Abstract
Rationale:
Menkes disease (MD), also known as Menkes kinky hair disease, is a fatal neurodegenerative disease caused by a defect in copper metabolism. The symptoms involve multiple organ systems, such as the brain, lung, gastrointestinal tract, urinary tract, connective tissue, and skin. There is currently no cure for this disease entity, and patients with the classic form of MD usually die from complications between 6 months and 3 years of age. Intracranial hemorrhage secondary to tortuous intracranial arteries is a well-known complication of MD, but spontaneous retroperitoneal hemorrhage, to the best of our knowledge, has never been reported in a patient with MD. Herein, we describe the first case of retroperitoneal hematoma as a complication of MD in a 4-year-old boy.
Patient concerns:
A 4-year-old Taiwanese male patient with MD was referred to the hospital and presented with a palpable epigastric mass.
Diagnoses:
On the basis of the findings of ultrasonography and enhanced computed tomography, the diagnosis was retroperitoneal hematoma.
Interventions:
Interventions included laparotomy with evacuation of the hematoma, manual compression, and suture of the bleeding vessels.
Outcomes:
There were no postoperative complications.
Lessons:
This case emphasizes that bleeding in patients with MD is possible at any site in the body owing to the unstable structure of the connective tissues. Timely diagnosis with proper imaging studies can lead to prompt and appropriate management and save patients from this life-threatening condition.
Keywords: copper, intracranial hemorrhage, Menkes kinky hair disease, retroperitoneal hematoma, vessel tortuosity
1. Introduction
Menkes disease (MD) is an X-linked disorder of copper metabolism with wide variability and clinical heterogeneity in its manifestations. Disrupted functions of copper-dependent enzymes, including cytochrome oxidase, dopamine beta-hydroxylase, lysyl oxidase (LO), sulfhydryl oxidase, superoxide dismutase, and tyrosinase, lead to the classic symptoms of MD. Early diagnosis is crucial; however, managing complications of the disease is equally important. Severe respiratory infections and neurological deterioration are the main causes of morbidity or mortality in MD.[1] Retroperitoneal hematoma is a rare entity in children, and most cases are caused by blunt abdominal trauma, malignancy, coagulopathies, or iatrogenic injuries.[2,3] We describe herein the first case of MD with retroperitoneal hematoma, manifesting as an epigastric mass, and the diagnosis was made with the aid of ultrasonography, computed tomography, and laparotomy. This case emphasizes that bleeding in patients with MD is possible at any site in the body due to the unstable structure of the connective tissues.
2. Case report
This study was approved by the Institutional Review Board (IRB) of MacKay Memorial Hospital (IRB number 17MMHIS146).
A 4-year-old Taiwanese boy initially presented at 5 months of age with focal seizure, hypotonia, and hypopigmented curly scalp hair, and the diagnosis of MD was confirmed by a molecular study at 7 months of age. Subsequently, copper-histidine injections were started. Other associated problems included global developmental delay, epilepsy, pectus excavatum, gastroesophageal reflux, bilateral nephrocalcinosis, vesicoureteral reflux (VUR), multiple urinary bladder diverticula, inguinal hernias, and osteopenia. He experienced cardiopulmonary resuscitation (CPR) 4 times during the ages of 3 to 4 years, and therapeutic hypothermia after CPR was performed in 1 episode. He was bedridden at home and dependent on a nasogastric tube feeding and tracheostomy ventilator. His regular medications included levetiracetam, topiramate, and vigabatrin for epilepsy, and prophylactic antibiotics for VUR.
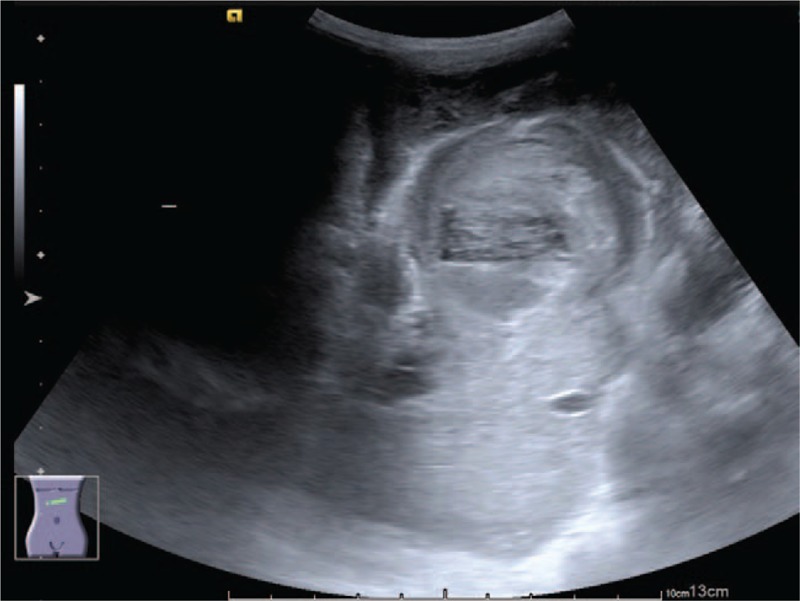
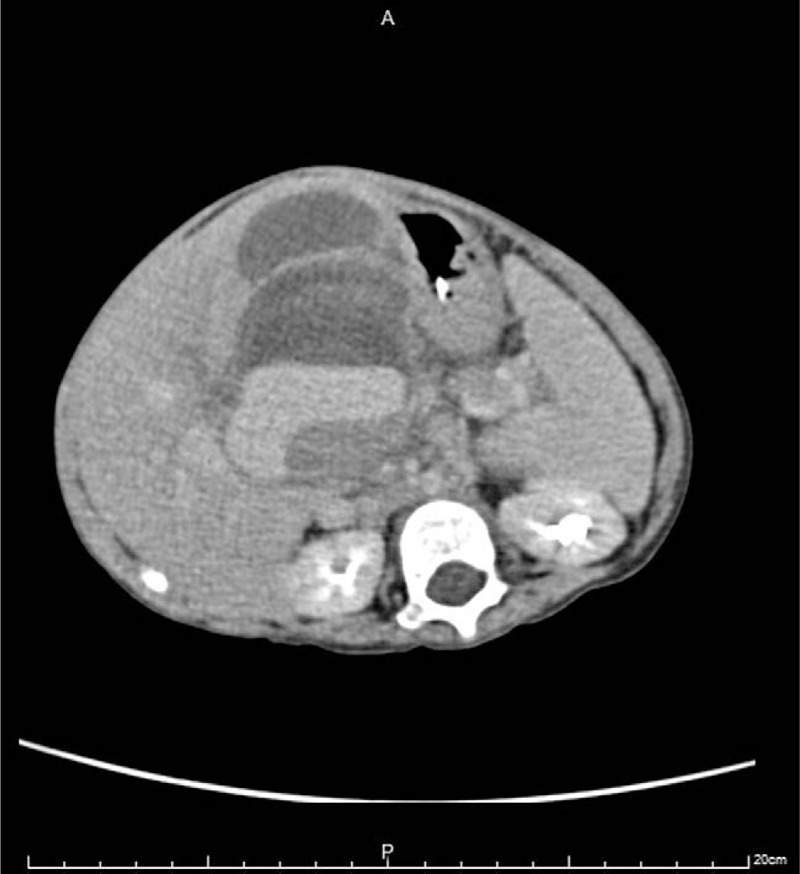
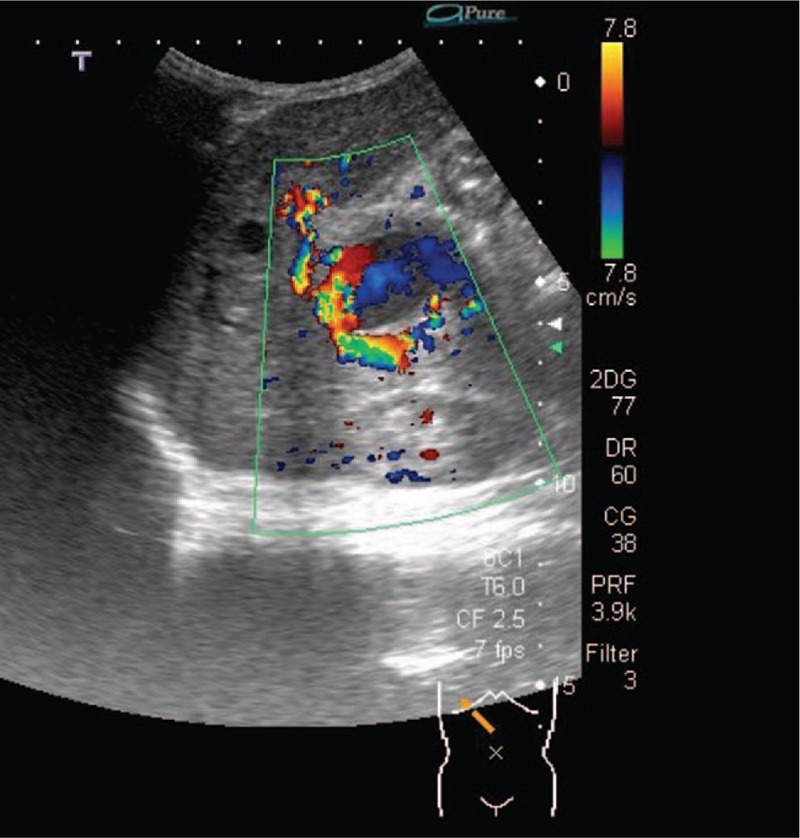
This time, he was referred to the hospital and presented with a palpable epigastric mass, and coffee-ground substance from the nasogastric tube was noted for 4 days. He had stool passage the day before admission, and there was no blood or mucus in his stool. He had no vomiting, diarrhea, cough, shortness of breath, or increase in oxygen demand. On physical examination, vital signs revealed the following: body temperature 38.1°C, blood pressure 105/51 mm Hg, heart rate 113 beats/min, and respiratory rate 36 breaths/min. He had a pale conjunctiva, and there was an elastic soft mass at the epigastrium measuring 6 cm × 5 cm. There was no abdominal muscle guarding, hepatosplenomegaly, skin bruises, or petechiae. Laboratory investigations indicated anemia, and other data are listed in Table 1. The follow-up hemoglobin level decreased from 7.8 to 6.8 g/dL within 20 hours; thus, 200 mL of packed red blood cells was transfused. Plain film of the abdomen showed no obvious signs of intestinal obstruction. The abdominal ultrasonogram revealed a hamburger-like mass at the subxiphoid area, consisting of hypoechoic “hamburger buns” and multilayered “hamburger filling” (Fig. 1). An enhanced computed tomography scan of the abdomen showed a mass containing 3 flat ovoid layers, 1 over another, the superficial 2 with low attenuation and the deepest with high attenuation (Fig. 2). The maximum diameters of the mass were 6.11 cm × 4.69 cm. On the next day, the follow-up abdominal ultrasonogram showed an increased thickness of the “bottom hamburger bun,” and a blood vessel was mapped by color Doppler, heading into the “bottom hamburger bun.” The ultrasonograms were suggestive of active intra-abdominal bleeding with a growing hematoma (Fig. 3). Emergent laparotomy was performed. A well-capsulated hematoma, composed of 3 chambers divided by pseudomembranes, was seen between the stomach and liver. The 2 superficial chambers were filled with necrotic tissues and blood clots, whereas the deepest chamber was impacted with blood clots and revealed multiple active bleeders (Fig. 4). One bleeder was identified as a branch of the left gastric artery. Manual compression, suture of the vessel, and surgical packing of the inner side of the capsule were performed to stop the bleeding.
Table 1.
Laboratory tests results upon admission.
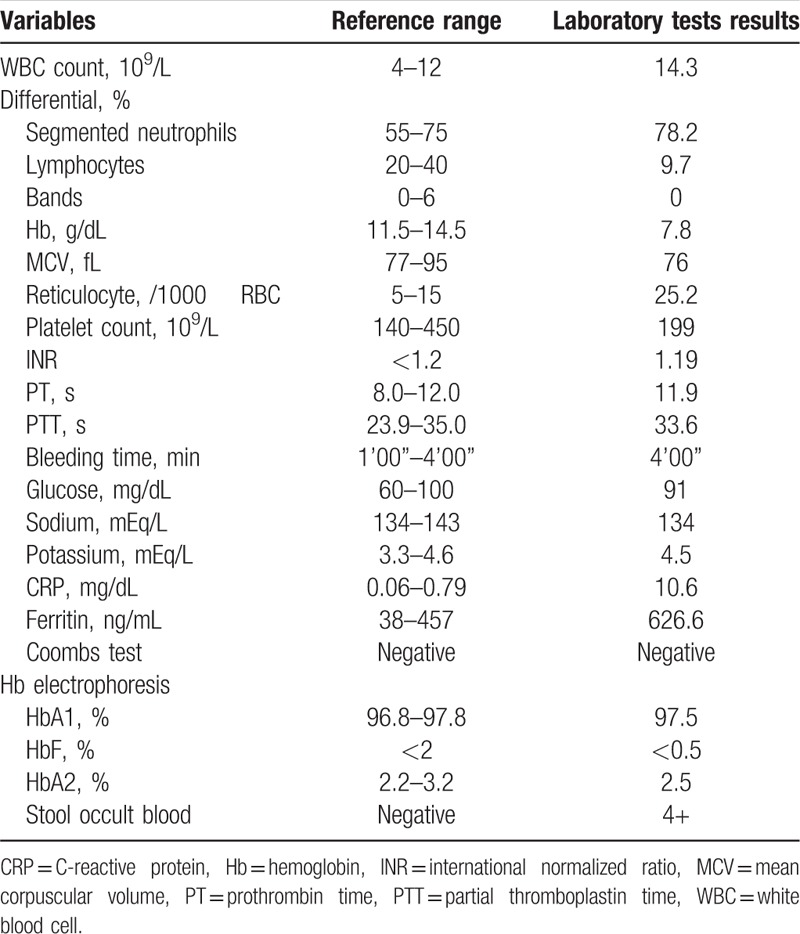
Figure 1.
Abdominal ultrasonography revealing a hamburger-like mass, consisting of hypoechoic “hamburger buns” and multilayered “hamburger filling.”
Figure 2.
Enhanced computed tomography scan of the abdomen showing a mass next to the stomach, containing 3 flat ovoid layers, the superficial 2 with low attenuation and the deepest with high attenuation.
Figure 3.
A blood vessel is mapped by color Doppler, heading into the “bottom hamburger bun.” The hypoechoic circular flow within the “hamburger bun” is also seen, indicating active bleeding with hematoma formation.
Figure 4.
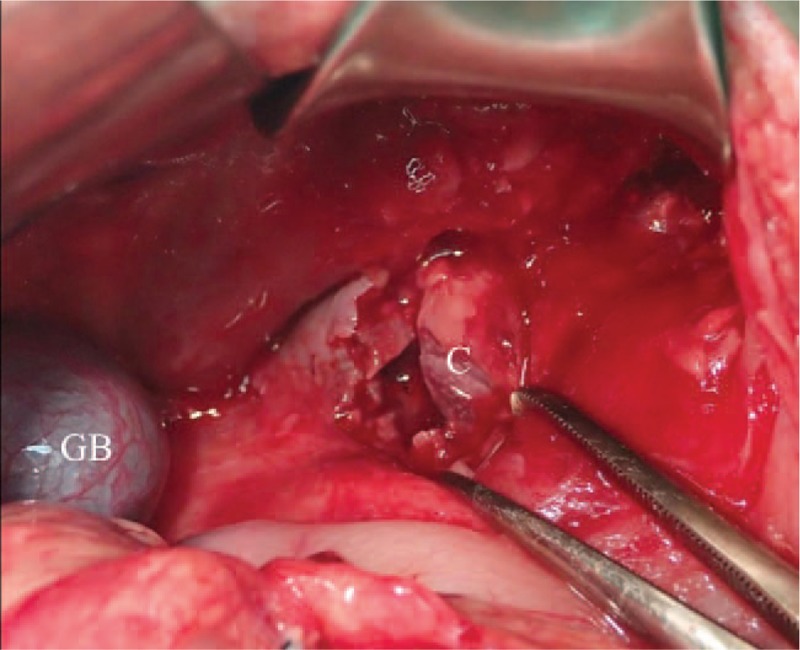
After removing the superficial 2 chambers of blood clots and necrotic tissues, the deepest chamber (C) with active bleeders is revealed.
The patient's postoperative course was smooth. Nasogastric feeding was started since postoperative day 3, and the patient was discharged 9 days postoperatively.
3. Discussion
MD is a lethal neurodegenerative disease caused by mutations of the ATP7A gene that lead to a defect in copper metabolism, contributing to low serum copper and ceruloplasmin levels. Copper is an essential element in the early stages of central nervous system development and in the biosynthesis and maintenance of bone and connective tissues.[4] As a result, clinical features of MD are diverse and multisystemic, including mental retardation, optic atrophy, convulsions, feeding difficulties, failure to thrive, hypothermia, hypotonia, apnea, infections, bladder diverticula, peculiar facies, kinky hair, hypopigmentation, bone changes, and cutis laxa.[5,6] Most patients die of complications before the age of 3 years. Copper-histidine therapy may be beneficial in preventing neurological deterioration; increasing tone; and improving sociocognitive milestones, hair changes, weight gain, and immunity in some patients, particularly when treatment is begun in the neonatal period or in the fetus.[7] However, the effects of long-term treatment are still limited, and the overall prognosis remains poor.[8]
It is worth mentioning that connective tissue disorders comprise a major part of symptoms, which are attributed to decreased activities of LO, the key copper-dependent enzyme that works in elastin and collagen cross-linking. Parenteral administration of copper-histidine cannot improve activities of LO because the administered copper is not transported to the Golgi apparatus, where LO combines with copper to function.[4,5] In patients with MD, defective elastic fibers within the internal elastic lamina, tunica media, and intimate layer of arteries and arterioles result in vascular tortuosity and ectasia, with greater predisposition to mucosal hemorrhage.[9]
In fact, brain magnetic resonance imaging (MRI) has become an adjunct diagnostic tool for MD, as increased cerebrovascular tortuosity seems to represent an early and reliable diagnostic biomarker of MD.[1,10] According to Manara et al,[11] elongated and thin-walled intracranial arteries appear on brain MRI scans at as early as disease onset in most patients with MD, whereas there is no evidence of significant vessel wall change evolution during the disease course.[12] In addition, epidural and subdural hemorrhages have been frequently reported in the literature.[5,13] One reported case in 1972 showed splitting, irregularity, and fragmentation of the internal elastic lamina of the systemic arteries upon necropsy, particularly in the mesenteric vessels and in the aorta and carotid, splenic, and renal arteries.[14] Nonetheless, there is a lack of literature depicting tortuosity of systemic vessels, and gastrointestinal bleeding or intra-abdominal bleeding has been scarcely reported.
Recurrent spontaneous subserosal hemorrhage of the ileum was reported in a 3-year-old patient with MD and led to intestinal obstructions.[15] Belsha et al[9] described a multilobulated hyperplastic polyp with extensive involvement of the antrum and pylorus of the stomach in a 5-month-old patient with MD, and it caused marked upper gastrointestinal tract bleeding. A hyperplastic polyp of such a large size in infancy is extremely rare, and it was hypothesized that mucosal redundancy owing to LO deficiency in combination with chronic pressure during gastric peristalsis at the pyloric outlet predisposed the patient to polyp formation.[9] To the best of our knowledge, spontaneous retroperitoneal hemorrhage in patients with MD has never been described; the cause of the hemorrhage and location and 3-chambered architecture of the hematoma in our case were also very unique. The common causes of retroperitoneal hemorrhage are blunt abdominal trauma, rupture of the aortic aneurysm, adrenal hemorrhage, renal hemorrhage, malignancy, coagulopathy (hemophilia or use of anticoagulants), or iatrogenic injuries (after femoral catheter insertion or bone marrow biopsy).[2,3] We postulated that the formation of 3 chambers of the hematoma in our patient was derived from intermittent bleeding of the friable and tortuous abdominal vessel(s) over time. Timely recognition of the hematoma by abdominal ultrasonography led to successful surgical management, and the patient returned to his usual state uneventfully. Unfortunately, there is currently no way to prevent a next episode of bleeding or any other life-threatening events in our patient.
This case highlights the importance of considering retroperitoneal hemorrhage as a diagnosis when a patient with MD presents with abdominal distention or even an abdominal mass. Abdominal ultrasonography is a useful, noninvasive, and irradiation-free modality that should be performed promptly in MD patients with gastrointestinal symptoms to differentiate surgical problems from nonsurgical ones. Since our patient survived several episodes of severe complications and exceeds the average life expectancy of MD, a variety of rare complications may provide unexpected challenges for clinicians in the future.
Footnotes
Abbreviations: CPR = cardiopulmonary resuscitation, IRB = Institutional Review Board, LO = lysyl oxidase, MD = Menkes disease, MRI = magnetic resonance imaging, VUR = vesicoureteral reflux.
The authors have no conflicts of interest to disclose.
References
- [1].Ojha R, Prasad AN. Menkes disease: what a multidisciplinary approach can do. J Multidiscip Healthc 2016;9:371–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Chenaitia H, Abrous K, Louis F, et al. WINFOCUS (World Interactive Network Focused On Critical UltraSound) Group France. Relevance of sonography for retroperitoneal hematoma. Am J Emerg Med 2011;29:827–8. [DOI] [PubMed] [Google Scholar]
- [3].Monib S, Ritchie A, Thabet E. Idiopathic retroperitoneal hematoma. J Surg Tech Case Rep 2011;3:49–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Kodama H, Fujisawa C, Bhadhprasit W. Pathology, clinical features and treatments of congenital copper metabolic disorders—focus on neurologic aspects. Brain Dev 2011;33:243–51. [DOI] [PubMed] [Google Scholar]
- [5].Kodama H, Fujisawa C, Bhadhprasit W. Inherited copper transport disorder: biochemical mechanisms, diagnosis, and treatment. Curr Drug Metab 2012;13:237–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Droms RJ, Rork JF, McLean R, et al. Menkes disease mimicking child abuse. Pediatr Dermatol 2017;34:e132–4. [DOI] [PubMed] [Google Scholar]
- [7].Yoganathan S, Sudhakar SV, Arunachal G, et al. Menkes disease and response to copper histidine: an Indian case series. Ann Indian Acad Neurol 2017;20:62–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Tümer Z, Petris M, Zhu S, et al. A 37-year-old Menkes disease patient-residual ATP7A activity and early copper administration as key factors in beneficial treatment. Clin Genet 2017;92:548–53. [DOI] [PubMed] [Google Scholar]
- [9].Belsha D, Narula P, Urs A, et al. Management of hyperplastic gastric polyp following upper gastrointestinal bleeding in infant with Menkes’ disease. World J Gastrointest Endosc 2017;9:341–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Kim OH, Suh JH. Intracranial and extracranial MR angiography in Menkes disease. Pediatr Radiol 1997;27:782–4. [DOI] [PubMed] [Google Scholar]
- [11].Manara R, D’Agata L, Rocco MC, et al. Neuroimaging changes in Menkes disease, part 1. AJNR Am J Neuroradiol 2017;38:1850–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Verma A, Menghani K. Menkes kinky hair disease. Indian Pediatr 2016;53:86. [PubMed] [Google Scholar]
- [13].Gandhi R, Kakkar R, Rajan S, et al. Menkes kinky hair syndrome: a rare neurodegenerative disease. Case Rep Radiol 2012;2012:684309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Danks DM, Campbell PE, Stevens BJ, et al. Menkes's kinky hair syndrome. An inherited defect in copper absorption with widespread effects. Pediatrics 1972;50:188–201. [PubMed] [Google Scholar]
- [15].Weng SC, Hsu CH, Wang NL, et al. Recurrent spontaneous subserosal hematoma of ileum causing intestinal obstruction in a patient with Menkes disease: a case report. Medicine (Baltimore) 2016;95:e4842. [DOI] [PMC free article] [PubMed] [Google Scholar]


