Supplemental Digital Content is available in the text
Keywords: configural processing, evoked power, face effect, face-specific processing, induced power, inversion effect, schizophrenia patients
Abstract
Schizophrenia is a complex disorder characterized by marked social dysfunctions, but the neural mechanism underlying this deficit is unknown. To investigate whether face-specific perceptual processes are influenced in schizophrenia patients, both face detection and configural analysis were assessed in normal individuals and schizophrenia patients by recording electroencephalogram (EEG) data. Here, a face processing model was built based on the frequency oscillations, and the evoked power (theta, alpha, and beta bands) and the induced power (gamma bands) were recorded while the subjects passively viewed face and nonface images presented in upright and inverted orientations. The healthy adults showed a significant face-specific effect in the alpha, beta, and gamma bands, and an inversion effect was observed in the gamma band in the occipital lobe and right temporal lobe. Importantly, the schizophrenia patients showed face-specific deficits in the low-frequency beta and gamma bands, and the face inversion effect in the gamma band was absent from the occipital lobe. All these results revealed face-specific processing in patients due to the disorder of high-frequency EEG, providing additional evidence to enrich future studies investigating neural mechanisms and serving as a marked diagnostic basis.
1. Introduction
Schizophrenia is a complex disorder characterized by marked social dysfunctions.[1] Individuals with schizophrenia have deficits in the processing and perception of sensory information and specific performance deficits in motivation, affect, and social skills.[2–4] Because of the social dysfunction in schizophrenia, studying society cognition allows for the identification of exceptions at the cognitive dysfunction and neurological abnormality levels. Identifying cognitive deficits may be helpful in diagnosing and describing the subtypes and predicting the treatment response and prognosis of schizophrenia. However, exploring social interactions is challenging because social interactions involve many overlapping processes. Because face processing is one of the most important processes during social interaction,[5] investigating the neural mechanism underlying face processing, particularly in schizophrenia patients, is important.
Face-specific processing includes the following 2 processes: featural processing and configural processing. More specifically, the objects of featural processing include details, such as the shape of the nose or eye, while configural processing focuses on the spatial distribution of the facial features and other components.[6–9] The face processing deficits in schizophrenia patients involve dysfunction in face-specific configural processing, which integrates information regarding the shapes of individual features and the relative distances between the features.[1,5,10] Several studies have demonstrated abnormalities in early visual face processing in schizophrenia at both the behavioral and physiological levels. Notably, behavioral studies have found that the deficits in facial identity recognition are reduced while performing simpler tasks in which other mnemonic and attentional demands are minimized, such as matching identically posed faces[11] or recognizing famous faces.[12] Several studies have found that the impairment worsens in association with the negative symptoms[13] and symptom severity.[14,15] Thus, the structural and functional abnormalities in the fusiform gyrus are likely responsible for at least some of the impairment in face identification observed in schizophrenia.[16–19] There is also evidence that schizophrenia patients display reduced inversion effects compared to controls.[20–22]
However, whether schizophrenia patients are impaired in the face inversion effect remains controversial because other studies failed to find these deficits.[23–25] In particular, several behavioral studies have reported that patients with schizophrenia are more impaired in discriminating faces that differ in configural information than in discriminating faces that differ in featural information.[22,26] These findings suggest that an underlying face processing abnormality exists that cannot be undetected using common behavioral measures. Although numerous neural abnormalities in regions associated with face processing have been described in schizophrenia, the impact of the basic visual facial processing abnormalities and the specificity of the impairment in facial processing have received little attention. No clear relationship between the facial recognition deficits and the symptom status has been identified.
Electrophysiological studies can provide valuable information regarding the neural activity underlying the stages of information processing. The temporal processing of faces is typically assessed using event-related potentials (ERPs).[27] The N170 component, which occurs within 130 to 200 milliseconds poststimulus onset, is one of the most often discussed ERP component related to face processing and maximal over posterior lateral electrode sites. N170 is the main evidence from the perspective of ERP; Bentin et al[6] found a strong negative component in subjects’ temporal occipital electrode 170 milliseconds after the face stimulus. Anaki et al[28] found that the amplitude of N170 is not affected by the familiarity of the subject with the face. But the latency of N170 exhibited a significant delay under the inverted face condition.[29] Subsequent studies have shown that the amplitude and latency of N170 are modulated by the inversion effect (the latency was delayed, and the amplitude increased).[30,31] Lee et al[32] also revealed that schizophrenia patients with wider neuropsychological impairments have a lower amplitude in N170 components than normal controls. Therefore, the determination of whether an object is a face or nonface occur early, and the structured coding of a face occurs much earlier than face recognition.[33] New evidence has shown that compared with healthy controls, schizophrenia patients exhibit a significant N170 latency sluggishness.[34] Because time-frequency (TF) analyses involve a 2-dimensional display mode to facilitate the observation of changes in the frequency components over time, TF analysis methods are frequently used to investigate nonstationary, nonlinear EEG signals. Since many brain functions are modulated by frequency specificity, many electrophysiological studies investigating face processing have focused on frequency oscillations. High-frequency oscillations are the most robust neural markers of face processing, particularly the induced gamma power, which is not necessarily phase locked to the stimuli onset.[35] Zion and colleagues further concluded that the induced power of the low-frequency γ band (25–50 Hz) significantly decreases after the inversion of face stimuli, while the induced power of the high-frequency γ band (50–70 Hz) was not modulated by inversion but was related to the subject's familiarity with the face stimulus in normal healthy individuals. Studies involving healthy participants found an enhanced induced gamma power during an epoch ranging between 200 and 400 milliseconds from the stimuli onset while processing recognizable objects vs. meaningless objects.[36,37] Additionally, the induced gamma power exhibited a reduction at approximately 150 to 250 milliseconds for inverted faces over upright faces in healthy individuals.[28] Furthermore, because several studies have reported that face processing reflected electrical activity in the high-frequency oscillation in healthy participants and the face-related activity observed in previous studies involving schizophrenia patients has been reported in N170, we hypothesized that the high-frequency components of face processing in schizophrenia may be damaged.
Here, we recorded event-related EEG data elicited by face and nonface stimuli presented in the upright and inverted orientations. We compared the evoked power and induced power in normal controls and schizophrenic patients under different stimuli conditions. During the indexes of interest, compared to the normal controls, we found deficits in both the face specificity and face inversion effect in the schizophrenia patients. High-frequency oscillation could be used as an electrophysiological marker in diagnosing and describing the subtypes and predicting the treatment response and prognosis in schizophrenia patients.
2. Methods
2.1. Participants
Twenty-one patients with schizophrenia and 21 normal controls participated in this study. The schizophrenia patients, who were recruited from Beijing Anding Hospital, were diagnosed based on the Structured Clinical Interview for Diagnostic and Statistical Manual of Mental Disorders. And the normal controls were recruited from the local community through local newspaper and poster advertisements. An initial screening interview was performed to exclude subjects who had any identifiable neurological disorder or head injury, any personal history of psychiatric disease, or a family history of psychiatric illness. All subjects had normal or corrected-to-normal vision and were right-handed. Their psychiatric symptoms were evaluated using the positive and negative syndrome scale (PANSS). None of the patients had a history of central nervous system diseases, alcohol or drug abuse, electroconvulsive therapy, mental retardation, or head injury with the loss of consciousness. The demographic and descriptive characteristics of the participants are shown in Supplementary Table S1. Moreover, all subjects signed a written informed consent form before their participation in the study. No significant group differences were observed in the gender distribution, age, or education level. All participants received financial compensation and provided written informed consent for their participation, and all methods were performed in accordance with the clinical–experimental guidelines for human experiments approved by the Institutional Review Board of Beijing Anding Hospital.
2.2. Procedures
We define the faces and nonface objects (tables) as nontarget stimuli, while pictures of flowers were defined as visual target stimuli. We used a passive visual detection paradigm in which the nontarget stimuli and visual target stimuli were randomly interspersed. Both the schizophrenia patients and normal subjects were asked to mentally count the target stimuli. The stimuli used in the study included 75 photographs of unfamiliar young faces, 75 photographs of tables, and 45 photographs of flowers, all in gray-scale measuring 10.58 × 12.70 cm. In total, 50% of the faces were male, 50% of the faces were female, and all faces lacked hair, eyeglasses, or other accessories. The following 5 stimulus conditions were used: upright faces, inverted faces, upright tables, inverted tables, and upright flowers. All images were equivalent in luminance and root-mean-squared (RMS) contrast using the Photoshop software system (Adobe Systems, Inc., San Jose, CA). The stimuli were presented at the center of a computer screen. The stimuli were viewed from a distance of 1.2 m, 5.05° vertically and 6.06° horizontally.
The study procedure consisted of stimuli presentations in 3 blocks; each block consisted of 20 upright faces, 20 upright tables, 20 inverted faces, 20 inverted tables, and 20 flowers as the target stimuli. All stimuli were pseudorandomized, and each stimulus was presented for 250 milliseconds with a random interstimulus interval range of 800 to 1200 milliseconds. The subjects were tasked with silently counting the occurrences of the target stimulus (flowers) and verbally reporting the number at the end of the session (Fig. 1A).
Figure 1.
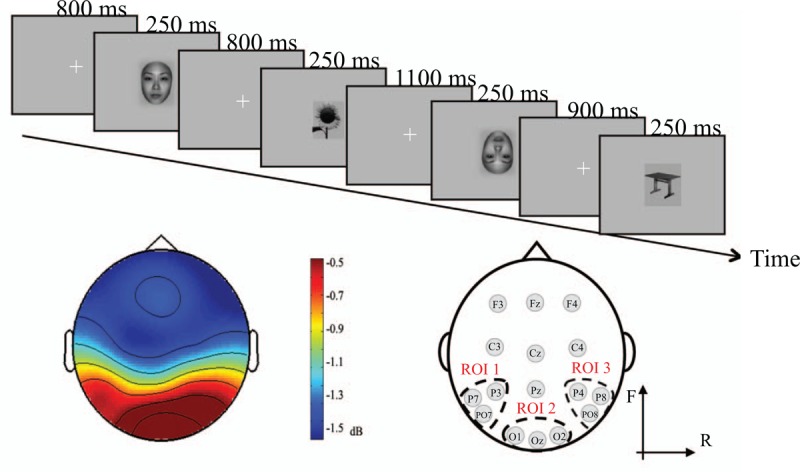
Flow diagram of the experiment (A). Spectral power distribution and method of ROI partitioning (B). The left portion of B shows the plot of the average energy in both the patients and normal controls. The right portion of B shows the distribution of the ROIs we defined. P3, P7, and PO7 fall within ROI1, which is located in the left temporal lobe; O1, Oz, and O2 fall within ROI2, which is located in the occipital region; and P4, P8, and PO8 fall within ROI3, which is located in the right temporal lobe.
2.3. EEG recording
A continuous electroencephalogram (EEG) was recorded for each subject at the P3, P7, PO7, O1, Oz, O2, P4, P8, and PO8 sites using Ag-AgCl active electrodes according to the 10 to 20 system.[38] According to the ERP characteristics of each electrode and scalp distribution, we divided the 9 electrodes into 3 region of interests (ROIs) (Fig. 1B), and the partitioning method used is shown in the figure below. Eye movements and blinks were monitored using bipolar horizontal and vertical electrooculography (EOG) derivations via the following 2 pairs of electrodes: one pair was attached to the external canthi, and the other pair was attached to the infraorbital and supraorbital regions of the left eye. Both EEG and EOG were sampled at 1200 Hz using a 0.05 to 100 Hz bandpass on the Cognitrace system (www.ant-neuro.com). The digitized EEG was saved and processed offline. The electrode impedance was maintained at less than 5 kΩ throughout the recording phase of the study.
The data analysis was performed using MATLAB R2013a (MathWorks, Inc., Natick, MA) with the following open source toolbox: EEGLAB (http://sccn.ucsd.edu/eeglab/, Swartz Center for Computational Neuroscience, La Jolla, CA).[39] The raw data were low-pass filtered offline at 100 Hz and subsequently high-pass filtered at 0.5 using a Hamming-windowed sinc finite impulse response (FIR) filter. The artifacts (e.g., eye artifacts, muscle artifacts, and electrocardiographic activity) were removed using an independent component analysis (ICA) procedure, and remaining artifacts exceeding ± 100 μV in amplitude or containing a change of over 100 μV within a period of 50 milliseconds were rejected. The artifact-free data were then segmented into epochs ranging from −300 milliseconds before to 400 milliseconds after the stimulus onset for all conditions. All participants showed a sufficient number of accepted epochs for the upright faces. The average acceptance rate did not significantly differ between the groups (schizophrenia patients 85.1%, 51.4 accepted epochs vs. normal control 85.2%, 51.5 accepted epochs, P > .1).
2.4. Time-frequency analysis
After the pretreatment, purified EEG signals were obtained from each group of experimental subjects. EEG signals induced by the same event were superimposed, averaged and then filtered through a 30 Hz low-pass filter, allowing us to obtain the ERP signal under each stimulation. A new estimation of the TF energy was performed based on the wavelet transformation of the artifact-free data, which provided a better compromise between the time and frequency resolutions than short-term Fourier transformations.[40,41] The artifact-free data were convoluted using complex Morlet wavelets, which have a Gaussian shape both in the time domain and the frequency domain at approximately its central frequency. The wavelet family used was defined by a constant ratio of 6, ranging from 20 to 100 Hz in 1 Hz steps. The TF energy was the squared norm of the convolution of a family of complex wavelets within each single trial data. The mean baseline log TF energy was subtracted from each spectral estimate, producing the baseline-normalized TF energy plot.
A TF analysis based on wavelet transformation (formula details shown in F(2−1)) can provide the variation in the EEG signal in both the time domain and the frequency domain simultaneously. And evoked and induced oscillations differ in their phase-relationships to the stimulus. Evoked oscillations are phase-locked to the stimulus, and the EEG data are first averaged over trials and then subject to TF analysis to give an event-related response. Whereas induced oscillations are not, the TF analysis is applied to each trial and the ensuing power is averaged across trials.[42] After obtaining the evoked power and induced power, we used the average energy at 300 milliseconds before the stimulus as the baseline and subtracted this value from the subsequent energy data to obtain the task-related evoked power and induced power. To study the phasic synchronization diversity of the different rhythms in the EEG data, we considered the evoked power and induced power characteristic indexes. The EEG TF characteristics were calculated and visualized using a TF map and a topographic map.
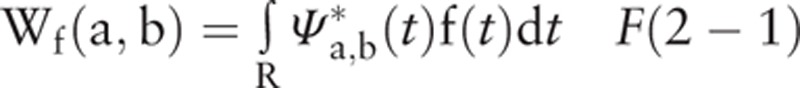 |
2.5. Statistical analysis
The TF map of each ROI was obtained by averaging the power values across all epochs and subjects. The choice of ROI partition was based on previous study findings. Most previous studies using the cortical EEG method have found that the GBA (gamma-band activity) was higher mainly at the midline cortical area (posterior > anterior) in face-related processing.[26,28,35,43–45]
The induced power and evoked power were statistically analyzed using a repeated measures analysis of variance (ANOVA) with the within-subject factors of stimulus types (face vs table), orientation (upright vs inverted), and brain ROI (ROI1, ROI2, and ROI3). A t test was performed to compare the groups. The P values were corrected by performing a Bonferroni adjustment for multiple comparisons. A Greenhouse-Geisser correction was adopted for the degrees of freedom. The statistical analyses were performed using SPSS 19 (SPSS, Chicago, IL). The methods used in the abovementioned statistical analyses have been verified by other experimental studies.[46]
3. Results
3.1. Division of frequency band in evoked power and induced power
Using the same ROI partitioning method used in the analysis of the TF energy, we can draw a conclusion regarding the contrast of the evoked power, induced power and phase-synchronization factors in the selected region (Fig. 2). As shown in Fig. 2, the phase synchronization of the low-frequency band below 30 Hz was good in the TF energy; thus, the evoked power can be used as an eigenvalue to improve the signal-noise ratio in the data analysis. The phase synchronization of the high-frequency band above 25 Hz was poor, but the induced power was high; thus, the induced power could be used as an eigenvalue for the analysis in the high-frequency band.
Figure 2.
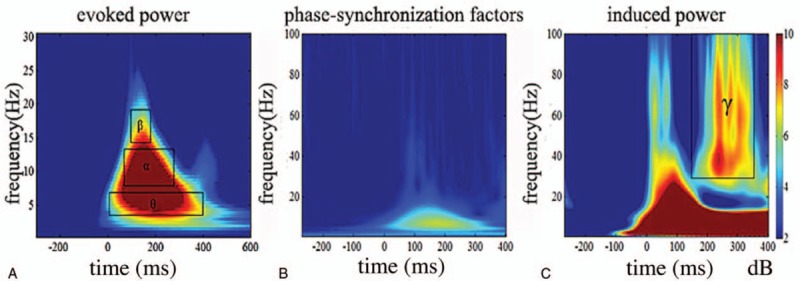
Contrast of evoked power (A), induced power (C), and phase-synchronization factors (B) in all subjects. The horizontal axis shows the time range, and the vertical axis shows the frequency range.
As shown in the Fig. 2 above, we can clearly observe that the evoked power in these subjects is mainly concentrated in the θ band, α band, and low-frequency β band. The energy of the θ wave (4–7 Hz) is mainly concentrated at 0 to 400 milliseconds after the stimulus, the α wave (8–14 Hz) is mainly concentrated at 50 to 300 milliseconds after the stimulus and the low-frequency β wave (15–20 Hz) is mostly concentrated at 100 to 200 milliseconds after the stimulus. We selected the average evoked power in the 3 TF windows as the object to analyze, and the characteristic TF window of face processing was found in the low-frequency TF energy. From the induced power shown in the figure above, a high-frequency induced power was observed in the frequency range of 150 to 350 milliseconds and 25 to 100 Hz after the stimulus. Thus, this TF window was selected as the main TF window of the induced power in the subjects.
3.2. Time-frequency energy
3.2.1. Within-group analysis
In the TF window of the θ frequency band (4–7 Hz), the average evoked power was unaffected by any intragroup factors; that is, this TF window was not the characteristic TF window of face processing or configural processing in the subjects. Therefore, the θ frequency band was not analyzed in this study. As observed in the results of the within-group analysis (Fig. 3A), in the α frequency band (8–14 Hz), both the schizophrenia patients and normal controls showed the face effect; that is, the energy induced by the nonface stimuli was significantly lower than that induced by the face stimuli, while in the β frequency band (15–20 Hz), only the normal controls showed the face effect (Fig. 3B; ROI1: F(1,20) = 12.582, P = .002; ROI2: F(1,20) = 12.337, P = .002; ROI3: F(1,20) = 11.587, P = .003). No significant difference was observed between the energy of face processing and nonface processing in the schizophrenia patients (ROI1: F(1,20) = 0.470, P = .501; ROI2: F(1,20) = 0.175, P = .680; ROI3: F(1,20) = 1.737, P = .202, all P > .05). Therefore, the schizophrenia patients did not display the face effect, and abnormities were observed in the bilateral temporal and occipital regions. No statistically significant differences were observed in the inversion effect in either the controls or patients in both the α and β frequency bands (α band: ROI1: F(1,20) = 0.475, P = .499; ROI2: F(1,20) = 1.324, P = .263; ROI3: F(1,20) = 2.092, P = .164; β band: ROI1: F(1,20) = 1.775, P = .198; ROI2: F(1,20) = 0.390, P = .539; ROI3: F(1,20) = 1.875, P = .186; all P > .05, as shown in Supplementary Fig. S1). In the γ frequency band (25–100 Hz; Fig. 3C), a significant face effect was observed in the normal controls but not in the schizophrenia patients, and the bilateral temporal and occipital regions in the schizophrenia patients were abnormal. We also found that the normal controls showed an inversion effect in the occipital region (ROI2: F(1,20) = 6.866, P = .016) and right temporal region (ROI3: F(1,20) = 5.682, P = .027); that is, the energy induced by the inverted images was significantly lower than that induced by the upright images. However, the inversion effect was found only in the right temporal region (ROI3: F(1,20) = 5.130, P = .035) in the schizophrenia patients, suggesting that the abnormalities in the occipital area (ROI2) disrupted configural processing in the schizophrenia patients. The sample characteristics and results of the with-group ANOVA are shown in Supplementary Table S2-1 and S2-2.
Figure 3.
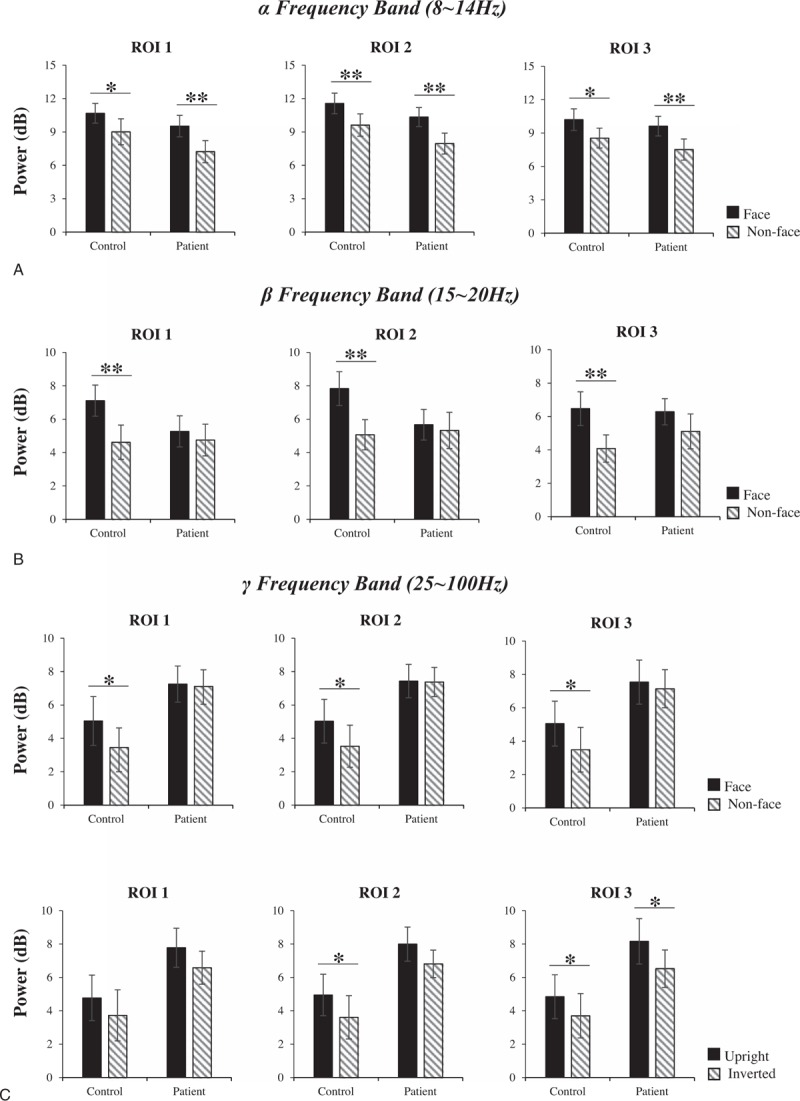
Within-group analysis of the evoked power in the α frequency band (A), β frequency band (B), and the induced power in the γ frequency band (C). In each graph, the horizontal axis shows the group name: control and patient, and the vertical axis shows the evoked power. The black box or diagonal stripes box represents the stimulus conditions. The bar graphs display the mean of standard error of the power for the controls or patients in different stimulus conditions. ∗P < .05, ∗∗P < .01.
3.2.2. Across-group analysis
To further analyze the across-group differences, we conducted independent samples t tests between the control and patient groups for ROI1, ROI2, and ROI3 under each of 4 stimulus conditions. Group effects were observed in the TF window of the γ band for all ROIs. However, although no significant group effects were found in either the α or β bands (all P > .05), the power of the α band in 4 stimulus conditions of each ROI in the normal controls is higher than that in the schizophrenia patients. And the power of the β band in face stimulus of ROI1 and ROI2 in the normal controls is higher than that in the schizophrenia patients. While the power of inverted nonface in the normal controls is lower than the schizophrenia patients in all ROIs, and this phenomenon was also observed in the upright nonface and face stimulus in ROI3 (see details in Supplementary Table S3-1 and S3-2 and Supplementary Fig. S2). As shown in Fig. 4A, the power of the γ band was significantly higher in the patients than in the controls. In ROI2, the induced power of the γ frequency band in the patients was significantly higher than that in the controls for both the upright nonface (t[40] = −2.548, P = .015) and inverted nonface (t[40] = −2.198, P = .034) stimuli, and significant differences were observed in the upright nonface stimuli in ROI1 (t[40] = −2.117, P = .041) and ROI3 (t[40] = −2.247, P = .030). The TF spectra of the γ band in the control and schizophrenia patients are shown in Fig. 4B. Significant differences were observed across the groups as shown in the TF energy marked by the red outlines. These results indicate that the damage to the high-frequency band could lead to face-specific deficits in schizophrenia.
Figure 4.
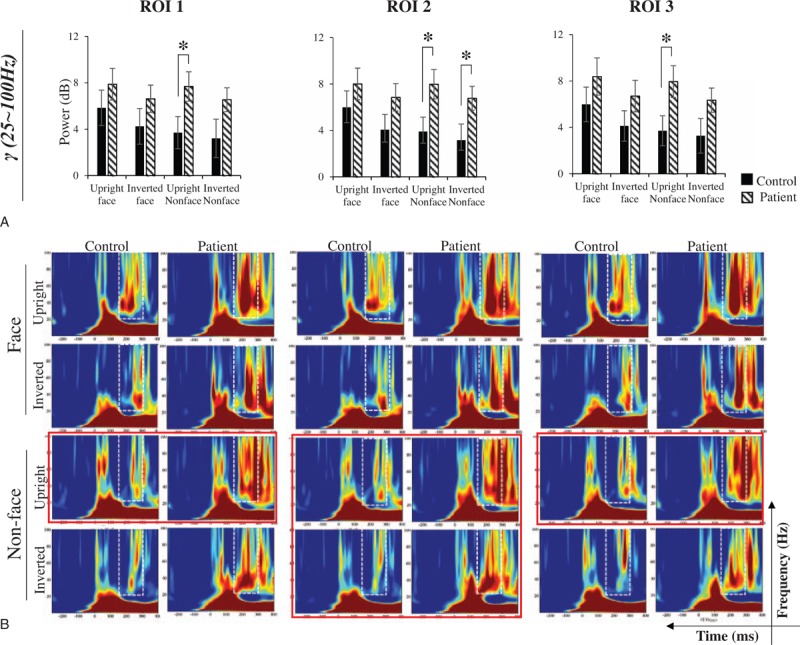
Across-group analysis of the induced power in the γ frequency band (A) and the induced power diagram in the γ frequency band (B). In graph A, the horizontal axis shows the stimulus condition, and the vertical axis shows the induced power. The black box represents the control group, while the white diagonal stripes box represents the patient group. In graph B, the horizontal axis shows the time range, the vertical axis shows the frequency range, and the box with the dotted line shows the induced power of the γ frequency band. Significant differences between the groups are outlined in red. The bar graphs display the mean of standard error of the induced power for each group in different stimulus conditions. ∗P < .05.
4. Discussion
Face processing is essential to social interaction and are thought to the main cognitive deficits of schizophrenia. The understanding neuronal mechanisms of oscillatory anomalies in face processing particularly for the face inversion effect is important. This study examined whether patients with schizophrenia display damage in the TF components of face processing in 4 stimulus conditions (upright face, inverted face, upright nonface, and inverted nonface). A model of the brain mechanism of face processing was established based on the frequency oscillations of the evoked power and the induced power. In this model, the evoked power in a subject's θ wave (4–7 Hz, 0–400 milliseconds), α wave (8–14 Hz, 50–300 milliseconds), and the low-frequency β wave (15–20 Hz, 100–200 milliseconds) were selected as characteristics of the evoked power, and the induced power of the γ wave (25–100 Hz, 150–350 milliseconds) was selected as the main feature of the induced power.
We found that the evoked power of the α wave and low-frequency β wave and the induced power of the γ wave under the condition of a face stimulus were significantly higher than those under the nonface condition, and the healthy subjects tend to adopt a “face priority processing” effect. The inversion effect was found only in the occipital and right temporal region of the γ band. However, the face effect was detected only in the α band in the schizophrenia patients, which is consistent with the results reported by Rousselet et al.[47] These authors reported that compared with images of objects, a 5 to 20 Hz oscillation has a larger amplitude in the time window 50 to 300 milliseconds after the onset of a face stimulus. Because the evoked power of α oscillatory responses to the presentation of a face is rarely reported, particularly in the complete dynamic of α activity,[48] this result enriched the sparse literature regarding the alpha band in face processing, particularly in schizophrenic patients. The face-specific deficits in schizophrenia, particularly in the low-frequency β and γ waves, and face inversion effect were absent from the γ band in the occipital lobe of schizophrenia patients.
Recent EEG studies investigating schizophrenia have focused on the gamma band due to its critical role in cognitive functions and, in general, support the finding of a gamma band reduction in schizophrenia. Zion-Golumbic et al[43] compared the induced power of the γ band (20–80 Hz) in response to human faces with that in response to ape faces, watches, hands, and buildings and found that the induced power of the γ band induced by the human face was significantly higher than that induced by the other types of stimulation. In 2010, the Lee et al found that patients have impaired gamma-band synchronization during face perception,[26] and in another article that investigated the source of the GBA in congenital prosopagnosia's magnetoencephalogram, indicating that the induced power of the γ band is not only caused by the fixation eye movement.[49] Similarly, in our γ band model (25–100 Hz, 150–350 milliseconds), the induced power in the γ band in the face stimulation in the control group was significantly higher than that in the nonface stimulation, and the energy in an upright stimulus stimulation was higher than that in inverted stimulation, which reflected that the induced power in this TF window was a specific feature of facial configural processing. More specifically, the induced power in the schizophrenia patients during this TF window was neither modulated by the stimulus type nor influenced by the direction of the stimulus, further supporting the ERP conclusion that schizophrenia induces high-frequency brain rhythm damage, resulting in patients with an impaired face-specific processing function and disordered high-frequency oscillations in face processing. Several behavioral studies have shown that in face and body processing, schizophrenia patients also present an inversion effect, and the impact of the inversion effect on accuracy is not significantly different from that in healthy adults, but the modulation of inversion in schizophrenia patients attenuates the response time.[22] In our study, the normal controls and schizophrenia patients both presented an inversion effect in the γ band, but the occipital area was deficient only in the schizophrenia patients. However, we found that the power of the γ band (25–100 Hz, 150–350 milliseconds) in the schizophrenia patients was higher than that in the controls in response to the face stimulus, which is inconsistent with most results published by others but is consistent with the results published by Lee et al in 2016 who found that early-stage (0–300 ms) GBA increased and late-stage (700–800 ms) GBA decreased in response to human faces compared to nonfacial stimuli in schizophrenia patients. Unsurprisingly, inconsistent and opposite findings typically exist due to the differences in the task category, target stimuli, and time window selected. Increased gamma has been described under working memory, somatosensory stimulation, visual recognition tasks, and unmediated conditions in schizophrenia patients.[50–52] The available literature appears to suggest that gamma band reductions occur during impaired cognitive functions, but occasionally, no abnormalities or even increased gamma-band activities are observed at rest or while performing less cognitively demanding tasks.[53]
As schizophrenia is a psychiatric disorder with significant sex differences, sex factors are also considered a very important factor in the study of subsequent mental disorder brain mechanisms. In this paper, faces were the nontarget stimuli in the experimental paradigm; thus, this article explores face processing under the nonattention condition. Nevertheless, studies investigating related behaviors have found that attention to an arrow is stronger than attention to a face upon guidance.[34] Thus, attention may be a necessary factor in exploring the mechanism of facial processing in the future. In summary, the present study demonstrates that healthy adults have a specific processing pattern that is more likely based on configural analysis during face processing. However, schizophrenia patients suffer from a lack of discriminant coding of face and nonface stimuli. People are known to more likely adopt a processing mode based on configural analysis for the cognitive processing of a familiar visual stimulus during the processing of visual stimuli; therefore, the lack of discriminant coding results in an impairment in facial recognition in schizophrenia patients. Furthermore, even with the limited sample size and our findings can serve as pilot data suggesting further research, this study still reveals that the effect of schizophrenia on brain processes is mainly focused on damage to the high-frequency brain rhythm. Therefore, a high-frequency brain rhythm can be used as a useful indicator to aid in the diagnosis of schizophrenia.
Supplementary Material
Acknowledgments
The authors thank the individuals who participated in our study and American Journal Experts providing language editing services.
Footnotes
Abbreviations: ANOVA = analysis of variance, EEG = electroencephalogram, EOG = electrooculography, ERP = event-related potential, FIR = finite impulse response, GBA = gamma-band activity, ICA = independent component analysis, PANSS = positive and negative syndrome scale, RMS = root-mean-squared, ROI = region of interest, TF = time-frequency.
Funding: This study was financially supported by the National Natural Science Foundation of China (Grant No. 81671776, No. 61727807, No. 61473043), the Beijing Municipal Science & Technology Commission (Grant No. Z161100002616020), Beijing Nova Program (Grant No. Z171100001117057).
ML and GP contributed equally to this study.
The authors have no conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
References
- [1].Bortolon C, Capdevielle D, Raffard S. Face recognition in schizophrenia disorder: a comprehensive review of behavioral, neuroimaging and neurophysiological studies. Neurosci Biobehav Rev 2015;53:79–107. [DOI] [PubMed] [Google Scholar]
- [2].Onitsuka T, Oribe N, Nakamura I, et al. Review of neurophysiological findings in patients with schizophrenia. Psychiatry Clin Neurosci 2013;67:461–70. [DOI] [PubMed] [Google Scholar]
- [3].Javitt DC, Freedman R. Sensory processing dysfunction in the personal experience and neuronal machinery of schizophrenia. Am J Psychiatry 2015;172:17–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Giersch A, Lalanne L, van Assche M, et al. On disturbed time continuity in schizophrenia: an elementary impairment in visual perception? Front Psychol 2013;4:281.doi: 10.3389/fpsyg.2013.00281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Marwick K, Hall J. Social cognition in schizophrenia: a review of face processing. Br Med Bull 2008;88:43–58. [DOI] [PubMed] [Google Scholar]
- [6].Bentin S, Allison T, Puce A, et al. Electrophysiological studies of face perception in humans. J Cogn Neurosci 1996;8:551–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Freire A, Lee K, Symons LA. The face-inversion effect as a deficit in the encoding of configural information: direct evidence. Perception 2000;29:159–70. [DOI] [PubMed] [Google Scholar]
- [8].Hole GJ, George PA, Eaves K, et al. Effects of geometric distortions on face-recognition performance. Perception 2002;31:1221–40. [DOI] [PubMed] [Google Scholar]
- [9].Yin RK. Looking at upside-down faces. J Exp Psychol 1969;81:141–5. [Google Scholar]
- [10].Darke H, Peterman JS, Park S, et al. Are patients with schizophrenia impaired in processing non-emotional features of human faces? Front Psychol 2013;4:529.doi: 10.3389/fpsyg.2013.00529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Hooker C, Park S. Emotion processing and its relationship to social functioning in schizophrenia patients. Psychiatry Res 2002;112:41–50. [DOI] [PubMed] [Google Scholar]
- [12].Whittaker JF, Deakin JF, Tomenson B. Face processing in schizophrenia: defining the deficit. Psychol Med 2001;31:499–507. [DOI] [PubMed] [Google Scholar]
- [13].Martin F, Baudouin JY, Tiberghien G, et al. Processing emotional expression and facial identity in schizophrenia. Psychiatry Res 2005;134:43–53. [DOI] [PubMed] [Google Scholar]
- [14].Penn DL, Combs DR, Ritchie M, et al. Emotion recognition in schizophrenia: further investigation of generalized versus specific deficit models. J. Abnorm Psychol 2000;109:512–6. [PubMed] [Google Scholar]
- [15].Sachs G, Steger-Wuchse D, Kryspin-Exner I, et al. Facial recognition deficits and cognition in schizophrenia. Schizophr Res 2004;68:27–35. [DOI] [PubMed] [Google Scholar]
- [16].Lee CU, Shenton ME, Salisbury DF, et al. Fusiform gyrus volume reduction in first-episode schizophrenia—a magnetic resonance imaging study. Arch Gen Psychiatry 2002;59:775–81. [DOI] [PubMed] [Google Scholar]
- [17].Quintana J, Wong T, Ortiz-Portillo E, et al. Right lateral fusiform gyrus dysfunction during facial information processing in schizophrenia. Biol Psychiatry 2003;53:1099–112. [DOI] [PubMed] [Google Scholar]
- [18].Honea R, Crow TJ, Passingham D, et al. Regional deficits in brain volume in schizophrenia: a meta-analysis of voxel-based morphometry studies. Am J Psychiatry 2005;162:2233–45. [DOI] [PubMed] [Google Scholar]
- [19].Onitsuka T, Niznikiewicz MA, Spencer KM, et al. Functional and structural deficits in brain regions subserving face perception in schizophrenia. Am J Psychiatry 2006;163:455–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Shin YW, Na MH, Ha TH, et al. Dysfunction in configural face processing in patients with schizophrenia. Schizophr Bull 2008;34:538–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Kim HS, Shin NY, Choi JS, et al. Processing of facial configuration in individuals at ultra-high risk for schizophrenia. Schizophr Res 2010;118:81–7. [DOI] [PubMed] [Google Scholar]
- [22].Soria Bauser D, Thoma P, Aizenberg V, et al. Face and body perception in schizophrenia: a configural processing deficit? Psychiatry Res 2012;195:9–17. [DOI] [PubMed] [Google Scholar]
- [23].Butler PD, Tambini A, Yovel G, et al. What's in a face? Effects of stimulus duration and inversion on face processing in schizophrenia. Schizophr Res 2008;103:283–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Schwartz BL, Marvel CL, Drapalski A, et al. Configural processing in face recognition in schizophrenia. Cogn Neuropsychiatry 2002;7:15–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Chambon V, Baudouin JY, Franck N. The role of configural information in facial emotion recognition in schizophrenia. Neuropsychologia 2006;44:2437–44. [DOI] [PubMed] [Google Scholar]
- [26].Lee SH, Kim DW, Kim EY, et al. Dysfunctional gamma-band activity during face structural processing in schizophrenia patients. Schizophr Res 2010;119:191–7. [DOI] [PubMed] [Google Scholar]
- [27].Palermo R, Rhodes G. Are you always on my mind? A review of how face perception and attention interact. Neuropsychologia 2007;45:75–92. [DOI] [PubMed] [Google Scholar]
- [28].Anaki D, Zion-Golumbic E, Bentin S. Electrophysiological neural mechanisms for detection, configural analysis and recognition of faces. Neuroimage 2007;37:1407–16. [DOI] [PubMed] [Google Scholar]
- [29].Picozzi M, Cassia VM, Turati C, et al. The effect of inversion on 3-to 5-year-olds’ recognition of face and nonface visual objects. J Exp Child Psychol 2009;102:487–502. [DOI] [PubMed] [Google Scholar]
- [30].Eimer M. Effects of face inversion on the structural encoding and recognition of faces. Evidence from event-related brain potentials. Brain Res Cogn Brain Res 2000;10:145–58. [DOI] [PubMed] [Google Scholar]
- [31].Minnebusch DA, Keune PM, Suchan B, et al. Gradual inversion affects the processing of human body shapes. Neuroimage 2010;49:2746–55. [DOI] [PubMed] [Google Scholar]
- [32].Lee SH, Kim EY, Kim S, et al. Event-related potential patterns and gender effects underlying facial affect processing in schizophrenia patients. Neurosci Res 2010;67:172–80. [DOI] [PubMed] [Google Scholar]
- [33].Olivares EI, Iglesias J, Saavedra C, et al. Brain signals of face processing as revealed by event-related potentials. Behav Neurol 2015;2015:514361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Yan T, Zhao S, Uono S, et al. Target object moderation of attentional orienting by gazes or arrows. Atten Percept Psychophys 2016;78:2373–82. [DOI] [PubMed] [Google Scholar]
- [35].Zion-Golumbic E, Bentin S. Dissociated neural mechanisms for face detection and configural encoding: evidence from N170 and induced gamma-band oscillation effects. Cereb Cortex 2007;17:1741–9. [DOI] [PubMed] [Google Scholar]
- [36].Bertrand O, Tallon-Baudry C. Oscillatory gamma activity in humans: a possible role for object representation. Int J Psychophysiol 2000;38:211–23. [DOI] [PubMed] [Google Scholar]
- [37].Herrmann CS, Munk MH, Engel AK. Cognitive functions of gamma-band activity: memory match and utilization. Trends Cogn Sci 2004;8:347–55. [DOI] [PubMed] [Google Scholar]
- [38].Guideline one: minimum technical requirements for performing clinical electroencephalography. American Electroencephalographic Society. J Clin Neurophysiol 1994;11:2–5. [PubMed] [Google Scholar]
- [39].Delorme A, Makeig S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J Neurosci Methods 2004;134:9–21. [DOI] [PubMed] [Google Scholar]
- [40].Makeig S. Auditory event-related dynamics of the EEG spectrum and effects of exposure to tones. Electroencephalogr Clin Neurophysiol 1993;86:283–93. [DOI] [PubMed] [Google Scholar]
- [41].Sinkkonen J, Tiitinen H, Naatanen R. Gabor filters—an informative way for analyzing event-related brain activity. J Neurosci Methods 1995;56:99–104. [DOI] [PubMed] [Google Scholar]
- [42].David O, Kilner JM, Friston KJ. Mechanisms of evoked and induced responses in MEG/EEG. Neuroimage 2006;31:1580–91. [DOI] [PubMed] [Google Scholar]
- [43].Zion-Golumbic E, Golan T, Anaki D, et al. Human face preference in gamma-frequency EEG activity. Neuroimage 2008;39:1980–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Keil A, Muller MM, Gruber T, et al. Effects of emotional arousal in the cerebral hemispheres: a study of oscillatory brain activity and event-related potentials. Clin Neurophysiol 2001;112:2057–68. [DOI] [PubMed] [Google Scholar]
- [45].Matsumoto A, Ichikawa Y, Kanayama N, et al. Gamma band activity and its synchronization reflect the dysfunctional emotional processing in alexithymic persons. Psychophysiology 2006;43:533–40. [DOI] [PubMed] [Google Scholar]
- [46].Zheng Y, Li H, Ning Y, et al. Sluggishness of early-stage face processing (N170) is correlated with negative and general psychiatric symptoms in schizophrenia. Front Hum Neurosci 2016;10:615.doi: 10.3389/fnhum.2016.00615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Rousselet GA, Husk JS, Bennett PJ, et al. Single-trial EEG dynamics of object and face visual processing. Neuroimage 2007;36:843–62. [DOI] [PubMed] [Google Scholar]
- [48].Guntekin B, Basar E. A review of brain oscillations in perception of faces and emotional pictures. Neuropsychologia 2014;58:33–51. [DOI] [PubMed] [Google Scholar]
- [49].Dobel C, Junghofer M, Gruber T. The role of gamma-band activity in the representation of faces: reduced activity in the fusiform face area in congenital prosopagnosia. PLoS ONE 2011;6:e19550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Spencer KM, Nestor PG, Perlmutter R, et al. Neural synchrony indexes disordered perception and cognition in schizophrenia. Proc Natl Acad Sci USA 2004;101:17288–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Lee KH, Williams LM, Breakspear M, et al. Synchronous gamma activity: a review and contribution to an integrative neuroscience model of schizophrenia. Brain Res Brain Res Rev 2003;41:57–78. [DOI] [PubMed] [Google Scholar]
- [52].Basar-Eroglu C, Brand A, Hildebrandt H, et al. Working memory related gamma oscillations in schizophrenia patients. Int J Psychophysiol 2007;64:39–45. [DOI] [PubMed] [Google Scholar]
- [53].Moran LV, Hong LE. High vs low frequency neural oscillations in schizophrenia. Schizophr Bull 2011;37:659–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


