Abstract
Background
Despite established guidelines for regular walking as a first line therapy for adults with peripheral arterial disease (PAD), most patients do not walk routinely. This paper presents the design specifications for a randomized clinical trial to examine the effectiveness of an internet-based walking program compared to a telephone intervention, or the combination (internet-based with telephone counseling) for promotion of regular walking in patients with PAD.
Methods
Sedentary adults with documented lower extremity PAD are being recruited from the University of Michigan Health System and the surrounding area. Participants are randomized to one of four arms in a 2×2 factorial design: 1) telephone counseling to promote walking, 2) an internet-based walking program with tailored step-count goals, 3) the combination of telephone counseling with the internet-based walking program, or 4) waitlist control. Participants receive a 4-month intervention phase, after which all participants are followed for an additional 8 months to assess long-term adherence to regular walking. Outcomes are assessed at baseline, 4 and 12 months. The primary outcome is walking distance assessed through a standardized treadmill protocol. Additional outcomes include change in step-counts measured with a commercial activity tracker, pain-free walking distance, and changes in health-related quality of life from baseline to follow-up.
Conclusion
Finding effective and feasible programs to promote walking among PAD patients is warranted. This study will add to current evidence regarding use of internet based programs with and without telephone counseling to promote regular walking in this population.
Keywords: Randomized clinical trial, Treatment intervention, Physical activity, Peripheral Arterial Disease
Introduction
Patients with peripheral arterial disease (PAD) are at increased risk for both cardiovascular and all-cause mortality; in addition, these patients exhibit significant functional impairment.1–4 Current estimates indicate that there are over 200 million patients with PAD world-wide, with over 8 million in the United States alone.5 Given the aging population, the number of adults with PAD is projected to increase over the next several decades.6 Patient morbidity and mortality associated with PAD results in over 4 billion dollars in treatment-related costs in the United States each year.7 Furthermore, these patients often report lower health-related quality of life (HRQOL) and show signs and symptoms of depression and/or anxiety.8, 9 The monetary and personal costs of PAD carry significant public health implications for which current treatment paradigms are inadequate.
Supervised walking programs are effective in reducing claudication symptoms and improving walking distance in patients with PAD.10–13 Trials using exercise interventions have demonstrated improvements in maximal walking which appear as effective or more so compared to pharmacotherapy. procedures.14–17 The Claudication: Exercise Versus Endoluminal Revascularization (CLEVER) study observed the greatest improvement in peak walking time for participants randomized to optimal medical therapy (OMT) with supervised exercise compared to OMT with stent revascularization or OMT alone.17
Although supervised exercise is a class 1 recommendation for PAD patients, most do not enroll in PAD exercise programs.18 Barriers to such programs include lack of third-party reimbursement, geographic distance to centers providing such programs, provider referral and patient motivation. Digital health programs to promote physical activity may be an effective alternative to supervised programs. Internet-mediated interventions can be delivered at low marginal per-participant cost and can be disseminated to any patient who has internet access.19–22 Internet-based programs to increase physical activity have been well studied in other chronic conditions,23–25 but have not been well evaluated in PAD patients.
The purpose of this paper is to describe the design, rationale, and outcomes of a 12-month randomized controlled trial that compares the efficacy of 1) telephone counseling to promote walking, 2) an internet based walking program with tailored step-count goals, 3) the combination of telephone counseling with the internet-based walking program, or 4) waitlist control. The primary outcomes of interest are change in maximal walking distance, health-related quality of life (HRQOL), and long-term adherence to regular walking.
Methods
Design & Specific Aims
This study is a randomized controlled trial with outcomes assessed at baseline, 4, and 12 months. (Figure 1)
Figure 1.
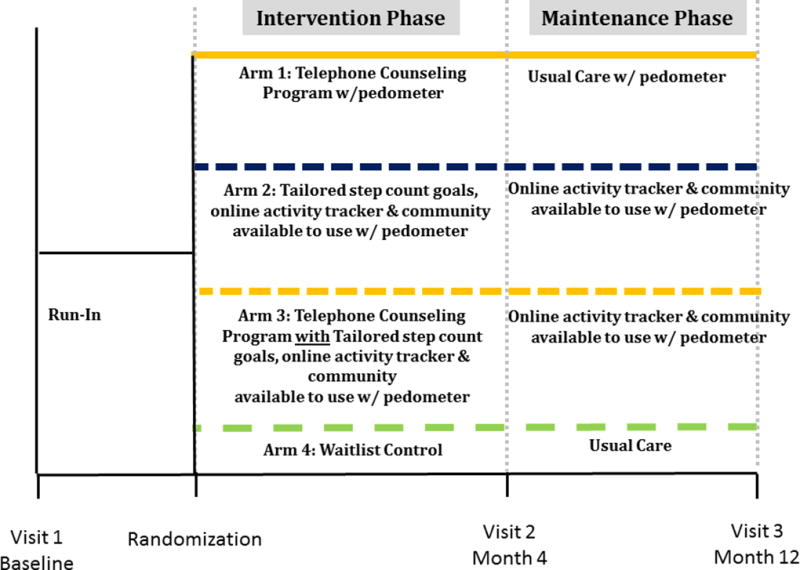
Study Protocol
The University of Michigan Medical School Institutional Review Board approved this study, and all participants provided their informed consent prior to the administration of any study procedures. Participants are randomized in a 2×2 factorial design to 1 of 4 conditions; Arm 1) telephone counseling with a pedometer (without tailored step-count goals); Arm 2) internet-based walking program with tailored step count goals, tailored messages, and an online community; Arm 3) the combination of the telephone counseling with the internet-based walking program with tailored step count goals, tailored messages, and an online community; or Arm 4) waitlist control. See Figure 1 for study design.
The specific aims are:
Aim 1: To test the effectiveness of a tailored internet-based walking program to improve maximal walking distance and health-related quality of life compared to a telephone counseling program, the combination of the internet-based program with telephone counseling, and usual care (waitlist control group) in patients with PAD.
Aim 2: To test the effectiveness of a tailored internet-based walking program to improve long-term adherence to walking in patients with PAD.
Study population
The study sample is comprised of adults 40 years or older with documented atherosclerotic PAD. Inclusion criteria for age are based on the prevalence of atherosclerotic PAD being extremely rare among younger adults. For this study, documented PAD included ankle-brachial indices< 0.9 or through imaging such as peripheral angiograms.7 We excluded individuals who report regular participation in structured exercise activities and those who are unable to walk for a short distance at baseline (defined as the ability to walk a grocery store aisle unassisted) to minimize ceiling and floor effects. Additional exclusion criteria include unstable cardiac conditions or recent cardiovascular events, including myocardial infarction or stroke within 3 months of enrollment and planned revascularization procedures. Lastly, participants must have access to email and the internet to allow them to use the Fitbit™ and access the internet-based intervention. See Table 1 for full eligibility requirements.
Table 1.
Inclusion and Exclusion Criteria
| Criteria | |
|---|---|
| Inclusion | Exclusion |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
PAD = peripheral arterial disease with a documented ankle brachial index of < 0.9 or documentation via imaging (MRI or CT scan, or angiogram);
inability to walk one city block;
a psychiatric disorder (including severe depression, psychosis, or dementia) which limits the patient’s ability to follow the study protocol; PA= physical activity, CVD =cardiovascular disease, TIA = transient ischemic stroke, MI = myocardial infarction, UA = unstable angina, HD = heart disease, NYHA = New York Heart Association, HF = heart failure
Recruitment
A challenge in many clinical trials is the adequate recruitment of participants. Our participants are primarily recruited through the PAD specialty clinic at the University of Michigan Health System (UMHS) and the larger health system. An estimated 4000 patient visits to UMHS are associated with a primary diagnosis of PAD. Despite this large number of patient visits, initial recruitment efforts were hampered by a higher than expected refusal rate. The most common reason for refusal was travel time to the health system if randomized to the supervised walking program. Indeed, less than 20% of PAD patients we approached lived within a 25-mile radius of our medical center. This barrier to recruitment is likely compounded by Michigan’s winters and the understandable reluctance of patients to travel during inclement weather. We considered using local community centers or gyms for the supervised walking program, but the projected costs and issues associated with participant safety were too great for that to be a feasible option. Consequently, we developed an alternative to the in-person supervised PAD rehab program in the form of telephone counseling. Since this design change, 28% of our study sample lives more than 1-hour driving distance from our center, and we have had 2 potential participants call from out-of-state and express willingness to fly to Michigan for the study visits.
We are using a combination of passive and active recruitment methods to reach out to potential participants. Passive approaches include distributing flyers throughout the main UMHS and satellite clinics, posting on Craigslist and newspaper advertisements, and creating a page on UMClinicalStudies.org, an information portal and search tool for individuals seeking clinical research opportunities. We also take advantage of the “Best Practice Alert” (BPA) system through the electronic medical records system (EPIC MiChart). A BPA is triggered and our study team is alerted when particular patient characteristics (e.g., age ≥ 40 years, PAD diagnosis) are noted for a patient visit, phone call or procedure. Our active approaches include weekly review of UMHS PAD clinic schedule for suitable patients. The study team then works with the clinic staff to find a time to meet with patients at some point during their visit. When we are unable to have face-to-face contact with a prospective participant (e.g. for patients who cancelled or no-showed to their clinic appointment, BPAs, patients at non-local clinics, etc.) we send an introduction letter, followed by two phone calls to screen for interest and eligibility. If the potential participant cannot be reached after two phone calls, they are deemed “no contact made/uninterested.” This is not necessarily a final status, as sometimes patients call us several months after we deemed them “no contact made.” Additionally, if these patients re-appear on the clinic schedule or via a BPA, and it’s been more than 6 months to a year since our last attempted contact, we send a second letter.
Screening
We use a tiered approach to screen potential participants for eligibility. Tier 1 involved a review of the medical record (if available) for primary criteria (age, PAD diagnosis, recent cardiovascular events, etc.). For Tier 2 we assessed for interest in the study and evaluate for criteria not readily available in the medical record (sedentary behavior, regular exercise habits, etc.). Finally, Tier 3 involved receiving medical clearance from a health care provider (e.g., PAD clinician, cardiologist, primary care physician). We attempted to obtain medical clearance for all interested and eligible individuals.
Study Procedures
Outcome assessments occur at baseline, 4-months (immediately post-intervention), and 12-months (study completion). At the baseline visit, participants complete a written consent followed by the treadmill testing for assessment of maximal walking distance (primary outcome) and pain-free walking distance. Baseline surveys, including HRQOL (primary outcome) are then completed followed by completion of a six-minute walk test. Resting and post-exercise ankle-brachial index (ABI) are completed at the time of treadmill testing. After an initial 1-week run-in-phase, the participant is randomized and the 4-month intervention phase begins. At the end of this 4-month intervention, the participant returns for the same assessments as completed at the baseline visit. The participant then completes the 8-month maintenance phase after which he/she returns to complete the 12-month visit. The same assessments as in the prior two visits are completed and an exit survey is also completed. The study activities for the internet walking program, the telephone counseling and the waitlist control are detailed in the section on interventions.
Randomization
Participants are randomized into 1 of 4 arms (internet walking program; telephone counseling; the combination of the two interventions; or waitlist control). We are using a stratified block randomization scheme based on age and maximal walking distance (from the baseline treadmill test); age greater than or equal to 70 and ability to walk more than 1500 feet at baseline, age less than 70 and ability to walk more than 1500 feet at baseline, age greater than or equal to 70 and inability to walk more than 1500 feet at baseline, and age less than 70 and inability to walk more than 1500 feet at baseline. Participants are randomized at the baseline visit and receive their study group assignment at the conclusion of this visit.
Run-in Period
A run-in period of 1-week, prior to randomization is included to assess adherence for wearing the pedometer, and to determine the first week’s step-count goals for participants randomized to the internet-based walking intervention arms. Adherence is defined as wearing the pedometer for 5 or more days with adequate data capture of step counts. If a participant wears the pedometer for < 5 days, they may try another week of the run-in prior to randomization. If the participant remains non-adherent to wearing the pedometer, he/she will not be randomized and will be excluded from the trial at that time.
Primary Outcomes
Maximal walking distance
Maximal walking distance is determined using the Gardner-Skinner protocol treadmill test, a validated measure of walking distance in PAD patients.26 This protocol is performed using a constant treadmill speed of 2 mph starting at 0% slope and increasing 2% in slope every 2 minutes. This protocol has been validated as an assessment of maximal walking distance and pain-free walking distance.26–29
Health-related Quality of Life (HRQOL)
HRQOL is assessed using a PAD-specific measure of HRQOL and two generic measures. The disease-specific measure is the Peripheral Artery Questionnaire (PAQ). The PAQ has 7 domains including, physical function, symptom stability, symptoms (frequency, and severity), satisfaction with treatment, quality of life, social function and which leg is most symptomatic. One point is assigned to the response correlated with the most limited function with additional point for each higher response in the item. Domain scores are calculated by summing the items within a domain and then subtracting the lowest possible score, dividing the range for the domain and then multiplying by 100. All domain specific scores range from 0 to 100, with higher scores indicating less limitation, greater quality of life, greater satisfaction with treatment or fewer symptoms, depending on the domain. The summary score is calculated by combining the scores for the following domains; physical limitation, symptom frequency and burden, social function and quality of life. All 7 PAQ domains have demonstrated internal consistency (0.80 to 0.94) and have acceptable test-retest reliability (mean difference ranged from 0.9 to 2.2).30 Construct validity has also been supported in patients with PAD.30 This PAQ is also responsive to clinical change in PAD,30 and is more sensitive than other measures of HRQOL (i.e., the SF 3631 or the Walking Impairment Questionnaire32).30
The EQ-5D-3L (EuroQOL, 1990)33 and Patient reported Outcomes Measurement Information System (PROMIS)34 were used to evaluate generic HRQOL. The EQ-5D-3L examines mobility, self-care, usual activities, pain/discomfort, and anxiety/depression.35 Participants rate each dimension on a 3-point scale: no problems, some problems, extreme problems. The individual scores for each dimension are combined to form a 5-digit description of current health state (e.g., 11232). These data can be presented descriptively by levels, e.g., no problem vs. any problems. This numeric health state can also be converted to a summary index using country-specific value sets and algorithms. This measure has been validated for use in cardiac patients.35
PROMIS was also used to evaluate 7 additional HRQOL domains (Physical Function, Ability to Participate In Social Roles and Activities, Satisfaction with Social Roles and Activities, Fatigue, Depression, Anxiety, and Self-Efficacy). PROMIS measures were designed to capture key symptoms and health concepts across different chronic conditions. Specifically, these measures were designed to capture the impact that a disease has on physical, mental and social well-being. PROMIS was developed using a general US population and clinical samples including COPD, DM, and CHD36 (n = 21,133 across all samples). PROMIS offers several advantages over more traditional measures of HRQOL. For example, PROMIS allows for cross-disease comparison and it utilizes computerized adaptive test (CAT) technology, a method whereby each individually administered item is selected based on the previous item response. CATs allow for the sensitive measurement of a broad range of symptomatology with the administration of a small subset of items (generally between 4–8 items) without losing the precision of a longer measure. The exact subset of items administered in a CAT depends upon Item Response Theory (IRT) calibrations.37 Using this methodological approach, items are arranged along a scale, e.g., from no symptoms to extreme symptoms. The dynamic nature of CAT allows for greater sensitivity across the disease spectrum than most traditional, static measures while still retaining the integrity of the full measure. CAT also provides better precision and lower standard error than static measures, even when the number of items administered for each is identical.38 In addition to CATs, each measure can also be administered as a calibrated short form (a static set of items). We administered both the CAT and SF version of these measures. PROMIS scores reflect a T-score metric, with a mean score of 50 and a SD of 10 (mean scores are relative to the general population).39 Higher scores indicate more of the construct being measured (e.g., higher Physical Function Scores indicate more/better Physical Function).
Step counts
Step-count data is collected through Fitbit™ (San Francisco, CA) pedometers for all participants except the control group.40 For participants randomized to the internet-based program (alone or in combination with telephone counseling), data on step counts are collected as daily and weekly counts. Tailored weekly-step count goals are based on the prior week’s step counts for these participants. This allows for weekly goals to be decreased following an illness or hospitalization; thus avoiding unrealistic goals which may reduce a participant’s motivation for walking.
Additional Measures
6-minute walk
Participants perform a six-minute walk test in accordance with the American Thoracic Society guidelines.41 The test is performed in a pre-marked hallway with a study team member giving scripted encouragement at one-minute intervals. Post-test vital signs are measured immediately upon the end of the test, and the total distance walked is measured and recorded.
ABIs are measured before the treadmill test and within 1–3 minutes post-exercise at Baseline, 4-month and 12-month assessments. The initial ABI is performed after the patient has been lying supine for at least 10 minutes. The ABI is obtained by determining the dorsalis pedis and posterior tibial arterial systolic blood pressure in both ankles and the brachial blood pressure in both arms, using a 5–7 MHz Doppler ultrasound instrument (Ultrasonic Doppler Flow Detector Model 811-B. Parks Medical Electronics, Inc. Aloha, OR). The ABI for each lower extremity is calculated by dividing each ankle reading by the higher of the 2 brachial readings. For participants with an ABI of >1.3 (non-compressible calcified arteries), toe-brachial index (TBI) in the great toe will be measured. The TBI is the ratio of the systolic blood pressure measured in the first toe divided by the systolic blood pressure in the arm.
Interventions
Conceptual Framework
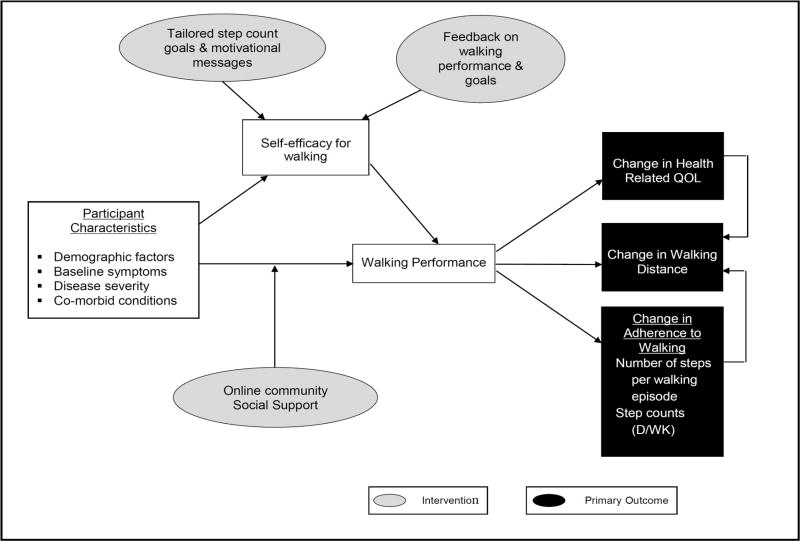
Given the potential for regular walking to improve physical function and HRQOL in patients with PAD, we developed an internet-based walking intervention with tailored step-count goals for patients with PAD. The conceptual framework, shown in Figure 2, outlines the components of the intervention and the expected association with the defined outcomes including maximal walking distance, HRQOL, and long-term adherence to walking. The components of the intervention were developed to improve self-efficacy for walking and overall walking performance, which are hypothesized to improved maximal walking distance, HRQOL and long-term adherence for walking. The efficacy of the internet-based walking intervention is compared to a telephone counseling program, the combination of the two (internet-based intervention with telephone counseling) or waitlist control (i.e. usual care) in a 2x2 factorial randomized controlled trial design. (Figure 1)
Figure 2.
Conceptual Model
QOL = quality of life; D/WK=day/week
Internet-based Walking Program
The primary intervention under evaluation is the internet-based walking program. It is a 2-pronged program that incorporates use of a pedometer with a study and disease-specific website. The website uses 1) tailored motivational messages, 2) step-count feedback, 3) individualized step count goals, and 4) an online forum. The motivational messaging includes disease-tailored weekly motivational and daily informational messages. Some of these messages contain disease-specific content related to PAD and associated risk factors, while other messages contain more generic information on walking and health. Participants are advised on walking according to the PAD Exercise Training Toolkit created by the Vascular Disease Foundation, the American Heart Association, and the American Association of Cardiovascular and Pulmonary Rehabilitation,42 (i.e., after a warm up they will walk until claudication symptoms occur and then rest until symptoms resolve, typically within 1–5 minutes), and continue the walk-rest pattern for 30–50 minutes daily.
Detailed step-count feedback is provided in real-time from the pedometer display throughout the day, and as a daily total on the study website (www.activitydaily.org). Participants are able to view a bar graph showing each of the last 7 days with an overlay of their personal step count goal. Participants will be encouraged to wear their pedometer during all walking hours during the study.
A major component of the program is the tailored step-count goals. Each week of the 4-month intervention phase, participants randomized to the internet-based arms receive a new tailored step-count goal. The algorithm for calculating step-count goals was developed based on a series of usability strides, pilot data, and randomized controlled trials among participants with chronic conditions including CAD.43, 44 The algorithm involves adding a fixed increment to the average step count based on uploaded pedometer data from the participants over the previous seven days. Every Sunday night, participants received an email with an individual step count goal for the upcoming week. Goals are increased or decreased based on the prior 7 days’ step counts. Thus following an illness, a participant’s goals will be lower than prior weeks to avoid unrealistic step-count goals which may lead to reductions in motivation for walking. The weekly step count goal is calculated as follows:
Goals are rounded to the nearest 100 and the maximum step count goal is 10,000 steps.
The internet-based program is provided to participants in 2 study arms (internet only) and (internet plus telephone counseling) who have access to separate online communities throughout the 12-month study. The inclusion of these communities aim to enhance social support thereby improving subject retention and long-term adherence to walking.23, 24 The online communities are open only to participants within the study arm in which they were randomized to. Participants are able to read and post messages on the online message boards. The message boards are moderated by study staff who evaluate postings for appropriateness, and health and safety issues. Study staff provides user support for the message boards, as well as initiate and participate in discussions. Periodic contests and games are posted to the forums to promote ongoing participation including the uploading of step counts and posting on the message boards. Participants in either the internet alone arm or the internet plus telephone arm will have ongoing access to the study website throughout the maintenance phase (months 8–12). During this time, they will continue to receive step count goals and can post to the online communities.
Telephone Counseling
We designed the telephone counseling arm to roughly mimic a more traditional supervised PAD rehabilitation program in that the exercise prescription was based on accumulating 30–50 minutes of walking at least 3–5 times per week. The intervention included weekly telephone appointments lasting 5–15 minutes. The content for the calls is standardized and is based on the material which we planned to use in the supervised PAD rehab program including information from the Vascular Disease Foundation, the American Heart Association, and the American Association of Cardiovascular and Pulmonary Rehabilitation.42 The intervention was led by two study staff (AMK and AKL). Interventionists were trained using a standardized script and role playing. The format for each call is the same for each subject: query number of days with 30 or more minutes of walking, query satisfaction with walking, query challenges and barriers and trouble shoot if necessary, goal setting for the next week, and assessing for adverse events and side effects. Participants in the telephone counseling receive pedometers and may use the real-time feedback, but do not have access to information or tools provided on the study website.
Waitlist Control
Participants randomized to a waitlist control group receive usual care. Participants are instructed to continue with their usual activities and recommendations from their health care team. These participants do not receive a pedometer nor do they have access to the internet-based program during their active enrollment. The study team follows up with these participants monthly by email to inquire about adverse events and any changes in management strategy for their PAD.
Study Completion
At the conclusion of the follow-up phase (i.e., 12 months), participants receiving the internet-based program will continue to have access to the study website, online community, and be able to upload their step counts until the study is completed. They will not receive goals or tailored tips. Participants in the usual care and telephone counseling groups will be offered access to the study website (and usual care participants will be given a pedometer).
Monitoring and Reporting of Adverse Events
All outcome assessments were conducted during routine office hours in an active outpatient clinic at UMHS. The treadmill tests are administered by certified exercise physiologists and overseen by an attending cardiologist. All safety procedures used for cardiac testing, including criteria for test termination, are used for the PAD treadmill testing. In the event of a medical event (EKG or abnormal hemodynamic changes, or reported symptoms), the attending clinic physician and the principal investigator are consulted and a decision is made regarding patient disposition (i.e., to home, follow-up with personal physician, to emergency department, etc.). Continued study involvement is suspended until the study team receives medical clearance from the individual’s care team. Serious adverse events are reported to the IRB per protocol, and the participant’s vascular specialist or cardiologist is alerted. Vital signs are assessed before and after the 6-minute walk and contraindications to testing per the ATS guidelines.
Additionally, each participant receives a monthly “health update” email in which they are asked about new or changed symptoms, emergency department and hospital visits and other changes in health status. We receive alerts via the electronic medical record anytime a participant is admitted to the UMHS, and we ask about adverse events with each encounter (visit, phone call, etc.) and encourage participants to contact us ad lib to report health status changes.
Analysis Plan
Power Calculations
Using results of prior PAD trials45–47 which compared supervised vs. non-supervised exercise programs, a two group Satterthwaite t-test with a 0.05 two-sided significance level will have >80% power to detect a difference in means of 225.2 meters (the difference between a Group 1 mean of 546.0 meters and a Group 2 mean of 320.8 meters). These results assume that the Group 1 standard deviation is 378.8 and the Group 2 standard deviation is 315.9 meters (ratio of Group 2 to Group 1 standard deviation is 1.20) when the sample sizes in each arm is 50. We also examined our power to detect differences in HRQOL using the PAQ based on work by Spertus et al.30 A sample size of 75 in each group will have 80% power to detect a difference in means of 12.50 assuming that the common standard deviation is 27.000 using a two group t-test with a 0.05 two-sided significance level.
Prior to analysis we will examine the distributions of all measures for deviations from normality and for violations of assumption of analyses. Preliminary analyses will include evaluation of baseline characteristics by group and a comparison of change from baseline to 4 months and 12 months for all primary outcome measures (maximal walking distance, HRQOL, step-counts). All analyses will use an intention-to-treat approach where the subject will contribute data to their assigned group regardless of the amount of data contributed (i.e., the duration or dose of participation). Maximal walking distance will be compared between the intervention groups and the control group using analysis of covariance with baseline step count as a covariate for two periods: one for 4-months and one for 12-months. Means and standard deviations of the within-person changes will be calculated. We will use a multi-variable general linear modeling approach with maximal walking distance as the dependent variable, and group assignment, baseline maximal walking distance, and baseline scores as the independent variables. Age, sex, disease severity, and current smoking status will be among some of the factors explored as potential confounders. We will run separate models for pain-free walking distance among subjects who report claudication symptoms at baseline. Finally, we will again use multi-variable general linear models with HRQOL at 4 and 12 months as the dependent variable and adjusted for baseline and for 12-months adjusted for the 4-months status. Specific domains within the HRQOL measures will be examined separately.
For aim 2 (examining the effectiveness of an automated internet-based walking program to improve adherence to long-term walking among patients with PAD) we will use a similar approach. We will examine change from 4-months, which corresponds to the completion of the intervention phase to 12-months. The primary outcome will be change in step-counts characterized by number of days that regular walking bouts occurred, and weekly step-counts. We will examine the data for correlations between frequency of participants downloading step-counts, frequency of interactions with the online community (postings), and measures of long-term adherence to walking. We will not have step counts for the control group, but will examine changes in treadmill measures of total walking distance and pain-free walking distance in addition to HRQOL (at 4 and 12 months) and self-reported physical activity from our surveys.
Discussion
Walking is a class one recommendation for patients with PAD, with evidence that supervised programs improve physical function, reduces claudication symptoms and improves maximal walking distance.7 Much of the literature supports supervised walking programs.10–13 A Cochrane review which included 22 trials noted improvements in maximal walking time of 5.12 minutes (95% confidence interval [CI] 4.51 to 5.72) compared to usual care or placebo.10 Improvements in pain-free walking distance were also observed with both supervised and home-based walking programs without increased CVD risk.28, 47 The Claudication: Exercise Versus Endoluminal Revascularization (CLEVER) study observed better peak walking distances at 6-months among patients randomized to a supervised walking program as compared to patients who received peripheral stenting. However, barriers to walking programs are numerous, and include financial barriers as most health insurances do not cover supervised PAD programs, and travel-related challenges. We hypothesized that an internet-based walking program would be well received by PAD patients as the barriers related to cost and geographic distance would be reduced. The current study employed a novel approach of leveraging the popularity of internet-based programming and the ease of telephone counseling as two options to promote walking within one’s own community.
Several studies have compared home-based exercise programs to supervised programs.47 Trials of home-based programs have significant limitations including a wide variation in the exercise recommendations and adherence rates. Perhaps more importantly, trials of home-based programs have relied primarily on participant’s self-report using paper diaries or periodic surveys to assess adherence to walking.28, 48 This may be an important limitation, given that there is data to suggest patient report of walking distance may not correlate well with actual walking distance.32, 49, 50 Thus, it is not surprising that at least one study of a home-based walking program has observed no increase in maximal walking distance compared to control.51 Use of a pedometer, which can download up to 41 days of walking data, provide for a detailed examination and a more detailed comparison of home-based versus supervised walking.
Digital health programs can reduce or eliminate barriers to participation observed in supervised exercise programs, while improving limitations observed in older studies of home-based exercise. Internet-based walking programs have been found to be effective in other chronic conditions including diabetes and heart disease.23–25 Furthermore they can be cost effective.19–22 Lastly, the number of Americans with internet access and who regularly use email and/or social media have increased dramatically, a trend which will likely continue in the future.52, 53
The Physical Activity Daily trial is one of the first to use the internet to deliver a walking program for patients with PAD. Use of commercially available activity trackers with tailored internet-based educational content and individual step-count goals enhance the reach of this program. We also incorporated an online community to provide peer-support to participants within the intervention arm. Use of an online community has been shown to improve long-term adherence to walking among subjects with chronic conditions such as CAD and DM.23 If effective, this program can be implemented in larger populations of PAD patients irrespective of geography and at potentially low-cost.
Acknowledgments
We would like to thank the participants of the PAD study for their time, and the clinical providers who care for PAD patients and who have provided enthusiastic support for the study.
Funding Source: This work was supported by the National Institutes of Health National Heart, Lung and Blood Institute (RO1AG045136).
Footnotes
Trial Registration No: NCT02767895
References
- 1.Heald CL, Fowkes FG, Murray GD, Price JF. Risk of mortality and cardiovascular disease associated with the ankle-brachial index: Systematic review. Atherosclerosis. 2006;189:61–9. doi: 10.1016/j.atherosclerosis.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 2.Fowkes FG, Murray GD, Butcher I, Heald CL, Lee RJ, Chambless LE, Folsom AR, Hirsch AT, Dramaix M, deBacker G, Wautrecht JC, Kornitzer M, Newman AB, Cushman M, Sutton-Tyrrell K, Lee AJ, Price JF, d'Agostino RB, Murabito JM, Norman PE, Jamrozik K, Curb JD, Masaki KH, Rodriguez BL, Dekker JM, Bouter LM, Heine RJ, Nijpels G, Stehouwer CD, Ferrucci L, McDermott MM, Stoffers HE, Hooi JD, Knottnerus JA, Ogren M, Hedblad B, Witteman JC, Breteler MM, Hunink MG, Hofman A, Criqui MH, Langer RD, Fronek A, Hiatt WR, Hamman R, Resnick HE, Guralnik J. Ankle brachial index combined with Framingham Risk Score to predict cardiovascular events and mortality: a meta-analysis. JAMA. 2008;300:197–208. doi: 10.1001/jama.300.2.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McDermott MM, Ferrucci L, Liu K, Guralnik JM, Tian L, Kibbe M, Liao Y, Tao H, Criqui MH. Women with peripheral arterial disease experience faster functional decline than men with peripheral arterial disease. J Am Coll Cardiol. 2011;57:707–14. doi: 10.1016/j.jacc.2010.09.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Writing Group M; Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, Das SR, de Ferranti S, Despres JP, Fullerton HJ, Howard VJ, Huffman MD, Isasi CR, Jimenez MC, Judd SE, Kissela BM, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Magid DJ, McGuire DK, Mohler ER, 3rd, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Rosamond W, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Woo D, Yeh RW, Turner MB American Heart Association Statistics C and Stroke Statistics S. Heart Disease and Stroke Statistics-2016 Update: A Report From the American Heart Association. Circulation. 2016;133:e38–60. doi: 10.1161/CIR.0000000000000350. [DOI] [PubMed] [Google Scholar]
- 5.Fowkes FG, Rudan D, Rudan I, Aboyans V, Denenberg JO, McDermott MM, Norman PE, Sampson UK, Williams LJ, Mensah GA, Criqui MH. Comparison of global estimates of prevalence and risk factors for peripheral artery disease in 2000 and 2010: a systematic review and analysis. Lancet. 2013;382:1329–40. doi: 10.1016/S0140-6736(13)61249-0. [DOI] [PubMed] [Google Scholar]
- 6.Roger VL, Go AS, Lloyd-Jones DM, Adams RJ, Berry JD, Brown TM, Carnethon MR, Dai S, de Simone G, Ford ES, Fox CS, Fullerton HJ, Gillespie C, Greenlund KJ, Hailpern SM, Heit JA, Ho PM, Howard VJ, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Makuc DM, Marcus GM, Marelli A, Matchar DB, McDermott MM, Meigs JB, Moy CS, Mozaffarian D, Mussolino ME, Nichol G, Paynter NP, Rosamond WD, Sorlie PD, Stafford RS, Turan TN, Turner MB, Wong ND, Wylie-Rosett J. Heart Disease and Stroke Statistics--2011 Update: A Report From the American Heart Association. Circulation. 2010 doi: 10.1161/CIR.0b013e3182009701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hirsch AT, Haskal ZJ, Hertzer NR, Bakal CW, Creager MA, Halperin JL, Hiratzka LF, Murphy WR, Olin JW, Puschett JB, Rosenfield KA, Sacks D, Stanley JC, Taylor LM, Jr, White CJ, White J, White RA, Antman EM, Smith SC, Jr, Adams CD, Anderson JL, Faxon DP, Fuster V, Gibbons RJ, Hunt SA, Jacobs AK, Nishimura R, Ornato JP, Page RL, Riegel B. ACC/AHA Guidelines for the Management of Patients with Peripheral Arterial Disease (lower extremity, renal, mesenteric, and abdominal aortic): a collaborative report from the American Associations for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (writing committee to develop guidelines for the management of patients with peripheral arterial disease)--summary of recommendations. J Vasc Interv Radiol. 2006;17:1383–97. doi: 10.1097/01.RVI.0000240426.53079.46. quiz 1398. [DOI] [PubMed] [Google Scholar]
- 8.Regensteiner JG, Hiatt WR, Coll JR, Criqui MH, Treat-Jacobson D, McDermott MM, Hirsch AT. The impact of peripheral arterial disease on health-related quality of life in the Peripheral Arterial Disease Awareness, Risk, and Treatment: New Resources for Survival (PARTNERS) Program. Vasc Med. 2008;13:15–24. doi: 10.1177/1358863X07084911. [DOI] [PubMed] [Google Scholar]
- 9.Smolderen KG, Hoeks SE, Pedersen SS, van Domburg RT, de L, II, Poldermans D. Lower-leg symptoms in peripheral arterial disease are associated with anxiety, depression, and anhedonia. Vasc Med. 2009;14:297–304. doi: 10.1177/1358863X09104658. [DOI] [PubMed] [Google Scholar]
- 10.Watson L, Ellis B, Leng GC. Exercise for intermittent claudication. Cochrane Database Syst Rev. 2008:CD000990. doi: 10.1002/14651858.CD000990.pub2. [DOI] [PubMed] [Google Scholar]
- 11.Hiatt WR, Regensteiner JG, Hargarten ME, Wolfel EE, Brass EP. Benefit of exercise conditioning for patients with peripheral arterial disease. Circulation. 1990;81:602–9. doi: 10.1161/01.cir.81.2.602. [DOI] [PubMed] [Google Scholar]
- 12.Hiatt WR, Wolfel EE, Meier RH, Regensteiner JG. Superiority of treadmill walking exercise versus strength training for patients with peripheral arterial disease. Implications for the mechanism of the training response. Circulation. 1994;90:1866–74. doi: 10.1161/01.cir.90.4.1866. [DOI] [PubMed] [Google Scholar]
- 13.Sanderson B, Askew C, Stewart I, Walker P, Gibbs H, Green S. Short-term effects of cycle and treadmill training on exercise tolerance in peripheral arterial disease. J Vasc Surg. 2006;44:119–27. doi: 10.1016/j.jvs.2006.03.037. [DOI] [PubMed] [Google Scholar]
- 14.Dawson DL, Cutler BS, Meissner MH, Strandness DE., Jr Cilostazol has beneficial effects in treatment of intermittent claudication: results from a multicenter, randomized, prospective, double-blind trial. Circulation. 1998;98:678–86. doi: 10.1161/01.cir.98.7.678. [DOI] [PubMed] [Google Scholar]
- 15.Money SR, Herd JA, Isaacsohn JL, Davidson M, Cutler B, Heckman J, Forbes WP. Effect of cilostazol on walking distances in patients with intermittent claudication caused by peripheral vascular disease. J Vasc Surg. 1998;27:267–74. doi: 10.1016/s0741-5214(98)70357-x. discussion 274–5. [DOI] [PubMed] [Google Scholar]
- 16.Gardner AW, Poehlman ET. Exercise rehabilitation programs for the treatment of claudication pain. A meta-analysis. JAMA. 1995;274:975–80. [PubMed] [Google Scholar]
- 17.Murphy TP, Cutlip DE, Regensteiner JG, Mohler ER, Cohen DJ, Reynolds MR, Massaro JM, Lewis BA, Cerezo J, Oldenburg NC, Thum CC, Goldberg S, Jaff MR, Steffes MW, Comerota AJ, Ehrman J, Treat-Jacobson D, Walsh ME, Collins T, Badenhop DT, Bronas U, Hirsch AT. Supervised Exercise Versus Primary Stenting for Claudication Resulting From Aortoiliac Peripheral Artery Disease: Six-Month Outcomes From the Claudication: Exercise Versus Endoluminal Revascularization (CLEVER) Study. Circulation. 2012;125:130–139. doi: 10.1161/CIRCULATIONAHA.111.075770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gerhard-Herman MD, Gornik HL, Barrett C, Barshes NR, Corriere MA, Drachman DE, Fleisher LA, Fowkes FG, Hamburg NM, Kinlay S, Lookstein R, Misra S, Mureebe L, Olin JW, Patel RA, Regensteiner JG, Schanzer A, Shishehbor MH, Stewart KJ, Treat-Jacobson D, Walsh ME. 2016 AHA/ACC Guideline on the Management of Patients With Lower Extremity Peripheral Artery Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2016 doi: 10.1161/CIR.0000000000000471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Warmerdam L, Smit F, van Straten A, Riper H, Cuijpers P. Cost-utility and cost-effectiveness of internet-based treatment for adults with depressive symptoms: randomized trial. J Med Internet Res. 2010;12:e53. doi: 10.2196/jmir.1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Andersson G, Bergstrom J, Carlbring P, Lindefors N. The use of the Internet in the treatment of anxiety disorders. Curr Opin Psychiatry. 2005;18:73–7. [PubMed] [Google Scholar]
- 21.Andersson G. Internet-based cognitive-behavioral self help for depression. Expert Rev Neurother. 2006;6:1637–42. doi: 10.1586/14737175.6.11.1637. [DOI] [PubMed] [Google Scholar]
- 22.Andersson G, Cuijpers P. Internet-based and other computerized psychological treatments for adult depression: a meta-analysis. Cogn Behav Ther. 2009;38:196–205. doi: 10.1080/16506070903318960. [DOI] [PubMed] [Google Scholar]
- 23.Resnick PJ, Janney AW, Buis LR, Richardson CR. Adding an online community to an internet-mediated walking program. Part 2: strategies for encouraging community participation. J Med Internet Res. 2010;12:e72. doi: 10.2196/jmir.1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Richardson CR, Buis LR, Janney AW, Goodrich DE, Sen A, Hess ML, Mehari KS, Fortlage LA, Resnick PJ, Zikmund-Fisher BJ, Strecher VJ, Piette JD. An online community improves adherence in an internet-mediated walking program. Part 1: results of a randomized controlled trial. J Med Internet Res. 2010;12:e71. doi: 10.2196/jmir.1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bain TM, Frierson GM, Trudelle-Jackson E, Morrow JR., Jr Internet reporting of weekly physical activity behaviors: the WIN Study. J Phys Act Health. 2010;7:527–32. doi: 10.1123/jpah.7.4.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gardner AW, Skinner JS, Cantwell BW, Smith LK. Progressive vs single-stage treadmill tests for evaluation of claudication. Med Sci Sports Exerc. 1991;23:402–8. [PubMed] [Google Scholar]
- 27.Degischer S, Labs KH, Hochstrasser J, Aschwanden M, Tschoepl M, Jaeger KA. Physical training for intermittent claudication: a comparison of structured rehabilitation versus home-based training. Vasc Med. 2002;7:109–15. doi: 10.1191/1358863x02vm432oa. [DOI] [PubMed] [Google Scholar]
- 28.Savage P, Ricci MA, Lynn M, Gardner A, Knight S, Brochu M, Ades P. Effects of home versus supervised exercise for patients with intermittent claudication. J Cardiopulm Rehabil. 2001;21:152–7. doi: 10.1097/00008483-200105000-00006. [DOI] [PubMed] [Google Scholar]
- 29.Gardner AW, Skinner JS, Vaughan NR, Bryant CX, Smith LK. Comparison of three progressive exercise protocols in peripheral vascular occlusive disease. Angiology. 1992;43:661–71. doi: 10.1177/000331979204300806. [DOI] [PubMed] [Google Scholar]
- 30.Spertus J, Jones P, Poler S, Rocha-Singh K. The peripheral artery questionnaire: a new disease-specific health status measure for patients with peripheral arterial disease. Am Heart J. 2004;147:301–8. doi: 10.1016/j.ahj.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 31.Ware JE, Jr, Gandek B. Overview of the SF-36 Health Survey and the International Quality of Life Assessment (IQOLA) Project. J Clin Epidemiol. 1998;51:903–12. doi: 10.1016/s0895-4356(98)00081-x. [DOI] [PubMed] [Google Scholar]
- 32.McDermott MM, Liu K, Guralnik JM, Martin GJ, Criqui MH, Greenland P. Measurement of walking endurance and walking velocity with questionnaire: validation of the walking impairment questionnaire in men and women with peripheral arterial disease. J Vasc Surg. 1998;28:1072–81. doi: 10.1016/s0741-5214(98)70034-5. [DOI] [PubMed] [Google Scholar]
- 33.EuroQol G. EuroQol--a new facility for the measurement of health-related quality of life. Health policy. 1990;16:199–208. doi: 10.1016/0168-8510(90)90421-9. [DOI] [PubMed] [Google Scholar]
- 34.Cella D, Riley W, Stone A, Rothrock N, Reeve B, Yount S, Amtmann D, Bode R, Buysse D, Choi S, Cook K, Devellis R, DeWalt D, Fries JF, Gershon R, Hahn EA, Lai JS, Pilkonis P, Revicki D, Rose M, Weinfurt K, Hays R. The Patient-Reported Outcomes Measurement Information System (PROMIS) developed and tested its first wave of adult self-reported health outcome item banks: 2005–2008. J Clin Epidemiol. 2010;63:1179–94. doi: 10.1016/j.jclinepi.2010.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dyer MT, Goldsmith KA, Sharples LS, Buxton MJ. A review of health utilities using the EQ-5D in studies of cardiovascular disease. Health and quality of life outcomes. 2010;8:13. doi: 10.1186/1477-7525-8-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hays RD, Bjorner JB, Revicki DA, Spritzer KL, Cella D. Development of physical and mental health summary scores from the patient-reported outcomes measurement information system (PROMIS) global items. Qual Life Res. 2009;18:873–80. doi: 10.1007/s11136-009-9496-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van der Linden WJ, Hambleton RK. Handbook of Modern Item Response Theory. New York: Springer-Verlag; 1997. [Google Scholar]
- 38.Lai JS, Cella D, Choi S, Junghaenel DU, Christoudolou C, Gershon R, Stone A. How item banks and its applications can influence measurement practice in rehabilitation medicine: A PROMIS fatigue item bank example. Arch Phys Med Rehabil. 2011;92:S20–S27. doi: 10.1016/j.apmr.2010.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rothrock NE, Hays RD, Spritzer K, Yount SE, Riley W, Cella D. Relative to the general US population, chronic diseases are associated with poorer health-related quality of life as measured by the Patient-Reported Outcomes Measurement Information System (PROMIS) J Clin Epidemiol. 2010;63:1195–204. doi: 10.1016/j.jclinepi.2010.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Holbrook EA, Barreira TV, Kang M. Validity and reliability of Omron pedometers for prescribed and self-paced walking. Med Sci Sports Exerc. 2009;41:670–4. doi: 10.1249/MSS.0b013e3181886095. [DOI] [PubMed] [Google Scholar]
- 41.Laboratories ATSCoPSfCPF. ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166:111–7. doi: 10.1164/ajrccm.166.1.at1102. [DOI] [PubMed] [Google Scholar]
- 42.AACVPR. VDFVatAAoCaPR. 2016 Vasculardisease.org/files/pad-exercise-training-toolkit.pdf.
- 43.Richardson CR, Brown BB, Foley S, Dial KS, Lowery JC. Feasibility of adding enhanced pedometer feedback to nutritional counseling for weight loss. J Med Internet Res. 2005;7:e56. doi: 10.2196/jmir.7.5.e56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Moy ML, Janney AW, Nguyen HQ, Matthess KR, Cohen M, Garshick E, Richardson CR. Use of pedometer and Internet-mediated walking program in patients with chronic obstructive pulmonary disease. J Rehabil Res Dev. 2010;47:485–96. doi: 10.1682/jrrd.2009.07.0091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cheetham DR, Burgess L, Ellis M, Williams A, Greenhalgh RM, Davies AH. Does supervised exercise offer adjuvant benefit over exercise advice alone for the treatment of intermittent claudication? A randomised trial. Eur J Vasc Endovasc Surg. 2004;27:17–23. doi: 10.1016/j.ejvs.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 46.Patterson RB, Pinto B, Marcus B, Colucci A, Braun T, Roberts M. Value of a supervised exercise program for the therapy of arterial claudication. J Vasc Surg. 1997;25:312–8. doi: 10.1016/s0741-5214(97)70352-5. discussion 318–9. [DOI] [PubMed] [Google Scholar]
- 47.Bendermacher BL, Willigendael EM, Teijink JA, Prins MH. Supervised exercise therapy versus non-supervised exercise therapy for intermittent claudication. Cochrane Database Syst Rev. 2006:CD005263. doi: 10.1002/14651858.CD005263.pub2. [DOI] [PubMed] [Google Scholar]
- 48.Nielsen SL, Larsen B, Prahl M, Jensen CT, Jensen BE, Wenkens V. Hospital training compared with home training in patients with intermittent claudication. Ugeskr Laeger. 1977;139:2733–6. [PubMed] [Google Scholar]
- 49.Watson CJ, Collin J. Estimates of distance by claudicants and vascular surgeons are inherently unreliable. Eur J Vasc Endovasc Surg. 1998;16:429–30. doi: 10.1016/s1078-5884(98)80012-9. [DOI] [PubMed] [Google Scholar]
- 50.Watson CJ, Phillips D, Hands L, Collin J. Claudication distance is poorly estimated and inappropriately measured. Br J Surg. 1997;84:1107–9. [PubMed] [Google Scholar]
- 51.Collins TC, Lunos S, Carlson T, Henderson K, Lightbourne M, Nelson B, Hodges JS. Effects of a home-based walking intervention on mobility and quality of life in people with diabetes and peripheral arterial disease: a randomized controlled trial. Diabetes Care. 2011;34:2174–9. doi: 10.2337/dc10-2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Eysenbach G. Medicine 2.0: social networking, collaboration, participation, apomediation, and openness. J Med Internet Res. 2008;10:e22. doi: 10.2196/jmir.1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hesse BW, Nelson DE, Kreps GL, Croyle RT, Arora NK, Rimer BK, Viswanath K. Trust and sources of health information: the impact of the Internet and its implications for health care providers: findings from the first Health Information National Trends Survey. Arch Intern Med. 2005;165:2618–24. doi: 10.1001/archinte.165.22.2618. [DOI] [PubMed] [Google Scholar]