Abstract
The AXR1 gene of Arabidopsis is required for many auxin responses. The highly branched shoot phenotype of mature axr1 mutant plants has been taken as genetic evidence for a role of auxin in the control of shoot branching. We compared the development of lateral shoots in wild-type Columbia and axr1-12 plants. In the wild type, the pattern of lateral shoot development depends on the developmental stage of the plant. During prolonged vegetative growth, axillary shoots arise and develop in a basal-apical sequence. After floral transition, axillary shoots arise rapidly along the primary shoot axis and grow out to form lateral inflorescences in an apical-basal sequence. For both patterns, the axr1 mutation does not affect the timing of axillary meristem formation; however, subsequent lateral shoot development proceeds more rapidly in axr1 plants. The outgrowth of lateral inflorescences from excised cauline nodes of wild-type plants is inhibited by apical auxin. axr1-12 nodes are resistant to this inhibition. These results provide evidence for common control of axillary growth in both patterns, and suggest a role for auxin during the late stages of axillary shoot development following the formation of the axillary bud and several axillary leaf primordia.
In plants, the shoot apex has an inhibitory effect on the development of lateral shoots. The theory that the plant hormone auxin (indole-3-acetic acid [IAA]) is a signal in this apical dominance remains a matter of debate. This theory is based on the effects of auxin application on decapitated plants and the discovery of basipetal auxin transport. The mechanism by which auxin acts is still obscure and is likely to be indirect (see Phillips, 1975; Trewavas, 1981; Cline, 1991, 1994).
The increasing number of mutations or transgenes that affect auxin content, transport, or sensitivity, especially in Arabidopsis, provides a different approach to investigate the role of auxin in apical dominance, and in particular which stages of lateral shoot development it regulates in vivo. Plants expressing the IAA biosynthetic genes from Agrobacterium tumefaciens have high endogenous IAA levels and increased apical dominance (Klee et al., 1987; Romano et al., 1993, 1995). The iaaL gene from Pseudomonas savastanoi, encoding an enzyme that conjugates IAA to Lys, has been transformed into tobacco, resulting in reduced levels of free IAA and reduced apical dominance (Romano et al., 1991).
Several mutants with altered auxin sensitivity have been produced in Arabidopsis. One such locus, AXR1, is defined by an allelic series of recessive mutations. The phenotype of axr1 mutant plants is pleiotropic, with defects in root, hypocotyl, stamen, and stem elongation, vascular development, lateral root formation, and root gravitropism. Leaf morphology is altered and shoot branching is increased at maturity (Estelle and Somerville, 1987; Lincoln et al., 1990). All axr1 tissues examined showed reduced auxin sensitivity in a variety of assays, including rapid gene induction by auxin (Abel et al., 1995; Timpte et al., 1995). This suggests that the AXR1 protein is required for auxin signaling. The AXR1 gene encodes a protein related to the amino-terminal half of ubiquitin-activating enzyme; sequencing of severe mutant alleles such as axr1-12 indicates complete loss of protein function (Leyser et al., 1993).
Although these mutant and transgenic lines suggest that branching is regulated by auxin in vivo, they were only investigated at a cursory level. Altered branching may be an indirect consequence of other auxin-regulated phenotypes. axr1-12 plants produce more branches than the wild type, and are less fertile (Lincoln et al., 1990). There is evidence that developing fruits limit lateral branching (Tamas et al., 1979; Hensel et al., 1994). Therefore, the increased branching in mature axr1 plants may be a consequence of their infertility.
The in vivo role of auxin is further called into question by examining the pattern of lateral shoot development in Arabidopsis. If apically derived auxin acts as an inhibitor of axillary branching, one might expect the lateral buds closest to the shoot apex to be the most repressed, giving a basal-apical (acropetal) sequence of bud growth. Arabidopsis shows this pattern during prolonged vegetative growth, e.g. in late-flowering ecotypes (Grbić and Bleecker, 1996). However, lateral inflorescences develop in an apical-basal (basipetal) sequence after floral transition (Alvarez et al., 1992; Hempel and Feldman, 1994).
To further assess the role of auxin in the regulation of Arabidopsis shoot branching, we compared axillary shoot development in the wild type and axr1-12 to determine the earliest stage when differences can be detected. In addition, we compared the auxin sensitivity of wild-type and axr1-12 lateral inflorescence outgrowth using nodes excised from the primary inflorescence. The axr1-12 mutation does not affect early stages of axillary shoot development; however, it promotes the subsequent growth of axillary shoots initiated by the acropetal and basipetal pattern. It also renders the outgrowth of isolated lateral inflorescences resistant to auxin inhibition. These results suggest a role for auxin in the stages of lateral shoot development following axillary meristem formation.
MATERIALS AND METHODS
Plants for Morphometric and Histological Analyses
Seeds of Arabidopsis (wild type and axr1-12, ecotype Columbia) were sown onto F2 compost (Levington Horticulture, Ipswich, UK) in shallow trays consisting of individual 4- × 4-cm pots (P40, Cookson Plantpak, Maldon, UK), with several seeds per pot. Compost for short-day-grown plants was treated with systemic insecticide (Intercept 70WG, Levington Horticulture) before sowing. After 2 to 5 d of cold treatment at 4°C, trays were transferred to 21°C, 8-h (short) or 16-h (long) photoperiods and watered with tap water. Light intensities were 130 and 50 μmol m−2 s−1 for the short and long photoperiod, respectively. Seedlings were thinned out to one per pot after germination.
Histology
Tissue was fixed overnight in 3% (v/v) formaldehyde/1.25% (v/v) glutaraldehyde in 0.05 m sodium phosphate buffer (pH 7.0), dehydrated in a graded ethanol series, transferred to Histoclear (National Diagnostics, Atlanta), and embedded in Paraplast Plus (Sigma-Aldrich, Poole, UK). Microtome sections (8 μm) were affixed to glass microscope slides covered with adhesive (1% [w/v] gelatin, 13% [v/v] glycerol), dewaxed in xylene, rehydrated in an ethanol series, stained in 0.025% (w/v) aqueous toluidine blue, dehydrated in an ethanol series followed by xylene, and coverslips were mounted with DPX (BDH Laboratory Supplies, Poole, UK).
Excised Node Experiments
Seeds were surface-sterilized for 15 min in 10% (v/v) Chloros bleach (Beveridge, Edinburgh, UK) followed by one wash with 70% (v/v) ethanol and five washes with sterile distilled water, and cold treated in water at 4°C for 3 d. Four individual seeds were then sown into sterile 1-L jars containing 50 mL of ATS medium (0.8% [w/v] agar, 1% [w/v] Suc, and mineral nutrients according to Wilson et al. [1990]). Jars were incubated at 22°C to 27°C in a 16-h photoperiod (50 μmol m−2 s−1) until the primary inflorescence elongated. To prepare split agar plates, 25-mL aliquots of ATS medium were dispensed into 9-cm Petri dishes and allowed to solidify for 45 to 60 min in a laminar flow hood with the lid open. A strip of agar about one-eleventh of the total by weight was then cut out along the diameter of the plate and removed, leaving a gap 6 to 8 mm wide. Dishes were further dried for 30 min. Half-plates containing the synthetic auxin 1-NAA were prepared by adding 100 μL of a 100 μm or 100 μL of a 1 mm 1-NAA stock to give approximate final concentrations of 1 or 10 μm, respectively. The added solution was spread over the agar surface until it was absorbed. Plates were prepared at least 1 d before node excision for diffusion of the applied 1-NAA into the agar layer. Stem sections consisting of a node with a visible axillary bud between parts of the apical and the basal internode and 8 to 15 mm long were excised from elongating primary inflorescences. The length of the bud was measured and the node was placed over the gap of a split plate, with the apical end inserted into one agar half and the basal end into the other. Plates containing up to three excised nodes were incubated in the conditions described above in near-vertical orientation. The lengths of the axillary shoots were measured daily with a metric ruler until d 8 following excision. Lengths were determined from the point where the adaxial side of the subtending petiole inserted into the stem to the tip of the axillary leaves when the bud was still closed, or to the inflorescence apex when the branch had started elongating.
RESULTS
Development of the Branched Inflorescence in Long Photoperiods
To establish when differences in branching between axr1-12 and wild-type plants first arise, the development of their shoot systems was studied over time. Neither wild-type nor axr1-12 rosettes showed visible axillary buds or branches during vegetative growth in long photoperiods. After the transition to reproductive growth, flower buds became visible in the center of the rosettes and the plants started to bolt, as defined by visible elongation of the lowermost internode of the primary inflorescence. The date of bolting was noted for each individual plant. Samples of five to eight plants of each genotype were removed and subjected to morphometric analysis covering a time course of 3-d intervals between 3 and 18 d after bolting. The leaves (excluding the cotyledons) were numbered in order of emergence, and first-order lateral development in each leaf axil was scored into three categories: (a) no axillary bud visible by eye, (b) visible bud, and (c) elongating inflorescence.
For laterals in category (c), inflorescence length was measured and the number of vegetative nodes determined. The presence of visible accessory inflorescences, which may arise between a lateral inflorescence and the subtending leaf in cauline nodes (Talbert et al., 1995), was noted. In total, 40 wild-type and 33 axr1-12 plants were analyzed. Wild-type plants bolted between d 28 and 37 after the end of cold treatment, with a mean ± se of 30.0 ± 0.4 d. axr1-12 plants bolted between d 29 and 35, on average slightly later than wild type (mean 32.0 ± 0.4 d). Wild-type plants produced between 13 and 24 leaves (rosette and cauline) before floral transition, with a mean ± se of 17.0 ± 0.4. With axr1-12, leaf numbers ranged from 16 to 27, with a mean ± se of 23.0 ± 0.5, significantly higher than wild type. Both mean rosette and mean cauline leaf numbers were higher for the mutant. Wild-type plants had on average 3.3 ± 0.2 cauline and 13.7 ± 0.3 rosette leaves. Mutant plants had on average 6.6 ± 0.2 cauline and 16.0 ± 0.4 rosette leaves.
In both the wild type and axr1-12, all of the cauline leaf axils on the primary inflorescence produced a lateral inflorescence. Some of these were clearly elongating on d 3 after the primary inflorescence started bolting and all were elongating by d 6. Due to the higher total cauline leaf number described above, axr1-12 plants had on average twice as many cauline inflorescences as the wild type.
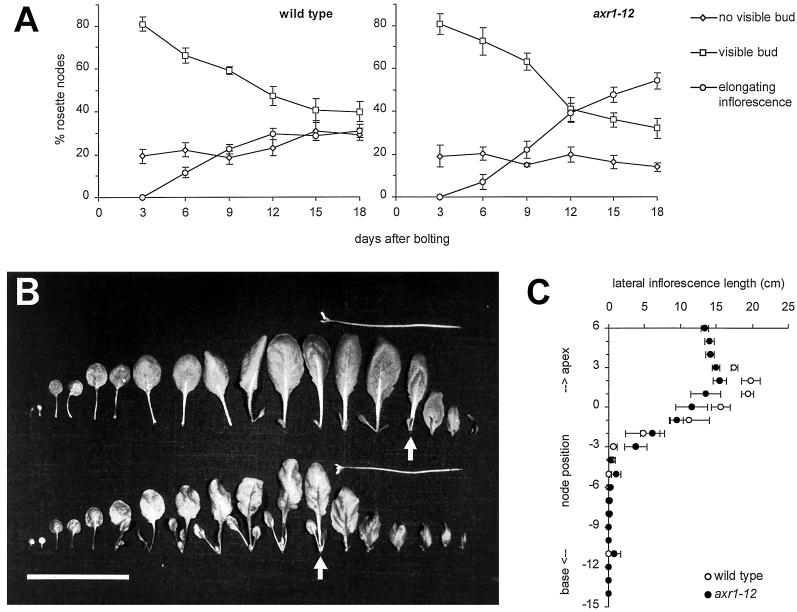
The time course of first-order lateral development in the rosettes of both genotypes is shown in Figure 1A. As the wild type and axr1-12 differed in the number of leaves (i.e. nodes) in the rosette, the three categories of laterals scored were expressed as proportions of the total rosette node number for each individual. In the wild type, the mean proportion of nodes with no visible bud varied between 19% and 31% without a clear trend between d 3 and 18 after bolting. The same was true for axr1-12, except that the percentages were lower (between 14% and 20%). The percentage of wild-type nodes with visible buds decreased from 81% to 40% between d 3 and 18, accompanied by a rise in the percentage of nodes with elongating inflorescences from 0% to 31%. In axr1-12, the proportion of nodes with visible buds decreased from 81% to 33% and the proportion of elongating inflorescences increased from 0% to 54%. The proportion of elongating inflorescences in the rosette was higher in axr1-12 than in the wild type from d 12 after bolting.
Figure 1.
Morphometric analysis of lateral shoot development of wild-type and axr1-12 plants grown in long photoperiods. A, Time course of lateral shoot development in rosette leaf axils. For each genotype, five to eight plants were sampled every 3 d between d 3 and 18 after bolting of the primary inflorescence, and their rosette leaf axils were examined. Lateral shoot development was scored into three developmental stages (no visible bud [⋄]; visible bud [□]; and elongating inflorescence [○]), and the proportion of laterals at each stage calculated for each plant. The mean proportions ± se for each time point are plotted. B, Lateral shoot development at consecutive nodes along the primary shoot axis, 6 d after bolting. For one wild-type (upper row) and one axr1-12 plant (lower row), cotyledons and leaves were dissected from the primary shoot axis with their attached axillary shoots and laid out in the sequence in which they developed (left to right = base to apex). The uppermost rosette leaves are marked by arrows. Bar = 5 cm. C, Mean lateral inflorescence lengths ± se at consecutive nodes along the primary shoot axis 18 d after the primary inflorescence started bolting. Five plants were analyzed for each genotype (wild type [○]; axr 1–12 [●]). Lateral inflorescence lengths for axils without a visible bud or with a vegetative bud were scored as zero. As the mean number of cauline leaves was 3 for wild-type and 6 for axr1-12 plants, plots for wild type were started three node positions basal from those of axr1-12. Thus, node position 0 represents on average the uppermost rosette leaf for both genotypes.
First-order lateral development in the rosettes of both genotypes displayed a basipetal gradient. At the time of bolting, a number of leaves at the base of the rosette had no visible buds in their axils. More apical leaves carried visible buds. During the 18 d after bolting, an increasing number of leaves in the most apical part of the rosette carried an elongating lateral inflorescence.
Figure 1B shows one representative plant for each genotype, dissected 6 d after bolting. Their cotyledons and leaves with attached axillary shoots were laid out in the sequence in which they had been produced by the primary shoot apex. The class “visible buds” in Figure 1A included very small buds with leaves just beginning to expand (which are not visible at the magnification of Fig. 1B), up to buds with several expanded leaves and visible flower buds. Although the proportions of “visible buds” did not differ between the wild type and axr1-12 during the 18 d following bolting (Fig. 1A), genotypes differed in the extent of bud growth as early as 6 d after bolting (Fig. 1B). The zone of nodes carrying buds showing significant leaf expansion, clearly seen at the magnification of Figure 1B, was extended to more basal node positions in axr1-12. Overall, axillary leaves had expanded further in the mutant than in the wild type, which was most obvious in the oldest one to two leaves of each bud, but also in the younger leaves in the center of the buds.
To compare inflorescence outgrowth in the wild type and axr1-12, the average first-order lateral inflorescence lengths were calculated for consecutive node positions along the primary shoot axis, starting with the uppermost cauline leaf node and proceeding basipetally. The distribution of inflorescence lengths is shown in Figure 1C for d 18 after bolting. Lateral inflorescence lengths for axils without a visible bud or with a vegetative bud were scored as zero. As the mean number of cauline leaves was three for wild-type and six for axr1-12 plants, plots for the wild type were started three node positions basal from those of axr1-12. Thus, node position 0 represents on average the uppermost rosette leaf for both genotypes. The wild-type inflorescence length distribution showed a characteristic pattern. Branches at the three most apical nodes (on average corresponding to the cauline nodes on the primary inflorescence) were of similar length. Mean inflorescence lengths then decreased progressively through up to four more basal node positions. No elongating inflorescences were found further toward the base. The inflorescence lengths of axr1-12 plants followed a comparable pattern, but the number of apical inflorescences of similar length was increased to six (corresponding to the mean number of cauline inflorescences in axr1-12) and the zone of decreasing length comprised up to six nodes. The apical-basal gradient of inflorescence length was less steep in the mutant. In apical nodes, the mean axr1-12 inflorescence length was clearly below that of wild type, while in the more basal nodes, the mean inflorescence length was slightly higher than that of the wild type. These inflorescence length distributions of the wild type and axr1-12 were established as early as 6 d after bolting and were maintained while the primary and lateral inflorescences elongated (data not shown).
Lateral inflorescence architecture displayed characteristic differences that were correlated with position along the shoot axis. Apical axillary meristems produced fewer leaves before floral transition than basal meristems. In wild-type plants leaf numbers ranged between two and four for the uppermost cauline inflorescence, and four to five for the lowermost. In axr1-12, the uppermost cauline inflorescences had two or three leaves and the lowermost had between six and 10 leaves. Leaf numbers increased further toward the lowermost rosette inflorescence, which had leaf numbers between nine and 11 for wild type and between 10 and 14 for axr1-12.
Between a lateral shoot and its subtending leaf, an accessory axillary meristem may form and develop into an accessory bud or inflorescence in Arabidopsis (Talbert et al., 1995). Accessory shoots were first seen in the axils of cauline leaves 6 d after bolting, occurring in 6.7% of the 20 wild-type cauline nodes and in 64% of the 39 axr1-12 cauline nodes examined. The percentages increased in both genotypes until d 18 after bolting, but remained clearly lower (27% of 15 nodes) in the the wild type than in axr1-12 (70% of 30 nodes). Thus, differences in accessory development in the two genotypes arose as early as 6 d after bolting. Accessory buds were not detected in rosette leaf axils of either wild type or axr1-12.
Development of Vegetative Lateral Shoots in Short Photoperiods
Our observations with plants grown in long photoperiods show that the axr1-12 mutation affects the growth of lateral shoots developing in the basipetal pattern early after floral transition. The development of lateral shoots initiated by the acropetal pattern was compared by dissecting wild-type and axr1-12 plants (six per genotype) that had undergone prolonged vegetative growth in 49 to 58 short photoperiods. Figure 2 shows one plant of each genotype dissected 49 d after sowing, with the oldest 27 leaves and attached axillary shoots laid out in the sequence of emergence. In the wild type, a small axillary bud not visible at the magnification of Figure 2 was associated with most leaves, and only a few buds scattered along the primary shoot axis showed some leaf expansion. In axr1-12, most buds showed considerable leaf expansion. Mutant bud development displayed a clear acropetal gradient, with bud size increasing toward the base, excluding some of the oldest juvenile leaves, where buds were either small or not visible.
Figure 2.
Lateral shoot development at consecutive nodes along the primary shoot axis in short photoperiods 49 d after the end of cold treatment. For one wild-type (upper row) and one axr1-12 plant (lower row), the oldest 27 leaves (without the cotyledons that had senesced) were dissected from the primary shoot axis with their attached axillary shoots and laid out in the sequence in which they developed (left to right = base to apex). The undissected apical parts of the rosettes are shown on the right. Bar = 5 cm.
Timing of Axillary Shoot Formation
The experiment described above shows that axr1-12 buds in the axils of rosette leaves are further developed than their wild-type counterparts. To determine whether axillary shoots were formed earlier in the mutant, we sectioned plants grown in long and short photoperiods and examined their leaf axils.
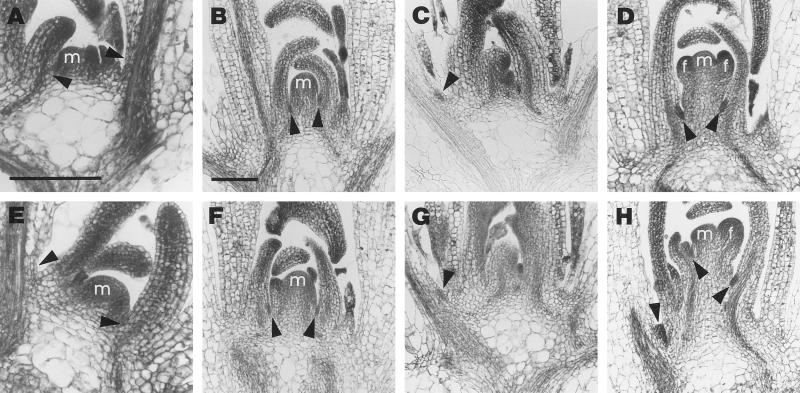
Plants grown in long days were fixed 15 and 20 d after the end of cold treatment, and longitudinal sections of both genotypes were compared. For both genotypes, we found vegetative plants and plants that had recently undergone floral transition among the plants fixed on d 15. Virtually all of the plants fixed on d 20 had undergone floral transition. During vegetative growth, the shoot apical meristem is relatively small and is situated directly on the short primary shoot axis (Vaughan, 1954; Miksche and Brown, 1965; Hempel and Feldman, 1994). For both genotypes, axillary meristems were not detected in the axils of either young or old leaves in vegetative plants (Fig. 3, A and E).
Figure 3.
Longitudinal sections of wild-type (A–D) and axr1-12 (E–H) shoots grown in long photoperiods stained with toluidine blue. Plants in A and C and E to G were fixed on d 15, and plants in B, D, and H were fixed on d 20 after the end of cold treatment. Bar in A (for A and E) and in bar in B (for B–D and F–H) = 100 μm. A and E, Vegetative shoots. m, Vegetative shoot apical meristem. Arrowheads point to the axils of leaf primordia and more mature leaves sectioned in a median plane and lacking axillary meristems. B and F, Shoots at floral transition. Elongation of the primary inflorescence is initiated by cell divisions in the region underlying the inflorescence meristem (m). Arrowheads mark clusters of meristematic cells in the axils of leaf primordia. C and G, Shoots at floral transition. Arrowheads point to meristematic cells at the base of the petiole of more mature leaves. D and H, Shoots whose inflorescence meristems (m) have produced flower primordia (f). Axillary meristematic regions (marked by arrowheads) are of increased size or have started to bulge out.
The early stages of reproductive growth are characterized by enlargement of the shoot apical meristem and by cell divisions in the rib meristem at its base, which is the first step in the formation of the primary inflorescence axis (Vaughan, 1954; Miksche and Brown, 1965; Hempel and Feldman, 1994). In both wild-type and axr1-12 plants at this stage, clusters of very small, strongly staining meristematic cells were observed at the base of young leaf primordia (Fig. 3, B and F), as well as at the base of the petiole of older leaves (Fig. 3, C and G). In plants in which the first flower primordia had been formed by the primary shoot apical meristem, the axillary meristematic zone had increased in size in both genotypes, but axillary leaf formation had not yet started (Fig. 3, D and H). The early stages of axillary shoot formation in axr1-12 (Fig. 3, E–H) were indistinguishable from those of the wild type (Fig. 3, A–D). Accessory axillary meristems at cauline nodes were observed in older plants of both genotypes, fixed at least 25 d after the end of cold treatment, but the frequencies were insufficient for a detailed comparison.
Plants grown in short photoperiods were fixed 37 d after the end of cold treatment, and series of transverse sections through six individual shoots were analyzed for each genotype. Leaves were numbered from apex to base, with 1 being the youngest leaf primordium. In both genotypes, the morphology of axillary shoots observed at consecutive node positions suggested that buds were formed and developed in an acropetal sequence. The axils of a number of the youngest leaf primordia close to the vegetative shoot apical meristem were morphologically indistinguishable from the rest of the leaf primordium. Further basal, the first indication of axillary bud formation was a region of strongly staining meristematic cells at the base of the leaf primordium. This increased in size in leaf primordia further basal until an axillary bud could be distinguished as a zone of meristematic cells bulging out from the petiole of the subtending leaf. Axillary buds at more basal node positions showed increasing numbers of axillary leaf primordia. The most apical node showing axillary cell division, a clear axillary bud, and a bud with at least one leaf primordium was determined for each series of sections (Table I). The mean node positions at which these stages were first observed were not significantly different between the wild type and axr1-12. We also counted axillary leaf primordia at nodes 32, 36, and 40. Node 40 was the oldest node for which an accurate determination could be made. Below this node, axillary buds were often sectioned in an oblique plane and leaf primordia could not be counted. The mean numbers of leaf primordia up to node 40 were slightly but not significantly higher for axr1-12 than for the wild type (data not shown).
Table I.
Early stages of axillary shoot formation in wild-type and axr1-12 plants grown in short photoperiods
| Most Apical Leaf Showing | Wild Type | axr1-12 |
|---|---|---|
| Axillary cell division zone | 16.5 ± 0.4 | 17.3 ± 1.5 |
| Axillary bud | 23.3 ± 0.5 | 23.2 ± 0.8 |
| Axillary bud plus leaf primordium | 29.2 ± 0.7 | 28.7 ± 0.5 |
Data are means ± se of six plants per genotype fixed 37 d after the end of cold treatment.
Thus, the pattern and timing of the early stages of axillary bud formation in axr1-12 was indistinguishable from that of the wild type in both long and short photoperiods.
Auxin Inhibition of Inflorescence Outgrowth from Excised Nodes
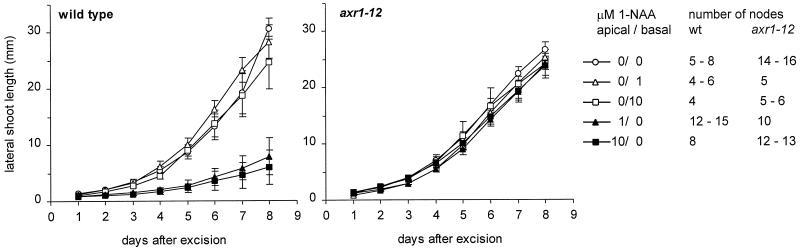
The above observations suggest that the axr1-12 mutation affects lateral shoot growth after axillary meristem formation. To determine whether auxin can regulate lateral shoot growth in Arabidopsis, we studied its effect on the outgrowth of cauline lateral inflorescences. Nodes whose lateral inflorescences had not yet started to elongate were excised from the primary inflorescence of plants grown in sterile conditions and placed with the cut ends of their apical and basal internodes contacting two separate slabs of agar medium in a Petri dish. This allowed us to apply auxin either apically or basally. We used the synthetic auxin 1-NAA in these experiments because of its greater stability compared with the natural auxin IAA. Nodes were excised as soon as internode elongation of the primary inflorescence permitted adjacent internodes of suitable length to be obtained. At this time, buds were typically between 0.5 and 2 mm long. Figure 4 shows results obtained using the lowermost cauline node.
Figure 4.
Effect of the synthetic auxin 1-NAA on lateral inflorescence outgrowth from excised nodes of the wild type and axr1-12. The lowermost cauline node was excised from the elongating primary inflorescence of axenically grown plants and inserted between two separate agar slabs. Slabs contained either no 1-NAA or 1 or 10 μm 1-NAA in contact with the apical or with the basal part of the stem. Mean lateral shoot lengths ± se were determined each day until d 8 following excision. Measurements for some lateral shoots that wilted and ceased elongating after d 5 were included in the earlier time points but were excluded from the later time points. Node numbers tested for each treatment are: ○, 0 μm 1-NAA apical/0 μm 1-NAA basal, 5 to 8 wild type and 14 to 16 axr1-12; ▵, 0 μm 1-NAA apical/1 μm 1-NAA basal, 4 to 6 wild type and 5 axr1-12; □, 0 μm 1-NAA apical/10 μm 1-NAA basal, 4 wild type and 5 to 6 axr1-12; ▴, 1 μm 1-NAA apical/0 μm 1-NAA basal, 12 to 15 wild type and 10 axr1-12; ▪, 10 μm 1-NAA apical/0 μm 1-NAA basal, 8 wild type and 12 to 13 axr1-12.
When excised wild-type nodes were inserted between two slabs of hormone-free agar, the lateral inflorescences elongated and reached a mean length of 5.5 mm on d 4 and 30.6 mm on d 8 after excision (Fig. 4, left). 1-NAA (1 and 10 μm) inhibited elongation when applied to the agar in contact with the apical internode. Elongation was completely inhibited until d 4 after excision. After this time, some buds started to elongate, especially with the lower 1-NAA concentration. 1-NAA (1 or 10 μm) had no effect when contacting the basal internode. When nodes excised from axr1-12 plants (Fig. 4, right), were tested in the absence of auxin or with 1 or 10 μm basal 1-NAA, lateral inflorescence outgrowth showed a time course very similar to that of the wild type under these treatments. However, in contrast to wild type, elongation of axr1-12 inflorescences was not significantly inhibited by apically applied 1-NAA at 1 or 10 μm.
DISCUSSION
To study the regulation of shoot branching, we characterized lateral shoot development in the Columbia ecotype of Arabidopsis grown in long and short photoperiods. Under either photoperiod, the development in Arabidopsis leaf axils follows a similar sequence from empty axil through vegetative axillary shoot to floral shoot. The branching patterns observed in Arabidopsis plants must reflect the rates of initiation and progress between these states. In this study we investigated the role of the AXR1 gene in these events.
Loss of AXR1 Function Does Not Affect Axillary Meristem Formation
In most respects, axillary meristem formation in the ecotype Columbia is identical to other ecotypes of Arabidopsis. In young leaf primordia the axils appear to be completely empty (Hempel and Feldman, 1994; Grbić and Bleecker, 1996; Fig. 3, A and E). The observed origin of the axillary meristem in the subtending leaf (Fig. 3, B, C, F, and G) is in accordance with clonal studies (Furner and Pumfrey, 1992; Irish and Sussex, 1992; Schnittger et al., 1996). A subepidermal origin with the epidermis being recruited later (Fig. 3C) is also consistent with clonal analysis (Schnittger et al., 1996). In contrast to Arabidopsis, the axillary meristems of some other angiosperm species (maize, sunflower) appear to arise in the primary shoot apical meristem above the nascent leaf primordia (Garrison, 1955; Johri and Coe, 1983; McDaniel and Poethig 1988; Jegla and Sussex, 1989; Sussex, 1989).
Studies of Arabidopsis ecotypes other than Columbia indicate that the sequence of axillary shoot formation along the primary shoot axis is acropetal during (prolonged) vegetative growth, but basipetal after floral transition (Hempel and Feldman, 1994; Grbić and Bleecker, 1996). Vegetative ecotype Columbia plants showed a clear acropetal pattern of axillary meristem formation, with detectable axillary cell divisions on average 17 nodes from the shoot apical meristem (Table I). After floral transition in long days, axillary cell divisions were seen all along the shoot axis (Fig. 3). Temporal resolution in our experiment may not have been sufficient to detect the basipetal sequence reported by Hempel and Feldman (1994) and Grbić and Bleecker (1996). In any case, floral transition coincides with a switch from the complete inhibition of axillary meristem development near the shoot apical meristem to the formation of axillary meristems in close proximity to it (from 17 nodes to less than three nodes distance in our experiments). This shows that axillary meristem formation is regulated independently of leaf age.
Early axillary shoot development in axr1-12 was indistinguishable from that of the wild type. In particular, the experiment in short photoperiods suggests that the axillary meristem and the first axillary leaf primordia were formed at a similar rate up to 40 nodes from the shoot apical meristem. Thus, AXR1 is not required to specify either of the opposing patterns of axillary meristem formation or the switch between them. AXR1 is also not involved in regulating the onset of axillary shoot formation in either pattern. Our observations were not sufficient to compare the formation of accessory axillary meristems in detail. They were detected in sections of both genotypes, which does not contradict the hypothesis that their formation is also unaffected by the axr1-12 mutation.
Loss of AXR1 Function Promotes Axillary Shoot Growth after Axillary Meristem Formation
After axillary meristem formation, lateral shoots develop in a manner similar to the primary shoot apical meristem. There appears to be an obligatory vegetative phase in that all of the axillary meristems initiate at least two leaves before undergoing floral transition, even if the primary shoot apical meristem is floral. Our results suggest that axillary shoot growth during these stages is repressed in the wild type, and that repression is relieved by the axr1-12 mutation (Figs. 1 and 2).
In short photoperiods, mutant buds were more advanced than those of the wild type before visible bolting of the primary shoot apex. Furthermore, differences between wild-type and axr1-12 lateral shoot development in long-day-grown plants were detected soon after floral transition and before significant fruit development (data not shown). Therefore, the effects of the axr1-12 mutation on lateral branching cannot just be the result of reduced fertility.
Effects of Loss of AXR1 Function Indicate That Auxin Regulates the Growth of Axillary Shoots after Axillary Meristem Formation
A role of auxin as a signal in apical dominance has been questioned for several species including Arabidopsis, because even high concentrations of auxin did not inhibit lateral outgrowth after decapitation (Cline, 1996). However, with Arabidopsis nodes excised from the primary inflorescence, the outgrowth of cauline inflorescences was completely inhibited for at least 4 d by 1-NAA applied apically at micromolar concentrations (Fig. 4). The lack of response to auxin in buds on decapitated plants in contrast to excised nodes was also observed in bean (Tamas et al., 1989). This could either reflect a variation in sensitivity to auxin or the presence of factors produced in the root that promote bud outgrowth.
Involvement of AXR1 in the control of apical dominance had been proposed by Estelle and Somerville (1987) and Lincoln et al. (1990), based on the bushy phenotype of mature axr1 mutant plants. The AXR1 gene has been isolated (Leyser et al., 1993) and our present understanding of the mechanism of action of the AXR1 protein suggests that it modulates the ubiquitin-mediated degradation of regulatory proteins (del Pozo et al., 1998; Leyser, 1998; Ruegger et al., 1998). Although all of the phenotypes of axr1 loss-of-function mutants may be explained in terms of reduced auxin sensitivity, AXR1 might regulate the degradation of other proteins not involved in the auxin response. However, it has been reported that increased branching conferred by the axr1-3 mutation is epistatic to the IAA-M auxin over-producing transgene (Romano et al., 1995). We show that detectably enhanced lateral growth after axillary meristem formation in vivo correlates with auxin resistance of isolated lateral inflorescences in axr1. This suggests an AXR1-mediated role for auxin in regulating Arabidopsis shoot branching after axillary meristem formation.
No differences in the timing or the pattern of axillary meristem formation between axr1-12 and wild type were detected. This may be because axillary meristem formation is auxin independent. Alternatively, auxin may regulate axillary meristem formation in an AXR1-independent manner. Analogy with yeast signaling systems suggests that the AXR1 protein may increase the efficiency of the degradative pathway, rather than being absolutely required for it (Leyser, 1998). AXR1 may therefore only be necessary for a subset of auxin signaling events, for example, those triggered by low levels of auxin.
The axr1-12 mutation affects axillary shoot growth independently of the prevailing pattern of axillary shoot formation and growth along the shoot axis. This suggests that growth is regulated by a common factor, and the fact that the axr1-12 mutant is auxin resistant suggests that this factor may be auxin. If this is the case, then the switch in the pattern of lateral shoot growth with floral transition might be due to a change in auxin distribution. At floral transition, the primary shoot apex ceases producing leaves and it is these that are thought to be the source of axillary growth inhibition and the site of auxin production rather than the apical meristem itself (White et al., 1975; Weiss and Shillo, 1988). With technical advances in auxin analysis (Uggla et al., 1996), detailed information on the temporal and tissue distribution of auxin in Arabidopsis may be obtained in the future.
Previous studies indicate that auxin does not act directly in the axillary bud, since apically applied radiolabeled IAA is not transported into inhibited buds (Hall and Hillman, 1975; Morris, 1977). To address the question of where auxin acts, we are currently expressing the wild-type AXR1 gene in an axr1-12 mutant background under the control of a variety of tissue-specific promoters. We hope to establish which tissues require the wild-type AXR1 protein to repress axillary shoot growth.
ACKNOWLEDGMENTS
We thank Dr. Voijslava Grbić for helpful discussions, Dr. Karin van de Sande, Dr. Jon Booker, and Stephen Day for critical reading of the manuscript, Megan Stark for photographic work, and the horticultural technicians at the University of York for excellent plant care.
Footnotes
This work was supported by a grant from the Biotechnology and Biological Sciences Research Council of the United Kingdom.
LITERATURE CITED
- Abel S, Nguyen MD, Theologis A. The PS-IAA4/5-like family of early auxin-inducible mRNAs in Arabidopsis thaliana. J Mol Biol. 1995;251:533–549. doi: 10.1006/jmbi.1995.0454. [DOI] [PubMed] [Google Scholar]
- Alvarez J, Guli CL, Yu X-H, Smyth DR. terminal flower: a gene affecting inflorescence development in Arabidopsis thaliana. Plant J. 1992;2:103–116. [Google Scholar]
- Cline MG. Apical dominance. Bot Rev. 1991;57:318–358. [Google Scholar]
- Cline MG. The role of hormones in apical dominance: new approaches to an old problem in plant development. Physiol Plant. 1994;90:230–237. [Google Scholar]
- Cline MG. Exogenous auxin effects on lateral bud outgrowth in decapitated shoots. Ann Bot. 1996;78:255–266. [Google Scholar]
- del Pozo JC, Timpte C, Tan S, Callis J, Estelle M. The ubiquitin-related protein RUB1 and auxin response in Arabidopsis. Science. 1998;280:1760–1763. doi: 10.1126/science.280.5370.1760. [DOI] [PubMed] [Google Scholar]
- Estelle MA, Somerville C. Auxin-resistant mutants of Arabidopsis thaliana with an altered morphology. Mol Gen Genet. 1987;206:200–206. [Google Scholar]
- Furner IJ, Pumfrey JE. Cell fate in the shoot apical meristem of Arabidopsis thaliana. Development. 1992;115:755–764. [Google Scholar]
- Garrison R. Studies in the development of axillary buds. Am J Bot. 1955;42:257–266. [Google Scholar]
- Grbić V, Bleecker AB. An altered body plan is conferred on Arabidopsis plants carrying dominant alleles of two genes. Development. 1996;122:2395–2403. doi: 10.1242/dev.122.8.2395. [DOI] [PubMed] [Google Scholar]
- Hall SM, Hillman JR. Correlative inhibition of lateral bud growth in Phaseolus vulgaris L: timing of bud growth following decapitation. Planta. 1975;123:137–143. doi: 10.1007/BF00383862. [DOI] [PubMed] [Google Scholar]
- Hempel FD, Feldman LJ. Bi-directional inflorescence development in Arabidopsis thaliana: acropetal initiation of flowers and basipetal initiation of paraclades. Planta. 1994;192:276–286. [Google Scholar]
- Hensel LL, Nelson MA, Richmond TA, Bleecker AB. The fate of inflorescence meristems is controlled by developing fruits in Arabidopsis. Plant Physiol. 1994;106:863–876. doi: 10.1104/pp.106.3.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irish VF, Sussex IM. A fate map of the Arabidopsis embryonic shoot apical meristem. Development. 1992;115:745–753. [Google Scholar]
- Jegla DE, Sussex IM. Cell lineage patterns in the shoot meristem of the sunflower embryo in the dry seed. Dev Biol. 1989;131:215–225. doi: 10.1016/s0012-1606(89)80053-3. [DOI] [PubMed] [Google Scholar]
- Johri MM, Coe EH. Clonal analysis of corn plant development. 1. The development of the tassel and the ear shoot. Dev Biol. 1983;97:154–172. doi: 10.1016/0012-1606(83)90073-8. [DOI] [PubMed] [Google Scholar]
- Klee HJ, Horsch RB, Hinchee MA, Hein MB, Hoffmann NL. The effects of overproduction of two Agrobacterium tumefaciens T-DNA auxin biosynthetic gene products in transgenic petunia plants. Genes Dev. 1987;1:86–96. [Google Scholar]
- Leyser HMO, Lincoln CA, Timpte C, Lammer D, Turner J, Estelle M. Arabidopsis auxin-resistance gene AXR1 encodes a protein related to ubiquitin-activating enzyme E1. Nature. 1993;364:161–164. doi: 10.1038/364161a0. [DOI] [PubMed] [Google Scholar]
- Leyser O. Auxin signalling: protein stability as a versatile control target. Curr Biol. 1998;8:R305–R307. doi: 10.1016/s0960-9822(98)70193-9. [DOI] [PubMed] [Google Scholar]
- Lincoln C, Britton JH, Estelle M. Growth and development of the axr1 mutants of Arabidopsis. Plant Cell. 1990;2:1071–1080. doi: 10.1105/tpc.2.11.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDaniel CN, Poethig RS. Cell-lineage patterns in the shoot apical meristem of the germinating maize embryo. Planta. 1988;175:13–22. doi: 10.1007/BF00402877. [DOI] [PubMed] [Google Scholar]
- Miksche JP, Brown JAM. Development of vegetative and floral meristems of Arabidopsis thaliana. Am J Bot. 1965;52:533–537. [Google Scholar]
- Morris DA. Transport of exogenous auxin in two-branched dwarf pea seedlings (Pisum sativum L.) Planta. 1977;136:91–96. doi: 10.1007/BF00387930. [DOI] [PubMed] [Google Scholar]
- Phillips IDJ. Apical dominance. Annu Rev Plant Physiol. 1975;26:341–367. [Google Scholar]
- Romano CP, Cooper ML, Klee HJ. Uncoupling auxin and ethylene effects in transgenic tobacco and Arabidopsis plants. Plant Cell. 1993;5:181–189. doi: 10.1105/tpc.5.2.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romano CP, Hein MB, Klee HJ. Inactivation of auxin in tobacco transformed with the indoleacetic acid-lysine synthetase gene of Pseudomonas savastanoi. Genes Dev. 1991;5:438–446. doi: 10.1101/gad.5.3.438. [DOI] [PubMed] [Google Scholar]
- Romano CP, Robson PRH, Smith H, Estelle M, Klee H. Transgene-mediated auxin overproduction in Arabidopsis: hypocotyl elongation phenotype and interactions with the hy6–1 hypocotyl elongation and axr1 auxin-resistant mutants. Plant Mol Biol. 1995;27:1071–1083. doi: 10.1007/BF00020881. [DOI] [PubMed] [Google Scholar]
- Ruegger M, Dewey E, Gray WM, Hobbie L, Turner J, Estelle M. The TIR1 protein of Arabidopsis functions in auxin response and is related to human SKP2 and yeast Grr1p. Genes Dev. 1998;12:198–207. doi: 10.1101/gad.12.2.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnittger A, Grini PE, Folkers U, Hülskamp M. Epidermal fate map of the Arabidopsis shoot meristem. Dev Biol. 1996;175:248–255. doi: 10.1006/dbio.1996.0112. [DOI] [PubMed] [Google Scholar]
- Sussex IM. Developmental programming of the shoot meristem. Cell. 1989;56:225–229. doi: 10.1016/0092-8674(89)90895-7. [DOI] [PubMed] [Google Scholar]
- Talbert PB, Adler HT, Parks DW, Comai L. The REVOLUTA gene is necessary for apical meristem development and for limiting cell divisions in the leaves and stems of Arabidopsis thaliana. Development. 1995;121:2723–2735. doi: 10.1242/dev.121.9.2723. [DOI] [PubMed] [Google Scholar]
- Tamas IA, Ozbun JL, Wallace DH, Powell LE, Engels CJ. Effect of fruits on dormancy and abscisic acid concentration in the axillary buds of Phaseolus vulgaris L. Plant Physiol. 1979;64:615–619. doi: 10.1104/pp.64.4.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamas IA, Schlossberg-Jacobs JL, Lim R, Friedman LB, Barone CC. Effect of plant growth substances on the growth of axillary buds in cultured stem segments of Phaseolus vulgaris L. Plant Growth Regul. 1989;8:165–183. [Google Scholar]
- Timpte C, Lincoln C, Pickett FB, Turner J, Estelle M. The AXR1 and AUX1 genes of Arabidopsis function in separate auxin response pathways. Plant J. 1995;8:561–569. doi: 10.1046/j.1365-313x.1995.8040561.x. [DOI] [PubMed] [Google Scholar]
- Trewavas A. How do plant growth substances work? Plant Cell Environ. 1981;4:203–228. [Google Scholar]
- Uggla C, Moritz T, Sandberg G, Sundberg B. Auxin as a positional signal in pattern formation in plants. Proc Natl Acad Sci USA. 1996;93:9282–9286. doi: 10.1073/pnas.93.17.9282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan JG. The morphology and growth of the vegetative and reproductive apices of Arabidopsis thaliana (L.) Heynh., Capsella bursa-pastoris (L.) Medic. and Anagallis arvensis L. J Linn Soc Bot. 1954;55:279–301. [Google Scholar]
- Weiss D, Shillo R. Axillary bud inhibition induced by young leaves or bract in Euphorbia pulcherrima Willd. Ann Bot. 1988;62:435–440. [Google Scholar]
- White JC, Medlow GC, Hillman JR, Wilkins MB. Correlative inhibition of lateral bud growth in Phaseolus vulgaris L.: isolation of indoleacetic acid from the inhibitory region. J Exp Bot. 1975;26:419–424. [Google Scholar]
- Wilson AK, Pickett FB, Turner JC, Estelle M. A dominant mutation in Arabidopsis confers resistance to auxin, ethylene and abscisic acid. Mol Gen Genet. 1990;222:377–383. doi: 10.1007/BF00633843. [DOI] [PubMed] [Google Scholar]