Abstract
Hepatic cytochrome P450 (CYP) 2E1 and CYP2A5 activate many important drugs and hepatotoxins. CYP2E1 is induced by alcohol, but whether CYP2A5 is upregulated by alcohol is not known. This article reviews recent studies on the induction of CYP2A5 by alcohol and the mechanism and role of reactive oxygen species (ROS) in this upregulation. Chronic feeding of ethanol to wild type mice increased CYP2A5 catalytic activity and protein and mRNA levels. This induction was blunted in CYP2E1 knockout mice and by a CYP2E1 inhibitor, but was restored in CYP2E1 knockin mice, suggesting a role for CYP2E1 in the induction of CYP2A5 by alcohol. Since CYP2E1 actively generates ROS, the possible role of ROS in the induction of CYP2A5 by alcohol was determined. ROS production was elevated by ethanol treatment. The antioxidants N-acetyl cysteine and vitamin C lowered the alcohol-induced elevation of ROS and blunted the alcohol-mediated induction of CYP2A5. These results suggest that ROS play a novel role in the crosstalk between CYP2E1 and CYP2A5. Alcohol treatment activated nuclear factor erythroid 2 (NFE2)-related factor 2 (Nrf2), a transcription factor which up-regulates expression of CYP2A5. The antioxidants blocked the activation of Nrf2. The alcohol-induced elevation of CYP2A5, but not CYP2E1, was lower in Nrf2 knockout mice. We propose that increased generation of ROS from the alcohol-induced CYP2E1 activates Nrf2, which subsequently up-regulates the expression of CYP2A5. Thus, a novel consequence of the alcohol-mediated induction of CYP2E1 and increase in ROS is the activation of redox-sensitive transcription factors, such as Nrf2, and expression of CYP2A5. Further perspectives on this alcohol-CYP2E1-ROS-Nrf2-CYP2A5 pathway are presented.
Keywords: Alcohol induction, Antioxidants, CYP2A5, CYP2E1, Nrf2, Reactive Oxygen species
1. INTRODUCTION
Cytochromes P450 2E1 (CYP2E1) and 2A5 (CYP2A5) are members of the CYP superfamily which metabolize many important drugs and toxins. For example, CYP2E1 can metabolize alcohol, acetaminophen, benzene, carbon tetrachloride (CCl4), and nitroamines, while CYP2A5 metabolizes nicotine, aflatoxin, and nitrosamines, among others. Both CYP2E1 and CYP2A5 are inducible enzymes. Many different compounds can increase their expression; for example, ethanol and pyrazole can induce CYP2E1, and pyrazole can induce CYP2A5. The ability of ethanol to induce CYP2A5 is a major focus of this review. As discussed below, CYP2E1 has been shown to be an active generator of reactive oxygen species (ROS) and plays an important role in the elevated production of ROS found after acute and chronic ethanol treatment. Nuclear factor erythroid 2 (NFE2)-related factor 2 (Nrf2) is an important transcription factor which upregulates many antioxidant genes and is protective against chemical toxicity and oxidative stress. Nrf2 also promotes the expression and upregulation of CYP2A5. Nrf2 is activated under conditions of elevated ROS. Alcohol can activate Nrf2, at least in part, via CYP2E1-generated ROS, and Nrf2 is protective against alcohol-induced liver injury and fat accumulation.
The goal of this review is to describe the mechanism by which alcohol upregulates the expression of CYP2A5 and explore the roles of CYP2E1, ROS, and Nrf2 in this mechanism. A series of possible future directions to further explore the interactions among the different components and significance of this alcohol-CYP2E1-ROS-Nrf2-CYP2A5 pathway conclude the review.
2. CYP2E1 AND ALCOHOLIC LIVER DISEASE
Cytochromes P450 are a superfamily of hemeproteins that metabolize various endogenous substrates such as steroids and fatty acids, and xenobiotics including drugs, toxins, and carcinogens [1]. CYP2E1 efficiently activates oxygen during consumption of NADPH provided by the NADPH-cytochrome P450 reductase, and this results in the production of ROS [2]. Microsomes from ethanol-treated animals displayed elevated rates of production of ROS and lipid peroxidation (LPO). At the microsomal level, increased generation of ROS by ethanol was thought to be through the induction of CYP2E1, because CYP2E1 exhibited enhanced NADPH oxidase activity as it is poorly coupled with NADPH-cytochrome P450 reductase [3, 4]. Microsomes from ethanol-treated animals, in which CYP2E1 was predominantly induced, displayed elevated rates of generation of hydrogen peroxide and superoxide radical, two major members of ROS. Increases in production of ROS after ethanol treatment were prevented by anti-CYP2E1 IgG, thus linking the generation of ROS to the induction of CYP2E1 [2, 5, 6].
CYP2E1 metabolizes many hepatotoxins to their active toxic forms. Examples include acetaminophen, benzene, CCl4, halogenated hydrocarbons, procarcinogens such as nitrosamines and azo compounds, ethanol, and other alcohols. Toxicity of these compounds is increased by chronic ethanol treatment because of the ethanol induction of CYP2E1 [7–9]. Besides its importance in drug and chemical and alcohol toxicity, CYP2E1 can be induced under a variety of metabolic and nutritional conditions such as diabetes, fasting, obesity, or high fat diets [2, 5, 6].
Many of the hepatic toxic effects of ethanol have been linked to its metabolism by CYP2E1. Studies in rodents with the intragastric ethanol infusion model showed that evident induction of CYP2E1 by chronic alcohol consumption parallels with significant alcoholic liver injury [10–12]. CYP2E1 inhibitors, such as diallyl sulfide (DAS) [13], phenethyl isothiocyanate (PIC) [14, 15], and chlormethiazole (CMZ) [16], blocked hepatic LPO and ameliorated alcohol-induced hepatic pathological changes in rats. Polyenylphosphatidylcholine (PPC), which was effective in suppressing alcohol-induced oxidative stress [17], also had an inhibitory effect on CYP2E1 [18]. Bardag-Gorce et al. [19] found that chronic ethanol-induced liver injury, oxidative stress, and decline in activity of the proteosome were blunted in CYP2E1 knockout (KO) mice.
We applied an oral feeding model instead of the intragastric infusion model to examine the role of CYP2E1 in alcoholic liver disease (ALD) and found that alcoholic steatosis and oxidative stress were only observed in wild type (WT) mice, but not in CYP2E1 KO mice [20]. Importantly, when the human CYP2E1 gene was reintroduced and expressed in CYP2E1 KO mice (humanized CYP2E1 transgenic mice; also known as knockin mice), ethanol-induced steatosis and oxidative stress were again observed [21]. These experiments suggest that CYP2E1 plays an important role in the development of ALD. The above considerations and the biochemical and toxicological properties of CYP2E1 and its contribution to alcohol-induced liver injury have been reviewed in detail [2, 5, 6, 22–24].
In addition to CYP2E1, gut-liver axis-mediated cytokines, such as tumor necrosis factor alpha (TNF-α), are generally considered as contributors to the development of ALD. Chronic alcohol consumption increases gut wall permeability, which results in elevated lipopolysaccharide (LPS) absorption into blood from the lumen of the intestine. In the liver, LPS activates Kupffer cells to produce and release TNF-α, which can subsequently cause liver injury. Pyrazole, a potent inhibitor of alcohol dehydrogenase and of the oxidation of ethanol, is a well characterized inducer of CYP2E1 [25, 26]. We used pyrazole as a CYP2E1 inducer to test whether CYP2E1 synergizes with LPS to enhance liver injury. CYP2E1 was induced by pyrazole in WT mice, but not in CYP2E1 KO mice, and pyrazole/LPS-induced liver injury was more severe in WT mice than in CYP2E1 KO mice, supporting the notion that CYP2E1 synergizes with LPS to enhance liver injury [27].
3. CYP2E1-CYP2A5 INTERACTIONS
Among many CYP2A subfamily members, CYP2A5 is expressed in the liver, kidney, lung, brain, olfactory mucosa, and small intestine, but not in the heart or spleen [28]. CYP2A6, the human ortholog to mouse CYP2A5, is expressed predominantly in the liver [29]. Coumarin, a plant alkaloid, is 7-hydroxylated by coumarin 7-hydroxylase (COH) which is encoded by the cyp2a5 gene in mouse and the cyp2a6 gene in human (for details about CYP2A5, refer to recent reviews [30, 31]). Besides CYP2E1, CYP2A6 was also found to be induced in liver samples from patients with alcoholic and nonalcoholic liver diseases [32]. As will be discussed below, CYP2A5 can be induced by alcohol in mice, and ethanol-mediated induction of CYP2A5 was CYP2E1-dependent; in human livers from ALD or cirrhosis patients, those with higher CYP2E1 expression also had elevated CYP2A6 expression. Thus, CYP2E1 and CYP2A5/6 seem to be interactive during their co-induction by ethanol; however, the mechanisms for these mutual interactions are not known.
As aforementioned, pyrazole is a potent inducer of CYP2E1, it is also an effective inducer of CYP2A5 [33]. While CYP2E1 was induced by pyrazole in WT mice, but not in CYP2E1 KO mice, CYP2A5 induction was also higher in WT mice than in CYP2E1 KO mice, and the constitutive expression of CYP2A5 was lower in CYP2E1 KO mice than in WT mice [27]. CMZ, an inhibitor of CYP2E1, was used in an attempt to exclude the possible effects of CYP2A5 in the pyrazole/LPS liver injury. However, CYP2A5 was also decreased when CYP2E1 was inhibited by CMZ; likewise, CYP2A5 induction by pyrazole was also lower in CYP2E1 KO mice [27]. Thus, CYP2E1 and CYP2A5 may both connect to pyrazole/LPS liver injury as there appear to be interactions between these two CYPs in the development of ALD as discussed below.
4. NRF2 SIGNALING
Nrf2 is a leucine zipper transcription factor which binds to the antioxidant response element (ARE) (also known as electrophile response element) of many genes to upregulate their expression [34–36]. Many of these gene products are involved in drug metabolism, such as CYP1A, CYP2A5, glutathione transferases (GST), drug transporters, alcohol dehydrogenase 7, aldehyde dehydrogenase 3, sulfotransferases, and glucuronyl transferases. Many others are involved in the detoxification and removal of ROS, such as superoxide dismutases (SOD), peroxiredoxins, sulfiredoxin, thioredoxins, glutathione peroxidases, glucose-6-phosphate dehydrogenase, NAD(P)H:quinone oxidoreductase, heme oxygenase 1 (HO-1), glutathione reductase, and the key enzyme in glutathione biosynthesis–gamma-glutamylcysteine synthetase catalytic subunit (GCSC) and regulatory subunit (GCSR) [37]. Activation of Nrf2 plays a major role in resistance to chemical toxicity and oxidative stress. For example, Nrf2 KO mice show increased sensitivity to chemical toxicity and pathologies associated with oxidative stress [38, 39]. Activation of Nrf2 by various chem-oprotective agents, such as sulforaphane, tert-butyl hydroquinone, and butylated hydroxyl anisole, affords protection against oxidative stress-induced toxicity [40]. Under basal cellular conditions, Nrf2 activation and translocation to the nucleus are suppressed by its binding to Keap1 (Kelch-like erythroid cell derived protein with CNC homology-associated protein 1). Modification of thiols of Nrf2 and/or Keap1 by oxidants and electrophiles dissociates Nrf2 from Keap1, followed by degradation of Keap1 by the proteasome complex and translocation of Nrf2 into the nucleus where it can dimerize with small Maf proteins to function as a transcription factor [34, 37]. Several recent reports have described the utility of Nrf2 in protection against liver diseases including alcohol and non-alcohol-induced steatohepatitis, liver fibrosis, hepatic iron toxicity, and acetaminophen-induced hepatotoxicity [38, 39, 41].
5. ETHANOL/CYP2E1-NRF2 INTERACTIONS
CYP2E1 was found to promote an initial upregulation of antioxidant defense in liver cells. Levels of reduced glutathione (GSH), catalase, GST, and HO-1 were elevated in HepG2 E47 cells which express CYP2E1 compared to the control C34 cells which do not express CYP2E1 [42, 43]. The upregulation of these antioxidant enzymes in the E47 cells was blunted by antioxidants including N-acetyl-L-cysteine (NAC) as well as by inhibitors of CYP2E1, which leads to the proposal that ROS generated from CYP2E1 in the E47 cells leads to the upregulation of these antioxidants [44, 45]. Similar results were obtained with hepatocytes isolated from rats with elevated levels of CYP2E1 [45]. These studies suggested that upregulation of antioxidant enzymes was an initial adaptive response of liver cells to the oxidative stress and toxicity promoted by CYP2E1.
Nrf2 regulates the expression of these antioxidant enzymes found to be elevated in liver cells with high expression of CYP2E1, and indeed Nrf2 was activated in the cells with high CYP2E1 expression [46, 47]. The increases in Nrf2 activation were blocked by lowering levels of ROS and by inhibitors of CYP2E1, thus implicating that CYP2E1-derived ROS contributed to the upregulation of Nrf2 in the liver cells. Therefore, the upregulation of Nrf2 in response to CYP2E1-derived ROS in the liver cells may result in elevated antioxidant defense and protection against CYP2E1 toxicity in these cells.
Treatments that elevated CYP2E1 levels in vivo also increased liver Nrf2 levels. Pyrazole is a well-known inducer of CYP2E1 [25]. Administration of pyrazole to rats and to mice elevated levels of hepatic CYP2E1 by about 2.5–3 fold. This was accompanied by a comparable increase in Nrf2 protein levels. Chronic ethanol feeding to rats and mice elevated CYP2E1 protein levels and catalytic activity, and these increases were accompanied by two-fold increases in Nrf2 protein and mRNA levels [46, 47].
Fetal alcohol spectrum disorder is a major birth defect caused by maternal intake of alcohol during pregnancy. Ethanol-induced oxidative stress has been implicated in the toxic actions of alcohol to the fetus [48]. This has led to investigation in vivo or in vitro as to whether activation of Nrf2 is protective against alcohol-induced fetal toxicity. In vivo, consumption of ethanol by the mother resulted in an elevated level of Nrf2 protein in mouse embryos [49]. This increase in Nrf2 elevated downstream targets of Nrf2 such as the antioxidant enzymes SOD, catalase, and glutathione peroxidase. Further elevation of Nrf2 levels and of these downstream targets by the Nrf2 inducer, 3H-1,2-dithiole-3-thione, lowered levels of ROS and protected the embryos against alcohol-induced apoptosis and toxicity. In vitro, addition of sulforaphane to neural crest cells activated Nrf2, followed by increases in Nrf2-regulated antioxidant enzymes [50]. As a result, ethanol-induced oxidative stress and apoptosis to the neural crest cells were prevented. In contrast, treating these cells with Nrf2 siRNA sensitized them to the effects of ethanol [50]. Ethanol caused an increase in Nrf2 protein levels in primary cortical neurons; however, GSH levels still declined, and apoptosis and cell death occurred [51]. Knockdown of Nrf2 exaggerated the decline in GSH and the occurrence of apoptosis caused by ethanol. Over-expression of Nrf2 via adenoviral delivery prevented the loss of GSH and the occurrence of apoptosis caused by ethanol in the neurons [51]. These and other studies show that upregulation of Nrf2 and antioxidant genes may be effective approaches to affording protection against alcohol actions in the embryo and brain cells.
Sulforaphane was recently shown to blunt binge ethanol-induced fatty liver in vivo and in vitro [52]. In an acute binge mouse model of alcohol administration, sulforaphane administered along with the binge alcohol lowered the alcohol-mediated elevation of oxidative stress in association with activation of Nrf2 and increased levels of GSH and HO-1. These changes prevented the increases in liver triglyceride levels. In vitro, addition of sulforaphane to HepG2 E47 cells activated Nrf2, elevated GSH levels, and lowered the increases in ROS produced when ethanol was added to these CYP2E1-expressing cells. The ethanol-induced increases in cellular triglycerides and lipid droplets were also decreased by sulforaphane addition. Thus, activation of Nrf2 by sulforaphane both in vivo and in vitro proved to be effective in protecting against ethanol-induced fat accumulation in liver cells [52].
Expression of Nrf2 was shown to protect against alcohol-induced liver injury in a study in which WT and Nrf2 KO mice were fed an ethanol-containing diet [53]. Increased mortality, fat accumulation, and liver injury occurred in the ethanol-fed Nrf2 KO mice compared to control diet-fed Nrf2 KO mice and ethanol-fed WT mice. In an interesting study, ethanol was acutely administered to WT mice, Nrf2 KO mice, Keap1 knockdown mice with elevated Nrf2, and hepatocyte Keap1 KO mice with maximal Nrf2 levels [54]. Ethanol-induced liver injury, steatosis, oxidative stress, and changes in mitochondrial GSH levels paralleled the Nrf2 levels as fat accumulation, hepatocyte damage, and lipid peroxidation were highest in the Nrf2 KO mice, intermediate in the WT mice, and lowest in the mice with high levels of Nrf2 due to the decline in Keap1. These studies clearly demonstrate that Nrf2 activation is effective in blunting ethanol-induced oxidative stress, fat accumulation, and liver injury [54]. Besides these in vivo studies, Nrf2 was protective against hepatotoxicity in vitro. For example, the flavonoid quercetin protected human hepatocytes against ethanol toxicity, and this protection was mediated via quercetin activating Nrf2 translocation into the nucleus, followed by induction of HO-1 [55]. As discussed below, another role of Nrf2 in ethanol/CYP2E1 actions is the induction of CYP2A5 by ethanol.
6. INDUCTION OF CYP2A5 BY ACUTE AND CHRONIC ETHANOL TREATMENT
Levels of several CYPs, notably, CYP2E1 and CYP2A6, were found to be increased in livers of patients with either alcoholic or non-alcoholic liver disease [32]. In vitro studies showed that ethanol treatment up-regulated CYP2A6 expression in human U937 monocytes [56]. Does ethanol induce CYP2A5? When drinking water containing ethanol was fed to mice, CYP2A5 was not induced [57]. We initiated studies to evaluate and characterize whether acute and/or chronic ethanol treatment can induce hepatic CYP2A5 and investigated the possible mechanisms for such an induction. Considering the well-known inducibility of CYP2E1 by ethanol, we were especially interested in knowing whether CYP2E1 and CYP2E1-derived ROS play a role in the actions of ethanol to induce CYP2A5. Results presented below are summarized from our previously published studies [58, 59].
6.1. Chronic Ethanol Induction of CYP2A5
After one week of ethanol feeding, CYP2E1 protein levels and catalytic activity (with para-nitrophenol as a substrate) were elevated 2–3 fold as compared to the dextrose controls, and this level of increase was maintained over the 3-week feeding period. CYP2A5 protein and catalytic activity (coumarin hydroxylation) were not altered after one week of ethanol feeding, relative to the pair-fed dextrose controls. However, both CYP2A5 protein and activity doubled by two weeks of ethanol feeding, and both were elevated about 4-fold after 3 weeks of feeding. Induction of CYP2E1 by chronic ethanol is largely via a posttranscriptional mechanism involving stabilization of the CYP2E1 protein by the ethanol substrate/ligand against proteasome-mediated degradation [60, 61], and this was confirmed in our further studies as CYP2E1 mRNA levels were not altered under conditions in which CYP2E1 protein and activity increased. However, at 3 weeks, ethanol did increase CYP2A5 mRNA levels approximating the 4-fold increase in CYP2A5 protein. Thus, while chronic ethanol feeding increases two important CYPs in the liver, the mechanism of induction for each CYP appears to differ. Interestingly, those zones of the liver from the ethanol-fed mice with the highest levels of CYP2E1 as detected immunohistochemically also contained the highest amounts of CYP2A5, i.e., CYP2E1 and CYP2A5 co-localized in the liver. With respect to the human liver, samples of human livers were obtained from our Medical School’s Department of Pathology and assayed for levels of CYP2E1 and CYP2A6 (the human ortholog of mouse CYP2A5) by immunoblots. One sample of liver was from a control healthy liver, 2 samples were from patients with ALD, and 4 samples were from patients with liver cirrhosis. The levels of both CYPs varied widely from liver to liver, e.g., CYP2E1 was elevated in one of the two livers from patients with ALD and in two of the four livers from patients with cirrhosis. The levels of CYP2A6 paralleled the levels of CYP2E1, being elevated in the one ALD patient with the higher CYP2E1 and the two cirrhotic patients with the higher CYP2E1. Those livers with lower CYP2E1 content also had lower CYP2A6 content. These studies suggest a close association between the induction of CYP2E1 and the induction of CYP2A5/2A6.
To examine the possible association between CYP2E1 and CYP2A5 more directly, we determined if chronic ethanol feeding induced CYP2A5 in CYP2E1 KO mice. These mice were developed by Dr. Frank Gonzalez’s group at the U.S. National Cancer Institute (NCI) [62] to allow evaluation of the role of CY2E1 in the biochemical and toxicological actions of ethanol. Whereas 3 weeks of ethanol feeding elevated CYP2A5 protein, mRNA, and activity by 4-fold in WT mice, there was no increase in CYP2A5 protein, mRNA, or activity in the CYP2E1 KO mice; basal levels of CYP2A5 and activity in the dextrose-treated mice were similar in the WT and the KO mice. Thus, failure to induce CYP2A5 in the CYP2E1 KO mice implicates a role for CYP2E1 in the induction of CYP2A5 by chronic ethanol feeding. These experiments were repeated in humanized CYP2E1 knockin (KI) mice, in which the human CYP2E1 is added back into the CYP2E1 KO mice to reestablish expression of human CYP2E1 and ascertain whether the absence of an activity in the CYP2E1 KO mice can be restored when CYP2E1 is restored [63]. Indeed, feeding the CYP2E1 KI mice ethanol chronically for 3 weeks resulted in an induction of CYP2A5 protein, mRNA, and catalytic activity. Ethanol elevated CYP2E1 protein and activity (but not mRNA) in the WT and the KI mice. These results indicate that while CYP2A5 is constitutively expressed in the absence of CYP2E1, induction of CYP2A5 by chronic ethanol requires CYP2E1.
To further validate the role of CYP2E1 in the induction of CYP2A5 by chronic ethanol feeding, the effect of CMZ, an effective chemical inhibitor of CYP2E1, was determined. CMZ has been previously used to block ethanol-induced liver pathology in the intragastric infusion model of chronic ethanol feeding [16] and to block ethanol-induced steatosis and oxidant stress in the Lieber-DeCarli liquid ethanol diet model [20]. Treatment with CMZ decreased the chronic ethanol-mediated induction of CYP2E1 and CYP2A5 comparably. Thus, either genetic knockout of CYP2E1 or chemical inhibition of CYP2E1 blocks ethanol-mediated induction of CYP2A5.
6.2. Acute Ethanol Induction of CYP2A5
We evaluated whether an acute ethanol induction model for CYP2A5 could be developed using the CYP2E1 KI mice with higher levels of CYP2E1 than the WT mice. CYP2E1 KI and KO mice were fed the Lieber-DeCarli liquid diet for 1, 2, and 3 days. CYP2E1 catalytic activity and protein level increased about 2- and 3- fold after 1 and 3 days of the ethanol feeding, respectively, in the KI mice. No CYP2E1 activity or protein was found in the CYP2E1 KO mice. With respect to CYP2A5, there was little increase in the catalytic activity or protein levels after 1 day of the ethanol feeding, but both activity and protein levels were increased 3- and 5-fold in the KI mice after 2 and 3 days of ethanol feeding, respectively. No such increases were found in the CYP2E1 KO mice. Thus, acute ethanol feeding can induce CYP2A5 when levels of CYP2E1 are high, and this induction occurs after CYP2E1 is initially and sufficiently elevated.
6.3. Mechanism of Induction of CYP2A5 by Ethanol and the Potentiation by CYP2E1
6.3.1. Role of ROS
Mechanistic studies initially focused on the possible role of ROS in the potentiation of ethanol-mediated induction of CYP2A5 by CYP2E1 since CYP2E1 produces ROS and contributes to the oxidative stress produced by acute and chronic ethanol treatment. CYP2E1 KI mice were fed with ethanol acutely for 2 days in the presence and absence of the antioxidants NAC or vitamin C. The acute ethanol treatment increased production of thiobarbituric acid-reactive substances (TBARS) as a reflection of LPO and caused a decline in hepatic levels of GSH, indices suggestive of enhanced oxidative stress. The treatments with either NAC or vitamin C prevented the increases in TBARS and the decline in GSH, thus blocking the acute ethanol-elevated oxidative stress. The acute ethanol feeding elevated the activity of both CYP2E1 and CYP2A5 and the protein levels of both CYPs about 3-fold. Treatment with the antioxidants blunted the increase in CYP2A5 activity by about 65–70% and also lowered the increase in CYP2A5 protein. However, the antioxidants had no effect on the induction of CYP2E1 activity or protein by the acute ethanol treatment. Thus, the induction of CYP2A5, but not CYP2E1, by acute ethanol involves, at least in part, a role for ROS.
Similar results were observed for chronic ethanol induction of CYP2A5. WT mice were fed ethanol for 3 weeks in the presence and absence of either NAC or vitamin C (given once daily via intraperitoneal injection). The chronic ethanol feeding elevated TBARS and lowered liver GSH, and these effects were prevented by the administration of either of the 2 antioxidants. CYP2E1 and CYP2A5 activity and content were increased 3-fold by the chronic ethanol feeding. Treatment with NAC or vitamin C almost totally blocked the induction of CYP2A5 by the chronic ethanol treatment. Similar to the acute ethanol model, the induction of CYP2E1 by chronic ethanol was not affected by the antioxidants.
6.3.2. Role of Nrf2
Expression of CYP2A5 and CYP2A6 are regulated by the redox-sensitive transcription factor Nrf2 [64–66], which can be elevated by pyrazole and ethanol, agents that increase CYP2E1 and ROS [46, 47]. In view of the induction of CYP2E1 by ethanol, the increase in ROS by ethanol, the blocking of ethanol-mediated induction of CYP2A5 by antioxidants, and the critical role of Nrf2 in regulating the expression of the cyp2a5 gene, a hypothesis was formulated in which ethanol-mediated induction of CYP2E1 leads to an increase in ROS, which then leads to the activation of Nrf2, which subsequently activates expression of CYP2A5. We evaluated whether the acute and chronic ethanol treatments activated Nrf2 and the role of ROS and CYP2E1 in such activation. One day treatment of CYP2E1 KI mice with ethanol resulted in an increase in Nrf2-DNA binding and hepatic Nrf2 levels and of nuclear levels of Nrf2, which were further elevated after 2 and 3 days of ethanol feeding. No such increases were found with the CYP2E1 KO mice. Thus, CYP2E1 was required for acute ethanol activation of Nrf2. The acute ethanol activation of Nrf2 was blocked when the KI mice were treated with NAC or vitamin C, indicating that CYP2E1-generated ROS are important in the acute ethanol activation of Nrf2. Similar results were found for the chronic ethanol feeding model as Nrf2 was activated in WT mice fed ethanol for 3 weeks. This activation was not found in the ethanol-fed CYP2E1 KO mice. Feeding ethanol chronically along with either NAC or vitamin C blunted activation of Nrf2. The induction of CYP2A5, but not CYP2E1, by either acute or chronic ethanol treatment was prevented under these conditions when activation of Nrf2 was blocked.
As discussed above, alcohol-induced liver injury was enhanced in Nrf2 KO mice [53]. The induction of CYP2A5 by chronic ethanol was determined in liver samples from Nrf2 KO mice kindly provided by Dr. Arndt Vogel [53]. Induction of CYP2A5 catalytic activity was lower in the Nrf2 KO mice (about 4-fold) as compared to the WT mice (about 9-fold), whereas induction of CYP2E1 was identical; the latter confirmed the results of Lamle et al. [53]. Thus, maximal induction of CYP2A5, but not CYP2E1, requires Nrf2.
7. CYP2A5 AND ALD
The experiments described above indicate that acute and chronic ethanol induction of CYP2A5 is strongly potentiated by CYP2E1. Is ethanol-mediated induction of CYP2E1 influenced by CYP2A5? Is there a role for ethanol-induced CYP2A5 in the development of ALD? To answer these questions, CYP2A5 KO mice were applied. The CYP2A5 KO mice were provided as a generous gift from Dr Xinxin Ding (SUNY College of Nanoscale Science and Engineering, Albany, NY, USA) [67]. These mice were fed ethanol chronically along with their genetic control WT mice. As expected, the ethanol feeding elevated CYP2A5 protein and activity in the WT mice, but not in the CYP2A5 KO mice. However, the ethanol feeding produced a comparable increase in CYP2E1 protein and catalytic activity in both the WT and the CYP2A5 KO mice as compared to dextrose-fed controls. Thus, the increase in CYP2E1 by ethanol is independent of the presence of CYP2A5, in contrast to the increase in CYP2A5 by ethanol which requires CYP2E1.
To examine the role of ethanol-induced CYP2A5 in the development of ALD, liver injury was evaluated. Our results suggest that in contrast to CYP2E1, CYP2A5 protects against, rather than promotes, the development of ALD. After ethanol feeding, serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST) were increased in CYP2A5 KO mice to a greater extent than in WT mice, and consistently, necroinflammation and steatosis were observed in liver sections upon hematoxylin and eosin staining and were more severe in CYP2A5 KO mice than in WT mice. Although hepatic GSH was decreased comparably in CYP2A5 KO mice and WT mice, TBARS, a marker of LPO, was increased by ethanol feeding more than 2-fold in CYP2A5 KO mice, but it was increased only 50% in WT mice [68]. Formation of 4-hydroxy-2-nonenal adducts (HNE) and 3-nitrotyrosine adducts (3-NT), markers of oxidative stress, were detected in liver sections from CYP2A5 KO mice to a greater extent than in those liver sections from WT mice [68]. These results suggest that CYP2A5 induction lowers alcohol-induced liver injury and oxidative stress, which is not surprising considering the facts that ethanol-mediated induction of CYP2A5 is regulated by the Nrf2 pathway and CYP2E1 up-regulates antioxidant genes. Thus, the ethanol-induced CYP2A5 may be among the panel of Nrf2-regulated antioxidant genes to protect against ALD.
Alcohol consumption can also induce liver fibrosis. Can CYP2A5 protect against liver fibrogenesis? Administration of CCl4 and thioacetamide (TAA) are two major models for the development of liver fibrosis. Interestingly, TAA induces CYP2A5 in the liver [69], but CCl4 has no inductive effect on CYP2A5 [70]. We found that TAA-induced liver fibrosis was enhanced in CYP2A5 KO mice while CCl4-induced liver fibrosis was comparable in WT and CYP2A5 KO mice [71]. These results suggest that CYP2A5 protects against TAA-induced liver fibrosis, but has no effect on CCl4-induced liver fibrosis.
8. CONCLUSIONS AND PERSPECTIVES
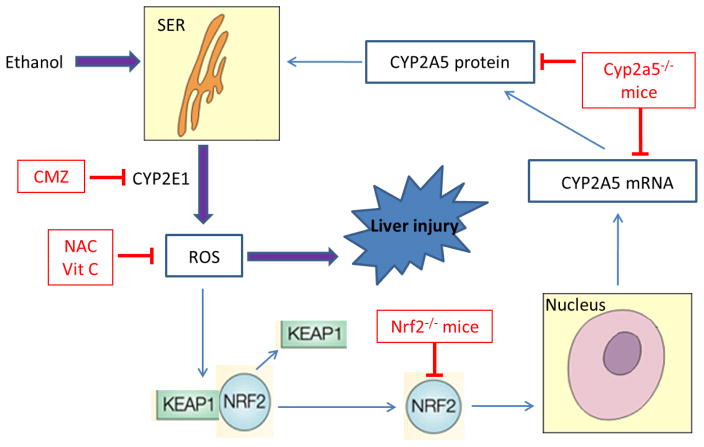
Based on the requirement for CYP2E1 for effective induction of CYP2A5 and Nrf2 by acute and chronic alcohol treatment, the requirement for Nrf2 for maximal induction of CYP2A5, and the prevention by antioxidants of this induction of Nrf2 and CYP2A5, the scheme shown in Figure 1 is proposed to reflect a mechanism for interactions among alcohol, CYP2E1, ROS, and Nrf2 in promoting the induction of CYP2A5. We suggest that increased generation of ROS produced from the induction of CYP2E1 by acute or chronic alcohol treatment activates Nrf2, and once activated, Nrf2 transcriptionally activates CYP2A5. While the increase in CYP2E1 by alcohol has generally been studied from either a drug metabolism point of view or a toxicological point of view, CYP2E1-generated ROS can also modulate transcription factor activity and thereby regulate expression of various genes. As summarized in this review, it appears novel that another consequence of the increase in CYP2E1-generated ROS and activation of Nrf2 is the induction of another member of the cytochrome P450 family, CYP2A5.
FIGURE 1. The Schema of hypotheses.
Ethanol causes proliferation of smooth endoplasmic reticulum (SER), which contains CYP2E1 and CYP2A5. CYP2E1-mediated ROS may activate redox sensitive transcription factor Nrf2, which in turn up-regulates CYP2A5. ROS derived from CYP2E1 contribute to liver injury. Vit C denotes vitamin C; for others in the figure, refer to the Abbreviations section.
Several directions for future research which may develop from these studies are the following:
Alcohol can cause an increase in levels of heme, and free heme can catalyze the production of ROS through the Fenton chemistry [72, 73]. Alcohol increases HO-1 levels, and this enzyme causes degradation of heme with the production of iron, carbon monoxide, and the bile pigments biliverdin and bilirubin [72]. Recently, bilirubin was identified as an endogenous substrate for CYP2A5, and CYP2A5 can function as a hepatic bilirubin oxidase to oxidize bilirubin to biliverdin [74, 75]. Both HO-1 and CYP2A5/6 are regulated by Nrf2, and it is possible that HO-1-mediated induction of bilirubin production, and CYP2A5 could control the intracellular levels of bilirubin [75].
Is alcohol a ligand or a substrate for oxidation by CYP2A5? Many substrates for cytochrome P450s induce their own metabolism via increasing the levels of the CYP enzyme which metabolizes that substrate. Alcohol induces CYP2E1 and binds to CYP2E1 producing a modified type II binding spectrum and being oxidized to acetaldehyde [7]. Could the alcohol induction of CYP2A5 reflect a fact that alcohol serves as a metabolic substrate for CYP2A5? This can be readily addressed using isolated microsomes expressing only CYP2A5 or microsomes from CYP2A5 KO mice or studying the oxidation of ethanol to acetaldehyde in the presence and absence of inhibitors of CYP2A5 or inhibitory CYP2A5 antibodies. Alternatively, ligands for CYP2E1, like alcohol, can increase the half-life of CYP2E1 protein by stabilizing the protein against proteasome-mediated degradation [60, 61]. Although, unlike CYP2E1, CYP2A5 is elevated by alcohol in mRNA levels suggesting transcriptional upregulation, the effect of alcohol as a possible ligand on the stability and half-life of CYP2A5 warrants investigation.
Does alcohol induce human CYP2A6, and if it does, is the mechanism similar to the alcohol induction of CYP2A5? CYP2A6, like CYP2A5, has been shown to be regulated by Nrf2 [66]. As mentioned above, there appears to be an association between levels of CYP2A6 and CYP2E1 in human livers from patients with ALD and with cirrhosis. Perhaps such livers can be assayed for levels of Nrf2. It is unclear whether incubation of human hepatocytes with ethanol causes induction of CYP2A6. The human monocyte cell line U937 exhibited higher levels of CYP2A6 in response to ethanol treatment [56]. Blood monocytes isolated from alcoholic patients may be a simple and feasible way to examine ethanol induction of CYP2A6 in humans.
What other transcription factors, besides Nrf2, play a role in the upregulation of expression of CYP2A5 by alcohol? Normally, Nrf2 heterodimerizes with small Maf proteins to function as a transcription factor. As discussed above, although lower, there was still significant induction of CYP2A5 by alcohol in Nrf2 KO mice. Besides Nrf2, other transcription factors are known to be capable of regulating the basal and the inducible expression of the CYP2A5 gene [31], and these include retinoid X receptor (RXR) alpha, coactivator PGC-1 alpha, and the aryl hydrocarbon receptor nuclear translocator [76, 77]. The effects of alcohol on these transcription factors should be assessed by chromatin immunoprecipitation (ChIP) experiments to identify transcription factor binding to the CYP2A5 promoter.
CYP2A5/2A6 is the major CYP for oxidation of nicotine to cotinine [67, 78]. Most alcoholics also smoke [79], and therefore it is likely that alcohol induction of CYP2A5/2A6 will accelerate blood nicotine clearance and increase the amount of tobacco smoking to maintain blood nicotine levels. We recently reported that intraperitoneal injection of nicotine increased chronic ethanol-induced steatosis and collagen deposition, but not inflammation [80]. Further studies on nicotine-alcohol biochemical and toxicological interactions would be of importance.
CYP2A5/2A6 oxidizes and activates many hepatocarcinogens [28]. One of the most significant diseases caused by alcohol is cancer as alcohol is considered a carcinogen for many tissues including the liver [81]. Several molecular mechanisms have been described for alcohol-mediated promotion of carcinogenesis [81]. One mechanism is CYP2E1-mediated activation of procarcinogens (e.g., nitrosamines) to the ultimate reactive carcinogens. Alcohol induction of CYP2A5/2A6 and the propensity of this CYP to oxidize and activate nitrosamines and other procarcinogens suggest that CYP2A5 may contribute to the actions of alcohol in promoting carcinogenesis. It will be interesting to detect whether CYP2A5 in the mouse lung and its orthologue CYP2A13 in the human lung are induced by alcohol and to evaluate whether alcohol synergizes with tobacco smoke to cause lung cancer.
An interesting speculation is the possibility that other CYPs which can be regulated by Nrf2 may be up-regulated by alcohol induction of CYP2E1 analogous to CYP2A5. Recently, cyp2b10 gene expression was shown to be increased by activators of Nrf2 such as phorone and phenobarbital in WT mice, but not in Nrf2 KO mice [82]. Alcohol has been shown to increase hepatic CYP2B, but we are not aware of any studies specifically evaluating cyp2b10.
Future studies will be required to evaluate the pathophysiological significance of CYP2A5, and its induction by acute and chronic alcohol treatment, to the biochemical and toxicological actions of alcohol on the liver and possibly on other tissues known to be affected by alcohol.
Acknowledgments
Studies from the authors’ laboratories were supported by the U.S. Public Health Service Grants AA-021362, AA-018790, and AA-020877 from the National Institute on Alcohol Abuse and Alcoholism at the National Institutes of Health (NIH), and ABMRF/The Foundation for Alcohol Research.
ABBREVIATIONS
- ALD
alcoholic liver disease
- CMZ
chlormethiazole
- CYP
cytochrome P450
- GSH
reduced glutathione
- GST
glutathione transferase
- HO-1
heme oxygenase 1
- KI
knockin
- KO
knockout
- LPO
lipid peroxidation
- LPS
lipopolysaccharide
- NAC
N-acetyl-L-cysteine
- Nrf2
nuclear factor erythroid 2 (NFE2)-related factor 2
- ROS
reactive oxygen species
- SOD
superoxide dismutase
- TAA
thioacetamide
- TBARS
thiobarbituric acid-reactive substances
- TNF-α
tumor necrosis factor alpha
- WT
wild type
References
- 1.Nebert DW, Nelson DR, Coon MJ, Estabrook RW, Feyereisen R, Fujii-Kuriyama Y, et al. The P450 superfamily: update on new sequences, gene mapping, and recommended nomenclature. DNA Cell Biol. 1991;10(1):1–14. doi: 10.1089/dna.1991.10.1. [DOI] [PubMed] [Google Scholar]
- 2.Lu Y, Cederbaum AI. CYP2E1 and oxidative liver injury by alcohol. Free Radic Biol Med. 2008;44(5):723–38. doi: 10.1016/j.freeradbiomed.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gorsky LD, Koop DR, Coon MJ. On the stoichiometry of the oxidase and monooxygenase reactions catalyzed by liver microsomal cytochrome P-450: products of oxygen reduction. J Biol Chem. 1984;259(11):6812–7. [PubMed] [Google Scholar]
- 4.Ekstrom G, Ingelman-Sundberg M. Rat liver microsomal NADPH-supported oxidase activity and lipid peroxidation dependent on ethanol-inducible cytochrome P-450 (P-450IIE1) Biochem Pharmacol. 1989;38(8):1313–9. doi: 10.1016/0006-2952(89)90338-9. [DOI] [PubMed] [Google Scholar]
- 5.Leung TM, Nieto N. CYP2E1 and oxidant stress in alcoholic and non-alcoholic fatty liver disease. J Hepatol. 2013;58(2):395–8. doi: 10.1016/j.jhep.2012.08.018. [DOI] [PubMed] [Google Scholar]
- 6.Caro AA, Cederbaum AI. Oxidative stress, toxicology, and pharmacology of CYP2E1. Annu Rev Pharmacol Toxicol. 2004;44:27–42. doi: 10.1146/annurev.pharmtox.44.101802.121704. [DOI] [PubMed] [Google Scholar]
- 7.Lieber CS. Cytochrome P-4502E1: its physiological and pathological role. Physiol Rev. 1997;77(2):517–44. doi: 10.1152/physrev.1997.77.2.517. [DOI] [PubMed] [Google Scholar]
- 8.Koop DR. Oxidative and reductive metabolism by cytochrome P450 2E1. FASEB J. 1992;6(2):724–30. doi: 10.1096/fasebj.6.2.1537462. [DOI] [PubMed] [Google Scholar]
- 9.Gonzalez FJ. Role of cytochromes P450 in chemical toxicity and oxidative stress: studies with CYP2E1. Mutat Res. 2005;569(1–2):101–10. doi: 10.1016/j.mrfmmm.2004.04.021. [DOI] [PubMed] [Google Scholar]
- 10.Morimoto M, Zern MA, Hagbjork AL, Ingelman-Sundberg M, French SW. Fish oil, alcohol, and liver pathology: role of cytochrome P450 2E1. Proc Soc Exp Biol Med. 1994;207(2):197–205. doi: 10.3181/00379727-207-43807. [DOI] [PubMed] [Google Scholar]
- 11.Nanji AA, Zhao S, Sadrzadeh SM, Dannenberg AJ, Tahan SR, Waxman DJ. Markedly enhanced cytochrome P450 2E1 induction and lipid peroxidation is associated with severe liver injury in fish oil-ethanol-fed rats. Alcohol Clin Exp Res. 1994;18(5):1280–5. doi: 10.1111/j.1530-0277.1994.tb00119.x. [DOI] [PubMed] [Google Scholar]
- 12.French SW, Morimoto M, Reitz RC, Koop D, Klopfenstein B, Estes K, et al. Lipid peroxidation, CYP2E1 and arachidonic acid metabolism in alcoholic liver disease in rats. J Nutr. 1997;127(5 Suppl):907S–11S. doi: 10.1093/jn/127.5.907S. [DOI] [PubMed] [Google Scholar]
- 13.Morimoto M, Hagbjork AL, Nanji AA, Ingelman-Sundberg M, Lindros KO, Fu PC, et al. Role of cytochrome P4502E1 in alcoholic liver disease pathogenesis. Alcohol. 1993;10(6):459–64. doi: 10.1016/0741-8329(93)90065-v. [DOI] [PubMed] [Google Scholar]
- 14.Morimoto M, Reitz RC, Morin RJ, Nguyen K, Ingelman-Sundberg M, French SW. CYP-2E1 inhibitors partially ameliorate the changes in hepatic fatty acid composition induced in rats by chronic administration of ethanol and a high fat diet. J Nutr. 1995;125(12):2953–64. doi: 10.1093/jn/125.12.2953. [DOI] [PubMed] [Google Scholar]
- 15.Albano E, Clot P, Morimoto M, Tomasi A, Ingelman-Sundberg M, French SW. Role of cytochrome P4502E1-dependent formation of hydroxyethyl free radical in the development of liver damage in rats intragastrically fed with ethanol. Hepatology. 1996;23(1):155–63. doi: 10.1002/hep.510230121. [DOI] [PubMed] [Google Scholar]
- 16.Gouillon Z, Lucas D, Li J, Hagbjork AL, French BA, Fu P, et al. Inhibition of ethanol-induced liver disease in the intragastric feeding rat model by chlormethiazole. Proc Soc Exp Biol Med. 2000;224(4):302–8. doi: 10.1046/j.1525-1373.2000.22435.x. [DOI] [PubMed] [Google Scholar]
- 17.Lieber CS. The discovery of the microsomal ethanol oxidizing system and its physiologic and pathologic role. Drug Metab Rev. 2004;36(3–4):511–29. doi: 10.1081/DMR-200033441. [DOI] [PubMed] [Google Scholar]
- 18.Aleynik MK, Leo MA, Aleynik SI, Lieber CS. Polyenylphosphatidylcholine opposes the increase of cytochrome P-4502E1 by ethanol and corrects its iron-induced decrease. Alcohol Clin Exp Res. 1999;23(1):96–100. [PubMed] [Google Scholar]
- 19.Bardag-Gorce F, Yuan QX, Li J, French BA, Fang C, Ingelman-Sundberg M, et al. The effect of ethanol-induced cytochrome p4502E1 on the inhibition of proteasome activity by alcohol. Biochem Biophys Res Commun. 2000;279(1):23–9. doi: 10.1006/bbrc.2000.3889. [DOI] [PubMed] [Google Scholar]
- 20.Lu Y, Zhuge J, Wang X, Bai J, Cederbaum AI. Cytochrome P450 2E1 contributes to ethanol-induced fatty liver in mice. Hepatology. 2008;47(5):1483–94. doi: 10.1002/hep.22222. [DOI] [PubMed] [Google Scholar]
- 21.Lu Y, Wu D, Wang X, Ward SC, Cederbaum AI. Chronic alcohol-induced liver injury and oxidant stress are decreased in cytochrome P4502E1 knockout mice and restored in humanized cytochrome P4502E1 knock-in mice. Free Radic Biol Med. 2010;49(9):1406–16. doi: 10.1016/j.freeradbiomed.2010.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bolt HM, Roos PH, Thier R. The cytochrome P-450 isoenzyme CYP2E1 in the biological processing of industrial chemicals: consequences for occupational and environmental medicine. Int Arch Occup Environ Health. 2003;76(3):174–85. doi: 10.1007/s00420-002-0407-4. [DOI] [PubMed] [Google Scholar]
- 23.Zhu H, Jia Z, Misra H, Li YR. Oxidative stress and redox signaling mechanisms of alcoholic liver disease: updated experimental and clinical evidence. J Dig Dis. 2012;13(3):133–42. doi: 10.1111/j.1751-2980.2011.00569.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.French SW. The importance of CYP2E1 in the pathogenesis of alcoholic liver disease and drug toxicity and the role of the proteasome. Subcell Biochem. 2013;67:145–64. doi: 10.1007/978-94-007-5881-0_4. [DOI] [PubMed] [Google Scholar]
- 25.Palakodety RB, Clejan LA, Krikun G, Feierman DE, Cederbaum AI. Characterization and identification of a pyrazole-inducible form of cytochrome P-450. J Biol Chem. 1988;263(2):878–84. [PubMed] [Google Scholar]
- 26.Winters DK, Cederbaum AI. Time course characterization of the induction of cytochrome P-450 2E1 by pyrazole and 4-methylpyrazole. Biochim Biophys Acta. 1992;1117(1):15–24. doi: 10.1016/0304-4165(92)90156-o. [DOI] [PubMed] [Google Scholar]
- 27.Lu Y, Cederbaum AI. Enhancement by pyrazole of lipopolysaccharide-induced liver injury in mice: role of cytochrome P450 2E1 and 2A5. Hepatology. 2006;44(1):263–74. doi: 10.1002/hep.21241. [DOI] [PubMed] [Google Scholar]
- 28.Su T, Ding X. Regulation of the cytochrome P450 2A genes. Toxicol Appl Pharmacol. 2004;199(3):285–94. doi: 10.1016/j.taap.2003.11.029. [DOI] [PubMed] [Google Scholar]
- 29.Raunio H, Rahnasto-Rilla M. CYP2A6: genetics, structure, regulation, and function. Drug Metabol Drug Interact. 2012;27(2):73–88. doi: 10.1515/dmdi-2012-0001. [DOI] [PubMed] [Google Scholar]
- 30.Kirby GM, Nichols KD, Antenos M. CYP2A5 induction and hepatocellular stress: an adaptive response to perturbations of heme homeostasis. Curr Drug Metab. 2011;12(2):186–97. doi: 10.2174/138920011795016845. [DOI] [PubMed] [Google Scholar]
- 31.Abu-Bakar A, Hakkola J, Juvonen R, Rahnasto-Rilla M, Raunio H, Lang MA. Function and regulation of the Cyp2a5/CYP2A6 genes in response to toxic insults in the liver. Curr Drug Metab. 2013;14(1):137–50. [PubMed] [Google Scholar]
- 32.Niemela O, Parkkila S, Juvonen RO, Viitala K, Gelboin HV, Pasanen M. Cytochromes P450 2A6, 2E1, and 3A and production of protein-aldehyde adducts in the liver of patients with alcoholic and non-alcoholic liver diseases. J Hepatol. 2000;33(6):893–901. doi: 10.1016/s0168-8278(00)80120-8. [DOI] [PubMed] [Google Scholar]
- 33.Kojo A, Viitala P, Pasanen M, Pelkonen O, Raunio H, Juvonen R. Induction of CYP2A5 by pyrazole and its derivatives in mouse primary hepatocytes. Arch Toxicol. 1998;72(6):336–41. doi: 10.1007/s002040050511. [DOI] [PubMed] [Google Scholar]
- 34.Ma Q. Role of nrf2 in oxidative stress and toxicity. Annu Rev Pharmacol Toxicol. 2013;53:401–26. doi: 10.1146/annurev-pharmtox-011112-140320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Baird L, Dinkova-Kostova AT. The cytoprotective role of the Keap1-Nrf2 pathway. Arch Toxicol. 2011;85(4):241–72. doi: 10.1007/s00204-011-0674-5. [DOI] [PubMed] [Google Scholar]
- 36.Niture SK, Kaspar JW, Shen J, Jaiswal AK. Nrf2 signaling and cell survival. Toxicol Appl Pharmacol. 2010;244(1):37–42. doi: 10.1016/j.taap.2009.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hayes JD, Dinkova-Kostova AT. The Nrf2 regulatory network provides an interface between redox and intermediary metabolism. Trends Biochem Sci. 2014;39(4):199–218. doi: 10.1016/j.tibs.2014.02.002. [DOI] [PubMed] [Google Scholar]
- 38.Klaassen CD, Reisman SA. Nrf2 the rescue: effects of the antioxidative/electrophilic response on the liver. Toxicol Appl Pharmacol. 2010;244(1):57–65. doi: 10.1016/j.taap.2010.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bataille AM, Manautou JE. Nrf2: a potential target for new therapeutics in liver disease. Clin Pharmacol Ther. 2012;92(3):340–8. doi: 10.1038/clpt.2012.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ma Q, He X. Molecular basis of electrophilic and oxidative defense: promises and perils of Nrf2. Pharmacol Rev. 2012;64(4):1055–81. doi: 10.1124/pr.110.004333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shin SM, Yang JH, Ki SH. Role of the Nrf2-ARE pathway in liver diseases. Oxid Med Cell Longev. 2013;2013:763257. doi: 10.1155/2013/763257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mari M, Cederbaum AI. CYP2E1 overexpression in HepG2 cells induces glutathione synthesis by transcriptional activation of gamma-glutamylcysteine synthetase. J Biol Chem. 2000;275(20):15563–71. doi: 10.1074/jbc.M907022199. [DOI] [PubMed] [Google Scholar]
- 43.Mari M, Cederbaum AI. Induction of catalase, alpha, and microsomal glutathione S-transferase in CYP2E1 overexpressing HepG2 cells and protection against short-term oxidative stress. Hepatology. 2001;33(3):652–61. doi: 10.1053/jhep.2001.22521. [DOI] [PubMed] [Google Scholar]
- 44.Schafer A, Galuppo P, Fraccarollo D, Vogt C, Widder JD, Pfrang J, et al. Increased cytochrome P4502E1 expression and altered hydroxyeicosatetraenoic acid formation mediate diabetic vascular dysfunction: rescue by guanylyl-cyclase activation. Diabetes. 2010;59(8):2001–9. doi: 10.2337/db09-1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nieto N, Mari M, Cederbaum AI. Cytochrome P450 2E1 responsiveness in the promoter of glutamate-cysteine ligase catalytic subunit. Hepatology. 2003;37(1):96–106. doi: 10.1053/jhep.2003.50003. [DOI] [PubMed] [Google Scholar]
- 46.Gong P, Cederbaum AI. Nrf2 is increased by CYP2E1 in rodent liver and HepG2 cells and protects against oxidative stress caused by CYP2E1. Hepatology. 2006;43(1):144–53. doi: 10.1002/hep.21004. [DOI] [PubMed] [Google Scholar]
- 47.Gong P, Cederbaum AI. Transcription factor Nrf2 protects HepG2 cells against CYP2E1 plus arachidonic acid-dependent toxicity. J Biol Chem. 2006;281(21):14573–9. doi: 10.1074/jbc.M600613200. [DOI] [PubMed] [Google Scholar]
- 48.Chen SY, Dehart DB, Sulik KK. Protection from ethanol-induced limb malformations by the superoxide dismutase/catalase mimetic, EUK-134. FASEB J. 2004;18(11):1234–6. doi: 10.1096/fj.03-0850fje. [DOI] [PubMed] [Google Scholar]
- 49.Dong J, Sulik KK, Chen SY. Nrf2-mediated transcriptional induction of antioxidant response in mouse embryos exposed to ethanol in vivo: implications for the prevention of fetal alcohol spectrum disorders. Antioxid Redox Signal. 2008;10(12):2023–33. doi: 10.1089/ars.2007.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen X, Liu J, Chen SY. Sulforaphane protects against ethanol-induced oxidative stress and apoptosis in neural crest cells by the induction of Nrf2-mediated antioxidant response. Br J Pharmacol. 2013;169(2):437–48. doi: 10.1111/bph.12133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Narasimhan M, Mahimainathan L, Rathinam ML, Riar AK, Henderson GI. Overexpression of Nrf2 protects cerebral cortical neurons from ethanol-induced apoptotic death. Mol Pharmacol. 2011;80(6):988–99. doi: 10.1124/mol.111.073262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhou R, Lin J, Wu D. Sulforaphane induces Nrf2 and protects against CYP2E1-dependent binge alcohol-induced liver steatosis. Biochim Biophys Acta. 2014;1840(1):209–18. doi: 10.1016/j.bbagen.2013.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lamle J, Marhenke S, Borlak J, von Wasielewski R, Eriksson CJ, Geffers R, et al. Nuclear factor-eythroid 2-related factor 2 prevents alcohol-induced fulminant liver injury. Gastroenterology. 2008;134(4):1159–68. doi: 10.1053/j.gastro.2008.01.011. [DOI] [PubMed] [Google Scholar]
- 54.Wu KC, Liu J, Klaassen CD. Role of Nrf2 in preventing ethanol-induced oxidative stress and lipid accumulation. Toxicol Appl Pharmacol. 2012;262(3):321–9. doi: 10.1016/j.taap.2012.05.010. [DOI] [PubMed] [Google Scholar]
- 55.Yao P, Nussler A, Liu L, Hao L, Song F, Schirmeier A, et al. Quercetin protects human hepatocytes from ethanol-derived oxidative stress by inducing heme oxygenase-1 via the MAPK/Nrf2 pathways. J Hepatol. 2007;47(2):253–61. doi: 10.1016/j.jhep.2007.02.008. [DOI] [PubMed] [Google Scholar]
- 56.Jin M, Kumar A, Kumar S. Ethanol-mediated regulation of cytochrome P450 2A6 expression in monocytes: role of oxidative stress-mediated PKC/MEK/Nrf2 pathway. PLoS One. 2012;7(4):e35505. doi: 10.1371/journal.pone.0035505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Honkakoski P, Autio S, Juvonen R, Raunio H, Gelboin HV, Park SS, et al. Pyrazole is different from acetone and ethanol as an inducer of the polysubstrate monooxygenase system in mice: evidence that pyrazole-inducible P450Coh is distinct from acetone-inducible P450ac. Arch Biochem Biophys. 1988;267(2):589–98. doi: 10.1016/0003-9861(88)90066-5. [DOI] [PubMed] [Google Scholar]
- 58.Lu Y, Zhuge J, Wu D, Cederbaum AI. Ethanol induction of CYP2A5: permissive role for CYP2E1. Drug Metab Dispos. 2011;39(2):330–6. doi: 10.1124/dmd.110.035691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lu Y, Zhang XH, Cederbaum AI. Ethanol induction of CYP2A5: role of CYP2E1-ROS-Nrf2 pathway. Toxicol Sci. 2012;128(2):427–38. doi: 10.1093/toxsci/kfs164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Song BJ, Gelboin HV, Park SS, Yang CS, Gonzalez FJ. Complementary DNA and protein sequences of ethanol-inducible rat and human cytochrome P-450s: transcriptional and post-transcriptional regulation of the rat enzyme. J Biol Chem. 1986;261(35):16689–97. [PubMed] [Google Scholar]
- 61.Song BJ, Veech RL, Park SS, Gelboin HV, Gonzalez FJ. Induction of rat hepatic N-nitrosodimethylamine demethylase by acetone is due to protein stabilization. J Biol Chem. 1989;264(6):3568–72. [PubMed] [Google Scholar]
- 62.Lee SS, Buters JT, Pineau T, Fernandez-Salguero P, Gonzalez FJ. Role of CYP2E1 in the hepatotoxicity of acetaminophen. J Biol Chem. 1996;271(20):12063–7. doi: 10.1074/jbc.271.20.12063. [DOI] [PubMed] [Google Scholar]
- 63.Cheung C, Yu AM, Ward JM, Krausz KW, Akiyama TE, Feigenbaum L, et al. The cyp2e1-humanized transgenic mouse: role of cyp2e1 in acetaminophen hepatotoxicity. Drug Metab Dispos. 2005;33(3):449–57. doi: 10.1124/dmd.104.002402. [DOI] [PubMed] [Google Scholar]
- 64.Abu-Bakar A, Lamsa V, Arpiainen S, Moore MR, Lang MA, Hakkola J. Regulation of CYP2A5 gene by the transcription factor nuclear factor (erythroid-derived 2)-like 2. Drug Metab Dispos. 2007;35(5):787–94. doi: 10.1124/dmd.106.014423. [DOI] [PubMed] [Google Scholar]
- 65.Lamsa V, Levonen AL, Leinonen H, Yla-Herttuala S, Yamamoto M, Hakkola J. Cytochrome P450 2A5 constitutive expression and induction by heavy metals is dependent on redox-sensitive transcription factor Nrf2 in liver. Chem Res Toxicol. 2010;23(5):977–85. doi: 10.1021/tx100084c. [DOI] [PubMed] [Google Scholar]
- 66.Yokota S, Higashi E, Fukami T, Yokoi T, Nakajima M. Human CYP2A6 is regulated by nuclear factor-erythroid 2 related factor 2. Biochem Pharmacol. 2011;81(2):289–94. doi: 10.1016/j.bcp.2010.09.020. [DOI] [PubMed] [Google Scholar]
- 67.Zhou X, Zhuo X, Xie F, Kluetzman K, Shu YZ, Humphreys WG, et al. Role of CYP2A5 in the clearance of nicotine and cotinine: insights from studies on a Cyp2a5-null mouse model. J Pharmacol Exp Ther. 2010;332(2):578–87. doi: 10.1124/jpet.109.162610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hong F, Liu X, Ward SS, Xiong H, Cederbaum AI, Lu Y. Absence of cytochrome P450 2A5 enhances alcohol-induced liver injury in mice. Dig Liver Dis. 2015;47(6):470–7. doi: 10.1016/j.dld.2015.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Salonpaa P, Krause K, Pelkonen O, Raunio H. Up-regulation of CYP2A5 expression by porphyrinogenic agents in mouse liver. Naunyn Schmiedebergs Arch Pharmacol. 1995;351(4):446–52. doi: 10.1007/BF00169087. [DOI] [PubMed] [Google Scholar]
- 70.Pellinen P, Stenback F, Rautio A, Pelkonen O, Lang M, Pasanen M. Response of mouse liver coumarin 7-hydroxylase activity to hepatotoxins: dependence on strain and agent and comparison to other monooxygenases. Naunyn Schmiedebergs Arch Pharmacol. 1993;348(4):435–43. doi: 10.1007/BF00171345. [DOI] [PubMed] [Google Scholar]
- 71.Hong F, Si C, Gao P, Cederbaum AI, Xiong H, Lu Y. The role of CYP2A5 in liver injury and fibrosis: chemical-specific difference. Naunyn Schmiedebergs Arch Pharmacol. 2016;389(1):33–43. doi: 10.1007/s00210-015-1172-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gozzelino R, Jeney V, Soares MP. Mechanisms of cell protection by heme oxygenase-1. Annu Rev Pharmacol Toxicol. 2010;50:323–54. doi: 10.1146/annurev.pharmtox.010909.105600. [DOI] [PubMed] [Google Scholar]
- 73.Lamsa V, Levonen AL, Sormunen R, Yamamoto M, Hakkola J. Heme and heme biosynthesis intermediates induce heme oxygenase-1 and cytochrome P450 2A5, enzymes with putative sequential roles in heme and bilirubin metabolism: different requirement for transcription factor nuclear factor erythroid- derived 2-like 2. Toxicol Sci. 2012;130(1):132–44. doi: 10.1093/toxsci/kfs237. [DOI] [PubMed] [Google Scholar]
- 74.Abu-Bakar A, Arthur DM, Aganovic S, Ng JC, Lang MA. Inducible bilirubin oxidase: a novel function for the mouse cytochrome P450 2A5. Toxicol Appl Pharmacol. 2011;257(1):14–22. doi: 10.1016/j.taap.2011.08.011. [DOI] [PubMed] [Google Scholar]
- 75.Kim SD, Antenos M, Squires EJ, Kirby GM. Cytochrome P450 2A5 and bilirubin: mechanisms of gene regulation and cytoprotection. Toxicol Appl Pharmacol. 2013;270(2):129–38. doi: 10.1016/j.taap.2013.04.013. [DOI] [PubMed] [Google Scholar]
- 76.Arpiainen S, Jarvenpaa SM, Manninen A, Viitala P, Lang MA, Pelkonen O, et al. Coactivator PGC-1alpha regulates the fasting inducible xenobiotic-metabolizing enzyme CYP2A5 in mouse primary hepatocytes. Toxicol Appl Pharmacol. 2008;232(1):135–41. doi: 10.1016/j.taap.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 77.Arpiainen S, Lamsa V, Pelkonen O, Yim SH, Gonzalez FJ, Hakkola J. Aryl hydrocarbon receptor nuclear translocator and upstream stimulatory factor regulate Cytochrome P450 2a5 transcription through a common E-box site. J Mol Biol. 2007;369(3):640–52. doi: 10.1016/j.jmb.2007.03.075. [DOI] [PubMed] [Google Scholar]
- 78.Benowitz NL, Swan GE, Jacob P, 3rd, Lessov-Schlaggar CN, Tyndale RF. CYP2A6 genotype and the metabolism and disposition kinetics of nicotine. Clin Pharmacol Ther. 2006;80(5):457–67. doi: 10.1016/j.clpt.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 79.Wall TL, Schoedel K, Ring HZ, Luczak SE, Katsuyoshi DM, Tyndale RF. Differences in pharmacogenetics of nicotine and alcohol metabolism: review and recommendations for future research. Nicotine Tob Res. 2007;9(Suppl 3):S459–74. doi: 10.1080/14622200701587045. [DOI] [PubMed] [Google Scholar]
- 80.Lu Y, Ward SC, Cederbaum AI. Nicotine enhances ethanol-induced fat accumulation and collagen deposition but not inflammation in mouse liver. Alcohol. 2013;47(5):353–7. doi: 10.1016/j.alcohol.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zakhari S, Vasiliou V, Guo QM. Alcohol and Cancer. Springer; New York, New York: 2011. [Google Scholar]
- 82.Ashino T, Ohkubo-Morita H, Yamamoto M, Yoshida T, Numazawa S. Possible involvement of nuclear factor erythroid 2-related factor 2 in the gene expression of Cyp2b10 and Cyp2a5. Redox Biol. 2014;2:284–8. doi: 10.1016/j.redox.2013.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]