Abstract
The regulation of Rubisco activity was investigated under high, constant photosynthetic photon flux density during the diurnal phases of Crassulacean acid metabolism in Kalanchoë daigremontiana Hamet et Perr. During phase I, a significant period of nocturnal, C4-mediated CO2 fixation was observed, with the generated malic acid being decarboxylated the following day (phase III). Two periods of daytime atmospheric CO2 fixation occurred at the beginning (phase II, C4–C3 carboxylation) and end (phase IV, C3–C4 carboxylation) of the day. During the 1st h of the photoperiod, when phosphoenolpyruvate carboxylase was still active, the highest rates of atmospheric CO2 uptake were observed, coincident with the lowest rates of electron transport and minimal Rubisco activity. Over the next 1 to 2 h of phase II, carbamylation increased rapidly during an initial period of decarboxylation. Maximal carbamylation (70%–80%) was reached 2 h into phase III and was maintained under conditions of elevated CO2 resulting from malic acid decarboxylation. Initial and total Rubisco activity increased throughout phase III, with maximal activity achieved 9 h into the photoperiod at the beginning of phase IV, as atmospheric CO2 uptake recommenced. We suggest that the increased enzyme activity supports assimilation under CO2-limited conditions at the start of phase IV. The data indicate that Rubisco activity is modulated in-line with intracellular CO2 supply during the daytime phases of Crassulacean acid metabolism.
Characterization of the diel regulation of carboxylation in Crassulacean acid metabolism (CAM) plants can be achieved by the framework first proposed by Osmond (1978). The model comprises four metabolic phases that encompass the temporal regulation of C4 and C3 carboxylation within the same cellular environment. Phase I represents nocturnal CO2 fixation mediated by PEP carboxylase (PEPC), resulting in the synthesis of the C4 product malate, which is stored overnight in the vacuole as malic acid. During the early morning, phase II marks the transition from C4 to C3 carboxylation and may be accompanied by considerable net atmospheric CO2 uptake. Recently, it has been demonstrated in some species that PEPC activity may remain active for 3 to 4 h during phase II (Borland et al., 1993; Roberts et al., 1997), with the carbon fixed accounting for a considerable proportion of the net daily carbon gain (Borland and Griffiths, 1996, 1997). Decarboxylation of malic acid and re-fixation of CO2 by Rubisco occurs behind closed stomata during phase III. Phase IV is often accompanied by an extended period of atmospheric CO2 uptake, which includes a shift from Rubisco to PEPC carboxylation toward the end of the day (Griffiths et al., 1990).
The four phases of CAM may additionally be characterized by CO2 supply. Whereas it is clear that decarboxylation generates very high internal partial pressures of CO2 during phase III (typically 1.8%–8%) (Cockburn et al., 1979; Spalding et al., 1979; Osmond et al., 1999), the low internal conductance of CO2 from the stomatal cavity to Rubisco active sites results in a very low pCO2 during atmospheric CO2 uptake (Maxwell et al., 1997). Therefore, during phases II and IV, the internal partial pressure of CO2 is limiting for Rubisco carboxylase activity, but is saturating for PEPC (Osmond et al., 1999). This raises the likelihood of considerable Rubisco oxygenase activity (Maxwell et al., 1998) and suggests that the fixation of CO2 by PEPC may confer an additional biochemical limitation to Rubisco carboxylase activity during phases II and IV (Borland et al., 1999).
The interplay between PEPC and Rubisco during phases II and IV of CAM is both intriguing and central to the correct operation of the pathway. However, of the two carboxylase enzymes that operate in CAM, only the diel regulation of PEPC activity is well understood. PEPC is present at night in a phosphorylated form that is insensitive to inhibition by malate. The enzyme is dephosphorylated in the light, with the decrease in carboxylase activity manifested as an increased sensitivity to malate inhibition (Carter et al., 1996). Equivalent investigations on diurnal Rubisco activity have been confounded by inherent problems of leaf acidity levels and long periods of stomatal closure that preclude conventional measurements of gas exchange or on-line carbon isotope discrimination techniques. Studies of Rubisco have been limited to ontogenetic changes in amount and activity during the development of CAM (Winter et al., 1982) or the response of Rubisco activity to elevated ambient CO2 at a single time point (Israel and Nobel, 1994).
In C3 plants, gas exchange is a good indicator of in vivo Rubisco activity (von Caemmerer and Farquhar, 1981; Woodrow and Berry, 1988), with enzyme activity modulated in response to environmental stimuli (Woodrow and Berry, 1988; Sage et al., 1990). Rubisco activity in C3 and C4 plants is modulated by carbamylation of active sites and, in many cases, by the binding of specific inhibitors to carbamylated sites (Portis, 1992, 1995). Activation of Rubisco sites requires the reversible binding of activator CO2, which is stabilized by the binding of Mg2+ to form an active, ternary complex (Lorimer and Miziorko, 1980; Andrews and Lorimer, 1987). Carbamylation may be inhibited by tight binding of RuBP to inactive sites (Brooks and Portis, 1988). Removal of this ligand and regulation of carbamylation requires the activity of the stromal protein Rubisco activase in a reaction that requires ATP and photosynthetic electron transport and is inhibited by ADP (Campbell and Ogren, 1990a, 1990b, 1992; Portis, 1992, 1995).
Carbamylation is light dependent (von Caemmerer and Edmondson, 1986; Hammond et al., 1998) and generally decreases in the presence of increased partial pressures of CO2 (Perchorowicz and Jensen, 1983; von Caemmerer and Edmondson, 1986; Mate et al., 1993). The presence of tight-binding sugar phosphate inhibitors, including CA1P, may also be significant in the regulation of Rubisco activity (Keys et al., 1995; Parry et al., 1997). CA1P binds tightly to carbamylated sites in low light and at night in a large number of species (Kobza and Seeman, 1989; Holbrook et al., 1994) and removal of this ligand is also facilitated by Rubisco activase (Portis, 1992, 1995; Wang and Portis, 1992; Salvucci and Ogren, 1996; Hammond et al., 1998).
To our knowledge, neither Rubisco activity nor the carbamylation state during the diurnal phases of CAM have been addressed. Whereas significant nocturnal inhibition of Rubisco activity, which is indicative of CA1P binding, has been detected in two obligate CAM species (Vu et al., 1984), it was not evident in a number of C3-CAM intermediates (Servaites et al., 1986). The time at which the measurements were made or whether any attempt was made to buffer leaf sap acidity was not clear in either study.
We have undertaken a study to investigate Rubisco activity during the diurnal phases of CAM. We have attempted to identify the significance of the carbamylation state and diurnal inhibitors to the in vivo regulation of Rubisco under the varying CO2 supply that is inherent to the CAM pathway. We have related these observations to measurements of gas exchange, leaf malate content, electron transport rate, and PEPC activation.
MATERIALS AND METHODS
Plant Material
Kalanchoë daigremontiana Hamet et Perr plants maintained under greenhouse conditions at Moorbank Botanical Gardens were transferred to a custom-made, controlled-environment chamber 1 week prior to experiments. During this time, the plants were watered daily and received a complete nutrient solution. Experiments were performed on the second fully expanded leaves. Incident PPFD was on average 500 μmol photon m−2 s−1 throughout a 12-h photoperiod. Day/night temperature and RH were 27°C/18°C and 45%/65%, respectively.
Gas Exchange and Chlorophyll Fluorescence
Simultaneous gas exchange and chlorophyll fluorescence were measured in situ in the growth chamber over a diel course for five leaves from five replicate plants using a portable infra-red gas analyzer (LI-6400, LI-COR, Lincoln, NE). Incident PPFD was 500 μmol photon m−2 s−1 with leaf temperature and vapor pressure deficit held at chamber conditions. Ambient CO2 was supplied at 380 μbar using a CO2 injector system (LI 6400–01, LI-COR).
The upper leaf chamber was fitted with a PAM-2000 adapter (LI 6400–06, LI-COR), which permitted simultaneous measurements of gas exchange and chlorophyll fluorescence using a PAM-2000 portable fluorimeter (Walz, Effeltrich, Germany). Following pre-dawn determination of the maximum quantum yield of PSII (Fv/Fm), measurements were made at 20-min intervals of the quantum yield of PSII photochemistry (ΦPSII = Fm′ − Fs/Fm′) (Genty et al., 1989) and nonphotochemical quenching (NPQ = Fm − Fm′/Fm′) (Bilger and Björkman, 1990). The apparent linear electron transport rate (ETR) was calculated as:
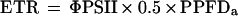 |
where PPFDa is absorbed light, calculated using an integrating sphere (Maxwell et al., 1997), and 0.5 is to correct for the proportion of light absorbed by PSII.
Titratable Acidity
Leaf disc samples for titratable acidity analyses were frozen prior to extraction in 4 mL of boiling water. A 1-mL aliquot was titrated against 10 mm NaOH with phenolphthalein as indicator.
Rubisco Carbamylation State and Activity
Leaf disc samples were snap-frozen in liquid nitrogen at intervals throughout the diurnal course. Carbamylation and activity assays were performed on extracts from the same leaf disc. Rubisco was extracted at 4°C by homogenizing leaf discs (5.3 cm2, approximately 600 mg fresh weight) in 2 mL of FF extraction buffer (350 mm HEPES-KOH, pH 8.0, 10 mm MgCl2, 5 mm EDTA, 14 mm β-mercaptoethanol, 3% [w/v] PVP 25, 15% [w/v] PEG 20,000, and 2.5% [v/v] Tween 20), 20 μL of 100 mm PMSF, and 200 mg of polyvinylpolypyrrolidone, and then centrifuged at 10,000 rpm for 30 s at 4°C. The extraction buffer was the most successful of a number tested, being well-buffered against leaf sap acidity, and did not result in any apparent malate-dependent inhibition of Rubisco activity. All extractions were undertaken at 4°C and were complete within 2 min of sampling. Carbamylation state was calculated by exchanging loosely bound 14CABP at noncarbamylated sites with an excess of 12CABP (Butz and Sharkey, 1989) according to the technique described by Ruuska et al. (1998).
Assays of initial activity were performed at 30°C, with 100 μL of supernatant added to 400 μL of assay buffer (166 mm Bicine-KOH, pH 8.0, 10 mm MgCl2, 5 mm DTT, and 25 mm NaH14CO3 [0.1 Ci/mol]). The reaction was initiated with the addition of RuBP to a final concentration of 0.5 mm and terminated after 1 min with 200 μL of 5 n HCl. Total extractable activity was measured by preincubating the sample for 8 min at 30°C prior to the addition of RuBP as described above, which was determined as the optimal time to consistently obtain maximum activity. The samples were dried overnight and resuspended in 100 μL of 50% (v/v) ethanol. Radioactivity of the sample was calculated using liquid scintillation techniques. Because considerable diurnal variation was observed in the total activity, the activation state of Rubisco was expressed as a percentage of the maximum rate observed during the day (obtained at 4 pm).
A number of checks were made for possible interference from malic acid and other metabolites that fluctuate over the diurnal phases of CAM. First, crude extracts were spiked with 200 mm malate. Second, activity was measured for a mixture composed of a 50-μL extract from both morning and afternoon samples. Finally, activity was measured before and after rapid desalting on Sephadex G25.
Apparent Activation State of PEPC
The extraction and assay of PEPC was modified from the method described by Nimmo et al. (1984). Leaves were homogenized in extraction buffer (200 mm Tris-HCl, pH 8.0, 2 mm EDTA, 1 mm DTT, 1 mm benzamidine, 10 mm malate, 2% [w/v] PEG 20,000, and 179 mm NaHCO3). The homogenate was filtered through three layers of muslin and centrifuged for 2 min at 10,000 rpm. The extract was then desalted into 50 mm Tris-HCl, pH 7.5, 1 mm DTT, 1 mm benzamidine, and 5% glycerol (w/v) using Sephadex G25 columns. All steps were carried out at 4°C and the extraction was completed within 5 min. The activity and apparent activation status of PEPC was determined as the Ki for malate using different malate concentrations in an assay mix containing Tris-HCl, pH 7.8, 5 mm MgCl2, 0.2 mm NADH, 10 mm NaHCO3, and 2 mm PEP. The assay was initiated by the addition of 100 μL of extract, and the change in A340 was monitored for 2 to 4 min at 30°C.
RESULTS
Gas Exchange and Chlorophyll Fluorescence
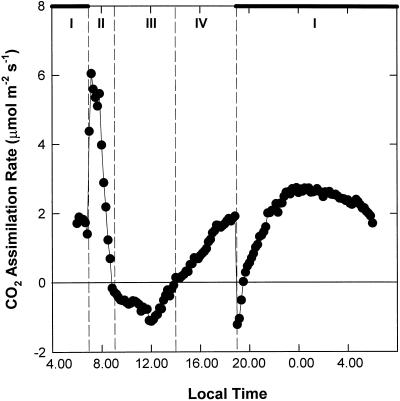
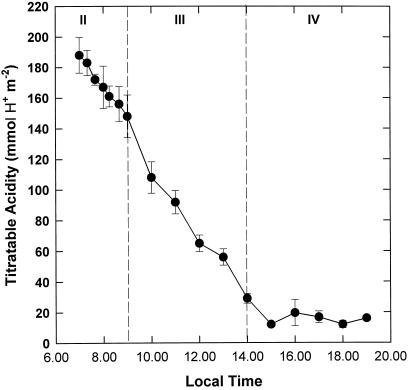
The four phases of CAM activity were characterized according to the observed pattern of diel CO2 assimilation (Fig. 1). Nocturnal CO2 uptake increased gradually over the first 2 h of the dark period to a maximum rate of 1.9 μmol CO2 m−2 s−1 at 11:30 pm during phase I. A pronounced phase II was observed, manifested as a short period of atmospheric CO2 uptake at the start of the photoperiod (7–9 am). Maximum rates of net CO2 uptake were observed during this phase (6.1 μmol CO2 m−2 s−1 at 7:10 am). Phase III was defined by stomatal closure and transition to CO2 release (Fig. 1, 9 am–2 pm). Phase IV marked a second, more prolonged period of atmospheric CO2 uptake from 2 to 7 pm, with a maximum rate of 2.0 μmol CO2 m−2 s−1, which was abruptly terminated at the start of the dark period (Fig. 1). Figure 2 illustrates the levels of titratable acidity in leaves of K. daigremontiana over the diurnal course depicted in Figure 1.
Figure 1.
Diel pattern of net atmospheric CO2 assimilation in leaves of K. daigremontiana over the four phases of CAM. Gas exchange was monitored continuously over a 24-h day/night cycle. Night is represented by the heavy bars. Data are the means of five replicates (se < 10%).
Figure 2.
Diurnal pattern of leaf sap titratable acidity in K. daigremontiana. Samples were taken at intervals over the daytime phases of CAM. The data are the means ± se of five replicates.
The dawn-dusk difference in acidity (ΔH+) was 178 mmol H+ m−2, which is typical for plants under the environmental conditions described. Decarboxylation commenced immediately at the start of the light period and continued throughout phases II and III (Fig. 2). However, two phases of decarboxylation were observed in leaves of K. daigremontiana: an initial slow release of CO2 early in the photoperiod (equivalent to an internal supply of 2.0 μmol CO2 m−2 s−1, assuming a stoichiometry of 2H+:1 malate:1 CO2) and a more accelerated rate of decarboxylation during phase III (3.5 μmol CO2 m−2 s−1). Levels of titratable acidity were low and relatively stable during phase IV (Fig. 2).
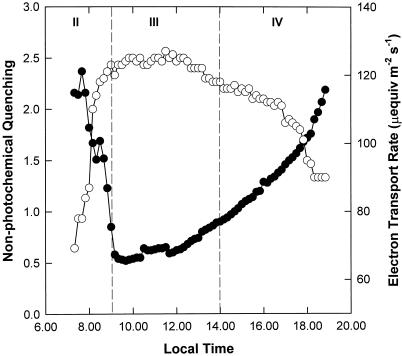
Measurements of diurnal chlorophyll fluorescence (Fig. 3) were made simultaneously with CO2 assimilation described above. The apparent rate of linear electron transport varied throughout the day even though the PPFD was constant. Rates were lowest during the first and last hour of the photoperiod (Fig. 3), coincident with maximum rates of CO2 fixation during phases II and IV, respectively (Fig. 1). From 8 am, the electron transport rate increased rapidly during phase II as CO2 uptake from the atmosphere decreased and decarboxylation of malate continued (Fig. 2). The highest sustained rates of electron transport were observed during phase III (maximum of approximately 127 μeq m−2 s−1) and a slow decline occurred toward the end of phase III and phase IV. Nonphotochemical quenching showed an inverse relationship with ETR, with the lowest levels during decarboxylation and the highest values at the beginning and end of the photoperiod (Fig. 3).
Figure 3.
Diurnal pattern of photosynthetic electron transport and nonphotochemical quenching. Chlorophyll fluorescence was measured throughout the photoperiod, simultaneous with the gas exchange shown in Figure 1. Measurements were made of ETR (○) and nonphotochemical quenching (●). The data are the means of five replicates (se ≤ 10%).
Carbamylation State and Carboxylase Activity
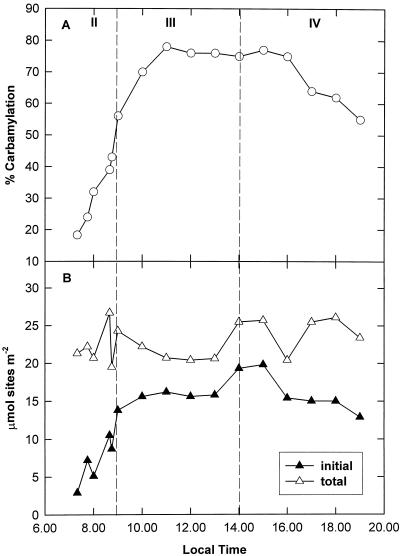
The carbamylation state of Rubisco was low at the start of the day (24% at 7:45 am) and increased rapidly during phase II (Fig. 4A), exhibiting a positive correlation with ETR (r2 =0.934). The maximum carbamylation state was achieved during phase III and was maintained at 70% to 80% throughout the remaining period of decarboxylation (Fig. 4A). At the end of the photoperiod, 55% of the Rubisco sites were carbamylated. The total number of active sites remained relatively constant over the diurnal course and particularly during phase II, when acidity levels in the leaves were highest (Fig. 4B). The initial number of active sites was, however, subject to diurnal regulation, being minimal at the beginning of the photoperiod and then undergoing a significant rise during phase II (Fig. 4B).
Figure 4.
Diurnal pattern of Rubisco carbamylation and the number of active sites in K. daigremontiana leaves. A, Carbamylation state was assessed at intervals over the diurnal course from the same extracts as described for activity in Figure 5. B, The initial (▴) and total (▵) micromolar content of active sites during the day was calculated using CABP binding.
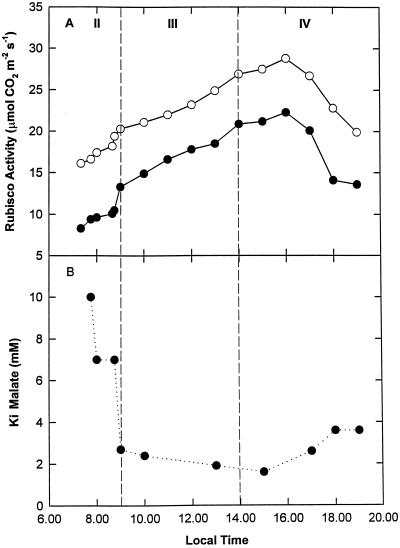
Initial and total Rubisco activity, together with the apparent activation state of PEPC, showed strong diurnal regulation (Fig. 5). The lowest activities of Rubisco occurred during early phase II and late phase IV (Fig. 5A), coincident with the highest apparent activity of PEPC (Fig. 5B). Rubisco activity increased throughout phases II and III, reaching a maximum early in phase IV at the point when atmospheric CO2 recommenced (Figs. 1 and 5A). Initial activity was consistently lower than total activity, with the greatest divergence observed during phases II and IV. The maximum total Rubisco activity occurred at 4 pm (28.8 μmol CO2 m−2 s−1), about 9 h into the photoperiod (Fig. 5A). PEPC showed the greatest sensitivity to malate inhibition (i.e. the lowest apparent activity) during phase III (Fig. 5B).
Figure 5.
Diurnal patterns of carboxylating enzyme activities in leaves of K. daigremontiana. Measurements were made of initial (●) and total (○) Rubisco activity (A) and the apparent activation state of PEPC (B).
To determine whether the levels of leaf sap acidity or malate had a direct inhibitory effect on Rubisco activity, a number of recovery assays were undertaken (Table I). The addition of malate had a negligible effect on total activity, whereas the mixture of morning and afternoon samples yielded a rate that was predicted for this combination. Desalting had no discernible effect on the activities of extracts prepared early or later in the photoperiod (data not shown).
Table I.
Total Rubisco activity following recovery assays
| Extract | Time | Total Activity | Percentage of 3 pm Control |
|---|---|---|---|
| μmol CO2 m−2 s−1 | |||
| Control | 9 am | 20.9 ± 0.23 | 75 |
| 3 pm | 27.2 ± 0.25 | 100 | |
| 200 mm Malate | 3 pm | 27.8 ± 0.28 | 102 |
| Control mix | 9 am + 3 pm | 23.5 ± 0.29 | 86 |
Rubisco total activities were measured for morning (9 am) and afternoon (3 pm) control extracts, or after spiking afternoon samples with 200 mm malate or in a mixture comprising 50% morning and 50% afternoon extract. The data are expressed as the means ± se of total Rubisco activity following an 8-min incubation (n = 5) and as mean percentages of the maximum total activity. Desalting morning and afternoon extracts had no discernible effect on either initial or total activities (data not shown).
DISCUSSION
We investigated diurnal regulation of Rubisco activity during CAM photosynthesis in the obligate CAM species K. daigremontiana under constant light intensity. Major changes in Rubisco activity were not directly attributable to light intensity, suggesting modulation by internal stimuli imposed by the diurnal phases of CAM. The experimental conditions revealed differences in photosynthesis, electron transport, and Rubisco activity, which were attributable to phase-dependent changes in intracellular pCO2. A relatively low but constant PPFD regime allowed chlorophyll fluorescence to be used diagnostically. Exceptionally high PPFD tends to obfuscate those diurnal changes in ETR and nonphotochemical quenching that were central to our observations.
High rates of gas exchange coupled to low Rubisco and high PEPC activities suggest that PEPC-mediated atmospheric CO2 uptake was maintained for at least the first hour of the photoperiod, as confirmed by earlier studies using on-line carbon isotope discrimination (Borland et al., 1993; Roberts et al., 1997). Carbamylation increased during phase II as apparent PEPC activity declined to a minimum, suggesting a tight co-regulation of both carboxylase enzymes, with a reduced likelihood that Rubisco and PEPC compete for CO2 during the early morning. High PEPC activity during the morning, coupled to limited Rubisco activity, would require little energetic input for carbon fixation from photophosphorylation (Winter and Smith, 1996), as evidenced by the low rates of whole-chain electron transport during maximal atmospheric CO2 uptake.
Decarboxylation began during phase II and was supported by increased rates of whole chain electron transport, which is required to supply ATP for the regeneration of PEP and increasing Rubisco activity (Winter and Smith, 1996). Thus, both the decrease in acidity and the increased ETR promoted carbamylation. We are uncertain whether the rise in intracellular pCO2 per se or a factor associated with electron transport represents the dominant regulatory factor. In C3 plants, carbamylation generally decreases in response to elevated CO2 (Perchorowicz and Jensen, 1983; von Caemmerer and Edmondson, 1986), which results in decreased activation levels as a result of RuBP (von Caemmerer and Edmondson, 1986) and/or Pi limitation (Sharkey, 1985). This is a common process of photosynthetic control that balances carboxylation with substrate availability (von Caemmerer and Farquhar, 1981; Sharkey, 1985; Sage et al., 1988, 1990). Chlorenchyma cells of CAM plants are apparently able to increase and maintain a high carbamylation state under high CO2 during phases II and III.
The requirement for electron transport for Rubisco activase activity has been established (Campbell and Ogren, 1990a, 1990b, 1992), and our data support this idea, because an increase in carbamylation occurred concomitant with the increase in ETR observed during phase II. The process that determines Rubisco activase activity in relation to ETR is unknown, but may relate to stromal ATP/ADP or transmembrane ΔpH (Portis, 1992, 1995). The data presented for K. daigremontiana suggest that ΔpH is not directly involved in modulating the increase in carbamylation state during phase II in this species, because the increase in activation occurred as the proton gradient was diminishing (i.e. lower nonphotochemical quenching). The relationship with stromal [ATP] is less clear. Reports indicate that under most conditions ATP/ADP is constant (Dietz and Heber, 1986; Brooks et al., 1988). While it may be predicted that ATP levels will rise during decarboxylation, consumption via gluconeogenesis should equally account for a greater proportion of this product of electron transport. Clearly, the functioning of Rubisco activase during CAM is an area that requires additional investigation.
Although maximal carbamylation had been attained, Rubisco activity continued to rise during phase III independent of ETR, suggesting a role for elevated CO2 in the modulation of Rubisco activity over this period. The increase in carbamylation over phase II and the first part of phase III was atypically slow compared with C3 plants (Portis, 1992, 1995; Salvucci and Ogren, 1996). The slow carbamylation of Rubisco was mirrored by a gradual rise in total activity of Rubisco and may be indicative of very slow removal of a daytime inhibitor (Parry et al., 1997), which again was presumably dependent on Rubisco activase. Strong nocturnal inhibition of Rubisco activity was observed, which implies a possible role for CA1P (Vu et al., 1984; Holbrook et al., 1994). However, other sugar phosphate inhibitors may also be involved (Keys et al., 1995). We are currently investigating both the nature of these putative inhibitory ligands and the relationship with Rubisco activase activity.
Given that the lowest Rubisco activities were found in the morning, when leaf-sap acidity content was highest, we went to considerable lengths to ensure that degradation of Rubisco did not occur as a consequence of acid release during protein extraction. We initially developed an extraction medium with high buffering capacity and then undertook a number of recovery assays. Additional evidence that malic-acid-dependent degradation did not occur was the stability of the total number of active sites, particularly when assayed early in phase II when acidity levels were highest.
Protracted carbamylation ensured that maximal Rubisco activity was observed during early phase IV when atmospheric CO2 uptake recommenced. During this time, internal pCO2 is exceptionally low (approximately 110 μbar) (Maxwell et al., 1997). We therefore postulate that a high Rubisco activity is required to maintain sink strength for CO2 under limiting conditions. Although this strategy will result in high rates of photorespiration (Maxwell et al., 1997, 1998), maintenance of light use minimizes photoinhibitory damage for a considerable period of the day. Equally, carbon fixed during phase IV is largely partitioned for growth. Therefore, maximal carboxylation at this time is advantageous (Borland et al., 1999). Toward the end of the day the Rubisco carbamylation state remained relatively high compared with early phase II as the apparent activation state of PEPC increased. Therefore, there is an increased possibility for futile cycling through C3 and C4 carboxylation during this period, in agreement with earlier observations based on gas exchange and on-line carbon isotope discrimination (Osmond et al., 1996; Borland and Griffiths, 1997).
We have investigated variations in Rubisco activity during the three phases of CAM photosynthesis and found that Rubisco activity is tightly regulated over the diurnal course in K. daigremontiana. Whereas carbamylation increased in line with a rise in internal CO2 during decarboxylation, maximum activity was delayed until later in the photoperiod, when actual atmospheric CO2 uptake occurs under limiting CO2 levels.
ACKNOWLEDGMENTS
We are grateful for the financial support from the Agricultural and Environmental Science Department, which brought Brent Helliker to Newcastle with his original ideas on the regulation of Rubisco. Susanne von Caemmerer maintained our self-belief with her enthusiasm for this work and many helpful discussions. Martin Parry kindly supplied the 14CABP necessary for the carbamylation measurements. We also thank Barry Osmond for his continued support and pioneering spirit.
Footnotes
The Natural Environment Research Council (NERC) provided support to K.M. (small grant no. GR8/03663), R.P.H. (UK studentship no. GT4/95/232), and A.R. (small grant no. GR9/2869). K.M. is in receipt of a Royal Society University Research Fellowship.
LITERATURE CITED
- Andrews TJ, Lorimer GH. Rubisco: structure, mechanisms and prospects for improvement. In: Hatch MD, Boardman NK, editors. Photosynthesis. The Biochemistry of Plants. Vol. 10. New York: Academic Press; 1987. pp. 131–218. [Google Scholar]
- Bilger W, Björkman O. Role of the xanthophyll cycle in photoprotection elucidated by measurements of light-induced absorbance changes, fluorescence and photosynthesis in Hedera canariensis. Photosynth Res. 1990;25:173–185. doi: 10.1007/BF00033159. [DOI] [PubMed] [Google Scholar]
- Borland AM, Griffiths H. Variations in the phases of Crassulacean acid metabolism and regulation of carboxylation patterns determined by carbon-isotope discrimination techniques. In: Winter K, Smith JAC, editors. Crassulacean Acid Metabolism. Biochemistry, Ecophysiology and Evolution. Berlin: Springer-Verlag; 1996. pp. 230–249. [Google Scholar]
- Borland AM, Griffiths H. A comparative study on the regulation of C3 and C4 carboxylation processes in the constitutive Crassulacean acid metabolism (CAM) plant Kalanchoë daigremontiana and the C3-CAM intermediate Clusia minor. Planta. 1997;201:368–378. doi: 10.1007/s004250050079. [DOI] [PubMed] [Google Scholar]
- Borland AM, Griffiths H, Broadmeadow MSJ, Fordham MC, Maxwell C. Short-term changes in carbon-isotope discrimination in the C3-CAM intermediate Clusia minor L. growing in Trinidad. Oecologia. 1993;95:444–453. doi: 10.1007/BF00321001. [DOI] [PubMed] [Google Scholar]
- Borland AM, Maxwell K, Griffiths H. Ecophysiology of the CAM pathway. In: Leegood RC, Sharkey TD, von Caemmerer S, editors. Advances in Photosynthesis: Photosynthesis, Physiology and Metabolism. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1999. (in press) [Google Scholar]
- Brooks A, Portis AR. Protein-bound ribulose-bisphosphate correlates with deactivation of ribulose bisphosphate carboxylase in leaves. Plant Physiol. 1988;87:244–249. doi: 10.1104/pp.87.1.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks A, Portis AR, Sharkey TD. Effects of irradiance and methyl viologen treatment on ATP, ADP, and activation of ribulose bisphosphate carboxylase in spinach leaves. Plant Physiol. 1988;88:850–853. doi: 10.1104/pp.88.3.850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butz ND, Sharkey TD. Activity ratios of ribulose-1,5-bisphosphate carboxylase accurately reflect carbamylation ratios. Plant Physiol. 1989;89:735–739. doi: 10.1104/pp.89.3.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell WJ, Ogren WL. A novel role for light in the activation of ribulose bisphosphate carboxylase/oxygenase. Plant Physiol. 1990a;92:110–115. doi: 10.1104/pp.92.1.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell WJ, Ogren WL. Electron transport through PSI stimulates light activation of ribulose bisphosphate carboxylase/oxygenase (Rubisco) by Rubisco activase. Plant Physiol. 1990b;94:479–484. doi: 10.1104/pp.94.2.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell WJ, Ogren WL. Light activation of Rubisco activase and thylakoid membranes. Plant Cell Physiol. 1992;33:751–756. [Google Scholar]
- Carter PJ, Fewson CA, Nimmo GA, Nimmo HG, Wilkins MB. Roles of circadian rhythms, light and temperature in the regulation of phosphoenolpyruvate carboxylase in Crassulacean acid metabolism. In: Winter K, Smith JAC, editors. Crassulacean Acid Metabolism. Biochemistry, Ecophysiology and Evolution. Berlin: Springer-Verlag; 1996. pp. 46–52. [Google Scholar]
- Cockburn W, Ting IP, Sternberg LO. Relationship between stomatal behaviour and internal carbon dioxide concentration in CAM plants. Plant Physiol. 1979;63:1029–1032. doi: 10.1104/pp.63.6.1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietz K-J, Heber U. Light and CO2 limitation of photosynthesis and states of the reactions of regenerating ribulose 1,5-bisphosphate or reducing 3-phosphoglycerate. Biochim Biophys Acta. 1986;848:392–401. [Google Scholar]
- Genty B, Briantais JM, Baker NR. The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochim Biophys Acta. 1989;990:87–92. [Google Scholar]
- Griffiths H, Broadmeadow MSJ, Borland AM, Hetherington CS. Short-term changes in carbon-isotope discrimination between C3 and C4 carboxylation during Crassulacean acid metabolism. Planta. 1990;181:604–610. doi: 10.1007/BF00193017. [DOI] [PubMed] [Google Scholar]
- Hammond ET, Andrews TJ, Woodrow IE. Regulation of ribulose-1,5-bisphosphate carboxylase/oxygenase by carbamylation and 2-carboxyarabinitol 1-phosphate in tobacco: insights from studies of antisense plants containing reduced amounts of Rubisco activase. Plant Physiol. 1998;118:1463–1471. doi: 10.1104/pp.118.4.1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holbrook GP, Campbell WJ, Rowland-Bamford A, Bowes G. Intraspecific variation in the light/dark modulation of ribulose 1,5-bisphosphate carboxylase-oxygenase activity in soybean. J Exp Bot. 1994;45:1119–1126. [Google Scholar]
- Israel AA, Nobel PS. Photosynthetic activities of carboxylating enzymes in the CAM species Opuntia ficus-indica grown under current and elevated CO2 concentrations. Photosynth Res. 1994;40:223–229. doi: 10.1007/BF00034772. [DOI] [PubMed] [Google Scholar]
- Keys AJ, Major I, Parry MAJ. Is there another player in the game of Rubisco regulation? J Exp Bot. 1995;46:1245–1251. [Google Scholar]
- Kobza J, Seeman JR. Light-dependent kinetics of 2-carboxyarabinitol 1-phosphate metabolism and ribulose-1,5-bisphosphate carboxylase activity in vivo. Plant Physiol. 1989;89:174–179. doi: 10.1104/pp.89.1.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorimer G, Miziorko H. Carbamate formation on the ε-amino group of a lysyl residue as the basis for the activation of ribulose bisphosphate carboxylase by CO2 and Mg2+ Biochemistry. 1980;19:5321–5328. doi: 10.1021/bi00564a027. [DOI] [PubMed] [Google Scholar]
- Mate CJ, Hudson GS, von Caemmerer S, Evans JR, Andrews TJ. Reduction of ribulose bisphosphate carboxylase activase levels in tobacco (Nicotiana tabacum) by antisense RNA reduces ribulose bisphosphate carboxylase carbamylation and impairs photosynthesis. Plant Physiol. 1993;102:1119–1128. doi: 10.1104/pp.102.4.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxwell K, Badger MR, Osmond CB. A comparison of CO2 and O2 exchange patterns and the relationship with chlorophyll fluorescence during photosynthesis in C3 and CAM plants. Aust J Plant Physiol. 1998;25:45–52. [Google Scholar]
- Maxwell K, von Caemmerer S, Evans JR. Is a low conductance to CO2 diffusion a consequence of succulence in plants with Crassulacean acid metabolism? Aust J Plant Physiol. 1997;24:777–786. [Google Scholar]
- Nimmo GA, Nimmo HG, Fewson CA, Wilkins MB. Diurnal changes in the properties of phosphoenolpyruvate carboxylase in Bryophyllum leaves: a possible covalent modification. FEBS Lett. 1984;178:199–203. [Google Scholar]
- Osmond CB. Crassulacean acid metabolism: a curiosity in context. Annu Rev Plant Physiol. 1978;29:379–414. [Google Scholar]
- Osmond B, Maxwell K, Popp M, Robinson S. On being thick: fathoming apparently futile pathways of photosynthesis and carbohydrate metabolism in succulent CAM plants. In: Bryant JA, Burrell MM, Kruger NJ, editors. Plant Carbohydrate Metabolism. Oxford: Bios Scientific Publishers; 1999. pp. 183–200. [Google Scholar]
- Osmond CB, Popp M, Robinson SA. Stoichiometric nightmares: studies in photosynthetic O2 and CO2 exchanges in CAM plants. In: Winter K, Smith JAC, editors. Crassulacean Acid Metabolism. Biochemistry, Ecophysiology and Evolution. Berlin: Springer-Verlag; 1996. pp. 46–52. [Google Scholar]
- Parry MAJ, Andralojc PJ, Parmar S, Keys AJ, Habash D, Paul MJ, Alred R, Quick WP, Servaites JC. Regulation of Rubisco by inhibitors in the light. Plant Cell Environ. 1997;20:528–534. [Google Scholar]
- Perchorowicz JT, Jensen R. Photosynthesis and activation of ribulose bisphosphate carboxylase in wheat seedlings. Plant Physiol. 1983;71:955–960. doi: 10.1104/pp.71.4.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portis AR. Regulation of ribulose 1,5-bisphosphate carboxylase/oxygenase activity. Annu Rev Plant Physiol Plant Mol Biol. 1992;43:415–437. [Google Scholar]
- Portis AR. The regulation of Rubisco by Rubisco activase. J Exp Bot. 1995;46:1281–1291. doi: 10.1093/jxb/erm240. [DOI] [PubMed] [Google Scholar]
- Roberts A, Borland AM, Griffiths H. Discrimination processes and shifts in carboxylation during the phases of Crassulacean acid metabolism. Plant Physiol. 1997;113:1283–1291. doi: 10.1104/pp.113.4.1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruuska SA, Andrews TJ, Badger MR, Hudson GS, Laisk A, Price GD, von Caemmerer S. The interplay between limiting processes in C3 photosynthesis studied by rapid response gas exchange using transgenic tobacco impaired in photosynthesis. Aust J Plant Physiol. 1998;25:859–870. [Google Scholar]
- Sage RF, Sharkey TD, Seemann JR. The in-vivo response of the ribulose 1,5-bisphosphate carboxylase activation state and the pool sizes of photosynthetic intermediates and elevated CO2 in Phaseolus vulgaris L. Planta. 1988;174:407–416. doi: 10.1007/BF00959528. [DOI] [PubMed] [Google Scholar]
- Sage RF, Sharkey TD, Seemann JR. Regulation of ribulose 1,5-bisphosphate carboxylase activity in response to light intensity and CO2 in the C3 annuals Chenopodium album L. and Phaseolus vulgaris L. Plant Physiol. 1990;94:1735–1742. doi: 10.1104/pp.94.4.1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvucci ME, Ogren WL. The mechanism of Rubisco activase: insights from studies of the properties and structure of the enzyme. Photosynth Res. 1996;47:1–11. doi: 10.1007/BF00017748. [DOI] [PubMed] [Google Scholar]
- Servaites JC, Parry MAJ, Gutteridge S, Keys AJ. Species variation in the predawn inhibition of ribulose 1,5-bisphosphate carboxylase oxygenase. Plant Physiol. 1986;82:1161–1163. doi: 10.1104/pp.82.4.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharkey TD. Photosynthesis in intact leaves of C3 plants: physics, physiology and rate limitations. Bot Rev. 1985;51:53–105. [Google Scholar]
- Spalding MD, Stumpf DK, Ku MSB, Burris RH, Edwards GE. Crassulacean acid metabolism and diurnal variations of internal CO2 and O2 concentrations in Sedum praealtum DC. Aust J Plant Physiol. 1979;6:557–567. [Google Scholar]
- von Caemmerer S, Edmondson DL. Relationship between steady-state gas exchange, in vivo ribulose bisphosphate carboxylase activity and some carbon reduction cycle intermediates in Raphanus sativus. Aust J Plant Physiol. 1986;13:669–688. [Google Scholar]
- von Caemmerer S, Farquhar GD. Some relationships between the biochemistry of photosynthesis and the gas exchange of leaves. Planta. 1981;89:376–387. doi: 10.1007/BF00384257. [DOI] [PubMed] [Google Scholar]
- Vu JCV, Allen LH, Bowes G. Light modulation of ribulose bisphosphate carboxylase activity in plants from different photosynthetic categories. Plant Physiol. 1984;76:843–845. doi: 10.1104/pp.76.3.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang ZY, Portis AR. Dissociation of ribulose-1,5-bisphosphate bound to ribulose-1,5-bisphosphate carboxylase/oxygenase and it's enhancement by ribulose-1,5-bisphosphate carboxylase/oxygenase activase-mediated hydrolysis of ATP. Plant Physiol. 1992;99:1348–1353. doi: 10.1104/pp.99.4.1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter K, Foster JG, Schmitt MR, Edwards GE. Activity and quantity of ribulose bisphosphate carboxylase- and phosphoenolpyruvate carboxylase-protein in two Crassulacean acid metabolism plants in relation to leaf age, nitrogen nutrition and point in time during a day/night cycle. Planta. 1982;154:309–317. doi: 10.1007/BF00393908. [DOI] [PubMed] [Google Scholar]
- Winter K, Smith JAC. Crassulacean acid metabolism: current status and perspectives. In: Winter K, Smith JAC, editors. Crassulacean Acid Metabolism. Biochemistry, Ecophysiology and Evolution. Berlin: Springer-Verlag; 1996. pp. 389–426. [Google Scholar]
- Woodrow IE, Berry JA. Enzymatic regulation of photosynthetic CO2 fixation in C3 plants. Annu Rev Plant Physiol Plant Mol Biol. 1988;39:533–594. [Google Scholar]