Abstract
Statins are known for their blood cholesterol-lowering effect and are widely used in patients with cardiovascular and metabolic diseases. Research over the past three decades shows that statins have diverse effects on different pathophysiological pathways involved in angiogenesis, inflammation, apoptosis, and anti-oxidation, leading to new therapeutic options. Recently, statins have attracted considerable attention for their immunomodulatory effect. Since immune reactivity has been implicated in a number of retinal diseases, such as uveitis, age-related macular degeneration (AMD) and diabetic retinopathy, there is now a growing body of evidence supporting the beneficial effects of statins in these retinopathies. This review evaluates the relationship between statins and the pathophysiological basis of these diseases, focusing on their potential role in treatment. A PubMed database search and literature review was conducted. Among AMD patients, there is inconsistent evidence regarding protection against development of early AMD or delaying disease progression; though they have been found to reduce the risk of developing choroidal neovascular membranes (CNV). In patients with retinal vein occlusion, there was no evidence to support a therapeutic benefit or a protective role with statins. In patients with diabetic retinopathy, statins demonstrate a reduction in disease progression and improved resolution of diabetic macular oedema (DMO). Among patients with uveitis, statins have a protective effect by reducing the likelihood of uveitis development.
Introduction
Background of statins
Statins, also known as 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitors, have long been used for controlling blood cholesterol levels and reducing the risk of cardiovascular morbidity and mortality. While their mechanism of action in vascular disease is established, evidence is accumulating regarding alternative immunomodulatory roles that may enhance their cardiovascular effect, as well as play a role in treating inflammatory diseases [1].
Statins were discovered in the early 1970s from Penicillium citrinium cultures [2]. Further research showed that these molecules strongly inhibit the rate-limiting enzyme in cholesterol biosynthesis, HMG-CoA reductase, which is responsible for the conversion of HMG-CoA to l-mevalonate and finally to farnesylpyrophosphate (FPP, the precursor for cholesterol synthesis) [3]. Another product of the L-mevalonate pathway is geranylgeranylpyrophspohate (GGPP), which is synthesised from FPP. FPP and GGPP are both essential isoprenoids and serve as lipid attachments for various intracellular proteins such as small guanosine triphosphatases (GTPases), which are thought to have an immunomodulatory effect by acting as adjuncts for different cell signalling processes. GTPases are key regulators in various biological function within the cell, such as gene transcription, and adhesion. Furthermore they serve as essential biochemicals for actin cytoskeleton which mediates a variety of essential biological functions, such as, cellular movement and division [4].
Early evidence of the immunosuppressive effect of statins comes from studies on c-reactive protein (CRP), a systemic inflammatory biomarker, in patients following myocardial infarcts. Individuals with elevated serum CRP levels >3 mg/L have an increased risk of cardiovascular events and use of statins results in a reduction of CRP levels independent of their effect on blood cholesterol levels [5, 6]. Endothelial function studies [7] as well as clinical trials from organ transplantation [8] and stroke prevention [9], support their additional immunomodulatory effect unrelated to lowering cholesterol. This has led to a recommendation to give cardiac transplant patients statins irrespective of their blood cholesterol levels [1, 10].
There is evidence supporting an inflammatory role in the pathophysiology of many retinal diseases and statins have been investigated in conditions such as cataract [11], glaucoma [12], diabetic retinopathy [13] and age-related macular degeneration (AMD) [14], with mixed results regarding their effect. There are also preliminary results suggesting that it may reduce the extent of scarring following rhegmatogenous retinal detachment [15].
Statin’s immunomodulatory effect
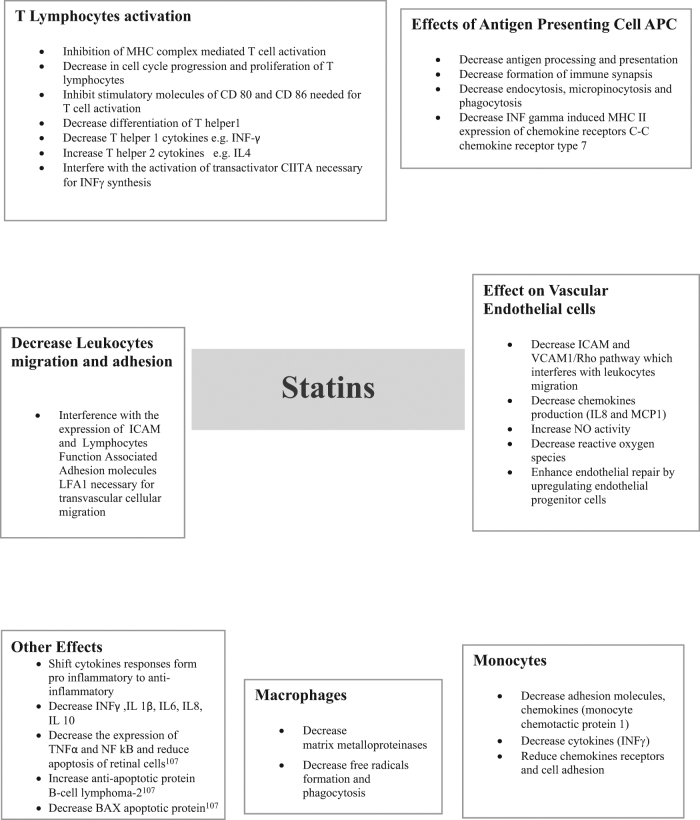
The immunomodulatory effect of statins is exerted either directly on cells, such as interfering with T lymphocyte proliferation [16, 17], inhibiting the expression of co-stimulatory molecules CD80 and CD86 on B cells [18], or through inhibition of cellular interactions and signalling molecules such as tumour necrosis factor α (TNF-α) [19], a potent proinflammatory cytokine that induces apoptosis of retinal cells [20]. By influencing the cytokine balance from proinflammatory to anti-inflammatory, statins modulate the immune response and achieve an immunosuppressive effect [18, 21]. Additionally, statins alter the interaction between the vascular endothelium and lymphocytes by blocking the intercellular adhesion molecule 1 (ICAM1) pathway, which facilities transvascular migration of lymphocytes [22], thereby reducing the number of lymphocytes reaching the sites of inflammation. For a full description of statin immunomodulatory pathways please refer to Fig. 1.
Fig. 1.
Statins immunomodulatory effects.
Age-related macular degeneration
While the pathophysiology of AMD is not completely understood, there are well-established risk factors such as increasing age, smoking, genetic predisposition and increased body mass index [23]. Pathological changes, including lipid accumulation under Bruch’s membrane, lipoprotein metabolism and inflammation, are thought to play a role in disease development and progression to the more advanced stages [24, 25]. Drusen, the hallmark of early AMD are lipid-rich deposits located between the retinal pigment epithelium (RPE) and Bruch’s membrane. The interaction between these lipids and reactive oxygen species results in the formation of peroxidised lipids [26], that trigger local inflammation through the upregulation of several inflammatory cytokines and VEGF, which predisposes to choroidal neovascularization or these lipid peroxides could lead to geographic atrophy by a direct toxic effect on the RPE cells [27].
Statins in AMD
The potential role for statins in the management of AMD is supported through several pathophysiological mechanisms. Statins can preserve the vascular supply to the outer retina, by reducing atherosclerotic changes, thus maintain the clearance of lipoproteins produced by the RPE and delaying the development of drusen [28, 29]. Their anti-inflammatory properties may further affect the inflammatory process underlying the development and progression of AMD [30], and their effect on induction of heme oxygenase 1 and downregulation of LDL and peroxidised lipids [31], may protect the outer retina, choroid, and RPE from oxidative damage [28]. Lastly, statins’ inhibit the activation of macrophages and the subsequent release of proinflammatory cytokines and matrix metalloproteinases (MMP 2), which are found in the CNV complex, delaying CNV development [28].
A meta-analysis of 15 studies examining the role of statins in AMD found a robust protective effect in early disease [32]. They reduced the risk of early AMD by 17%, whereas in late AMD, their use was associated with a reduced risk of CNV development, but with no effect on the development of geographic atrophy (GA). The Blue Mountain Eye Study that followed 3654 people at 10 year intervals showed that statin users had a reduced risk of developing soft drusen, but did not affect the incidence of progressing to early AMD [33]. This was replicated in the Beaver Dam Eye Study and the Rotterdam Eye Study, where no association was found between statin use and developing AMD at 5 year follow-up [34, 35]. However, statins users in the Beaver Dam Eye Study were 32% less likely to develop soft indistinct drusen, 36% less likely to have large drusen (≥125 μm) and 71% less likely to present with late disease compared to non-statin users [34]. In-line with these results, Maguire and colleagues examined 744 patients for the development of advanced AMD (GA or neovascularaiztion) and found no protective effect with statins after 5 year follow-up [36]. Several studies examined the effect of statins on development of disease specific features and progression to advanced disease. A cross-sectional study with a mean duration of statin intake of 61.1 months found that statins were associated with a lower risk of AMD in subjects’ ≥68 years old, which was more pronounced the longer statins were used [37]. An open-label study examined the effect of high-dose (80 mg) atorvastatin in patients with high-risk features for progression to advanced AMD (presence of many large soft drusenoiod deposits) and showed a trend towards reduced number of drusenoid RPE detachments and a visual gain of 3 letters on average after 1 year follow-up [14]. Two randomised controlled trials aimed to accurately determine the effect of statins on AMD patients. The first examined the effect of 20 mg of simvastatin on 30 subjects for three months but did not show a statistically significant effect on vision [38]. In the second study, 114 subjects were randomised to either 40 mg simvastatin or placebo. The results showed a four-fold decrement in the risk of disease progression in the treated group, and was even more prominent among patients with the complement factor H (CFH) allele [39], where the odds ratio for disease progression was 0.08. In 2015 a Cochrane systematic review and meta-analysis concluded that there is inadequate evidence to support the role of statins in prevention or alteration of AMD progression [40]. Tables 1 and 2 summarise the trials that offer support or otherwise for the protective role of statins in AMD.
Table 1.
Clinical trials that do not support the role of statins in AMD
| Trial | Design | Follow-up | Drug | Results |
|---|---|---|---|---|
| Martini et al. [38] | Randomised control trial 30 subjects | 3 months | 20mg simvastatin | No statistically significant effect on vision after three months of simvastatin use. |
| McGwin et al. [95] | Case-control study 2755 (390 AMD versus 2365 controls) were analysed | 3 years | Statins | No association between statin use and AMD. |
| Smeeth et al. 2009 [94] | Population-based | Median of 4.4 years | Statins | Statin may increase risk of AMD. |
| Smeeth et al. [96] | Population-based/ Case-control study | Median of 4 years | Statins | Statin use did not decrease risk of AMD. |
| Peiretti et al. [97] | Case-control study | Statin use of ≥ 3 years | Statins | Statins use may increase risk of AMD. |
| Klein et al. [34] | Cohort study population-based | Over 5 years | Statins | No association between statin use and 5 year incidence of AMD. |
| ALIENOR [98] | Population-based 963 elderly subjects | 9 years | Statins | HDL was significantly associated with increased risk of early AMD. Other serum lipids (LDL, triglyceride) and statin use did not show a statistically significant correlation with risk of AMD. |
| Rottredam eye study [35] | Population-based cohort study 3434 patients | 5 years | Statins | No association between statin use and AMD. |
| Beaver dam eye study [34] | Population-based cohort study 2780 patients | 5 years | Statins | No association between statin use and AMD. |
| Meta-analysis of three trials (Beaver dam eye study, blue mountains eye study and Rotterdam study) [99] | Population-based cohort study 6950 patients | Up to 20 years | Statins | Found no association between serum lipids, and statin use with the incidence or progression of AMD. |
| Complication of Age-related Macular Degeneration Prevention Trail (CAPT) [36] | Randomised clinical trial 744 subjects | 5 years | Statins | No strong protective effect of statins was found on the development of advanced AMD. |
| Gehlbach et al. [40] | Cochrane review | Statins | Insufficient evidence to conclude that statins have a role in prevention or alteration of AMD course. |
Table 2.
Clinical trials that support the role of statins in AMD
| Trial | Design | Follow-up | Drug | Results |
|---|---|---|---|---|
| Vavvas et al. [14] | Open-label prospective 26 subjects | 1 year | 80 mg atorvastatin daily | Regression of drusenoid RPE detachment in 10 patients and vision gain of 3.3 letters. None developed advanced AMD (Geographic atrophy or CNV). |
| Barbosa et al. [37] | Cross-sectional 5604 subject | 2005-2008 | Statins | Possible beneficial effect of statins use for AMD prevention in subject’s ≥ 68 years of age. The benefit was greater with the longer use of statins. |
| Guymer et al. [39] | Randomised controlled trial 114 subjects | 3 years | 40 mg simvastatin/placebo | Four-fold decrease in AMD progression in simvastatin group compared to placebo and greater benefit was observed in CFH gene carriers. |
| The Blue Mountains Eye Study [33] | Population-based cohort study, 3654 at baseline | 5 and 10 years | Statins | Statins users had reduced risk of development of indistinct soft drusen, but there was no significant association between statins and the incidence of early AMD. |
Diabetic retinopathy
Diabetic retinopathy (DR) is a frequent complication of diabetes and increases in prevalence with disease duration. The most common causes of vision loss in diabetes are macular oedema and complications related to proliferative diabetic retinopathy. There is mounting evidence regarding endothelial dysfunction in diabetes and inflammatory processes leading to the development of DR [41]. High glucose levels lead to activation of protein kinase C and induction of the aldose reductase pathway with overproduction of free radicals, endothelial cell injury and upregulation of proinflammatory cytokines such as TNF-α, IL-1, ICAM1 and IL-6 [42, 43]. This can lead to breakdown of the blood retinal barrier (BRB), resulting in loss of vascular integrity, which results in increased permeability, leading to macular oedema as well as compromised blood supply and retinal ischaemia. [44]. More advanced, proliferative diabetic retinopathy (PDR) is partly mediated by transforming growth factor β2 (TGF β2), which is upregulated in diabetes. TGF β is responsible for the transformation of RPE and hyalocytes into myofibroblastic cells and induces contraction of fibrocellular complexes, which predisposes to the development of vitreous haemorrhages and tractional retinal detachment [45], resulting in vision loss. Hypercholesterolemia, in addition to hyperglycaemia, is regarded as a strong risk factor for endothelial dysfunction [46], leading to diabetic macular oedema [47]. Total cholesterol and LDL were found to be significantly higher in subjects with clinically significant macular oedema [48–50] and were a significant risk factor for retinal hard exudate formation [50].
Statins have a role in maintaining the stability of the BRB by reducing reactive oxygen species, increasing nitrous oxide (NO) levels and increasing the numbers of endothelial progenitor cells (EPC), which are thought to play a role in maintaining a functional BRB [51]. This results in improvement in vascular resistance, blood flow velocity and retinal perfusion of subjects with diabetic retinopathy [52]. Moreover, Atorvastatin was found to have pleiotropic functions on RPE cells, including anti-proliferation, anti-contraction, and anti-adhesion [53]. In PDR, statins are known to suppress TGF β and have been shown to suppress the formation of retinal neovascular tufts and delay the progression of PDR in animal models [54].
Statin clinical trials in diabetic retinopathy
The association between serum cholesterol and DR was first reported by Dornan et al. [55]. Extensive evidence linking elevated serum lipids and the increased risk of hard exudates comes from the early treatment diabetic retinopathy study [49] and the Wisconsin epidemiology study of diabetic retinopathy [56]. High serum cholesterol levels were found to be a significant contributor to the extent of developing retinal hard exudates, and patients with higher serum cholesterol levels at baseline had a 50% risk of vision loss compared to those with lower lipid levels [50, 57]. The diabetic control and complication trial [58] showed that serum lipids, mainly the triglycerides to HDL ratio and LDL levels were risk factors for DMO and hard exudate formation. The study highlighted the potential role of lipid-lowering agents in reducing the risk of DMO. Furthermore, treatment with statins resulted in a reduction of the amount of lipids within the retina, seen on histopathological examination of retinae from patients with high serum cholesterol and DMO [59]. However, other studies looking at the total cholesterol level as a risk factor for developing DR were less conclusive [60]. While the Chennai urban rural epidemiology study [61] found total cholesterol to be an independent risk factor for the development of DR, cholesterol was found to have a protective effect in the Singapore Malay eye study [62]. In yet another study, blood triglyceride and LDL cholesterol levels were significantly associated with DR and diabetic macular oedema (DMO), respectively [63].
Statins and other lipid-lowering drugs have been extensively studied as treatments for diabetic retinopathy. A study on the use of pravastatin demonstrated a reduction in hard exudates in all patients and reduced numbers of microaneurysms in 67% of patients [64]. Simvastatin 20 mg was reported to delay the progression of DR compared with placebo treated controls [65]. Similarly, treatment with atorvastatin resulted in a 66% reduction in hard exudates and leakage on fluorescein angiography among eyes with DMO and dyslipidaemia [66]. However, it was not found to be beneficial in DR in 30 diabetic subjects with normal serum lipid levels [67]. The combination of atorvastatin and fibrates demonstrated a significant reduction in macular oedema in 47 diabetic patients with non-proliferative DR [68], as well as reducing the rate of recurrent vitreous haemorrhage in eyes with PDR [69].
Two randomised controlled trials, the fenofibrate intervention and event lowering in diabetes (FIELD) and action to control cardiovascular risk in diabetes (ACCORD) examined the effect of fenofibrate, a non-statin drug that reduces triglycerides and LDL cholesterol, alone or together with simvastatin, respectively. In both studies, over 5 year follow-up a significantly positive effect was found in reducing DR progression, and the addition of simvastatin augmented the control of the DR [70–73]. This led to less need for other treatment measures such as focal laser in the FIELD study. These effects were cholesterol independent and may be attributed to anti-inflammatory, anti-apoptotic and vasodilatory effects, which are NO mediated [74, 75]. Finally, the improved effect observed by combining statins and fenofibrate may be due to a synergistic action that is independent of their cholesterol-lowering effect. Despite the positive effect observed on DMO, statins were less effective in preventing the occurrence of mild DR and no recommendations could be made regarding initiating lipid-lowering drugs in patients with normal serum lipid levels. Tables 3 and 4 summarise the trials that offer support or otherwise for the protective role of statins in DR.
Table 3.
Clinical trials that support the role of statins in DR
| Trial | Design | Follow-up | Drug | Results |
|---|---|---|---|---|
| FIELD study [70] | Randomised control trial 9795 Subjects | 5 years | Fenofibrate 200 mg/placebo | The rate of first laser treatment for diabetic retinopathy was significantly lower in fenofibrate group compared to placebo. P value< 0.0002 |
| ACCORD-EYE [73] | Randomised control trial 2856 Subjects | 4 years | Fenofibrate +simvastatin/simvastatin +placebo | Fenofibrate + simvastatin group has more reduction in the progression of diabetic retinopathy compared to simvastatin alone. |
| Denniston et al. [69] | Retrospective trial of 79 eyes of 54 patients | – | Statin user versus nonusers | Statins are associated with lower rate of vitreous haemorrhage at 1 year. |
| Sen et al. [65] | Randomised control trial 50 patients | 6 months | Simvastatin 20 mg versus placebo | Simvastatin slowed the progression of diabetic retinopathy. However, visual acuity improvement was not statistically significant between the two groups. |
| Panagiotoglou et al. [100] | Open-label study 18 subjects | 1 year | Atorvastatin | Reduced the severity of hard exudates and fluorescein leakage in diabetic maculopathy. |
| Gordon et al. [64] | Open-label study on 6 patients | 1 year | Pravastatin | Reduced hard exudates and had beneficial effect on non-proliferative DR. |
| Gupta et al. [66] | Randomised control trial 30 patients | 18 weeks | Atorvastatin | Reduction of hard exudate and sub foveal lipid migration following laser treatment for CSMO. |
| Nielsen et al. [101] | Population-based study 15679 | 13 years | Statins | Patients had lower cumulative incidence of DR (statins are potentially protective against DR). |
| Gaede et al. [102] | Randomised control trial 160 patients | 7.8 years | Statin versus placebo | Statins reduced risk of DR and reduced need for laser photocoagulation. |
Table 4.
Clinical trials that do not support the role of statins in DR
| Trial | Design | Follow-up | Drug | Results |
|---|---|---|---|---|
| Narang et al. [67] | Randomised control trial 30 patients | 6 months | Atorvastatin | No relation between statin use and reduction in hard exudates, macular oedema or visual acuity in subjects with normal serum lipid levels. |
| Fried et al. [103] | Randomised control trial 39 patients | 2 years | Simvastatin/diet versus diet alone | No difference in retinopathy status between the two groups. |
| Liinamaa et al. [104] | Case-control study | — | Statins | Statins are associated with significantly higher levels of VEGF in patients with PDR. |
| Collaborative Atorvastatin Diabetes Study CARDS Trial [105] | Randomised control trial 2838 subjects with Type 2 DM | 3.9 years | Atorvastatin/placebo | No added benefits of statin in DR. Treatment group needed less laser but was not statistically significant. |
| Zhang et al. [106] | Case-control study 114 lipid- lowering agents users versus 570 controls | — | Statin and non-statin lipid-lowering agents | No association between statins use and DR. |
Retinal vein occlusion
Retinal vein occlusion (RVO) is the second most common cause of retinal vascular disease after DR [76]. Vision loss is secondory to macular oedema and/or retinal ischaemia, which can lead to neovascularisation and high risk of tractional retinal detachment and neovascular glaucoma. Atherosclerosis, among other cardiovascular risk factors such as hyperlipdeima, diabetes and hypertension, contributes to the pathophysiology of RVO [77]. Hyperlipideamia was more than two-fold more common in patients with all types of RVO compared to controls [78]. Raised serum lipids may change plasma viscosity and alter platelet function, which predispose to thrombosis and blood stasis [79]. A number of inflammatory cytokines and growth factors may also be upregulated in response to the onset of retinal ischaemia [80], which may predispose to a state of chronic inflammation [81]. Statins, in addition to their anti-inflammatory action, were shown to have a potential protective effect on the microvascular endothelium in retinal ischaemia models [82]. While low doses promoted vascular repair mechanism through upregulating VEGF and NO levels, higher doses resulted in cell death due to depletion of intracellular cholesterol and disruption of key structures within the cell.
There is little clinical evidence regarding the role of statins in the management of RVO, or in preventing its development. A retrospective study of RVO patients, as well as patients with a high risk of developing RVO (defined as those having hypertension and primary open angle glaucoma), no clear preventative or therapeutic benefit could be found after 43 months follow-up [83].
Uveitis
Uveitis mainly affects young people and carries a significant risk of vision loss [84, 85]. It can be associated with an underlying systemic disease, though most cases remain idiopathic. Corticosteroids represent the mainstay of the treatment. However, steroids are not ideal for long-term therapy because they are associated with a wide array of side effects, particularly increasing total serum cholesterol levels and induction of diabetes and hypertension, thus increasing their cardiovascular risks. Similarly, other immunosuppressive drugs may also have significant side effects including increasing cardiovascular morbidity [86].
Statins have been shown to have a number of anti-inflammatory and immunomodulatory effects, specifically their ability to reduce key inflammatory cytokines such as IL-6, IL8 and TNF α, as well as prevent leukocyte adhesion to vascular endothelium [5, 22]. The role of statins has been extensively studied in a mouse model of experimental autoimmune uveoretinitis, where it was found that they reduce the clinical and histological scores of inflammation and inhibit T lymphocyte recruitment into the retina [87]. In a mouse model of acute autoimmune retinal disease, statins were found to inhibit leukocyte infiltration into the retina and reduce retinal vascular leakage [88]. Treatment with statins also resulted in improved vascular stability in a rodent model of lipopolysaccharides-induced liver microvascular dysfunction [89].
Clinical studies in multiple sclerosis (MS) patients showed a positive effect from simvastatin on brain atrophy, suggesting these drugs cross the blood–brain barrier and can play a role in controlling inflammatory diseases [90]. In rheumatoid arthritis statins have a potential benefit in controlling disease activity manifesting as improvement in disease activity scores and reducing the number of tender and swollen joints [91]. Ocular involvement is common in many of these conditions and statins may offer an additional treatment approach to such patients. In a retrospective population-based study, use of statins was found to have a protective effect on uveitis development, over a period of 2 years [92]. The study identified 108 incident cases of uveitis with an incidence of 19% among statin users, compared to 30% in patients not treated with statins. Another study also reported a two-fold reduction in the risk of ocular inflammatory disease in male patients who used statins, compared to a control group, over a five year period [93]. Although this finding did not reach statistical significance, the risk reduction was greater with longer duration of statin use. These studies suggest that statins have potential benefits in patients with ocular inflammatory disease.
To address the question of whether there could be therapeutic benefits of statins with respect to disease control and dose of anti-inflammatory medications in patients with a diagnosis of uveitis, there is an ongoing phase II RCT comparing the effect of simvastatin 80 mg once daily vs. placebo among patients with sight-threatening uveitis. This may support the role of simvastatin in treating such patients, beyond reducing the cardiovascular risks related to long-term exposure to systemic immunosuppressive drugs and corticosteroids, especially given the relatively low side effect profile of statins, when compared to other immunosuppressive medications [94].
Summary
The potential role of statins in ocular disease is supported by the understanding of their mechanism of action, not only in reducing blood cholesterol levels, but also through direct vascular and anti-inflammatory mechanisms. However, the summation of clinical studies offers conflicting results and the use of these drugs remain inconclusive. This may be related to the different treatment regimens and follow-up lengths used in the various studies, with good support for reducing the risk of CNV in AMD patients, as well as reducing the progression of DR and promoting the resolution of DMO in patients with dyslipidaemia. In RVO, the effect of statins was not obvious and there is limited data in other ocular diseases. As such, at this time there remains no clear recommendation to add statins, purely for their anti-inflammatory and immunomodulatory effects to the treatment of patients with ocular diseases. However, when other risk factors, such as hyperlipidaemia, diabetes and hypertension exist, statins remain an invaluable option to decrease cholesterol and reduce cardiovascular risk factors. There are encouraging results that support the role of statins in ocular diseases and further studies may provide clearer evidence in support of this treatment and offer patients additional benefits from this class of medications.
Methodology
In August 2017 we performed a comprehensive literature review using the PubMed online database. We used the following terms: ‘statins immunomodulatory effect’; ‘statins and retinal disease’; ‘statins and the eye’; ‘statins and age-related macular degeneration’; ‘statins and AMD’; ‘statins and diabetic retinopathy’; ‘statins and retinal vein occlusion’; and ‘statins and uveitis’. We only included papers written in English. There were no date limits. We reviewed all articles and included those of interest focusing on animal models, laboratory research and clinical studies. We manually searched the reference lists of these manuscripts for additional studies.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.Jain MK, Ridker PM. Anti-inflammatory effects of statins: clinical evidence and basic mechanisms. Nat Rev Drug Discov. 2005;4:977–87. doi: 10.1038/nrd1901. [DOI] [PubMed] [Google Scholar]
- 2.Endo A, Kuroda M, Tsujita Y. ML-236A, ML-236B, and ML-236C, new inhibitors of cholesterogenesis produced by Penicillium citrinium. J Antibiot. 1976;29:1346–8. doi: 10.7164/antibiotics.29.1346. [DOI] [PubMed] [Google Scholar]
- 3.Graaf MR, Richel DJ, van Noorden CJ, Guchelaar HJ. Effects of statins and farnesyltransferase inhibitors on the development and progression of cancer. Cancer Treat Rev. 2004;30:609–41. doi: 10.1016/j.ctrv.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 4.Hall A. Rho GTPases and the actin cytoskeleton. Science. 1998;279:509–14. doi: 10.1126/science.279.5350.509. [DOI] [PubMed] [Google Scholar]
- 5.Musial J, Undas A, Gajewski P, Jankowski M, Sydor W, Szczeklik A. Anti-inflammatory effects of simvastatin in subjects with hypercholesterolemia. Int J Cardiol. 2001;77:247–53. doi: 10.1016/s0167-5273(00)00439-3. [DOI] [PubMed] [Google Scholar]
- 6.Albert MA, Glynn RJ, Ridker PM. Plasma concentration of C-reactive protein and the calculated Framingham Coronary Heart Disease Risk Score. Circulation. 2003;108:161–5. doi: 10.1161/01.CIR.0000080289.72166.CF. [DOI] [PubMed] [Google Scholar]
- 7.Jarvisalo MJ, Toikka JO, Vasankari T, Mikkola J, Viikari JS, Hartiala JJ, et al. HMG CoA reductase inhibitors are related to improved systemic endothelial function in coronary artery disease. Atherosclerosis. 1999;147:237–42. doi: 10.1016/s0021-9150(99)00189-6. [DOI] [PubMed] [Google Scholar]
- 8.Kobashigawa JA, Katznelson S, Laks H, Johnson JA, Yeatman L, Wang XM, et al. Effect of pravastatin on outcomes after cardiac transplantation. New Engl J Med. 1995;333:621–7. doi: 10.1056/NEJM199509073331003. [DOI] [PubMed] [Google Scholar]
- 9.Sever PS, Dahlof B, Poulter NR, Wedel H, Beevers G, Caulfield M, et al. Prevention of coronary and stroke events with atorvastatin in hypertensive patients who have average or lower-than-average cholesterol concentrations, in the Anglo-Scandinavian Cardiac Outcomes Trial--Lipid Lowering Arm (ASCOT-LLA): a multicentre randomised controlled trial. Drugs. 2004;64(Suppl 2):43–60. doi: 10.2165/00003495-200464002-00005. [DOI] [PubMed] [Google Scholar]
- 10.Kittleson MM, Kobashigawa JA. Statins in heart transplantation. Clin transpl. 2013:135-43. [PubMed]
- 11.Erie JC, Pueringer MR, Brue SM, Chamberlain AM, Hodge DO. Statin use and incident cataract surgery: a case-control study. Ophthalmic Epidemiol. 2016;23:40–5. doi: 10.3109/09286586.2015.1077258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Talwar N, Musch DC, Stein JD. Association of daily dosage and type of statin agent with risk of open-angle glaucoma. JAMA Ophthalmol. 2017;135:263–7. doi: 10.1001/jamaophthalmol.2016.5406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang W, Yan H. Simvastatin increases circulating endothelial progenitor cells and reduces the formation and progression of diabetic retinopathy in rats. Exp eye Res. 2012;105:1–8. doi: 10.1016/j.exer.2012.09.014. [DOI] [PubMed] [Google Scholar]
- 14.Vavvas DG, Daniels AB, Kapsala ZG, Goldfarb JW, Ganotakis E, Loewenstein JI, et al. Regression of some high-risk features of age-related macular degeneration (AMD) in patients receiving intensive statin treatment. EBioMedicine. 2016;5:198–203. doi: 10.1016/j.ebiom.2016.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tuuminen R, Haukka J, Loukovaara S. Statins in rhegmatogenous retinal detachment are associated with low intravitreal angiopoietin-2, VEGF and MMP-2 levels, and improved visual acuity gain in vitrectomized patients. Graefe's Arch Clin Exp Ophthalmol. 2015;253:1685–93. doi: 10.1007/s00417-014-2873-2. [DOI] [PubMed] [Google Scholar]
- 16.Chakrabarti R, Engleman EG. Interrelationships between mevalonate metabolism and the mitogenic signaling pathway in T lymphocyte proliferation. J Biol Chem. 1991;266:12216–22. [PubMed] [Google Scholar]
- 17.Cuthbert JA, Lipsky PE. A product of mevalonate proximal to isoprenoids is the source of both a necessary growth factor and an inhibitor of cell proliferation. Trans Assoc Am Physicians. 1991;104:97–106. [PubMed] [Google Scholar]
- 18.Youssef S, Stuve O, Patarroyo JC, Ruiz PJ, Radosevich JL, Hur EM, et al. The HMG-CoA reductase inhibitor, atorvastatin, promotes a Th2 bias and reverses paralysis in central nervous system autoimmune disease. Nature. 2002;420:78–84. doi: 10.1038/nature01158. [DOI] [PubMed] [Google Scholar]
- 19.Sadeghi MM, Tiglio A, Sadigh K, O'Donnell L, Collinge M, Pardi R, et al. Inhibition of interferon-gamma-mediated microvascular endothelial cell major histocompatibility complex class II gene activation by HMG-CoA reductase inhibitors. Transplantation. 2001;71:1262–8. doi: 10.1097/00007890-200105150-00014. [DOI] [PubMed] [Google Scholar]
- 20.Robinson R, Ho CE, Tan QS, Luu CD, Moe KT, Cheung CY, et al. Fluvastatin downregulates VEGF-A expression in TNF-alpha-induced retinal vessel tortuosity. Invest Ophthalmol Vis Sci. 2011;52:7423–31. doi: 10.1167/iovs.11-7912. [DOI] [PubMed] [Google Scholar]
- 21.Kwak B, Mulhaupt F, Myit S, Mach F. Statins as a newly recognized type of immunomodulator. Nat Med. 2000;6:1399–402. doi: 10.1038/82219. [DOI] [PubMed] [Google Scholar]
- 22.Yoshida M, Sawada T, Ishii H, Gerszten RE, Rosenzweig A, Gimbrone MA, et al. HMG-CoA reductase inhibitor modulates monocyte–endothelial cell interaction under physiological flow conditions in vitro. Arterioscler, Thromb, Vasc Biol. 2001;21:1165–71. doi: 10.1161/hq0701.092143. [DOI] [PubMed] [Google Scholar]
- 23.Al-Zamil WM, Yassin SA. Recent developments in age-related macular degeneration: a review. Clin Interv Aging. 2017;12:1313–30. doi: 10.2147/CIA.S143508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miller JW. Age-related macular degeneration revisited--piecing the puzzle: the LXIX Edward Jackson memorial lecture. Am J Ophthalmol. 2013;155:1–35. doi: 10.1016/j.ajo.2012.10.018. [DOI] [PubMed] [Google Scholar]
- 25.Ding X, Patel M, Chan CC. Molecular pathology of age-related macular degeneration. Progress Retin eye Res. 2009;28:1–18. doi: 10.1016/j.preteyeres.2008.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Curcio CA, Johnson M, Rudolf M, Huang JD. The oil spill in ageing Bruch membrane. Br J Ophthalmol. 2011;95:1638–45. doi: 10.1136/bjophthalmol-2011-300344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Spaide RF, Ho-Spaide WC, Browne RW, Armstrong D. Characterization of peroxidized lipids in Bruch's membrane. Retina. 1999;19:141–7. doi: 10.1097/00006982-199902000-00010. [DOI] [PubMed] [Google Scholar]
- 28.Guymer RH, Chiu AW, Lim L, Baird PN. HMG CoA reductase inhibitors (statins): do they have a role in age-related macular degeneration? Surv Ophthalmol. 2005;50:194–206. doi: 10.1016/j.survophthal.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 29.Friedman E. Update of the vascular model of AMD. Br J Ophthalmol. 2004;88:161–3. doi: 10.1136/bjo.2003.036277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Penfold PL, Madigan MC, Gillies MC, Provis JM. Immunological and aetiological aspects of macular degeneration. Progress Retin eye Res. 2001;20:385–414. doi: 10.1016/s1350-9462(00)00025-2. [DOI] [PubMed] [Google Scholar]
- 31.Curcio CA, Messinger JD, Sloan KR, McGwin G, Medeiros NE, Spaide RF. Subretinal drusenoid deposits in non-neovascular age-related macular degeneration: morphology, prevalence, topography, and biogenesis model. Retina. 2013;33:265–76. doi: 10.1097/IAE.0b013e31827e25e0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ma L, Wang Y, Du J, Wang M, Zhang R, Fu Y. The association between statin use and risk of age-related macular degeneration. Sci Rep. 2015;5:18280. doi: 10.1038/srep18280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tan JS, Mitchell P, Rochtchina E, Wang JJ. Statins and the long-term risk of incident age-related macular degeneration: the Blue Mountains Eye Study. Am J Ophthalmol. 2007;143:685–7. doi: 10.1016/j.ajo.2006.11.021. [DOI] [PubMed] [Google Scholar]
- 34.Klein R, Klein BE, Tomany SC, Danforth LG, Cruickshanks KJ. Relation of statin use to the 5-year incidence and progression of age-related maculopathy. Arch Ophthalmol. 2003;121:1151–5. doi: 10.1001/archopht.121.8.1151. [DOI] [PubMed] [Google Scholar]
- 35.van Leeuwen R, Tomany SC, Wang JJ, Klein R, Mitchell P, Hofman A, et al. Is medication use associated with the incidence of early age-related maculopathy? Pooled findings from 3 continents. Ophthalmology. 2004;111:1169–75. doi: 10.1016/j.ophtha.2003.10.024. [DOI] [PubMed] [Google Scholar]
- 36.Maguire MG, Ying GS, McCannel CA, Liu C, Dai Y. Statin use and the incidence of advanced age-related macular degeneration in the Complications of Age-related Macular Degeneration Prevention Trial. Ophthalmology. 2009;116:2381–5. doi: 10.1016/j.ophtha.2009.06.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barbosa DT, Mendes TS, Cintron-Colon HR, Wang SY, Bhisitkul RB, Singh K, et al. Age-related macular degeneration and protective effect of HMG Co-A reductase inhibitors (statins): results from the National Health and Nutrition Examination Survey 2005-2008. Eye. 2014;28:472–80. doi: 10.1038/eye.2014.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Martini E, Scorolli L, Burgagni M, Fessehaie S. Valutazione degli effetti retinici della somministrazione di simvastatina in pazienti affetti da degenerazione maculare senile. Ann Ottalmol e Clin Ocul. 1991;117:1121–6. [Google Scholar]
- 39.Guymer RH, Baird PN, Varsamidis M, Busija L, Dimitrov PN, Aung KZ, et al. Proof of concept, randomized, placebo-controlled study of the effect of simvastatin on the course of age-related macular degeneration. PloS ONE. 2013;8:e83759. doi: 10.1371/journal.pone.0083759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gehlbach P, Li T, Hatef E. Statins for age-related macular degeneration. Cochrane Database Syst Rev. 2016:Cd006927. [DOI] [PMC free article] [PubMed]
- 41.Landmesser U, Hornig B, Drexler H. Endothelial dysfunction in hypercholesterolemia: mechanisms, pathophysiological importance, and therapeutic interventions. Semin Thromb Hemost. 2000;26:529–37. doi: 10.1055/s-2000-13209. [DOI] [PubMed] [Google Scholar]
- 42.Tang J, Kern TS. Inflammation in diabetic retinopathy. Progress Retin eye Res. 2011;30:343–58. doi: 10.1016/j.preteyeres.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gustavsson C, Agardh CD, Hagert P, Agardh E. Inflammatory markers in nondiabetic and diabetic rat retinas exposed to ischemia followed by reperfusion. Retina. 2008;28:645–52. doi: 10.1097/IAE.0b013e31815ec32d. [DOI] [PubMed] [Google Scholar]
- 44.Joussen AM, Poulaki V, Mitsiades N, Kirchhof B, Koizumi K, Dohmen S, et al. Nonsteroidal anti-inflammatory drugs prevent early diabetic retinopathy via TNF-alpha suppression. FASEB J. 2002;16:438–40. doi: 10.1096/fj.01-0707fje. [DOI] [PubMed] [Google Scholar]
- 45.Kita T, Hata Y, Arita R, Kawahara S, Miura M, Nakao S, et al. Role of TGF-beta in proliferative vitreoretinal diseases and ROCK as a therapeutic target. Proc Natl Acad Sci USA. 2008;105:17504–9. doi: 10.1073/pnas.0804054105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rangaswamy S, Penn MS, Saidel GM, Chisolm GM. Exogenous oxidized low-density lipoprotein injures and alters the barrier function of endothelium in rats in vivo. Circ Res. 1997;80:37–44. doi: 10.1161/01.res.80.1.37. [DOI] [PubMed] [Google Scholar]
- 47.Ding J, Wong TY. Current epidemiology of diabetic retinopathy and diabetic macular edema. Curr Diabetes Rep. 2012;12:346–54. doi: 10.1007/s11892-012-0283-6. [DOI] [PubMed] [Google Scholar]
- 48.Jew OM, Peyman M, Chen TC, Visvaraja S. Risk factors for clinically significant macular edema in a multi-ethnics population with type 2 diabetes. Int J Ophthalmol. 2012;5:499–504. doi: 10.3980/j.issn.2222-3959.2012.04.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chew EY, Klein ML, Ferris FL, 3rd, Remaley NA, Murphy RP, Chantry K, et al. Association of elevated serum lipid levels with retinal hard exudate in diabetic retinopathy. Early Treatment Diabetic Retinopathy Study (ETDRS) Report 22. Arch Ophthalmol. 1996;114:1079–84. doi: 10.1001/archopht.1996.01100140281004. [DOI] [PubMed] [Google Scholar]
- 50.Klein BE, Moss SE, Klein R, Surawicz TS. The Wisconsin epidemiologic study of diabetic retinopathy. XIII. Relationship of serum cholesterol to retinopathy and hard exudate. Ophthalmology. 1991;98:1261–5. doi: 10.1016/s0161-6420(91)32145-6. [DOI] [PubMed] [Google Scholar]
- 51.Fadini GP, Baesso I, Albiero M, Sartore S, Agostini C, Avogaro A. Technical notes on endothelial progenitor cells: ways to escape from the knowledge plateau. Atherosclerosis. 2008;197:496–503. doi: 10.1016/j.atherosclerosis.2007.12.039. [DOI] [PubMed] [Google Scholar]
- 52.Ozkiris A, Erkilic K, Koc A, Mistik S. Effect of atorvastatin on ocular blood flow velocities in patients with diabetic retinopathy. Br J Ophthalmol. 2007;91:69–73. doi: 10.1136/bjo.2006.098285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wu WC, Lai YH, Hsieh MC, Chang YC, Wu MH, Wu HJ, et al. Pleiotropic role of atorvastatin in regulation of human retinal pigment epithelial cell behaviors in vitro. Exp eye Res. 2011;93:842–51. doi: 10.1016/j.exer.2011.09.016. [DOI] [PubMed] [Google Scholar]
- 54.Kawahara S, Hata Y, Kita T, Arita R, Miura M, Nakao S, et al. Potent inhibition of cicatricial contraction in proliferative vitreoretinal diseases by statins. Diabetes. 2008;57:2784–93. doi: 10.2337/db08-0302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dornan TL, Carter RD, Bron AJ, Turner RC, Mann JI. Low density lipoprotein cholesterol: an association with the severity of diabetic retinopathy. Diabetologia. 1982;22:167–70. doi: 10.1007/BF00283746. [DOI] [PubMed] [Google Scholar]
- 56.Klein BE, Klein R, Moss SE. Is serum cholesterol associated with progression of diabetic retinopathy or macular edema in persons with younger-onset diabetes of long duration? Am J Ophthalmol. 1999;128:652–4. doi: 10.1016/s0002-9394(99)00222-6. [DOI] [PubMed] [Google Scholar]
- 57.Chowdhury TA, Hopkins D, Dodson PM, Vafidis GC. The role of serum lipids in exudative diabetic maculopathy: is there a place for lipid lowering therapy? Eye. 2002;16:689–93. doi: 10.1038/sj.eye.6700205. [DOI] [PubMed] [Google Scholar]
- 58.Miljanovic B, Glynn RJ, Nathan DM, Manson JE, Schaumberg DA. A prospective study of serum lipids and risk of diabetic macular edema in type 1 diabetes. Diabetes. 2004;53:2883–92. doi: 10.2337/diabetes.53.11.2883. [DOI] [PubMed] [Google Scholar]
- 59.Cusick M, Chew EY, Chan CC, Kruth HS, Murphy RP, Ferris FL., 3rd Histopathology and regression of retinal hard exudates in diabetic retinopathy after reduction of elevated serum lipid levels. Ophthalmology. 2003;110:2126–33. doi: 10.1016/j.ophtha.2003.01.001. [DOI] [PubMed] [Google Scholar]
- 60.Hove MN, Kristensen JK, Lauritzen T, Bek T. The prevalence of retinopathy in an unselected population of type 2 diabetes patients from Arhus County, Denmark. Acta Ophthalmol Scand. 2004;82:443–8. doi: 10.1111/j.1600-0420.2004.00270.x. [DOI] [PubMed] [Google Scholar]
- 61.Rema M, Premkumar S, Anitha B, Deepa R, Pradeepa R, Mohan V. Prevalence of diabetic retinopathy in urban India: the Chennai Urban Rural Epidemiology Study (CURES) eye study, I. Invest Ophthalmol Vis Sci. 2005;46:2328–33. doi: 10.1167/iovs.05-0019. [DOI] [PubMed] [Google Scholar]
- 62.Wong TY, Cheung N, Tay WT, Wang JJ, Aung T, Saw SM, et al. Prevalence and risk factors for diabetic retinopathy: the Singapore Malay Eye Study. Ophthalmology. 2008;115:1869–75. doi: 10.1016/j.ophtha.2008.05.014. [DOI] [PubMed] [Google Scholar]
- 63.Rema M, Srivastava BK, Anitha B, Deepa R, Mohan V. Association of serum lipids with diabetic retinopathy in urban South Indians--the Chennai Urban Rural Epidemiology Study (CURES) eye study--2. Diabet Med: a J Br Diabet Assoc. 2006;23:1029–36. doi: 10.1111/j.1464-5491.2006.01890.x. [DOI] [PubMed] [Google Scholar]
- 64.Gordon B, Chang S, Kavanagh M, Berrocal M, Yannuzzi L, Robertson C, et al. The effects of lipid lowering on diabetic retinopathy. Am J Ophthalmol. 1991;112:385–91. doi: 10.1016/s0002-9394(14)76244-0. [DOI] [PubMed] [Google Scholar]
- 65.Sen K, Misra A, Kumar A, Pandey RM. Simvastatin retards progression of retinopathy in diabetic patients with hypercholesterolemia. Diabetes Res Clin Pract. 2002;56:1–11. doi: 10.1016/s0168-8227(01)00341-2. [DOI] [PubMed] [Google Scholar]
- 66.Gupta A, Gupta V, Thapar S, Bhansali A. Lipid-lowering drug atorvastatin as an adjunct in the management of diabetic macular edema. Am J Ophthalmol. 2004;137:675–82. doi: 10.1016/j.ajo.2003.11.017. [DOI] [PubMed] [Google Scholar]
- 67.Narang S, Sood S, Kaur B, Singh R, Mallik A, Kaur J. Atorvastatin in clinically-significant macular edema in diabetics with a normal lipid profile. Nepal J Ophthalmol. 2012;4:23–8. doi: 10.3126/nepjoph.v4i1.5846. [DOI] [PubMed] [Google Scholar]
- 68.Ilyina Y, Bezditko P, Mohamed AS, Zavoloka O, Zubkova D. Statins and fibrates as the treatment of nonproliferative diabetic retinopathy in type 2 diabetes mellitus. Spektrum Augenheilkd. 2016;30:111–6. [Google Scholar]
- 69.Denniston AK, Banerjee S, Gibson JM, Dodson PM. Cardiovascular therapies and their role in diabetic eye disease. Diabet Med. 2005;22:665–6. doi: 10.1111/j.1464-5491.2005.01484.x. [DOI] [PubMed] [Google Scholar]
- 70.Keech AC, Mitchell P, Summanen PA, O'Day J, Davis TM, Moffitt MS, et al. Effect of fenofibrate on the need for laser treatment for diabetic retinopathy (FIELD study): a randomised controlled trial. Lancet. 2007;370:1687–97. doi: 10.1016/S0140-6736(07)61607-9. [DOI] [PubMed] [Google Scholar]
- 71.Buse JB, Bigger JT, Byington RP, Cooper LS, Cushman WC, Friedewald WT, et al. Action to control cardiovascular risk in diabetes (ACCORD) trial: design and methods. Am J Cardiol. 2007;99(12a):21i–33i. doi: 10.1016/j.amjcard.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 72.Group AS, Group AES. Effects of medical therapies on retinopathy progression in type 2 diabetes. New Engl J Med. 2010;363:233. doi: 10.1056/NEJMoa1001288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chew EY, Davis MD, Danis RP, Lovato JF, Perdue LH, Greven C, et al. The effects of medical management on the progression of diabetic retinopathy in persons with type 2 diabetes: the Action to Control Cardiovascular Risk in Diabetes (ACCORD) Eye Study. Ophthalmology. 2014;121:2443–51. doi: 10.1016/j.ophtha.2014.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Omae T, Nagaoka T, Tanano I, Kamiya T, Yoshida A. Fenofibrate, an anti-dyslipidemia drug, elicits the dilation of isolated porcine retinal arterioles: role of nitric oxide and AMP-activated protein kinase. Invest Ophthalmol Vis Sci. 2012;53:2880–6. doi: 10.1167/iovs.11-8841. [DOI] [PubMed] [Google Scholar]
- 75.Garcia-Ramirez M, Hernandez C, Palomer X, Vazquez-Carrera M, Simo R. Fenofibrate prevents the disruption of the outer blood retinal barrier through downregulation of NF-kappaB activity. Acta Diabetol. 2016;53:109–18. doi: 10.1007/s00592-015-0759-3. [DOI] [PubMed] [Google Scholar]
- 76.Prisco D, Marcucci R. Retinal vein thrombosis: risk factors, pathogenesis and therapeutic approach. Pathophysiol Haemost Thromb. 2002;32:308–11. doi: 10.1159/000073587. [DOI] [PubMed] [Google Scholar]
- 77.Rehak M, Wiedemann P. Retinal vein thrombosis: pathogenesis and management. J Thromb Haemost. 2010;8:1886–94. doi: 10.1111/j.1538-7836.2010.03909.x. [DOI] [PubMed] [Google Scholar]
- 78.O'Mahoney PR, Wong DT, Ray JG. Retinal vein occlusion and traditional risk factors for atherosclerosis. Arch Ophthalmol. 2008;126:692–9. doi: 10.1001/archopht.126.5.692. [DOI] [PubMed] [Google Scholar]
- 79.Dodson PM, Galton DJ, Hamilton AM, Blach RK. Retinal vein occlusion and the prevalence of lipoprotein abnormalities. Br J Ophthalmol. 1982;66:161–4. doi: 10.1136/bjo.66.3.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lee WJ, Kang MH, Seong M, Cho HY. Comparison of aqueous concentrations of angiogenic and inflammatory cytokines in diabetic macular oedema and macular oedema due to branch retinal vein occlusion. Br J Ophthalmol. 2012;96:1426–30. doi: 10.1136/bjophthalmol-2012-301913. [DOI] [PubMed] [Google Scholar]
- 81.Noma H, Funatsu H, Mimura T, Harino S, Hori S. Aqueous humor levels of vasoactive molecules correlate with vitreous levels and macular edema in central retinal vein occlusion. Eur J Ophthalmol. 2010;20:402–9. doi: 10.1177/112067211002000222. [DOI] [PubMed] [Google Scholar]
- 82.Medina RJ, O'Neill CL, Devine AB, Gardiner TA, Stitt AW. The pleiotropic effects of simvastatin on retinal microvascular endothelium has important implications for ischaemic retinopathies. PloS ONE. 2008;3:e2584. doi: 10.1371/journal.pone.0002584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Matei VM, Xia JY, Nguyen C. Poor outcomes despite aspirin or statin use in high-risk patients with retinal vein occlusion. Graefe'S Arch Clin Exp Ophthalmol. 2017;255:761–6. doi: 10.1007/s00417-016-3569-6. [DOI] [PubMed] [Google Scholar]
- 84.Tomkins-Netzer O, Talat L, Bar A, Lula A, Taylor SR, Joshi L, et al. Long-term clinical outcome and causes of vision loss in patients with uveitis. Ophthalmology. 2014;121:2387–92. doi: 10.1016/j.ophtha.2014.07.007. [DOI] [PubMed] [Google Scholar]
- 85.Rychwalski PJ, Cruz OA, Alanis-Lambreton G, Foy TM, Kane RE. Asymptomatic uveitis in young people with inflammatory bowel disease. J Am Assoc Pediatr Ophthalmol Strabismus. 1997;1:111–4. doi: 10.1016/s1091-8531(97)90009-4. [DOI] [PubMed] [Google Scholar]
- 86.Murphy CC, Greiner K, Plskova J, Duncan L, Frost NA, Forrester JV, et al. Cyclosporine vs tacrolimus therapy for posterior and intermediate uveitis. Arch Ophthalmol. 2005;123:634–41. doi: 10.1001/archopht.123.5.634. [DOI] [PubMed] [Google Scholar]
- 87.Kohno H, Sakai T, Saito S, Okano K, Kitahara K. Treatment of experimental autoimmune uveoretinitis with atorvastatin and lovastatin. Exp Eye Res. 2007;84:569–76. doi: 10.1016/j.exer.2006.11.011. [DOI] [PubMed] [Google Scholar]
- 88.Gegg ME, Harry R, Hankey D, Zambarakji H, Pryce G, Baker D, et al. Suppression of autoimmune retinal disease by lovastatin does not require Th2 cytokine induction. J Immunol. 2005;174:2327–35. doi: 10.4049/jimmunol.174.4.2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.La Mura V, Pasarin M, Meireles CZ, Miquel R, Rodriguez-Vilarrupla A, Hide D, et al. Effects of simvastatin administration on rodents with lipopolysaccharide-induced liver microvascular dysfunction. Hepatology. 2013;57:1172–81. doi: 10.1002/hep.26127. [DOI] [PubMed] [Google Scholar]
- 90.Chataway J, Schuerer N, Alsanousi A, Chan D, MacManus D, Hunter K, et al. Effect of high-dose simvastatin on brain atrophy and disability in secondary progressive multiple sclerosis (MS-STAT): a randomised, placebo-controlled, phase 2 trial. Lancet. 2014;383:2213–21. doi: 10.1016/S0140-6736(13)62242-4. [DOI] [PubMed] [Google Scholar]
- 91.McCarey DW, McInnes IB, Madhok R, Hampson R, Scherbakov O, Ford I, et al. Trial of atorvastatin in rheumatoid arthritis (TARA): double-blind, randomised placebo-controlled trial. Lancet. 2004;363:2015–21. doi: 10.1016/S0140-6736(04)16449-0. [DOI] [PubMed] [Google Scholar]
- 92.Borkar DS, Tham VM, Shen E, Parker JV, Uchida A, Vinoya AC, et al. Association between statin use and uveitis: results from the Pacific Ocular Inflammation study. Am J Ophthalmol. 2015;159:707–13. doi: 10.1016/j.ajo.2015.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yunker JJ, McGwin G, Jr, Read RW. Statin use and ocular inflammatory disease risk. J Ophthalmic Inflamm Infect. 2013;3:8. doi: 10.1186/1869-5760-3-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Smeeth L, Douglas I, Hall AJ, Hubbard R, Evans S. Effect of statins on a wide range of health outcomes: a cohort study validated by comparison with randomized trials. Br J Clin Pharmacol. 2009;67:99–109. doi: 10.1111/j.1365-2125.2008.03308.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.McGwin G, Jr, Modjarrad K, Hall TA, Xie A, Owsley C. 3-hydroxy-3-methylglutaryl coenzyme a reductase inhibitors and the presence of age-related macular degeneration in the Cardiovascular Health Study. Arch Ophthalmol. 2006;124:33–7. doi: 10.1001/archopht.124.1.33. [DOI] [PubMed] [Google Scholar]
- 96.Smeeth L, Cook C, Chakravarthy U, Hubbard R, Fletcher AE. A case control study of age related macular degeneration and use of statins. Br J Ophthalmol. 2005;89:1171–5. doi: 10.1136/bjo.2004.064477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Peiretti E, Mandas A, Abete C, Vinci M, Piludu S, Casu M, et al. Age-related macular degeneration and cognitive impairment show similarities in changes of neutral lipids in peripheral blood mononuclear cells. Exp eye Res. 2014;124:11–6. doi: 10.1016/j.exer.2014.04.017. [DOI] [PubMed] [Google Scholar]
- 98.Cougnard-Gregoire A, Delyfer MN, Korobelnik JF, Rougier MB, Le Goff M, Dartigues JF, et al. Elevated high-density lipoprotein cholesterol and age-related macular degeneration: the Alienor study. PloS ONE. 2014;9:e90973. doi: 10.1371/journal.pone.0090973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Klein R, Myers CE, Buitendijk GH, Rochtchina E, Gao X, de Jong PT, et al. Lipids, lipid genes, and incident age-related macular degeneration: the three continent age-related macular degeneration consortium. Am J Ophthalmol. 2014;158:513–24. doi: 10.1016/j.ajo.2014.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Panagiotoglou TD, Ganotakis ES, Kymionis GD, Moschandreas JA, Fanti GN, Charisis SK, et al. Atorvastatin for diabetic macular edema in patients with diabetes mellitus and elevated serum cholesterol. Ophthalmic Surg Lasers Imaging. 2010;41:316–22. doi: 10.3928/15428877-20100430-04. [DOI] [PubMed] [Google Scholar]
- 101.Nielsen SF, Nordestgaard BG. Statin use before diabetes diagnosis and risk of microvascular disease: a nationwide nested matched study. Lancet Diabetes Endocrinol. 2014;2:894–900. doi: 10.1016/S2213-8587(14)70173-1. [DOI] [PubMed] [Google Scholar]
- 102.Gaede P, Lund-Andersen H, Parving HH, Pedersen O. Effect of a multifactorial intervention on mortality in type 2 diabetes. New Engl J Med. 2008;358:580–91. doi: 10.1056/NEJMoa0706245. [DOI] [PubMed] [Google Scholar]
- 103.Fried LF, Forrest KY, Ellis D, Chang Y, Silvers N, Orchard TJ. Lipid modulation in insulin-dependent diabetes mellitus: effect on microvascular outcomes. J Diabetes Complicat. 2001;15:113–9. doi: 10.1016/s1056-8727(01)00140-4. [DOI] [PubMed] [Google Scholar]
- 104.Liinamaa MJ, Savolainen MJ. High vitreous concentration of vascular endothelial growth factor in diabetic patients with proliferative retinopathy using statins. Ann Med. 2008;40:209–14. doi: 10.1080/07853890701749209. [DOI] [PubMed] [Google Scholar]
- 105.Colhoun HM, Betteridge DJ, Durrington PN, Hitman GA, Neil HA, Livingstone SJ, et al. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): multicentre randomised placebo-controlled trial. Lancet. 2004;364:685–96. doi: 10.1016/S0140-6736(04)16895-5. [DOI] [PubMed] [Google Scholar]
- 106.Zhang J, McGwin G., Jr. Association of statin use with the risk of developing diabetic retinopathy. Arch Ophthalmol. 2007;125:1096–9. doi: 10.1001/archopht.125.8.1096. [DOI] [PubMed] [Google Scholar]
- 107.Zhang Y, Zhang Z, Yan H. Simvastatin inhibits ischemia/reperfusion injury-induced apoptosis of retinal cells via downregulation of the tumor necrosis factor-alpha/nuclear factor-kappaB pathway. Int J Mol Med. 2015;36:99–405. doi: 10.3892/ijmm.2015.2244. [DOI] [PMC free article] [PubMed] [Google Scholar]