Abstract
Investigating the complex interplay between blood cells and the endothelium is crucial in understanding the pathophysiology of many diseases. Observation of the in vivo vasculature is difficult due to the complexities of vessel geometry, limited visualization capability, as well as variability and complexity inherent to biologic systems. Therefore, in vitro systems serve as ideal tools to study these cellular interactions. Microfluidic technologies are an ideal tool for recapitulating the vasculature in vivo as they can be used to fabricate fluidic channels on the size scale capillaries using gas permeable, biologically inert, and optically transparent substrates. Microfluidic channels can be vascularized by coating the inner surface of the microchannels with a confluent monolayer of endothelial cells, representing a reductionist, tightly controlled, in vitro model of the microvasculature. In this review, we present advances in the field of “endothelialized” microfluidics, focusing specifically on non-traditional fabrication and endothelialization techniques. We then summarize the various applications of endothelialized microfluidics, and speculate on the future directions of the field, including the exciting applications to personalized medicine.
Introduction
Studying the cellular interactions which occur within the vasculature is of utmost importance in understanding the pathophysiology of various vascular etiologies such as sickle cell disease, malaria, and stroke[1–3]. These cellular interactions are controlled, in part, by the hemodynamic and rheological forces present within the vasculature[4]. For example, platelets have been shown to preferentially aggregate and activate in regions of high shear stress created by vascular abnormalities such as stenotic lesions[5, 6]. Furthermore, many underlying physiological complications that arise from these disease states have origins in the microvasculature, such as pain crisis and stroke in sickle cell disease[7]. Therefore, a complete investigation of the pathophysiology of various vascular diseases requires characterization of these hemodynamic forces and the impact that they have on cellular interactions within the microvasculature.
Due to the complexity of in vivo models, in which microvascular geometries and system inputs cannot be readily controlled, reductionist, in vitro representations of the microvasculature are necessary to fully characterize the pathophysiology of various diseases. To that end, microfluidic technologies have been developed to address this need (Fig. 1) [8–11]. In this review, we will present advances in the field of “endothelialized” microfluidics in which endothelial cells are seeded onto microchannels in order to recapitulate the in vivo microvasculature and investigate various vascular and hematological processes. We will particularly highlight recent advances in the field that utilize alternatives to the traditional photolithography-based microfluidic paradigm.
Fig. 1. Top view of a microfluidic device filled with dye to accentuate channels.
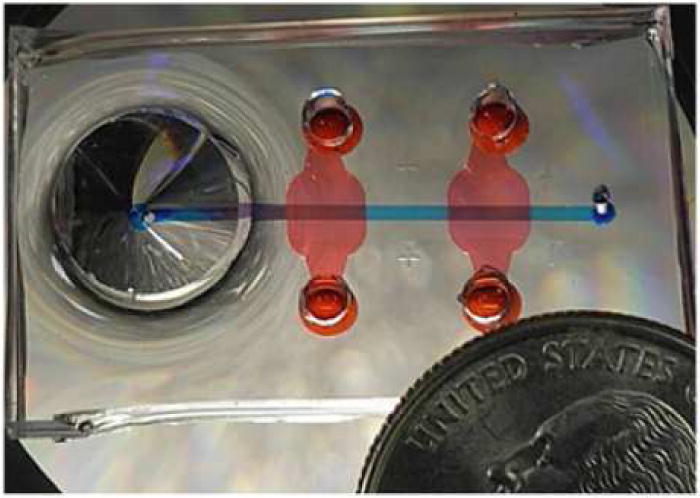
Image reproduced, with permission, from reference 10.
Microfluidic techniques for recapitulating in vivo vasculature
Microfluidics refers to the fabrication of small channels (channel width on the scale of microns) for the purpose of fluid flow[12]. These techniques are particularly useful for conducting biological assays and studying biological processes and phenomena for a number of reasons. First, the geometry of microfluidic devices can be very tightly controlled during fabrication. In the context of the microvasculature, tight geometric control facilitates investigation of biological interactions at specific geometric structures within the microvasculature (stenosis, aneurysm, vascular bifurcations, etc.) [13–15]. Importantly, microfluidic devices can be fabricated using microscopy compatible, transparent substrates, allowing for facile imaging of microvascular processes[16, 17]. Additionally, these devices may be constructed out of biologically inert materials, ensuring that the microvascular process of interest can be easily studied with minimal interference[18]. Finally and most importantly, microfluidic devices allow for control of the system inputs, which represent a significant improvement over in vivo models due to the inherent complexity of biological systems. These reductionist systems allow for isolation of specific microvascular processes for investigation.
The most common microfluidic technique used to develop in vitro vascular models is known as soft lithography[19, 20]. Adapted from the semiconductor industry, soft lithography utilizes the patterned exposure of a photosensitive material to develop a master mold which serves as the geometric structure of the devices. Plasma bonding and replica molding of the transparent, biologically inert, flexible silicone, gas permeable polymer polydimethylsiloxane (PDMS) is the most common technique used to create microfluidic channels[21]. These techniques are very robust and repeatable; however, the photolithography process requires specialized engineering training and expensive equipment. This labor intensive fabrication process precludes rapid iteration and testing of novel microvascular models. This represents a significant barrier to entry of the field to many researchers trying to study microvascular phenomena. Thus, a need exists for techniques utilizing in vitro microfluidics that avoid complex fabrication.
To that end, many innovative techniques have been developed that do not require photolithography to recapitulate microvascular environments. One such technique, 3D printing, allows for the development of 3 dimensional objects layer by layer. Once a desired geometry is designed in a computer aided design (CAD) program, microfluidic channels themselves may be constructed in one step, rather than fabrication of a master mold which must then be cast in PDMS[22]. Liu et al. describe a system in which a proprietary material was used to print channels which were subsequently combined with endothelialized inserts to study the interactions between blood vessel walls and red blood cells (RBCs) [23]. Gross et al. describe a method in which PDMS is coated on the inside of a 3D printed channel, which was subsequently used for endothelial cell culture, combining the benefits of PDMS with those of 3D printing[24]. Drolet et al. present a technique in which a 3D printer is modified to print a dissolvable sugar glass (Fig. 2), which may be encased in a biocompatible scaffold and dissolved, defining the microchannel geometry which can then be endothelialized[25]. Microfibers represent another technique used to create 3D structures for investigating microvascular cellular processes due to their ease of preparation and assembly, utilizing techniques such as multiple laminar flows and electrospinning[26]. Cheng et al. report a system in which hollow, bio-functional microfibers were formed using multiple laminar flows (a quick-gelling, biologically active alginate sheath flow surrounding a poly(vinyl alcohol) inner phase fluid) which were then endothelialized[27].
Fig. 2. Production of a microfluidic device using 3D printing of sugar glass.
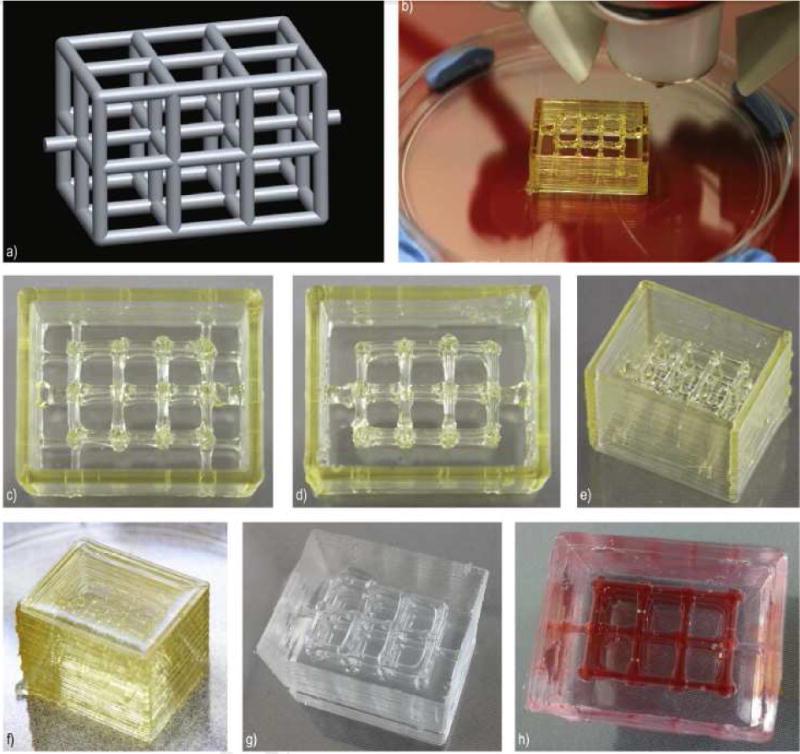
a) CAD model of the desired microfluidic channel. b) Device in the process of printing. c–e) Completed view of the sugar glass structure. f) Sugar glass structure after casting in PDMS g) microfluidic chambers after casting with PDMS and dissolution of sugar glass channels. h) Red Dye added for channel visualization. Image reproduced, with permission, from reference 25.
One non-traditional microfabrication technique of particular importance is the “do-it-yourself” method developed by Mannino et al[28]. Rather than utilizing microfabrication facilities or expensive 3D printing equipment, this technique relies on off-the-shelf materials alone to generate robust, repeatable microfluidic channels. This technique utilizes poly(methyl methacrylate) (PMMA) optical fiber (500μm diameter encased in, and subsequently removed from, PDMS in order to form microchannels that mimic optical fiber can be made easily to create different geometries found in the environment of the microvasculature such as stenoses, aneurysms, and bifurcations (Fig. 3B). This technique represents a significant benefit over photolithography because it obviates the need for expensive microfabrication equipment and experience. Furthermore, the resultant structures have round lumens, a feature which is atypical of microfluidic devices and is more physiologically accurate[29, 30]. It is important to note that the choice of substrate material used and the microvascular geometries generated by these non-traditional fabrication processes is crucial to the success of these devices, as substrate mechanical properties and geometry-mediated fluid shear stress experienced by endothelial cells have been shown to significantly impact surface protein expression as well as permeability of the endothelial monolayer in the context of microfluidics[28, 31, 32].
Fig. 3. Endothelialized microfluidics can be developed using off-the-shelf laboratory materials.
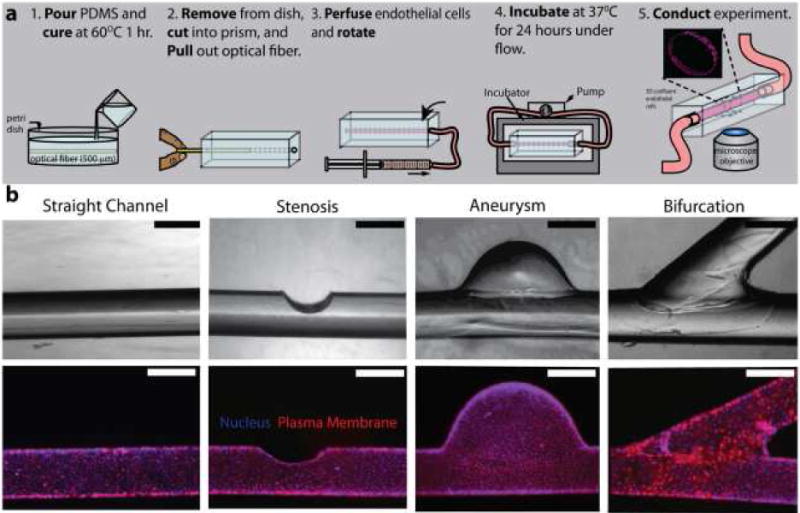
a) Fabrication process flow of this “do-it-yourself” endothelialized microfluidic device. b) Different microvascular geometries created via slight alterations in the fabrication protocol. Scale bars represent 500 μm. Image reproduced, with permission, from reference 28.
Endothelialization techniques within microfluidic devices
While creating physical channels is a crucial step in the development of in vitro microvascular models, these models must seamlessly incorporate the biological components of blood vessels found in vivo to accurately recapitulate the microvasculature. Endothelialization is the key element of any in vitro microfluidic vasculature model. Endothelialization refers to the process of lining the microchannel lumen with a 3-dimensional monolayer of endothelial cells. Endothelial cells line the inner surface of blood vessels and play a key role in the barrier function of the microvasculature. Additionally, endothelial cells are a primary contributor to the cellular interactions that occur within the microvasculature[33–35]. Endothelialization of microfluidic channels typically involves the deposition of an extracellular matrix (ECM) protein, along the inner surface of the microchannels to provide the endothelial cells with a basement layer to adhere to[36, 37]. The primary ECM proteins collagen, fibronectin, and laminin have all been used to promote endothelial attachment and healthy endothelial phenotype in microfluidic devices fabricated from multiple substrates (e.g. Polystyrene and PDMS) [38, 39] After the basement ECM layer is deposited within the microchannels, endothelial cells are perfused through the microchannels, where they are allowed to adhere to, and spread across the microchannel walls. The cells are cultured until they spread and form a monolayer uniformly across the entire inner surface of the microchannels.
Several unique approaches have been developed to endothelialise microfluidic devices. Rotational seeding was utilized by Mannino et al. to endothelialized larger microfluidic devices (500+ μm diameter) [28]. Typically, in microfluidic systems, gravity plays a smaller role in endothelial spreading than capillary action (i.e. cells will spread in all directions within channels regardless of orientation with respect to gravity) [40]. However, in larger microfluidic devices (on the order of 500μm diameter channels) gravity plays a larger role, and rotation about the central axis of the microchannel becomes crucial to ensure successful seeding of endothelial cells and formation of the endothelial monolayer. Hewes et al. reports a technique in which an inkjet printer was modified to bioprint free-standing micro vessels by continuously printing endothelial cell-laden alginate drops into a cross-linker bath in a circular pattern[41]. This technique represents a novel microfluidic fabrication method, as well as a novel endothelialization technique, as the endothelial cells required to recapitulate the microvasculature are already present in the structural material used to create the microfluidic device.
Applications of endothelialized microfluidics
Endothelialized microfluidic techniques have a wide variety of applications in biomedical engineering and medicine. In addition to the examples previously presented, endothelialized microfluidic technology has been used to study a variety of cellular interactions that occur in the microvasculature, such as leukocyte-endothelial and RBC-endothelial interactions in vitro [42, 43]. Furthermore, endothelialized microfluidic technology has been utilized to investigate microvascular phenomena implicated in diverse pathologies. Jain et al. report a novel microfluidic device containing a chemically preserved endothelium that can be used to evaluate platelet aggregation and thrombosis under different physiological and pharmacological conditions after prolonged storage[44]. The ability to successfully evaluate thrombosis after prolonged storage potentially allows this technology to function in point-of-care settings. Furthermore, endothelial monolayers have been developed within ECM-based hydrogels to study a variety of microvascular processes. In order to study the spatiotemporal effects of chemotherapy delivery within a tumor, “do-it-yourself” endothelialized microfluidics have been utilized by fabricating a micro vessel within a tumor cell laden hydrogel (Fig. 4A–C) [45]. Chemotherapy can then be perfused though this “do-it-yourself” tumor-on-a-chip and the effects of the drug on the cancer cells within the model can be visualized in real time, something that was previously impossible with typical in vivo tumor models. Additionally, Bischel et al. report the development of a hydrogel-based microfluidic device that can be used to study angiogenesis in vitro, enabling the creation of new microchannels within microfluidic devices as well as the observation of this process (Fig. 4D–E) [46].
Fig. 4. Vascular networks can be generated within hydrogels.
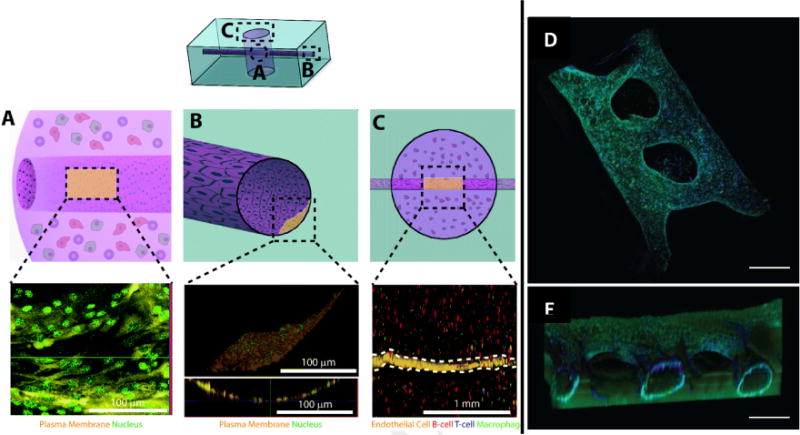
A–C) Endothelial cells can be successfully cultured within microchannels traversing a tumor cell-laden hydrogel. Image reproduced, with permission, from reference 45 D–E) In vitro angiogenesis assay featuring endothelial channels which are encouraged to invade the surrounding hydrogel. Scale bars represent 500 μm. Image reproduced, with permission, from reference 46.
The composition of the endothelial monolayer may also be varied in order to study the vasculature of specific regions in the body. In this case, endothelial cells may be co-cultured with organspecific cells to investigate organ-specify microvascular cellular interactions. Chonan et al. report a microfluidic system in which glioma initiating cells are co-cultured with endothelial cells in order to the invasive properties of glioblastoma[47]. Wang et al. and Pradhakarpandian et al. report co-cultured microfluidic systems that employs co-culture to simulate the highly selective barrier function of the blood brain barrier (BBB) [48, 49]. Jain et al. have recently demonstrated co-culture of vascular lumen and alveolar epithelial cells under whole blood perfusion, and shown organ-level responses to pulmonary injury, vascular inflammation and thrombus formation [50]. As these studies indicate, co-culture of endothelial cells with organ specific cells alters endothelial function to more accurately recapitulate the organ of interest. For example, co-culture of endothelial cells with alveolar epithelial cells as well as astrocytes and pericytes had a significant impact on the permeability of the endothelial monolayer[48, 50]. When co-cultured with astrocytes and pericytes, endothelial permeability decreased, due to the fact that these cell types maintain the highly impermeable function of the blood-brain barrier[48]. When co-cultured with alveolar epithelial cells in the presence of lipopolysaccharide endotoxin, endothelial permeability increased due to activation of the epithelial cells and subsequent interaction with the endothelium rather than interaction with the endothelium alone[50]. Overall, as the pathophysiology of many disease states profoundly impacts the vasculature, these models serve as key test beds for elucidating the complex role of the microvasculature in disease.
Conclusion and Future Directions
Endothelialized microfluidics offer advantages over traditional in vivo and in vitro approaches to studying the microvasculature, including the ability to isolate the system of interest from the variability and confounding factors found in vivo, as well as the ability to tightly control the geometry of the desired system. These methods have recently been adapted for a myriad of applications in the microvascular space, by utilizing cutting edge advances in microfabrication technology. While traditional photolithography-based approaches to developing these microfluidic models offer the ability to accurately and repeatably generate channels with tightly controlled geometries, they suffer from the time, training, cost, and equipment requirements necessary to develop them. In order to minimize the impact of these drawbacks, non-traditional fabrication techniques utilizing diverse tools such as 3D-printing, electrospininning, inkjet printing, and “do-it-yourself” replica molding have been used to generate endothelialized microfluidic. While these techniques do not require specialized cleanroom equipment and training like traditional photolithography, they are not without drawbacks. These techniques also come with their own associated costs and specialized equipment, and suffer from channel resolution and repeatability issues with respect to traditional photolithography. Overall, these methods represent a crucial tool in improving our understanding of microvascular processes and phenomena.
Going forward, endothelialized microfluidic technology is being adapted to address a wide range of problems. As fabrication methods used to develop these technologies improve, fabrication of more complex vascular geometries (e.g. complex tortuosity of blood vessels found in vivo) will be enabled[51]. Fabrication of complex vascular geometries enables more accurate recapitulation of the in vivo environment, which further enables more physiologically relevant research. Furthermore, the increased sophistication of fabrication techniques may enable microfluidic advances into the field of personalized medicine. For example, endothelialized microfluidic techniques (e.g. 3D printing) may be used in conjunction with clinical imaging (e.g. computed tomography, magnetic resonance imaging, and angiography) to develop patient specific microvascular models which can then be used as a test-bed for potential therapies[52, 53]. Additionally, these technologies may be personalized via culture with patient-derived cells in order to study the patient-specific impact of treatment approaches[54]. Finally, the addition of multi-cell co-cultures increases the number of vascular environments and pathophysiologies that may be studied using these techniques[55, 56]. Overall, culturing the inner surface of microfluidic devices with endothelial cells has enabled researchers to observe and investigate microvascular phenomena which were previously inaccessible via traditional in vivo and in vitro models. As this field continues to grow and mature using sophisticated, yet non-traditional, fabrication techniques, researchers will have unparalleled access to investigate disease pathophysiology and test potential clinical therapies in vitro.
Highlights.
Microfluidics can be endothelialized to recapitulate the microvasculature in vitro.
Fabrication techniques advances have increased the accessibility of microfluidics.
Varied endothelialization techniques enable the study of a multiple diseases.
These techniques have exciting implications in personalized medicine.
Acknowledgments
R.G.M and W.A.L conducted the research and wrote the review. N.K and A.J edited the review and provided valuable input and feedback. Financial support for this work was provided by a National Science Foundation Graduate Research Fellowship DGE-1650044 (to R.G.M).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
(•) – of special interest (••) – of outstanding interest
- 1.Barabino G, McIntire L, Eskin S, Sears D, Udden M. Endothelial cell interactions with sickle cell, sickle trait, mechanically injured, and normal erythrocytes under controlled flow. Blood. 1987;70:152–7. [PubMed] [Google Scholar]
- 2.Wautier J-L, Wautier M-P. Molecular basis of erythrocyte adhesion to endothelial cells in diseases. Clin Hemorheol Micro. 2013;53:11–21. doi: 10.3233/CH-2012-1572. [DOI] [PubMed] [Google Scholar]
- 3.Merkel K, Ginsberg P, Parker J, Post M. Cerebrovascular disease in sickle cell anemia: a clinical, pathological and radiological correlation. Stroke. 1978;9:4552. doi: 10.1161/01.str.9.1.45. [DOI] [PubMed] [Google Scholar]
- 4.Suresh S. Mechanical response of human red blood cells in health and disease: some structure-property-function relationship. Journal of materials research. 2006;21:1871–1877. [Google Scholar]
- 5.Ku D, Flannery C. Development of a flow-through system to create occluding thrombus. Biorheology. 2007;44:273–84. [PubMed] [Google Scholar]
- 6.Nesbitt WS, Westein E, Tovar-Lopez FJ, Tolouei E, Mitchell A, Fu J, Carberry J, Fouras A, Jackson SP. A shear gradient-dependent platelet aggregation mechanism drives thrombus formation. Nat Med. 2009;15:665–73. doi: 10.1038/nm.1955. [DOI] [PubMed] [Google Scholar]
- 7.Kaul, Fabry, Nagel Microvascular sites and characteristics of sickle cell adhesion to vascular endothelium in shear flow conditions: pathophysiological implications. Proc National Acad Sci. 1989;86:3356–3360. doi: 10.1073/pnas.86.9.3356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tsai M, Kita A, Leach J, Rounsevell R, Huang JN, Moake J, Ware RE, Fletcher DA, Lam WA. In vitro modeling of the microvascular occlusion and thrombosis that occur in hematologic diseases using microfluidic technology. J Clin Invest. 2012;122:408–18. doi: 10.1172/JCI58753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miller J, Stevens K, Yang M, Baker B, Nguyen D-H, Cohen D, Toro E, Chen A, Galie P, Yu X, et al. Rapid casting of patterned vascular networks for perfusable engineered three-dimensional tissues. Nature Materials. 2012;11:768–774. doi: 10.1038/nmat3357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Song J, Cavnar S, Walker A, Luker K, Gupta M, Tung Y-C, Luker G, Takayama S. Microfluidic Endothelium for Studying the Intravascular Adhesion of Metastatic Breast Cancer Cells. Plos One. 2009;4:e5756. doi: 10.1371/journal.pone.0005756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sei Y, Ahn S, Virtue T, Kim T, Kim Y. Detection of frequency-dependent endothelial response to oscillatory shear stress using a microfluidic transcellular monitor. Sci Reports. 2017;7:10019. doi: 10.1038/s41598-017-10636-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Whitesides G. The origins and the future of microfluidics. Nature. 2006;442:368–373. doi: 10.1038/nature05058. [DOI] [PubMed] [Google Scholar]
- 13.Markl M, Wegent F, Zech T, Bauer S. In vivo wall shear stress distribution in the carotid artery effect of bifurcation geometry, internal carotid artery stenosis, and recanalization therapy [Internet] Circulation: Cardiovascular Imaging. 2010;3:647–655. doi: 10.1161/CIRCIMAGING.110.958504. [DOI] [PubMed] [Google Scholar]
- 14.Boussel L, Rayz V, McCulloch C, Martin A, Acevedo-Bolton G, Lawton M, Higashida R, Smith W, Young W, Saloner D. Aneurysm growth occurs at region of low wall shear stress: patient-specific correlation of hemodynamics and growth in a longitudinal study. Stroke; a journal of cerebral circulation. 2008;39:2997–3002. doi: 10.1161/STROKEAHA.108.521617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meng H, Wang Z, Hoi Y, Gao L, Metaxa E, Swartz DD, Kolega J. Complex hemodynamics at the apex of an arterial bifurcation induces vascular remodeling resembling cerebral aneurysm initiation. Stroke. 2007;38:1924–1931. doi: 10.1161/STROKEAHA.106.481234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beebe D, Mensing G, Walker G. Physics and applications of microfluidics in biology. Annu Rev Biomed Eng. 2002;4:261–286. doi: 10.1146/annurev.bioeng.4.112601.125916. [DOI] [PubMed] [Google Scholar]
- 17.Hou X, Zhang Y, Santiago G, Alvarez M, Ribas J, Jonas S, Weiss P, Andrews A, Aizenberg J, Khademhosseini A. Interplay between materials and microfluidics. Nature Reviews Materials. 2017;2:17016. doi: 10.1038/natrevmats.2017.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stone HA, Stroock AD, Ajdari A. Engineering flows in small devices: microfluidics toward a lab-on-a-chip. Annu Rev Fluid Mech. 2004;36:381–411. [Google Scholar]
- 19.Qin D, Xia Y, Whitesides GM. Soft lithography for micro- and nanoscale patterning. Nat Protoc. 2010;5:491–502. doi: 10.1038/nprot.2009.234. [DOI] [PubMed] [Google Scholar]
- 20.Kane R, Takayama S, Ostuni E, Ingber D, Whitesides G. Patterning proteins and cells using soft lithography. Biomaterials. 1999;20:2363–2376. doi: 10.1016/s0142-9612(99)00165-9. [DOI] [PubMed] [Google Scholar]
- 21.Jo B-H, Lerberghe LM, Motsegood KM, Beebe DJ. Three-dimensional micro-channel fabrication in polydimethylsiloxane (PDMS) elastomer. J Microelectromech S. 2000;9:76–81. [Google Scholar]
- 22.Huber B, Engelhardt S, Meyer W, Krüger H. Blood-vessel mimicking structures by stereolithographic fabrication of small porous tubes using cytocompatible polyacrylate elastomers, biofunctionalization and endothelialization. Journal of functional biomaterials. 2016;7:11. doi: 10.3390/jfb7020011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu Y, Chen C, Summers S, Medawala W. C-peptide and zinc delivery to erythrocytes requires the presence of albumin: implications in diabetes explored with a 3D-printed fluidic device. Integrative Biology. 2015;7:534–543. doi: 10.1039/c4ib00243a. [DOI] [PubMed] [Google Scholar]
- 24.Gross B, Anderson K, Meisel J, McNitt M, Spence D. Polymer Coatings in 3D-Printed Fluidic Device Channels for Improved Cellular Adherence Prior to Electrical Lysis. Anal Chem. 2015;87:6335–6341. doi: 10.1021/acs.analchem.5b01202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25•.Bégin-Drolet A, Dussault M-A, Fernandez S, Larose-Dutil J, Leask R, Hoesli C, Ruel J. Design of a 3D printer head for additive manufacturing of sugar glass for tissue engineering applications. Addit Manuf. 2017;15:29–39. This work presents a technology in which specialized 3D printing of sugar glass is used to generate microchannels. Channels are defined by sugar glass, where they are then cast in PDMS and dissolved to create microchannel lumens. [Google Scholar]
- 26.Fioretta E, Simonet M, Smits A, Baaijens F, Bouten C. Differential Response of Endothelial and Endothelial Colony Forming Cells on Electrospun Scaffolds with Distinct Microfiber Diameters. Biomacromolecules. 2014;15:821–829. doi: 10.1021/bm4016418. [DOI] [PubMed] [Google Scholar]
- 27.Cheng Y, Yu Y, Fu F, Wang J, Shang L, Gu Z, Zhao Y. Controlled Fabrication of Bioactive Microfibers for Creating Tissue Constructs Using Microfluidic Techniques. Acs Appl Mater Interfaces. 2016;8:1080–1086. doi: 10.1021/acsami.5b11445. [DOI] [PubMed] [Google Scholar]
- 28••.Mannino R, Myers D, Ahn B, Wang Y, Rollins M, Gole H, Lin A, Guldberg R, Giddens D, Timmins L, et al. Do-it-yourself in vitro vasculature that recapitulates in vivo geometries for investigating endothelial-blood cell interactions. Scientific Reports. 2015;5:12401. doi: 10.1038/srep12401. This work utilized off-the-shelf laboratory products to generate endothelialized microfluidic devices capable of recapitulating various microvascular gerometries. This technology was used to investigate cellular interactions within the vasculature within the context of sickle cell disease. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang X, Forouzan O, Burns J, Shevkoplyas S. Traffic of leukocytes in microfluidic channels with rectangular and rounded cross-sections. Lab on a Chip. 2011;11:3231. doi: 10.1039/c1lc20293f. [DOI] [PubMed] [Google Scholar]
- 30.Young E, Beebe D. Fundamentals of microfluidic cell culture in controlled microenvironments. Chemical Society reviews. 2010;39:1036–48. doi: 10.1039/b909900j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.LaValley DJ, Zanotelli MR, Bordeleau F, Wang W, Schwager SC, Reinhart-King CA. Matrix Stiffness Enhances VEGFR-2 Internalization, Signaling, and Proliferation in Endothelial Cells. Converg Sci Phys Oncol. 2017 doi: 10.1088/2057-1739/aa9263. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Buchanan C, Verbridge S, Vlachos P, Rylander M. Flow shear stress regulates endothelial barrier function and expression of angiogenic factors in a 3D microfluidic tumor vascular model. Cell Adhesion Migr. 2014;8:517–524. doi: 10.4161/19336918.2014.970001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pober J, Sessa W. Evolving functions of endothelial cells in inflammation. Nature Reviews Immunology. 2007;7:803–815. doi: 10.1038/nri2171. [DOI] [PubMed] [Google Scholar]
- 34.Chien S. Effects of Disturbed Flow on Endothelial Cells. Annals of Biomedical Engineering. 2008;36:554–562. doi: 10.1007/s10439-007-9426-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Malek A, Jackman R, Rosenberg R, Izumo S. Endothelial expression of thrombomodulin is reversibly regulated by fluid shear stress. Circulation Research. 1994;74:852860. doi: 10.1161/01.res.74.5.852. [DOI] [PubMed] [Google Scholar]
- 36.Myers DR, Sakurai Y, Tran R, Ahn B, Hardy ET, Mannino R, Kita A, Tsai M, Lam WA. Endothelialized microfluidics for studying microvascular interactions in hematologic diseases. Journal of visualized experiments: JoVE. 2011 doi: 10.3791/3958. [no volume] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Farcas M, Rouleau L, Fraser R, Leask R. The development of 3-D, in vitro, endothelial culture models for the study of coronary artery disease. BioMedical Engineering OnLine. 2009;8:30. doi: 10.1186/1475-925X-8-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tran R, Ahn B, Myers D, Qiu Y, Sakurai Y, Moot R, Mihevc E, Spencer H, Doering C, Lam W. Simplified prototyping of perfusable polystyrene microfluidics. Biomicrofluidics. 2014;8:046501. doi: 10.1063/1.4892035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dominical V, Vital D, O’Dowd F, Saad S, Costa F, Conran N. In vitro microfluidic model for the study of vaso-occlusive processes. Exp Hematol. 2015;43:223–228. doi: 10.1016/j.exphem.2014.10.015. [DOI] [PubMed] [Google Scholar]
- 40.Squires T, Quake S. Microfluidics: Fluid physics at the nanoliter scale. Rev Mod Phys. 2005;77:977–1026. [Google Scholar]
- 41.Hewes S, Wong A, Searson P. Bioprinting microvessels using an inkjet printer. Bioprinting. 2017;7:14–18. [Google Scholar]
- 42.Soroush F, Zhang T, King D, Tang Y, Deosarkar S, Prabhakarpandian B, Kilpatrick L, Kiani M. A novel microfluidic assay reveals a key role for protein kinase C δ in regulating human neutrophil–endothelium interaction. J Leukocyte Biol. 2016;100:1027–1035. doi: 10.1189/jlb.3MA0216-087R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Seo J, Conegliano D, Farrell M, Cho M, Ding X, Seykora T, Qing D, Mangalmurti N, Huh D. A microengineered model of RBC transfusion-induced pulmonary vascular injury. Sci Reports. 2017;7:3413. doi: 10.1038/s41598-017-03597-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44•.Jain A, Meer A, Papa A-L, Barrile R, Lai A, Schlechter B, Otieno M, Louden C, Hamilton G, Michelson A, et al. Assessment of whole blood thrombosis in a microfluidic device lined by fixed human endothelium. Biomed Microdevices. 2016;18:73. doi: 10.1007/s10544-016-0095-6. This work presents a thrombosis assay within a microfluidic device that utilizes chemically preserved endothelium. This preservation capability allows for transit and storage capabilities, highlighting the point-of-care capabilities of endothelialized microfluidics. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45••.Mannino R, Santiago-Miranda A, Pradhan P, Qiu Y, Mejias J, Neelapu S, Roy K, Lam W. 3D microvascular model recapitulates the diffuse large B-cell lymphoma tumor microenvironment in vitro. Lab Chip. 2016;17:407–414. doi: 10.1039/c6lc01204c. This work utilized a “do-it-yourself” fabrication method to generate an endothelialized microchannel traversing a tumor cell-laden hydrogel. This work enables investigation of the kinetics of drug delivery within tumors. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46•.Bischel L, Young E, Mader B, Beebe D. Tubeless microfluidic angiogenesis assay with three-dimensional endothelial-lined microvessels. Biomaterials. 2013;34:1471–1477. doi: 10.1016/j.biomaterials.2012.11.005. This work presents a microfluidic angiogenesis assay where endothelialized microchannels are fabricated within a hydrogel and are monitored as new vessels grow into the hydrogel. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chonan Y, Taki S, Sampetrean O, Saya H, Sudo R. Endothelium-induced three-dimensional invasion of heterogeneous glioma initiating cells in a microfluidic coculture platform. Integr Biol. 2017;9:762–773. doi: 10.1039/c7ib00091j. [DOI] [PubMed] [Google Scholar]
- 48.Wang J, Khafagy E-S, Khanafer K, Takayama S, ElSayed M. Organization of Endothelial Cells, Pericytes, and Astrocytes into a 3D Microfluidicin VitroModel of the Blood–Brain Barrier. Mol Pharm. 2016;13:895–906. doi: 10.1021/acs.molpharmaceut.5b00805. [DOI] [PubMed] [Google Scholar]
- 49.Prabhakarpandian B, Shen MC, Nichols JB, Mills IR. SyM-BBB: a microfluidic blood brain barrier model. Lab on a Chip. 2013;13:1093–1101. doi: 10.1039/c2lc41208j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jain A, Barrile R, Meer A, Mammoto A, Mammoto T, Ceunynck K, Aisiku O, Otieno M, Louden C, Hamilton G, et al. Primary Human Lung Alveolus-on-a-chip Model of Intravascular Thrombosis for Assessment of Therapeutics. Clin Pharmacol Ther. 2017 doi: 10.1002/cpt.742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hong G, Lee J, Robinson J, Raaz U, Xie L, Huang N, Cooke J, Dai H. Multifunctional in vivo vascular imaging using near-infrared II fluorescence. Nat Med. 2012;18:1841–1846. doi: 10.1038/nm.2995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lan Q, Chen A, Zhang T, Li G, Zhu Q, Fan X, Ma C, Xu T. Development of Three-Dimensional Printed Craniocerebral Models for Simulated Neurosurgery. World Neurosurg. 2016;91:434–442. doi: 10.1016/j.wneu.2016.04.069. [DOI] [PubMed] [Google Scholar]
- 53.Mazzoli A. Selective laser sintering in biomedical engineering. Medical Biological Eng Comput. 2013;51:245–256. doi: 10.1007/s11517-012-1001-x. [DOI] [PubMed] [Google Scholar]
- 54.Bhatia SN, Ingber DE. Microfluidic organs-on-chips. Nature biotechnology. 2014;32:760–772. doi: 10.1038/nbt.2989. [DOI] [PubMed] [Google Scholar]
- 55.Adriani G, Ma D, Pavesi A, Kamm R, Goh E. A 3D neurovascular microfluidic model consisting of neurons, astrocytes and cerebral endothelial cells as a blood–brain barrier. Lab Chip. 2016;17:448–459. doi: 10.1039/c6lc00638h. [DOI] [PubMed] [Google Scholar]
- 56.Virumbrales-Muñoz M, Ayuso J, Olave M, Monge R, Miguel D, Martínez-Lostao L, Gac S, Doblare M, Ochoa I, Fernandez L. Multiwell capillarity-based microfluidic device for the study of 3D tumour tissue-2D endothelium interactions and drug screening in co-culture models. Sci Reports. 2017;7:11998. doi: 10.1038/s41598-017-12049-4. [DOI] [PMC free article] [PubMed] [Google Scholar]


