Abstract
Type 2 diabetes is associated with higher pulse pressure. In this study, we assessed and compared effects of classic diabetes treatments on pulse pressure (PP), systolic blood pressure (SBP), and diastolic blood pressure (DBP) in patients with type 2 diabetes.
In a retrospective cohort study, 718 non-hypertensive patients with type 2 diabetes were selected and divided into 4 groups including metformin, insulin, glibenclamide+metformin, and metformin+insulin. They were followed for 4 consecutive visits lasting about 45.5 months. Effects of drug regimens on pulse and blood pressure over time were assessed separately and compared in regression models with generalized estimating equation method and were adjusted for age, duration of diabetes, sex, smoking, and body mass index (BMI).
Studied groups had no significant change in PP, SBP, and DBP over time. No significant difference in PP and DBP among studied groups was observed (PP:P = 0.090; DBP:P = 0.063). Pairwise comparisons of PP, SBP, and DBP showed no statistically significant contrast between any 2 studied groups. Interactions of time and treatment were not different among groups.
Our results demonstrate patients using metformin got higher PP and SBP over time. Averagely, pulse and blood pressure among groups were not different. Trends of variation in pulse and blood pressure were not different among studied diabetes treatments.
Keywords: cardiovascular safety, glibenclamide, insulin, metformin, pulse pressure, systolic and diastolic blood pressure, type 2 diabetes mellitus
1. Introduction
Pulse pressure (PP), defined as the difference between systolic blood pressure (SBP) and diastolic blood pressure (DBP) is the clinical manifestation of arterial stiffness.[1] The systolic component of a wide PP increases the cardiac demand by exerting higher afterload on the heart which results in myocardial hypertrophy; while its diastolic element limits cardiac supply by decreasing coronary perfusion.[2] Hence, wide PP is associated with the incidence of cardiovascular disease (CVD) besides the occurrence of cerebrovascular disease and nephropathy.[3–10] PP is known to be higher in diseases involving vascular system such as the type 2 diabetes mellitus (DM2). People with DM2 have increased arterial stiffness resulting in a wide PP compared with their non-diabetic peers.[7] The risk of CVD is higher in DM2 patients; furthermore, increase in their PP is an additional risk factor for CVD incidence and has a positive association with mortality.[11]
Drugs used to control the blood glucose in DM2 also could modify blood pressure as their side effects[12–14]; however, outcomes are not the same. For instance, while metformin could reduce blood pressure, insulin treatment does not have the similar effect.[15–19] Also, even there is not a consensus over the general effect of some antidiabetic agents like metformin and insulin on blood pressure.[19–23]
Although several works have been done on the effect of different glucose-lowering therapies on blood pressure; little is known about their action on PP. Along with their study, Skov et al[24] demonstrate that there is no statistically significant difference between PP of diabetic patients treated with insulin and its combinations with metformin (Met) or Rosiglitazone or both. We have postulated there should not be any difference between the effect of studied hypoglycemic treatments on pulse and blood pressure of diabetic patients. However, yet, there is not a comprehensive study comparing classic treatments applied in DM2. Moreover, there are some confounding factors need to be studied. Therefore, in the current study, we assessed and compared the effect of glucose-lowering regimens including metformin, Met+insulin, glibenclamide+metformin (Glb+Met), and insulin on diabetic patients PP, SBP, and DBP considering confounding factors over 45.5 months of follow-up.
2. Materials and methods
2.1. Study sample
This study is a part of an open prospective cohort conducted in the diabetes clinic of Valiasr hospital (Tehran, Iran). Data collection for this cohort started from 2008. Patients with DM2 who had attended Valiasr diabetes clinic have been enrolled in the original cohort. In the current study, we selected 718 diabetic non-hypertensive patients who had been already using metformin, Glb+Met, Met+insulin, or insulin only from the ongoing cohort study.
We excluded patients with primary hypertension as well as patients treated with lipid-lowering therapies other than statins. For avoiding the bias resulted by excluding normotensive people who got hypertensive later along follow-up, we traced those individuals who were totally 13 people (6 patients on metformin, 2 metformin and glibenclamide combination, 1 using the combination of insulin and metformin, and 1 patient using insulin). Patients with uncontrolled hypothyroidism or clinical hyperthyroidism were not included in the study as well as patients with any other interfering endocrinological disease. Those individuals with any history of myocardial infarction, percutaneous coronary intervention, stent placement, CCU admission, and cerebrovascular accidents were excluded from the study.
Patients were followed at the Valiasr hospital from the date of enrollment (the baseline visit) every year for 3 consecutive visits until October 2015 which had been set as the end of the follow-up. At each follow-up session, we included only patients who did not have any change in the drug which had been previously used.
Before enrollment, written informed consents were taken from all participants. The ethics committee of the Tehran University of Medical Sciences approved the study protocol.
2.2. Clinical and laboratory measurements
All the patient's characteristics, including age, duration of DM2 diagnosis, sex, body weight, height, body mass index (BMI), blood pressure, total cholesterol, high-density lipoprotein cholesterol, low-density lipoprotein (LDL) cholesterol, triglycerides, HbA1c, fasting blood glucose (FBS), 1hour post-prandial glucose (1hppg), creatinine concentration, and medication (antihypertensive drug, cholesterol-lowering drug, and antidiabetic drug) were extracted from the computerized hospitalization records at the baseline and during the follow-up.
Age, medication, and the duration of DM2 have been obtained from the participants through the interview at the first visit. Weight, height, and the waist circumference were measured at the baseline. Systolic blood pressure and diastolic blood pressure measurements were performed on the arm of seated participants after 10 minutes of resting using standard mercury sphygmomanometer. The measurement was repeated after 15 minutes and the average was reported. We calculated eGFR using the Cockcroft and Gault equation, based on age, sex, weight, and serum creatinine.[25]
After 12 hours of fasting, venous blood samples were collected for the biochemical analysis. FBS and 1hppg were measured by the glucose oxidase method. HbA1c was measured by high-performance liquid chromatography. Measurement of serum creatinine was performed by Jaffe method. Plasma total cholesterol, triglyceride, high-density lipoprotein cholesterol (HDL), low-density lipoprotein cholesterol (LDL) were determined using direct enzymatic method (Parsazmun, Karaj, Iran).
2.3. Outcome measures and definitions
The primary outcome of this study is PP, which is defined as the difference between SBP and DBP. We calculated PP using SBP and DBP, measured at the baseline and during each follow-up.
The diagnosis of DM2 was made based on fasting blood glucose >126 or 2-hour postprandial glucose >200 or randomized blood glucose >200 or HbA1c >6.5.[26] People with hypertension were defined as those who were taking antihypertensive medications. BMI was computed as weight in kilograms divided by height per square meter (kg/m2).
2.4. Statistical analysis
Statistical analysis was carried out using Stata (version 12; Stata Corp LP, College Station, TX) for Windows. Baseline patients’ characteristics were presented as mean (SD) for continuous variables and number (percentage) for categorical variables. Chi-squared test or one-way ANOVA was used as appropriate to evaluate group differences.
To compare PP, SBP, and DBP among treatment groups, regression models with generalized estimating equation method were used taking into account the correlation between repeated blood pressure measurements of the same subjects. Treatments as indicator variables were main predictors of the model. In the model, the variable “time” was defined as months since the baseline visit. Following, chi-squared test was carried out for comparing treatments’ effect in general. For comparing trends in blood and pulse pressure variation over time in each group, the interaction of treatments and time was compared with and without adjustment for covariates by introducing their product term in regression models with generalized estimating equation method. Age, duration of diabetes, BMI, sex, and smoking were selected as covariates. Post hoc analysis was used as appropriate for pairwise comparison following regression analysis with Sidak correction.
In order to assess change in pulse and blood pressure over time, regression models with generalized estimating equation method were used; in which PP, SBP, and DBP in each studied groups were compared among visits by adjusting for duration of diabetes.
The sample size was calculated assuming 5 mmHg as the least detectable variation of pulse and blood pressure (equivalent of 0.36 standard deviation), type 1 error of 0.05, and 0.5 as the correlation among our repeated measures. Although here by having the least total sample size of 120 as the number of patients in the last visit, this study offers the power of 0.99.
A P value <.05 (2-sided) was set as the significance threshold.
3. Results
3.1. Patients
Seven hundred eighteen diabetic normotensive patients using metformin (189 patients), metformin and glibenclamide (360 patients), metformin and insulin (63 patients), or insulin only (106 patients) were selected from the running cohort study. One hundred seventy two of 718 subjects were selected for the second visit; others were excluded due to either lack of the data or change in the antidiabetic agent. Similarly, the third visit included 138 and the last visit consisted of 120 subjects. The median time gap between our baseline visit and the first, second, and then last follow-up were 12, 23, and 35.5 months, respectively. October 2015 was set as the end of the follow-up.
3.2. Baseline characteristics
Table 1 shows baseline characteristics in each studied group. The median age of the study sample was 45.5 years. There was no sex composition difference among studied groups. The baseline PP had no significant variation among study groups. At the baseline, patients on Glb+Met treatment had higher SBP and patients treated with Glb+Met had higher DBP compared with patients using insulin (95% confidence interval [CI] = 0.40–11.36, P = .028; 95%CI = 0.59–7.50, P = .012, respectively). There was no statistically significant difference among study groups regarding the proportion of smokers.
Table 1.
Baseline characteristics of studied groups.

3.3. PP, SBP, and DBP change over time in each group
PP, SBP, and DBP of patients over visits are shown in Table 2 and illustrated in Fig. 1A–C. Despite steady blood pressure of other groups, PP (Χ2 = 13.47; P = .003) and SBP (Χ2 = 8.45; P = .037) of patients treated with Met+insulin increased over time. Notably, in this group, PP and SBP alteration were limited to the contrast between first and third visit (PP: 95%CI = 1.38, 19.34; P = .014; SBP: 95%CI = −1.32, 22.30; P = .111) which did not define a specific change. Notably, including normotensive patients who got hypertensive later had no statistically significant result in this comparison.
Table 2.
PP, SBP, and DBP among studied groups at each visit.

Figure 1.

PP, SBP, and DBP of patients in each visit are illustrated in plots (A)–(C), respectively. DBP = diastolic blood pressure, Glb = glibenclamide, Met = metformin, PP = pulse pressure, SBP = systolic blood pressure.
3.4. PP, SBP, and DBP comparison among studied groups
As shown in Table 3, without adjustment PP (P < .05), SBP (P < .01), and DBP (P < .05) all had significant differences among studied groups. After adjustment for covariates variation of PP among groups disappeared (P = .090); but, the difference in DBP remained with a trend to statistical significance (P = .063) and SBP still varied among studied groups (P < .05). Pairwise comparison of PP and SBP among studied groups showed no significant contrast between any 2 groups; but revealed higher DBP in Glb+Met group compared with the insulin group with a trend to statistical significance (95% Cl: −6.26, 0.31; P = .098). Table 4 shows the complete pairwise comparison of pulse and blood pressure among studied groups.
Table 3.
Results of comparison of PP, SBP, and DBP among studied groups.
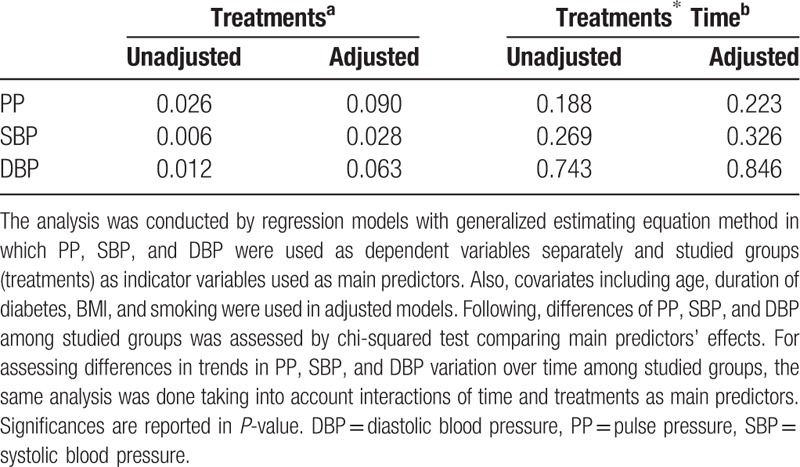
Table 4.
Results of pairwise comparison of studied groups regarding PP, SBP, and DBP.

With and without adjustment, treatments and time had no interaction in predicting PP, SBP, and DBP which showed trends in blood and pulse pressure variation over time among studied groups had no difference. Again including normotensive patients who got hypertensive later had no statistically significant result in these comparisons.
4. Discussion
Higher PP in DM2 increases the risk of CVD and its mortality.[3,6,27] DM2 is associated with metabolic syndrome and other cardiovascular risk factors; hence, cardiovascular safety of different glucose-lowering therapies is a critical issue and several studies have already been done comparing cardiovascular outcomes of different antidiabetic treatments.[28–31]
There have not been enough studies comparing the outcome of different modalities of glucose-lowering therapy regarding PP. In this study, we have demonstrated pulse and blood pressure of DM2 patients treated with classic glucose-lowering treatments including Met, Glb+Met, Met+insulin, and insulin had no significant change over time. Importantly, we have shown there was no difference in PP, SBP, and DBP among patients treated with these medications.
Many studies have reported metformin reduces cardiovascular events and mortality compared with other antidiabetic agents.[29,32,33] However, the role of blood pressure in this risk reduction has not been elucidated yet. Although some authors have reported a decrease in SBP or DBP by using metformin,[34,35] there are studies reporting a nonsignificant effect of this medication on patients blood pressure.[36,37] We did not find any significant change of PP, SBP, and DBP in patients treated with metformin over follow-up. There have not been enough studies on the effect of metformin on PP; however, Wulffelé et al[37] demonstrated a decrease in nocturnal PP of diabetic patients including both normotensive and hypertensive cases after 16 weeks treatment with metformin.
It seemed PP and SBP of patients treated with Met+insulin increased over time, but this variation was only between first and third visit which could not reliably define a trend. Formerly, Mourão-Júnior et al[38] demonstrated the addition of metformin to diabetic patients controlled with insulin had no effect on blood pressure. Studies should be done for clarifying the effect of adding insulin to metformin or vice versa on blood pressure and most importantly pulse pressure.
Among mechanisms in which sulfonylurea could lead to the higher CVD morbidity, Williams has shown glibenclamide increases nocturnal SBP in diabetic patients. We did not find any study assessing mainly the effect of sulfonylurea on PP in DM2; although St John Sutton et al[44] provided data showing a statistically significant decrease in diabetic patients PP after 52 weeks treatment with Glyburide. Also, we observed no change in SBP, DBP, and PP of diabetic patients treated with Glb+Met. Similarly, Herman et al[47] had found no blood pressure difference among patients treated with different combinations of metformin and Glyburide. Again, there has been a lack of studies about the effect of Glb+Met combination treatment on PP.
There are studies reporting increased cardiovascular events and mortality by insulin treatment in DM2 patients especially by applying intensive blood glucose control.[49–51] On another hand, some studies have reported an insignificant association between insulin use and cardiovascular morbidity and mortality.[52,53] Here we focused on the role of pulse and blood pressure and found no significant change in SBP and DBP of diabetic patients treated with insulin; however, DBP in patients treated with insulin in this study was lower than patients on Glb+Met with a trend to statistical significance. There are studies showing blood pressure elevation secondary to insulin initiation in diabetic patients[21,19];however, our finding is consistent with Flores et al[20] which reported no rise in blood pressure secondary to insulin therapy. Furthermore, Rowe et al[54] had already demonstrated insulin infusion could lead to increase in PP; although, we observed no difference in PP of patients treated with insulin over the follow-up.
As noted before, there have not been enough studies comparing these classic glucose-lowering treatments according to their effect on PP. Skov et al[24] already demonstrated that effect of different combinations of insulin, metformin, or Rosiglitazone on PP did not vary. We also have shown there is no difference in trends of PP, SBP, and DBP variation over time among studied groups which has not been reported before.
In our study, after adjustment for covariates and pairwise comparisons, no significant contrast between any 2 groups was observed. However, in crude analysis SBP, DBP, and PP were different among treatment groups. The effect of adjustment showed covariates confounding effects and could be explained by following facts. First, with prolonged duration of DM2, vessels would be exposed to hyperglycemic media longer which facilitates arterial stiffening. Also, duration of diabetes itself has shown to be associated with pulse pressure.[55] Second, hypoglycemic medications such as metformin are selected based on patients’ characteristics such as BMI[56] which is formerly reported to have a positive association with pulse pressure.[57] Together, different BMI, DM2 duration, and age of patients among this study groups could lead to a significant crude result.
This study is among few studies comparing PP of diabetic patients treated with different antidiabetic regimens. Our study is highlighted by having a large and targeted sample size and using generalized estimating equation analysis. Notably, we excluded hypertensive patients as well as patients treated with lipid-lowering therapies other than statins due to the interference their various treatments could cause. Including normotensive people who got hypertensive later along follow-up had no statistically significant outcome, showing this exclusion had not biased the final result. However, we had some limitations. First, studied groups were not randomized; although, we adjusted results for some known covariates to compensate for the lack of randomization in this prospective cohort study. Furthermore, randomized clinical trial (RCT) design limits the follow-up time and restricts trends study of pulse and blood pressure, an analysis requiring long follow-up period. Second, we had no control group to compare treatment groups with; consequently, we were not able to demonstrate each drug net effect on patients’ blood pressure. Although we evaluated and compared the effect of 4 antidiabetic medication regimen on pulse pressure over time, further studies needed to investigate other cardiovascular safety issues.
5. Conclusion
There was no statistically significant difference in PP, SBP, and DBP among patients treated with classic antidiabetic regimens including metformin, Glb+Met, Met+insulin, and insulin alone. Trends in PP, SBP, and DBP variation over time were not different among studied glucose lowering modalities. These hypoglycemic regimens also did not affect PP, DBP, and SBP over time. However, further studies are needed to improve our understanding of these findings.
Uncited references
Footnotes
Abbreviations: CVD = cardiovascular disease, DBP = diastolic blood pressure, DM2 = type 2 diabetes mellitus, Glb = glibenclamide, Met = metformin, PP = pulse pressure, SBP = systolic blood pressure.
Funding: This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
The authors report no conflicts of interest.
References
- [1].Safar ME, et al. Pulse pressure, arterial stiffness, and end-organ damage. Curr Hypertens Rep 2012;14:339–44. [DOI] [PubMed] [Google Scholar]
- [2].Safar ME. Systolic blood pressure, pulse pressure and arterial stiffness as cardiovascular risk factors. Curr Opin Nephrol Hypertens 2001;10:257–61. [DOI] [PubMed] [Google Scholar]
- [3].Glasser SP, et al. Is pulse pressure an independent risk factor for incident acute coronary heart disease events? The REGARDS study. Am J Hypertens 2014;27:555–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Darne B, et al. Pulsatile versus steady component of blood pressure: a cross-sectional analysis and a prospective analysis on cardiovascular mortality. Hypertension 1989;13:392–400. [DOI] [PubMed] [Google Scholar]
- [5].Franklin SS, et al. Is pulse pressure useful in predicting risk for coronary heart disease? The Framingham heart study. Circulation 1999;100:354–60. [DOI] [PubMed] [Google Scholar]
- [6].Lee ML, Rosner BA, Weiss ST. Relationship of blood pressure to cardiovascular death: the effects of pulse pressure in the elderly. Ann Epidemiol 1999;9:101–7. [DOI] [PubMed] [Google Scholar]
- [7].Schram MT, et al. Diabetes, pulse pressure and cardiovascular mortality: the Hoorn Study. J Hypertens 2002;20:1743–51. [DOI] [PubMed] [Google Scholar]
- [8].Safar ME, et al. Central pulse pressure and mortality in end-stage renal disease. Hypertension 2002;39:735–8. [DOI] [PubMed] [Google Scholar]
- [9].Knudsen ST, et al. Ambulatory pulse pressure, decreased nocturnal blood pressure reduction and progression of nephropathy in type 2 diabetic patients. Diabetologia 2009;52:698–704. [DOI] [PubMed] [Google Scholar]
- [10].Liu FD, et al. Pulse pressure as an independent predictor of stroke: a systematic review and a meta-analysis. Clin Res Cardiol 2016;105:677–86. [DOI] [PubMed] [Google Scholar]
- [11].Cockcroft JR, et al. Pulse pressure predicts cardiovascular risk in patients with type 2 diabetes mellitus. Am J Hypertens 2005;18:1463–7. [DOI] [PubMed] [Google Scholar]
- [12].Charles MA, et al. Treatment with metformin of non-diabetic men with hypertension, hypertriglyceridaemia and central fat distribution: the BIGPRO 1.2 trial. Diabetes Metab Res Rev 2000;16:2–7. [DOI] [PubMed] [Google Scholar]
- [13].Preiss D, et al. Metformin for non-diabetic patients with coronary heart disease (the CAMERA study): a randomised controlled trial. Lancet Diabetes Endocrinol 2014;2:116–24. [DOI] [PubMed] [Google Scholar]
- [14].Bird ST, et al. Polycystic ovary syndrome and combined oral contraceptive use: a comparison of clinical practice in the United States to treatment guidelines. Gynecol Endocrinol 2013;29:365–9. [DOI] [PubMed] [Google Scholar]
- [15].Koren S, et al. The effect of sitagliptin versus glibenclamide on arterial stiffness, blood pressure, lipids, and inflammation in type 2 diabetes mellitus patients. Diabetes Technol Ther 2012;14:561–7. [DOI] [PubMed] [Google Scholar]
- [16].Orchard TJ, et al. Long-term effects of the Diabetes prevention program interventions on cardiovascular risk factors: a report from the DPP Outcomes Study. Diabet Med 2013;30:46–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Wilding J, et al. Glycated hemoglobin, body weight and blood pressure in Type 2 diabetes patients initiating dapagliflozin treatment in primary care: a retrospective study. Diabetes Ther 2016;7:695–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Wulffele MG, et al. The effect of metformin on blood pressure, plasma cholesterol and triglycerides in type 2 diabetes mellitus: a systematic review. J Intern Med 2004;256:1–4. [DOI] [PubMed] [Google Scholar]
- [19].Persson SU. Blood pressure reactions to insulin treatment in patients with type 2 diabetes. Int J Angiol 2007;16:135–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Flores L, et al. Insulin therapy in type 2 diabetic patients: effects on arterial blood pressure and endothelin-1 plasma levels. Diabetes Res Clin Pract 1998;41:151–5. [DOI] [PubMed] [Google Scholar]
- [21].Randeree HA, et al. Effect of insulin therapy on blood pressure in NIDDM patients with secondary failure. Diabetes Care 1992;15:1258–63. [DOI] [PubMed] [Google Scholar]
- [22].Williams S, et al. Effects of glibenclamide on blood pressure and cardiovascular responsiveness in non-insulin dependent diabetes mellitus. J Hypertens 1998;16:705–11. [DOI] [PubMed] [Google Scholar]
- [23].Zhou L, et al. Effects of metformin on blood pressure in nondiabetic patients: a meta-analysis of randomized controlled trials. J Hypertens 2017;35:18–26. [DOI] [PubMed] [Google Scholar]
- [24].Skov V, et al. Metformin, but not rosiglitazone, attenuates the increasing plasma levels of a new cardiovascular marker, fibulin-1, in patients with type 2 diabetes. Diabetes Care 2014;37:760–6. [DOI] [PubMed] [Google Scholar]
- [25].Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron 1976;16:31–41. [DOI] [PubMed] [Google Scholar]
- [26].Diagnosis and classification of diabetes mellitus. Diabetes Care 2010;33(Suppl):S62–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Palmieri V, et al. Association of pulse pressure with cardiovascular outcome is independent of left ventricular hypertrophy and systolic dysfunction: the Strong Heart Study. Am J Hypertens 2006;19:601–7. [DOI] [PubMed] [Google Scholar]
- [28].Cefalu WT, et al. Beyond metformin: safety considerations in the decision-making process for selecting a second medication for type 2 diabetes management: reflections from a diabetes care editors’ expert forum. Diabetes Care 2014;37:2647–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Wurm R, et al. Cardiovascular safety of metformin and sulfonylureas in patients with different cardiac risk profiles. Heart 2016;102:1544–51. [DOI] [PubMed] [Google Scholar]
- [30].Goldfine AB. Assessing the cardiovascular safety of diabetes therapies. N Engl J Med 2008;359:1092–5. [DOI] [PubMed] [Google Scholar]
- [31].Alberti K, et al. Harmonizing the metabolic syndrome a joint interim statement of the international diabetes federation task force on epidemiology and prevention; national heart, lung, and blood institute; American heart association; world heart federation; international atherosclerosis society; and international association for the study of obesity. Circulation 2009;120:1640–5. [DOI] [PubMed] [Google Scholar]
- [32].Evans JM, et al. Risk of mortality and adverse cardiovascular outcomes in type 2 diabetes: a comparison of patients treated with sulfonylureas and metformin. Diabetologia 2006;49:930–6. [DOI] [PubMed] [Google Scholar]
- [33].Johnson JA, et al. Decreased mortality associated with the use of metformin compared with sulfonylurea monotherapy in type 2 diabetes. Diabetes Care 2002;25:2244–8. [DOI] [PubMed] [Google Scholar]
- [34].Landin K, Tengborn L, Smith U. Treating insulin resistance in hypertension with metformin reduces both blood pressure and metabolic risk factors. J Intern Med 1991;229:181–7. [DOI] [PubMed] [Google Scholar]
- [35].Giugliano D, et al. Metformin improves glucose, lipid metabolism, and reduces blood pressure in hypertensive, obese women. Diabetes Care 1993;16:1387–90. [DOI] [PubMed] [Google Scholar]
- [36].Nagi DK, Yudkin JS. Effects of metformin on insulin resistance, risk factors for cardiovascular disease, and plasminogen activator inhibitor in NIDDM subjects: a study of two ethnic groups. Diabetes Care 1993;16:621–9. [DOI] [PubMed] [Google Scholar]
- [37].Wulffelé MG, et al. Does metformin decrease blood pressure in patients with Type 2 diabetes intensively treated with insulin? Diabet Med 2005;22:907–13. [DOI] [PubMed] [Google Scholar]
- [38].Mourão-Júnior CA, et al. Effects of metformin on the glycemic control, lipid profile, and arterial blood pressure of type 2 diabetic patients with metabolic syndrome already on insulin. Braz J Med Biol Res 2006;39:489–94. [DOI] [PubMed] [Google Scholar]
- [39].Hong J, et al. Effects of metformin versus glipizide on cardiovascular outcomes in patients with type 2 diabetes and coronary artery disease. Diabetes Care 2013;36:1304–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Jorgensen CH, et al. Effects of oral glucose-lowering drugs on long term outcomes in patients with diabetes mellitus following myocardial infarction not treated with emergent percutaneous coronary intervention--a retrospective nationwide cohort study. Cardiovasc Diabetol 2010;9:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].King P, Peacock I, Donnelly R. The UK Prospective Diabetes Study (UKPDS): clinical and therapeutic implications for type 2 diabetes. Br J Clin Pharmacol 1999;48:643–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Gerstein HC, et al. Effects of intensive glycaemic control on ischaemic heart disease: analysis of data from the randomised, controlled ACCORD trial. Lancet 2014;384:1936–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Monami M, et al. Are sulphonylureas all the same? A cohort study on cardiovascular and cancer-related mortality. Diabetes Metab Res Rev 2007;23:479–84. [DOI] [PubMed] [Google Scholar]
- [44].St John Sutton M, et al. A comparison of the effects of rosiglitazone and glyburide on cardiovascular function and glycemic control in patients with type 2 diabetes. Diabetes Care 2002;25:2058–64. [DOI] [PubMed] [Google Scholar]
- [45].Mogensen UM, et al. Cardiovascular safety of combination therapies with incretin-based drugs and metformin compared with a combination of metformin and sulphonylurea in type 2 diabetes mellitus--a retrospective nationwide study. Diabetes Obes Metab 2014;16:1001–8. [DOI] [PubMed] [Google Scholar]
- [46].Ekstrom N, et al. Cardiovascular safety of glucose-lowering agents as add-on medication to metformin treatment in type 2 diabetes: report from the Swedish National Diabetes Register. Diabetes Obes Metab 2016;18:990–8. [DOI] [PubMed] [Google Scholar]
- [47].Hermann LS, et al. Therapeutic comparison of metformin and sulfonylurea, alone and in various combinations. A double-blind controlled study. Diabetes Care 1994;17:1100–9. [DOI] [PubMed] [Google Scholar]
- [48].Ferrannini E, DeFronzo RA. Impact of glucose-lowering drugs on cardiovascular disease in type 2 diabetes. Eur Heart J 2015;36:2288–96. [DOI] [PubMed] [Google Scholar]
- [49].Gamble JM, et al. Insulin use and increased risk of mortality in type 2 diabetes: a cohort study. Diabetes Obes Metab 2010;12:47–53. [DOI] [PubMed] [Google Scholar]
- [50].Group T.A.t.C.C.R.i.D.S.. Effects of intensive glucose lowering in Type 2 diabetes. N Engl J Med 2008;358:2545–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Gerstein HC, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 2008;358:2545–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Gamble JM, et al. Association of insulin dosage with mortality or major adverse cardiovascular events: a retrospective cohort study. Lancet Diabetes Endocrinol 2017;5:43–52. [DOI] [PubMed] [Google Scholar]
- [53].Siraj ES, et al. Insulin dose and cardiovascular mortality in the ACCORD trial. Diabetes Care 2015;38:2000–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Rowe JW, et al. Effect of insulin and glucose infusions on sympathetic nervous system activity in normal man. Diabetes 1981;30:219–25. [DOI] [PubMed] [Google Scholar]
- [55].Cockcroft JR, et al. Pulse pressure predicts cardiovascular risk in patients with type 2 diabetes mellitus. Am J Hypertens 2005;18:1463–7. discussion 1468-9. [DOI] [PubMed] [Google Scholar]
- [56].Hollander P. Anti-diabetes and anti-obesity medications: effects on weight in people with diabetes. Diabetes Spectrum 2007;20:159–65. [Google Scholar]
- [57].Kwagyan J, et al. The impact of body mass index on pulse pressure in obesity. J Hypertens 2005;23:619–24. [DOI] [PubMed] [Google Scholar]


