Abstract
Hepatosplenic Gamma Delta T cell lymphoma (γδHSTL) is a rare, highly aggressive, and rapidly lethal T cell lymphoma which manifests 18F-FDG PET/CT findings that can mimic benign conditions. Patients with γδHSTL present with unexplained symptoms of a hematologic malignancy like the B symptoms of lymphoma including weight loss, fevers, and night sweats, as well as, splenomegaly and hepatomegaly. Thrombocytopenia, anemia, or neutropenia are also common due to spleen, liver and bone marrow involvement. The peripheral blood, however, typically does not show abnormal T cells. The clinical and 18F-FDG PET/CT findings are presented for 3 patients with γδHSTL. Patients with γδHSTL may have a normal 18F-FDG PET/CT or an 18F-FDG PET/CT with any combination of the three findings: splenomegaly with intense FDG uptake; hepatomegaly with increased FDG uptake; and diffuse, increased FDG uptake in the bone marrow. Importantly, lymphadenopathy is usually absent, and most patients show morphologically normal lymph nodes with normal FDG uptake. Due to the aggressive nature of the disease, γδHSTL is a critical diagnosis to consider in patients who present with clinical signs of suspected hematologic malignancy and variable 18F-FDG PET/CT findings. The absence of lymphadenopathy and normal FDG uptake in lymph nodes are typical pertinent negative findings that differentiate γδHSTL from other lymphomas. A bone or liver biopsy is frequently necessary to establish the diagnosis and should be recommended.
Keywords: Hepatosplenic Gamma-Delta T-cell lymphoma, 18F-FDG PET/CT, non-Hodgkin lymphoma, pancytopenia, hepatosplenomegaly, hematology and oncology, anemia, myelodysplastic syndrome, chemotherapy, infection
Introduction
Hepatosplenic Gamma-Delta T-Cell Lymphoma (γδHSTL) is a very rare, highly aggressive, and rapidly lethal T-cell lymphoma [1-4]. In a multicenter study performed in 2008, γδHSTL accounted for only 1.4% of the total number of peripheral T-Cell and natural killer/T-cell Lymphomas, which are themselves rare forms of non-Hodgkin’s Lymphoma [5]. γδHSTL is largely therapy resistant although some studies demonstrate improved mortality rates with intense induction chemotherapy and allogenic bone marrow transplantation [6,7]. Studies demonstrate a devastating overall 5-year survival rate of 7% and a median survival time of 16 months [5,8].
γδHSTL typically occurs in younger men (68%) with a median age of onset of 34 years [5,9-12]. Patients may commonly present with B symptoms of lymphoma including fever, weight loss, and night sweats as well as fatigue, abdominal pain, and sometimes jaundice [9,10]. Physical exam findings include splenomegaly and hepatomegaly in 50% of patients [9]. Lymphadenopathy is typically absent on exam [10]. Laboratory findings include pancytopenia as well as elevated alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase, and lactate dehydrogenase [10]. Twenty percent of patients with γδHSTL have a history of chronic immune suppression related to solid organ transplant and autoimmune disorders [12]. A few reported cases suggest an association with Epstein-Barr virus [14]. 18F-FDG PET/CT findings may be non-specific and variable. Characteristic findings include splenomegaly with increased FDG uptake, hepatomegaly with increased FDG uptake, and diffusely increased FDG uptake in the bone marrow.
The purpose of this study is to review the clinical presentation and imaging findings of γδHSTL, a rare and aggressive lymphoma that can mimic benign conditions on imaging and potentially delay diagnosis.
Case 1
A 59-year-old man with no past medical history presented with light stools, dark urine, jaundice, and abdominal pain. Computed tomography (CT) of the abdomen, right upper quadrant ultrasound (US), and HIDA scan were reported to be unremarkable, and the patient was discharged home.
A month later, the patient presented with persistent jaundice, 20-pound weight loss, new abdominal swelling, and edema. The patient was pancytopenic (WBC 2.2 K, hemoglobin 10.2 K, platelet count 31 K) and had transaminitis (ALT 165; AST 190), hyperbilirubinemia (total bilirubin 10.8), and elevated alkaline phosphatase (130). Ultrasound demonstrated splenomegaly and ascites. 18F-FDG PET/CT demonstrated splenomegaly with normal FDG activity of the liver (SUVmax 2.8), spleen (SUVmax 2.1), and lymph nodes (Figure 1).
Figure 1.
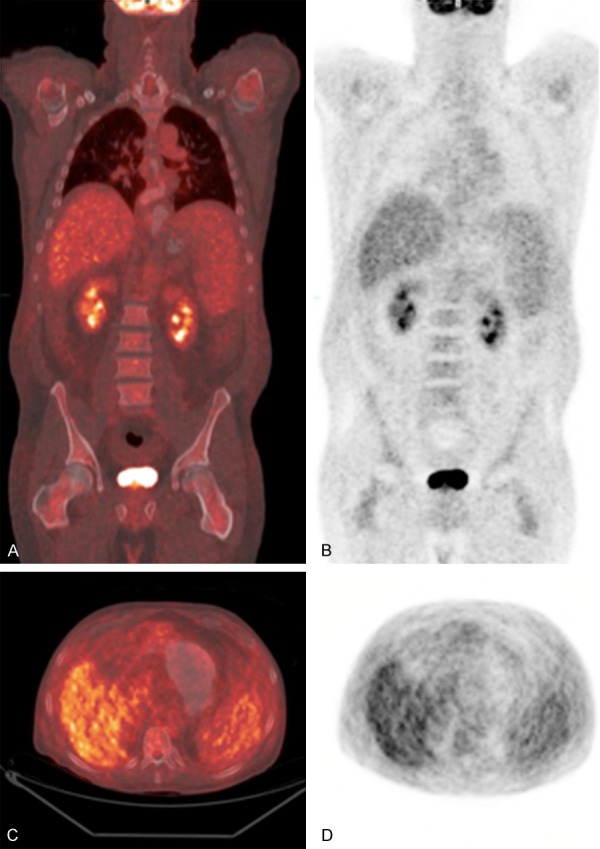
59-year-old man with γδHSTL at initial presentation. Coronal 18F-FDG PET/CT fusion (A) and 18F-FDG PET (B) demonstrating splenomegaly with normal FDG activity in the liver, spleen, and bone marrow. Axial 18F-FDG PET/CT fusion (C) and 18F-FDG PET (D) demonstrating splenomegaly and normal FDG activity in the liver, spleen, and bone marrow.
Transjugular liver biopsy demonstrated extensive T-cells with positive CD3, CD20, CD7, and T1A1 expression and negative CD5 expression, compatible with hepatosplenic T-Cell Lymphoma. Bone marrow biopsy were consistent with liver biopsy results and subsequent flow cytometry showed that 12% of T-cells expressed TCR Gamma/Delta chains.
Despite two regimens of chemotherapy (Cyclophosphamide and Prednisone; ASHAP [Adriamycin, Solumedrol, High-dose Ara-C, Platinum]), the disease progressed, and the patient died two months after diagnosis.
Case 2
A 20-year-old man with a history of G-6-PD deficiency and sickle cell trait, presented with fatigue, malaise, decreased appetite, palpitations and elevated blood pressure (BP 170/100). On exam, the patient was hypertensive (193/116) and had splenomegaly and scattered petechiae. Laboratory data demonstrated leukopenia (white blood cell count 3.1 K), thrombocytopenia (platelet count 28 K), and transaminitis (AST 83; ALT 100). CT of the abdomen and pelvis demonstrated splenomegaly. The patient was followed for four weeks for suspected infectious mononucleosis and chronic hemolysis due to G-6-PD deficiency.
A transjugular liver biopsy was performed for worsening transaminitis and demonstrated T-cell lymphoma, suspicious for hepatosplenic T-cell lymphoma (CD3+, CD2+, CD7+, CD5-, T1A1 unsatisfactory). Subsequent bone marrow biopsy demonstrated mature T-cell lymphoma with flow cytometry showing abnormal T-cell population with TCR of gamma/delta subtype, compatible with γδHSTL.
18F-FDG PET/CT was performed at baseline and on two subsequent follow up visits (Figure 2). The initial 18F-FDG PET/CT showed an enlarged spleen with diffuse and focally increased FDG activity (SUVmax 3.6) as well as diffusely increased FDG activity in the bone marrow (SUVmax 3.8). The liver (SUVmax 2.2) and lymph nodes were normal.
Figure 2.
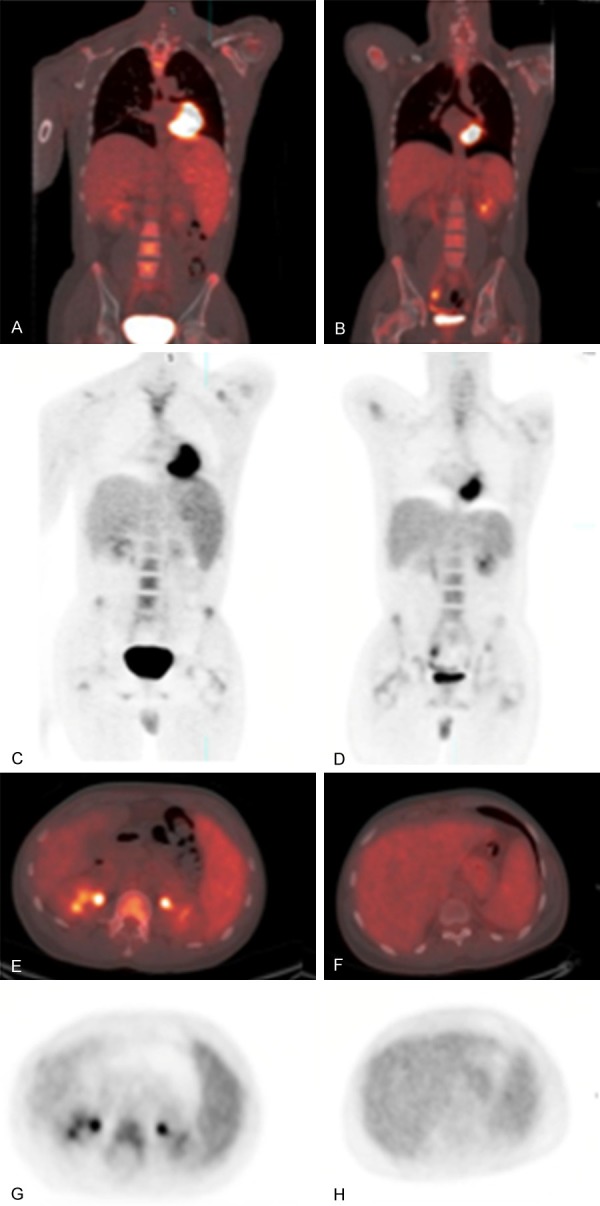
20-year-old man with γδHSTL. Initial presentation (coronal 18F-FDG PET/CT fusion (A) and 18F-FDG PET (C); axial 18F-FDG PET/CT fusion (E) and 18F-FDG PET (G)) and follow up (coronal 18F-FDG PET/CT fusion (B) and 18F-FDG PET (D); axial 18F-FDG PET/CT fusion (F) and 18F-FDG PET (H)). Note improving splenomegaly and lower FDG uptake in spleen after treatment, correlating with temporary clinical improvement.
After chemotherapy (EPOCH [Etoposide, Prednisone, Oncovin, Cyclophosphamide, Hydroxydaunorubicin] and DHAP [Dexamethasone, High dose cytarabine, cisplatin]), the patient was clinically in remission. Subsequent 18F-FDG PET/CT demonstrated decreased FDG activity in the spleen (SUVmax 2.9) and diffuse bone marrow activity (SUVmax 3.3).
Case 3
A 70-year-old man with past medical history of diabetes mellitus type II and hypertension presented with a one week history of progressive fatigue, fever, hypotension, imbalance, and weight loss. Physical exam demonstrated splenomegaly and initial laboratory work up showed pancytopenia (white blood cell count 2.4 K, hemoglobin 11.3 K, platelet count 51 K). CT of the chest, abdomen, and pelvis showed splenomegaly.
Bone marrow biopsy showed atypical intermediate to large T-cells with positive CD3 and CD7 expression constituting 20-30% of total cellularity. Phenotypically abnormal gamma-delta T-cells constituted 14% of the cell population. CD4, CD5, CD8, and CD20 expression were predominantly negative.
18F-FDG PET/CT was performed at baseline and showed a markedly enlarged spleen with diffusely increased 18F-FDG uptake (SUVmax 9.0) and diffuse 18F-FDG uptake in the bone marrow (Figure 3).
Figure 3.
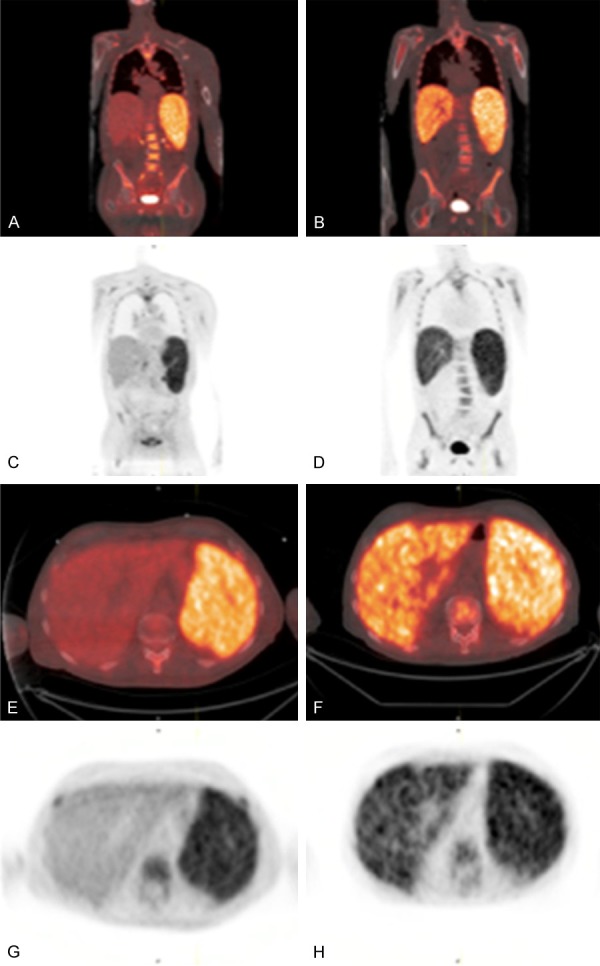
70-year-old man with γδHSTL. Initial presentation with splenomegaly and diffuse, increased FDG activity in the spleen and bone marrow (coronal 18F-FDG PET/CT fusion (A) and 18F-FDG PET (C); axial 18F-FDG PET/CT fusion (E) and 18F-FDG PET (G)). Follow up with newly developed diffuse, increased FDG activity in the liver, with persistent increased spleen and bone marrow FDG uptake (coronal 18F-FDG PET/CT fusion (B) and 18F-FDG PET (D); axial 18F-FDG PET/CT fusion (F) and 18F-FDG PET (H)).
After 1 month of EPOCH chemotherapy, the patient experienced worsening pancytopenia, fever and hypotension, concerning for disease progression. Subsequent 18F-FDG PET/CT showed an interval increase in size of the spleen without change in 18F-FDG uptake (SUVmax 9.0) and diffuse increase in 18F-FDG uptake in the liver (SUVmax 5.4 compared to SUVmax 3.1 previously), demonstrating an imaging correlate to clinically rapidly progressing disease (Figure 3). There was diffuse 18F-FDG uptake in the bone marrow and again no evidence of hypermetabolic lymphadenopathy. Persistent disease progression was noted despite a cycle of R-ESHAP chemotherapy (Rituximab, Etoposide, Solumedrol, high dose Cytarabine, Platinum). The patient was referred to palliative care and home hospice care.
Discussion
Myeloproliferative Disorder constitutes a group of clonal hematopoietic stem cell disorders that lead to increased bone marrow production of cells belonging to the granulocytic, erythroid, and megakaryocytic lineage [15,16]. 18F-FDG PET/CT may demonstrate diffusely increased bone marrow uptake, splenomegaly with increased FDG uptake, and hepatomegaly with increased FDG uptake [17].
Granulocyte-macrophage colony-stimulating factor (GM-CSF) is a bone marrow stimulant administered to patients treated with cytotoxic chemotherapy. Multiple studies have shown that GM-CSF increases glucose metabolism in macrophages and can lead to diffusely increased FDG uptake in the bone marrow and spleen [18].
Multiple nonspecific infectious and inflammatory processes have shown increased FDG uptake in the body. While not entirely known, some believe that the increased FDG uptake in the bone marrow in the setting of fevers, bacteremia, or viral infections may be due to interleukin dependent upregulation of glucose transporters [19].
To our knowledge, the only prior report of γδHSTL imaging with FDG PET/CT showed diffuse increased activity in the spleen, liver and bone marrow [20]. Our cases, however, show a variable pattern of FDG uptake at initial diagnosis and after chemotherapy. Our 3 cases also illustrate the diagnostic challenges posed by γδHSTL, due to the variability in presentation that makes it a difficult disease to categorize amongst the better understood lymphomas [21]. Firstly, our patients represent a wide range in age (20-70 years) although literature suggests that this disease process typically affects younger men. Secondly, the three patients present with varied and vague symptoms, some of which do not classically correlate with that of T-cell lymphoma. Thirdly, the 18F-FDG PET/CT findings are also varied and only one of the three patients demonstrated the classic imaging findings of γδHSTL.
In case 1, imaging findings demonstrated only splenomegaly and otherwise normal FDG activity, mimicking a pattern of benign disease processes. Despite the normal FDG PET findings, the patient rapidly progressed despite chemotherapy and expired within 2 months of diagnosis. In case 2, the initial splenomegaly and increased FDG activity in the spleen responded with a decrease in size and FDG uptake that correlated with clinical response to chemotherapy. In case 3, the splenomegaly and increased FDG activity in the spleen persisted after therapy and the interval development of abnormal increased FDG uptake in the liver correlated with clinical progression.
Conclusion
In summary, the clinical presentation and imaging findings in these 3 cases were not specific. However, γδHSTL is a critical diagnosis to consider in patients who present with clinical signs of suspected hematologic malignancy and variable 18F-FDG PET/CT findings including the more characteristically described pattern of splenomegaly and increased FDG uptake in the liver, spleen, and bone marrow. Rapid diagnosis after liver or bone marrow biopsy may influence treatment. Improvement or worsening in FDG PET/CT in the liver or spleen could potentially aid in assessing response to therapy.
Disclosure of conflict of interest
None.
References
- 1.Yabe M, Medeiros L, Tang G, Wang S, Ahmed S, Nieto Y, Hu S, Bhagat G, Oki Y, Patel K, Routbort M, Luthra R, Fanale M, Bueso-Ramos C, Jorgensen J, Vega F, Chen W, Hoehn D, Konoplev S, Milton D, Wistuba I, Li S, You M, Young K, Miranda R. Prognostic factors of hepatosplenic T-cell lymphoma. Am J Surg Pathol. 2016;40:676–688. doi: 10.1097/PAS.0000000000000614. [DOI] [PubMed] [Google Scholar]
- 2.Cingam S, Patel S, Koshy N. A case of hepatosplenic t cell lymphoma - a rare, agrressive tumor of the young. J La State Med Soc. 2017;169:49–50. [PubMed] [Google Scholar]
- 3.Raza A, Ewton A. Hepatosplenic t-cell lymphoma. Blood. 2014;124:677. doi: 10.1182/blood-2014-05-575449. [DOI] [PubMed] [Google Scholar]
- 4.Calvaruso M, Gulino A, Buffa S, Guarnotta C, Franco G, Cacciatore M, Bonura M, Franco V, Florena A. Challenges and new prospects in hepatosplenic γδ T-cell lymphoma. Leuk Lymphoma. 2014;55:2457–2465. doi: 10.3109/10428194.2014.889821. [DOI] [PubMed] [Google Scholar]
- 5.Vose J, Armitage J, Weisenburger D International T-Cell Lymphoma Project. International peripheral t-cell and natural killer/t-cell lymphoma study: pathology findings and clinical outcomes. J. Clin. Oncol. 2008;26:4124–4130. doi: 10.1200/JCO.2008.16.4558. [DOI] [PubMed] [Google Scholar]
- 6.Ashmore P, Patel M, Vaughan J, Wiggill T, Willem P, van den Berg E, Philip V, Lakha A. Hepatosplenic t-cell lymphoma: a case series. Hematol Oncol Stem Cell Ther. 2015;8:78–84. doi: 10.1016/j.hemonc.2014.09.006. [DOI] [PubMed] [Google Scholar]
- 7.Saste A, Arias-Stella J, Kuriakose P. Progression of a hepatosplenic gamma delta T-cell leukemia/lymphoma on hyperCVAD/MTX and ara-C: literature review and our institutional treatment approach. Clin Case Rep. 2015;4:67–71. doi: 10.1002/ccr3.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Otero H, Jagannathan J, Prevedello L, Johnston C, Ramaiya N, Van den Abbeele A, DiPiro P. CT and pet/ct findings of t-cell lymphoma. AJR Am J Roentgenol. 2009;193:349–358. doi: 10.2214/AJR.08.1398. [DOI] [PubMed] [Google Scholar]
- 9.Visnyei K, Grossbard M, Shapira I. Hepatosplenic γδ t-cell lymphoma: an overview. Clin Lymphoma Myeloma Leuk. 2013;13:360–369. doi: 10.1016/j.clml.2013.03.011. [DOI] [PubMed] [Google Scholar]
- 10.Shi Y, Wang E. Hepatosplenic t-cell lymphoma: a clinicopathologic review with an emphasis on diagnostic differentiation from other t-cell/natural killer-cell neoplasms. Arch Pathol Lab Med. 2015;139:1173–1180. doi: 10.5858/arpa.2014-0079-RS. [DOI] [PubMed] [Google Scholar]
- 11.Ferreri A, Govi S, Pileri S. Hepatosplenic gamma-delta T-cell lymphoma. Crit Rev Oncol Hematol. 2012;83:283–292. doi: 10.1016/j.critrevonc.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 12.Foppoli M, Ferreri A. Gamma-delta t-cell lymphomas. Eur J Haematol. 2014;94:206–218. doi: 10.1111/ejh.12439. [DOI] [PubMed] [Google Scholar]
- 13.Belhadj K, Reyes F, Farcet JP, Tilly H, Bastard C, Angonin R, Deconinck E, Charlotte F, Leblond V, Labouyrie E, Lederlin P, Emile JF, Delmas-Marsalet B, Arnulf B, Zafrani ES, Gaulard P. Hepatosplenic γδ T-cell lymphoma is a rare clinicopathologic entity with poor outcome: report on a series of 21 patients. Blood. 2003;102:4261–4269. doi: 10.1182/blood-2003-05-1675. [DOI] [PubMed] [Google Scholar]
- 14.Tsunematsu S, Natsuizaka M, Fujita H, Otsuka N, Terashita K, Sato F, Kobayashi T, Nakai M, Tsukuda Y, Horimoto H, Sho T, Suda G, Nakanishi M, Hashino S, Chuma M, Sakamoto N. Hepatosplenic gamma-delta t-cell lymphoma associated with Epstein-barr virus. Intern Med. 2014;53:2079–2082. doi: 10.2169/internalmedicine.53.2236. [DOI] [PubMed] [Google Scholar]
- 15.Agool A, Glaudemans A, Boersma H, Dierckx R, Vellenga E, Slart R. Radionuclide imaging of bone marrow disorders. Eur J Nucl Med Mol Imaging. 2010;38:166–178. doi: 10.1007/s00259-010-1531-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Inoue K, Okada K, Harigae H, Taki Y, Goto R, Kinomura S, Kato S, Kaneta T, Fukuda H. Diffuse bone marrow uptake on F-18 FDG PET in patients with myelodysplastic syndromes. Clin Nucl Med. 2006;31:721–723. doi: 10.1097/01.rlu.0000242685.55001.67. [DOI] [PubMed] [Google Scholar]
- 17.Saboo SS, Krajewski KM, O’Regan KN, Giardino A, Brown JR, Ramaiya N, Jagannathan JP. Spleen in haematological malignancies: spectrum of imaging findings. Br J Radiol. 2012;85:81–92. doi: 10.1259/bjr/31542964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Basu S, Baghel N. Intense FDG uptake in the spleen due to recent granulocyte-macrophage colony-stimulating factor administration: follow-up scan clarifying the situation. J Cancer Res Ther. 2011;7:228. doi: 10.4103/0973-1482.82933. [DOI] [PubMed] [Google Scholar]
- 19.Vos FJ, Bleeker-Robers CP, Delsing CE, Kullberg BJ, Oyen WJ. Bone-marrow uptake of 18F-FDG during fever. Lancet Infect Dis. 2010;10:509–510. doi: 10.1016/S1473-3099(10)70137-7. [DOI] [PubMed] [Google Scholar]
- 20.Quak E, Salaun V, Le Naoures C, Fruchart C. 18f-fdg pet/ct in hepatosplenic gamma-delta t-cell lymphoma. Clin Nucl Med. 2015;40:730–731. doi: 10.1097/RLU.0000000000000876. [DOI] [PubMed] [Google Scholar]
- 21.Sindhu H, Chen R, Chen H, Wong J, Chaudhry R, Xu Y, Wang J. Gamma-delta (γδ) T-cell lymphoma - another case unclassifiable by World Health Organization classification: a case report. J Med Case Rep. 2017;11:163. doi: 10.1186/s13256-017-1312-5. [DOI] [PMC free article] [PubMed] [Google Scholar]


