Abstract
As HIV-infected patients grow older, some accumulate multiple health problems earlier than the noninfected ones in particular frailty phenotypes. Patients with frailty phenotype are at higher risk of adverse outcomes (worsening mobility, disability, hospitalization, and death within three years).
Our study aimed to evaluate prevalence of frailty in elderly HIV-infected patients and to assess whether frailty is associated with HIV and geriatric factors, comorbidities, and precariousness in a French cohort of older HIV infected.
This 18-month cross-sectional multicenter study carried in 2013 to 2014 had involved 502 HIV-infected patients aged 50 years and older, cared in 18 HIV-dedicated hospital medical units, located in South of France.
Prevalence of frailty was 6.3% and of pre-frailty 57.2%. Low physical activity and weakness were the main frailty markers, respectively 49.4% and 19.9%. In univariate models, precariousness, duration of HIV antiretroviral treatment >15 years, 2 comorbidities or more, risk of depression, activities of daily living disability, and presence of pain were significantly associated with frail and pre-frail phenotype. Multivariate logistic regression analyses showed that only pain was significantly different between frail and pre frail phenotype versus non frail phenotype (odds ratio = 1.2; P = .002).
Our study is the first showing a significant association between pain and frailty phenotype in older patients infected by HIV. As frailty phenotype could be potentially reversible, a better understanding of the underlying determinant is warranted. Further studies are needed to confirm these first findings.
Keywords: frailty phenotype, geriatric assessment, HIV-aging, pain, precariousness
1. Introduction
The increasing life-span of people living with HIV (PHIV) presents new challenges related to aging.[1] As PHIV grow older, some experience multimorbitidy, polypharmacy, altered physical function earlier than the noninfected ones.[2–5]
These problems are not totally explained by age, severity of the HIV disease or duration, or toxicity of antiretroviral drugs. Literature suggests that frailty phenotype could be a marker of this variability between PHIV.[6] Using data from the Cardiovascular Health Study, Fried et al identified 5 frailty markers: nutrition, weakness, slowness, energy, and physical activity.[7] They reported that older persons with at least 3 of the 5 frailty markers have a significantly increased risk of suffering from adverse outcomes such as falls, worsening mobility, disability, hospitalization, and death within 3 years. Moreover, the presence of at least one of these markers confers an increased risk of adverse outcomes.[7,8] Since 2001, in various context and population, such as cancer, cardiovascular diseases and osteoporosis, the Fried phenotype has demonstrated its capacity to predict adverse outcomes.[9] Desquilbet et al first has shown that HIV infection was associated with an earlier occurrence of a phenotype related to frailty.[3]
VISAGE is a French multidisciplinary study group focusing on elderly PHIV.[10,11] Our study “VISAGE-3” aimed to evaluate prevalence of frailty in elderly PHIV and to assess whether frailty is associated with HIV and geriatric factors, comorbidities, and deprivation in a French cohort of older PHIV.
2. Methods
2.1. Study design
This 18-month cross-sectional observational multicenter study carried in 2013 to 2014 involved PHIV aged 50 years and older, cared in 18 HIV-dedicated hospital medical units, located in south of France. All patients provided a written consent for their participation to the study. Patients unable to answer a questionnaire or unable to do walking tests were excluded from the study.
Age, sex, body mass index (BMI), HIV data, socioeconomics and behavior factors, geriatric assessment, comorbidities, and frailty markers were collected by questionnaires and measures.
2.2. HIV data
Duration on HIV, CDC stage, last and nadir CD4 cell count, undetectable last viral load, and start of antiretroviral therapy (>15 years) were collected.
2.3. Socioeconomic and behavior factors
Level of education, incomes and professional activity, smoking, alcohol and drugs consumption were collected. To assess deprivation, the French social validated EPICES (Evaluation of Precarity and Inequalities in Health Examination Centers) score was used.[12] This score is calculated according to an algorithm based on the responses to 11-item questionnaire exploring socioeconomic individual deprivation. It varies from 0 (the least deprived) to 100 (the most deprived). A deprivation state is defined as a score ≥30.17.[13]
2.4. Comorbidities
Number and type of comorbidities were collected. Hepatitis C or B, cancers (acquired immune deficiency syndrome [AIDS] or not AIDS-related), cardiovascular diseases (atrial fibrillation, cardiac failure, coronary disease, ischemic cerebrovascular diseases), chronic kidney disease, chronic obstructive pulmonary disease, lipodystrophy, dyslipidemia, diabetes, hypertension, psychiatric, and osteoarthritis diseases were recorded from the medical chart.
2.5. Geriatric assessment
The functional status was assessed using 6 tasks of the Katz index of activities of daily living (ADL). Disability was defined as the need for assistance to complete at least one ADL.[14] The 4-item Geriatric Depression Scale (mini GDS) was used to screen a risk of depression. A score of ≥1 indicated a risk of depression.[15] Patients who had experienced ≥1 falls in the previous 6 months were considered to have a positive history of falls. Visual analogic scale (VAS, from 0 to 10) was used to assess pain.
2.6. Frailty markers
The 5 frailty markers adapted from the Fried phenotype were recorded: nutrition, energy, weakness, physical activity, and slowness.
-
-
Nutritional status was assessed by the question: “In the last year, have you lost more than 4 kilograms unintentionally”. An affirmative answer to the question indicated a positive marker of frailty for nutrition.[7]
-
-
Energy was assessed using a visual scale ranging from 0 (no energy) to 10 (full of energy). A score <3 indicated a positive marker of frailty for energy.[8]
-
-
Weakness was assessed by the maximal value of 3 measurements of grip strength (in kilograms) in the dominant hand using a Jamar handheld dynamometer. The lowest quintile by sex and BMI was considered a positive marker of frailty for weakness.[7]
-
-
Physical activity was assessed by a validated self-report question from the Canadian Study of Health and Aging Risk Factor Questionnaire.[16] No exercise or a low level of exercise was considered a positive marker of frailty for physical activity.
-
-
Slowness was assessed by gait speed (time to walk 4 m). Score under 0.8 m/s indicated positive marker for slowness.[7,17]
Patients who had ≥3 markers were classified as frail, patients with 1 or 2 markers as pre-frail, and patients with no markers as not-frail.[7]
2.7. Data analysis
Sample characteristics were detailed using mean/standard deviations for quantitative variables, and frequencies for qualitative variables. Two groups of individuals were constituted: “non-frail” (no marker of Fried phenotype); “frail and pre-frail” (≥1 marker). Comparisons between the 2 groups were performed using Student ttests for quantitative variables, and χ2 or Fisher exact tests for frequencies. Multivariate analysis using logistic regression models was performed to determine variables potentially linked to frail profile, using a forward stepwise approach. Variables relevant to the models were selected on their clinical interest and/or a threshold P value ≤.2 during univariate analysis. Variables selected were age, sex, school diploma, deprivation, start of HIV therapy, comorbidities, depression, disability, and pain (P < .20). The final model expressed the odds ratios and 95% confidence intervals. All the tests were 2-sided. Statistical significance was defined as P < .05. The statistical analyses were performed using the SPSS version 17.0 software package (SPSS Inc, Chicago, IL).
2.8. Ethical statement
All the participants gave their written informed consent to participate. The study was promoted by the Clinical Research Department of Assistance Publique-Hôpitaux de Marseille (AP-HM) and approved by the French Consultative Committee for the Protection of Persons consenting to biomedical research (CCPP South Mediterranean Marseille I; registration number: 2011-A01679–32) and by the French Agency of Sanitary Security for Health Products (ANSM; registration number: B111670–40).
3. Results
A total of 509 PHIV were screened among whom 502 were included: 365 (72.7%) men and 137 (27.3%) women. The 7 patients excluded because of a lack of data were 5 men and 2 women.
Sixty percent of patients were between 50 and 59 years. HIV-infection lifetime was ≥25 years for one-fourth of the PHIV. Nadir CD4 count was <200 cells/mm3 for almost half the PHIV and 438 (87.3%) had undetectable viral load. Almost one-fourth (23.7%) was at AIDS stage. Concerning the weight, on-third (34.4%) were overweight or obese (BMI ≥ 25). Tobacco consumption was high (61.5%). Almost half (49.0%) of PHIV had deprivation.
The prevalence of frailty and pre-frailty were 6.3% and 57.2%, respectively. Low physical activity and weakness were the main frailty markers, respectively, 49.4% and 19.9% (Table 1).
Table 1.
Baseline frailty's 5 markers.
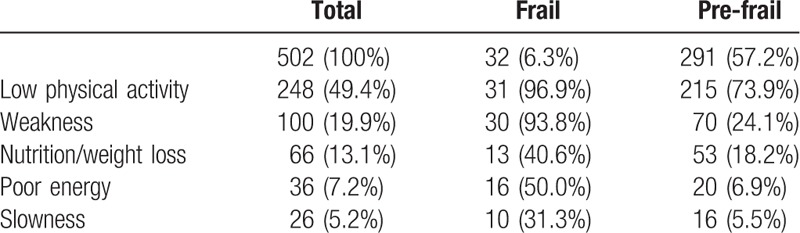
The 3 main comorbidities were: dyslipidemia (36.7%), lipodystrophy (30.3%), and hepatitis B or C (26.1%). More than half (60.4%) had ≥2 or comorbidities and more than one-third had ≥3 or more comorbidities.
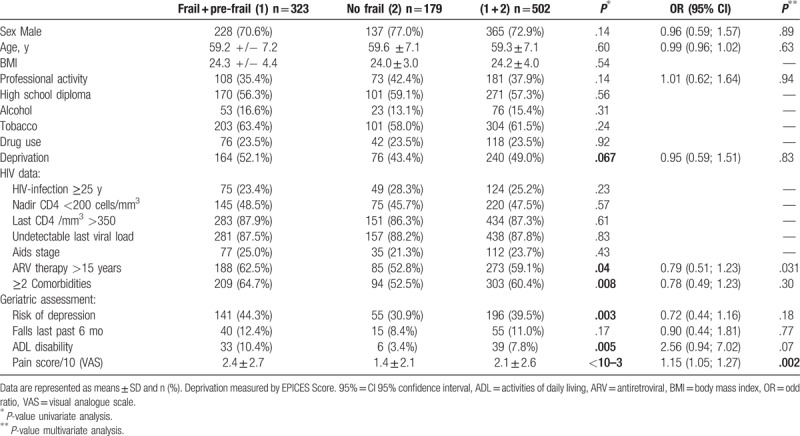
In univariate model, deprivation, start of antiretroviral therapy >15 years, ≥2 or more comorbidities, lipodystrophy, risk of depression, ADL disability, and presence of pain were significantly associated with frail and pre-frail phenotype (Table 2). Multivariate logistic regression analyses (67.1% of prediction; chi-square = 26.954, df = 10, P = .003; the -2 log likelihood = 508.009, Cox and Snell R Square at 0.06) with age and variables P < .20 showed that only pain was significantly different between frail and pre-frail phenotype versus non-frail phenotype (P = .002) (Table 2).
Table 2.
Associations of sociodemographic, HIV infection, number of comorbidities, health baseline characteristics with frailty and pre-frailty.
4. Discussion
4.1. Main findings
In this study, two-thirds of PHIV had at least 1 frailty markers. All previous studies in HIV population, except the one performed by Kooij et al in 2016 had focused on presence of ≥3 markers.[6,18] However, in her cohort, Fried has emphasized that the pre-frail group (1 or 2 frailty markers) was also at risk for these outcomes (intermediate risk) and at risk for subsequent frailty. Then, assessing the presence of any frailty markers seems to be meaningful in PHIV, especially as all studies on the prevalence of the frailty phenotype demonstrate that it occurs about 10 years earlier than in the general population.[3]
Using the phenotype approach, previous studies have shown that frailty phenotype is frequently associated in HIV infected patients with several comorbidities such as HCV co-infection, diabetes or kidney disease, cognitive impairment, depressive symptoms, with low socio-economic status (shorter formal education, unemployed, or with lower incomes) and HIV measures (current and nadir CD4 cell count, detectable HIV RNA viral load, duration on HAART therapy).[19–28] These data are the basis of our starting hypothesis.
In the AGEHIV cohort, Kooij et al did not find any relationship between frailty and duration of VIH, nadir of CD4, last CD4 level, antiretroviral exposure as in our study, although their population was younger (mean age 52.8 years, one-third <50) and had low BMI.[18] In our study, only presence of pain was significantly associated with the presence of any frailty markers.
In a previous study of our group, we found that >60% of PHIV used paracetamol regularly suggesting that pain is a major concern in this population.[20,21] Recent literature questioned the role of pain in frailty phenotype.[29,30] In a cross-sectional study including 252 community dwelling elderly, Coelho et al[31] found that pain predicted 5.8% of the variance of frailty, 5.9% of the variance of physical frailty, 4.0% of the variance of psychological frailty, and intensity of pain was significantly associated with an increase of frailty. In a literature review, Nessighaoui et al, found 12 cross-sectional studies which directly examined the relationship between frailty and pain. Only one did not found a link between frailty and pain.[32] This study used the frail index instead of frailty phenotype which may explain different results because of a different assessment tool. Today no study has found this relationship in PHIV.
Frailty syndrome is usually considered a reversible condition, thus amenable of specific preventive interventions.[33] Extensive literature focused on nutrition and physical activities.[34,35] However, persistent pain in older adult population is very common and has multiple determinants. Pain may represent a relevant risk factor, easily accessible, as well as a potential target for interventions. Longitudinal studies are required to better understand the possible association between pain and frailty in PHIV.
4.2. Strengths and limitations
Our study presents several strengths. We used validated self-report and performance tests. We explored original domains such as deprivation, geriatric assessment and pain related to frailty which is a growing concern among PHIV. However, our study has potential limitations. We have selected patients for whom the frailty criteria were measurable, as described by Fried. This limit is inherent to the measurement tool. Nevertheless, excluding frail patients, especially those who were unable to answer a questionnaire or unable to do walking test, avoid to overestimate the prevalence of frailty. Our study was performed exclusively in the South of France. Although frailty prevalence is likely to vary across Europe, it is the first time that it is estimated on a regional scale in France and on PHIV. The lack of a reference group is explained by the preliminary nature of our report which is the starting point of a longitudinal study aiming to estimate the evolution of frailty over the years in this cohort.
5. Conclusion
Our study is the first to describe a link between pain and frailty in older HIV patients. It is a new additional marker of frailty in HIV patients. This observation should be confirmed by further studies. It would be interesting to have more practical frailty scores in order to perform them during routine medical examination. As reduced physical activity concerns half of our cohort, we could hypothesize that increasing physical activity by pain reduction could reverse the frail phenotype in most HIV patients.
Acknowledgments
Authors wrote this article on behalf of the Visage group, and wish to thank the following persons for their active participation in this study: Allegre T., Chadapaud S., Cohen-Valensi R., Granet P., De Jaureguiberry J.P., Pellissier L, Pichancourt G., Philibert P, Tollinchi F. All participating people gave permission to be named in this article.
Footnotes
Abbreviations: ADL = activities of daily living, BMI = body mass index, EPICES = Evaluation of Precarity and Inequalities in Health Examination Centers score, GDS = Geriatric Depression Scale, HIV = human immunodeficiency virus, PHIV = people living with HIV, VAS = visual analogic scale.
Funding: This work was supported by University Hospital Center AP-HM, Marseille, France and Gilead Science. The funders had no role in the design, methods, subject recruitment, data collection, analysis and preparation of the article.
The authors report no conflicts of interest.
Contributor Information
Collaborators: the Visage group
References
- [1].Deeks SG, Lewin SR, Havlir DV. The end of AIDS: HIV infection as a chronic disease. Lancet 2013;382:1525–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Calcagno A, Nozza S, Muss C, et al. Ageing with HIV: a multidisciplinary review. Infection 2015;43:509–22. [DOI] [PubMed] [Google Scholar]
- [3].Desquilbet L, Jacobson LP, Fried LP, et al. HIV-1 infection is associated with an earlier occurrence of a phenotype related to frailty. J Gerontol A Biol Sci Med Sci 2007;62:1279–86. [DOI] [PubMed] [Google Scholar]
- [4].Detels R, Jacobson L, Margolick J, et al. The multicenter AIDS Cohort Study, 1983 to …. Public Health 2012;126:196–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Oursler KK, Goulet JL, Crystal S, et al. Association of age and comorbidity with physical function in HIV-infected and uninfected patients: results from the Veterans Aging Cohort Study. AIDS Patient Care STDs 2011;25:13–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Brothers TD, Kirkland S, Guaraldi G, et al. Frailty in People Aging With Human Immunodeficiency Virus (HIV) Infection. J Infect Dis 2014. [DOI] [PubMed] [Google Scholar]
- [7].Fried LP, Tangen CM, Walston J, et al. Frailty in older adults evidence for a phenotype. J Gerontol A Biol Sci Med Sci 2001;56:M146–57. [DOI] [PubMed] [Google Scholar]
- [8].Bandeen-Roche K, Xue Q-L, Ferrucci L, et al. Phenotype of frailty: characterization in the women's health and aging studies. J Gerontol A Biol Sci Med Sci 2006;61:262–6. [DOI] [PubMed] [Google Scholar]
- [9].Retornaz Frédérique, Karunananthan Sathya, Sourial Nadia, et al. « Fragilité et traitement des maladies chroniques », dans La fragilité des personnes âgées. Définitions, controverses et perspectives d’action. Rennes, Presses de l’EHESP, « Hors collection », 2013, p. 123–138. DOI: 10.3917/ehesp.bela.2013.01.0123. URL: https://www.cairn.info/la-fragilite-des-personnes-agees--9782810901234-page-123.htm. [Google Scholar]
- [10].Darque A, Enel P, Ravaux I, et al. Drug interactions in elderly individuals with the human immunodeficiency virus. J Am Geriatr Soc 2012;60:382–4. [DOI] [PubMed] [Google Scholar]
- [11].Enel P, Retornaz F, Philibert P, Ravaux I, Antolini MS, Petit N, et al. Drug Interaction Rate No Higher With HIV Than Without in French Group Over 60. Conference Reports for NATAP. Second International Workshop on HIV and Aging, 2011, Oct 27–28, Baltimore, MD.[cited 2016 Mar 14]. [Google Scholar]
- [12].Labbe E, Blanquet M, Gerbaud L, et al. A new reliable index to measure individual deprivation: the EPICES score. Eur J Public Health 2015;25:604–9. [DOI] [PubMed] [Google Scholar]
- [13].Labbé E, Moulin JJ, Guéguen R, Gerbau DL, et al. Un indicateur de mesure de la précarité et de la santé sociale: le score EPICES. L’expérience des Centres d’examens de santé de l’Assurance maladie. La Revue de l’Ires 2007;1:3–49. [Google Scholar]
- [14].Katz S. Assessing self-maintenance: activities of daily living, mobility, and instrumental activities of daily living. J Am Geriatr Soc 1983;31:721–7. [DOI] [PubMed] [Google Scholar]
- [15].Yesavage JA, Sheikh JI. Geriatric Depression Scale (GDS). Recent evidence and development of a shorter version. Clin Gerontol 1986;5:165–73. [Google Scholar]
- [16].Davis HS, MacPherson K, Merry HR, et al. Reliability and validity of questions about exercise in the canadian study of health and aging. Int Psychogeriatr 2001;13(S1):177–82. [DOI] [PubMed] [Google Scholar]
- [17].Studenski S, Perera S, Patel K, et al. Gait speed and survival in older adults. JAMA J Am Med Assoc 2011;305:50–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Kooij KW, Wit FWNM, Schouten J, et al. HIV infection is independently associated with frailty in middle-aged HIV type 1-infected individuals compared with similar but uninfected controls. AIDS 2016;30:241–50. [DOI] [PubMed] [Google Scholar]
- [19].Ianas V, Berg E, Mohler MJ, et al. Antiretroviral therapy protects against frailty in HIV-1 infection. J Int Assoc Provid AIDS Care JIAPAC 2013;12:62–6. [DOI] [PubMed] [Google Scholar]
- [20].Althoff KN, Jacobson LP, Cranston RD, et al. Age, comorbidities, and AIDS predict a frailty phenotype in men who have sex with men. J Gerontol A Biol Sci Med Sci 2014;69A:189–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Önen NF, Agbebi A, Shacham E, et al. Frailty among HIV-infected persons in an urban outpatient care setting. J Infect 2009;59:346–52. [DOI] [PubMed] [Google Scholar]
- [22].Marquine MJ, Umlauf A, Rooney A, et al. The Veterans Aging Cohort Study (VACS) Index is associated with concurrent risk for neurocognitive impairment. J Acquir Immune Defic Syndr 19992014;65:190–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Piggott DA, Muzaale AD, Mehta SH, et al. Frailty, HIV infection, and mortality in an aging cohort of injection drug users. PLos One 2013;8:e54910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Erlandson KM, Allshouse AA, Jankowski CM, et al. A comparison of functional status instruments in HIV-infected adults on effective antiretroviral therapy. HIV Clin Trials 2012;13:324–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Terzian AS, Holman S, Nathwani N, et al. Factors associated with preclinical disability and frailty among HIV-infected and HIV-uninfected women in the era of cART. J Womens Health 20022009;18:1965–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Pathai S, Gilbert C, Weiss HA, et al. Frailty in HIV-infected adults in South Africa. J Acquir Immune Defic Syndr 19992013;62:43–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Adeyemi O, Livak B. Higher Veterans Aging Cohort Study (VACS) Index scores in HIV-positive adults with CD4 counts <200 cells/mm3 despite viral suppression: JAIDS. J Acquir Immune Defic Syndr 2013;63:e78–81. [DOI] [PubMed] [Google Scholar]
- [28].Desquilbet L, Margolick JB, Fried LP, et al. Relationship between a frailty-related phenotype and progressive deterioration of the immune system in HIV-infected men. J Acquir Immune Defic Syndr 19992009;50:299–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Nessighaoui H, Lilamand M, Patel KV, et al. Frailty and pain: two related conditions. J Frailty Aging 2015;4:144–8. [DOI] [PubMed] [Google Scholar]
- [30].Wade KF, Lee DM, McBeth J, et al. Chronic widespread pain is associated with worsening frailty in European men. Age Ageing 2016;45:268–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Coelho T, Paul C, Gobbens RJ, et al. Multidimensional frailty and pain in community dwelling elderly. Pain Med 2017;18:693–701. [DOI] [PubMed] [Google Scholar]
- [32].Shega JW, Andrew M, Kotwal A, et al. Relationship between persistent pain and 5-year mortality: a population-based prospective cohort study. J Am Geriatr Soc 2013;61:2135–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Gill TM, Gahbauer EA, Allore HG, et al. Transitions between frailty states among community-living older persons. Arch Intern Med 2006;166:418–23. [DOI] [PubMed] [Google Scholar]
- [34].Botros D, Somarriba G, Neri D, et al. Interventions to address chronic disease and HIV: strategies to promote exercise and nutrition among HIV-infected individuals. Curr HIV/AIDS Rep 2012;9:351–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Gomes-Neto M, Conceição CS, Oliveira Carvalho V, et al. A systematic review of the effects of different types of therapeutic exercise on physiologic and functional measurements in patients with HIV/AIDS. Clinics (Sao Paulo) 2013;68:1157–67. [DOI] [PMC free article] [PubMed] [Google Scholar]


