 1841–1849 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ray, A. et al A novel TLR‐9 agonist C792 inhibits plasmacytoid dendritic cell‐induced myeloma cell growth and enhance cytotoxicity of bortezomib. Leukemia 28, 1716–1724 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Glavey, S. et al Dissecting the mechanisms of activity of SLAMF7 and the targeting antibody elotuzumab in multiple myeloma. Blood 124, 3431 (2014).25267198 [Google Scholar]
- 27. Guillerey, C. & Smyth, M.J. NK cells and cancer immunoediting. Curr. Top. Microbiol. Immunol. 395, 115–145 (2016). [DOI] [PubMed] [Google Scholar]
- 28. Bernal, M. et al Changes in activatory and inhibitory natural killer (NK) receptors may induce progression to multiple myeloma: implications for tumor evasion of T and NK cells. Hum. Immunol. 70, 854–857 (2009). [DOI] [PubMed] [Google Scholar]
- 29. Fionda, C. , Soriani, A. , Zingoni, A. , Santoni, A. & Cippitelli, M. NKG2D and DNAM‐1 ligands: molecular targets for NK cell‐mediated immunotherapeutic intervention in multiple myeloma. Biomed. Res. Int. 2015, 178698 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sarkar, S. et al Optimal selection of natural killer cells to kill myeloma: the role of HLA‐E and NKG2A. Cancer Immunol. Immunother. 64, 951–963 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cruz‐Munoz, M.E. , Dong, Z. , Shi, X. , Zhang, S. & Veillette, A. Influence of CRACC, a SLAM family receptor coupled to the adaptor EAT‐2, on natural killer cell function. Nat. Immunol. 10, 297–305 (2009). [DOI] [PubMed] [Google Scholar]
- 32. Veillette, A. & Guo, H. CS1, a SLAM family receptor involved in immune regulation, is a therapeutic target in multiple myeloma. Crit. Rev. Oncol. Hematol. 88, 168–177 (2013). [DOI] [PubMed] [Google Scholar]
- 33. Tai, Y.T. et al Anti‐CS1 humanized monoclonal antibody HuLuc63 inhibits myeloma cell adhesion and induces antibody‐dependent cellular cytotoxicity in the bone marrow milieu. Blood 112, 1329–1337 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Dimopoulos, M.A. et al ELOQUENT‐2 update: a phase 3, randomized, open‐label study of elotuzumab in combination with lenalidomide/dexamethasone in patients with relapsed/refractory multiple myeloma ‐ 3‐year safety and efficacy follow‐up. Blood 126, 28 (2015). [Google Scholar]
- 35. Jakubowiak, A. et al Randomized phase 2 study of elotuzumab plus bortezomib/dexamethasone (Bd) versus Bd for relapsed/refractory multiple myeloma. Blood 127, 2833–2840 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. ClinicalTrials.gov . An investigational immuno‐therapy trial of pomalidomide and low‐dose dexamethasone with or without elotuzumab to treat refractory and relapsed and refractory multiple myeloma (ELOQUENT‐3). https://clinicaltrials.gov/ct2/show/NCT02654132?term=02654132&rank=1 (2017).
- 37. Jagannath, S. et al Single‐arm, phase 2 study of elotuzumab in combination with pomalidomide and dexamethasone in patients with multiple myeloma who are relapsed/refractory to lenalidomide: initial safety data. Clin. Lymphoma Myeloma Leuk. 17, e127 (2017). [Google Scholar]
- 38. Dimopoulos, M.A. et al Long‐term follow‐up on overall survival from the MM‐009 and MM‐010 phase III trials of lenalidomide plus dexamethasone in patients with relapsed or refractory multiple myeloma. Leukemia 23, 2147–2152 (2009). [DOI] [PubMed] [Google Scholar]
- 39. Marshall, M.A. , Ribas, A. & Huang, B. Evaluation of baseline serum C‐reactive protein (CRP) and benefit from tremelimumab compared to chemotherapy in first‐line melanoma. J. Clin. Oncol. 28(suppl 15), 2609 (2010). [Google Scholar]
- 40. Small, E.J. et al Placebo‐controlled phase III trial of immunologic therapy with sipuleucel‐T (APC8015) in patients with metastatic, asymptomatic hormone refractory prostate cancer. J. Clin. Oncol. 24, 3089–3094 (2006). [DOI] [PubMed] [Google Scholar]
- 41. Wolchok, J.D. et al Guidelines for the evaluation of immune therapy activity in solid tumors: immune‐related response criteria. Clin. Cancer Res. 15, 7412–7420 (2009). [DOI] [PubMed] [Google Scholar]
- 42. Dimopoulos, M.A. et al Phase 3 ELOQUENT‐2 study: extended 4‐year follow‐up of elotuzumab plus lenalidomide/dexamethasone vs lenalidomide/dexamethasone in relapsed/refractory multiple myeloma. Haematologica 102, 167–168 (2017). [Google Scholar]
- 43. Hoos, A. et al A clinical development paradigm for cancer vaccines and related biologics. J. Immunother. 30, 1–15 (2007). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information
Supporting information
Supporting information
Supporting information


