Abstract
The cerebrospinal fluid (CSF) offers a window into the workings of the brain and blood-brain barrier (BBB). Molecules that enter into the central nervous system (CNS) by passive diffusion or receptor-mediated transport through the choroid plexus often appear in the CSF prior to acting within the brain. Other molecules enter the CNS by passing through the BBB into the brain’s interstitial fluid prior to appearing in the CSF. This pattern is also often observed for molecules synthesized by neurons or glia within the CNS. The CSF is therefore an important conduit for the entry and clearance of molecules into/from the CNS and thereby constitutes an important window onto brain activity and barrier function. Assessing the CSF basally, under experimental conditions, or in the context of challenges or metabolic diseases can provide powerful insights about brain function. Here, we review important findings made by our labs, as influenced by the late Randall Sakai, by interrogating the CSF.
Keywords: Cerebrospinal fluid, Blood-brain barrier, Food intake, Behavioral endocrinology, Satiation signals, Insulin
1. History
It is a great honor to be asked to contribute to this series of articles commemorating the career of Randall Sakai. While each of us knew and admired Randall in many ways, one of us (SCW) knew him particularly well and collaborated with him and his students and fellows for many years in Cincinnati. In fact, their careers followed a parallel course. Both Randall and SCW received Bachelor’s degrees in zoology at the University of Washington (UW), albeit several years apart, and both began their research career in Washington’s Department of Psychology, each exploring aspects of salt and water balance and associated behaviors. Richard Weisinger and SCW, co-graduate students at UW in the late 1960s, investigated causal mechanisms of sodium appetite in rats [1,2]. In a parallel manner, and several years later after SCW had joined the faculty at UW, Randall worked in the lab of John Simpson while an undergraduate, also investigating mechanisms of sodium appetite [3] before moving to the University of Pennsylvania to conduct his doctoral work with Alan Epstein and continuing to investigate salt and water balance and producing a wealth of important papers on the topic.
The parallel interests continued in that early on, both Randall [3] and SCW [4–7] each recognized the importance of interrogating the cerebrospinal fluid (CSF) to understand brain mechanisms underlying ingestive behavior, and similarly, that both understood the value of administering biologically active compounds directly into the CSF and assessing behavior [8–11]. This research strategy continued throughout both of their research careers.
Randall completed a post-doc with Bruce McEwen at the Rockefeller University where he conducted his seminal work on corticoid receptors and stress behavior, and where he began his work on social stressors utilizing the visible burrow system. When Alan Epstein died, Randall joined the faculty at the University of Pennsylvania and we later recruited him to Cincinnati where he further expanded his work on stress and social hierarchies. With his graduate students and post-doctoral fellows, he also branched out into several other fields including a return to the basics of salt and water balance and behavior. In recent years, SCW and Randall in fact each collaborated with their trainees in this area [12–15], including accessing the brain via the CSF [16].
Based on the parallel histories, and the joint emphasis upon the CSF and ingestive behavior, we thought it appropriate to commemorate Randall by briefly reviewing where our latest efforts in utilizing the CSF to understand ingestive behavior have progressed. While several techniques have been used historically to obtain clean (i.e., free of blood cells) CSF samples from small rodents, we recently improved and then standardized the procedure for rats [17] and then modified it for mice [18]. Several features of the technique are noteworthy. First, the animal need not be sacrificed, and can continue to participate in experiments and even have the CSF resampled after an appropriate interval. Second, we devised a method to be absolutely certain that there is no blood contamination; i.e., we are able to assess the CSF sample for the presence of apolipoprotein B, a plasma protein that is never found in the CSF physiologically [17]. More recently, we developed a more rapid method using a microplate to precisely determine the level of blood contamination in CSF samples spectrophotometrically in <10 min using only a 2-μL volume of CSF [19]. This approach uses a blood standard curve to assess the absorbance resulting from hemoglobin contamination, which is not detectable in pure CSF samples from healthy rodents [19], and is much faster than traditional hemocytometric counting and which has greater precision. Samples with >0.001% blood contamination are excluded from data analysis. Typically, >95% of samples have no detectable contamination once the technique is mastered. Finally, and perhaps most importantly, the collection technique is easy to use and provides ample CSF for most purposes within a few minutes.
2. Insulin and the CSF
Our lab has a long history of investigating insulin and the brain using the CSF as a convenient interface. In an early series of experiments using dogs we found that when plasma insulin is experimentally increased, CSF insulin also increases after a short lag; but perhaps more importantly, we also observed that insulin was present in the CSF at all times, even in fasting conditions when basal plasma insulin is low [20], a finding that was also made for humans [21]. Unlike other tissues, the brain was not considered to utilize insulin for glucose metabolism [22], and further, it was a common thought at the time that peptides such as insulin should not be able to cross the blood-brain barrier. For these reasons, we pondered the question as to why insulin is present in the CNS. One possibility that we proposed and pursued was that since insulin is secreted by the pancreas into the blood in direct proportion to the amount of stored body fat, perhaps the level of insulin entering the brain functions as a key afferent signal informing the brain as to how much stored energy is available, thereby influencing food intake. Support for this concept was first demonstrated by our lab using baboons [10] and later using rodents [23,24]. The weight-suppressing effects of intranasal insulin (which specifically targets the CSF and hence the central nervous system) were later demonstrated clinically in humans [25]. These discoveries established a new paradigm for our understanding of energy homeostasis [26,27], one which has been rigorously investigated in the past several decades and which easily accommodated other blood-borne signals secreted in the periphery in proportion to body fat content and entering the brain to influence food intake, including leptin and amylin [28,29]. Many reviews have been written about the catabolic actions of insulin in the brain [28,30–32].
3. Energy balance and the blood-brain barrier (BBB)
According to the World Health Organization, obesity now affects over 600 million adults worldwide [33], implying that a major shift in energy balance has occurred over the last few decades, one that favors greater energy intake relative to energy expenditure. Under conditions of stable body weight, energy balance is maintained by finely-tuned regulatory systems that are orchestrated as the brain integrates sensory information concerning body fat, food that is being consumed and processed in the gut, circulating nutrients, tissue needs, and so on. Much of the requisite information emanates from outside the brain, with signals being conveyed via nerves, such as the afferent vagus from the digestive tract and related viscera, or else as circulating signals including many hormones and nutrients [34].
To be efficacious at influencing the brain, peripherally-originating signals must either stimulate peripheral afferent nerves proceeding to the brain or else negotiate the BBB. One example of the former is cholecystokinin (CCK), which is secreted from I-cells in the duodenum in response to nutrients in the intestinal lumen [35]. While some of the released CCK enters the blood as a hormone acting on distant organs (e.g., eliciting bile flow from the liver), CCK also is thought to act locally on CCK receptors located on sensory nerve endings in the wall of the intestine [36,37]. Another example is glucagon-like peptide-1 (GLP-1), which in addition to being a hormonal incretin for pancreatic insulin secretion, is also considered a satiation signal that contributes to limiting meal size [38]. It is secreted from L-cells in the intestinal wall in response to nutrients and other signals in the intestinal lumen, and it is thought to act on local sensory nerve endings [38] and also to enter the hepatic portal circulation, where before entering the general circulation, it stimulates GLP-1 receptors on afferent vagal branches located in the wall of the hepatic portal vein [39].
Apolipoprotein A-IV (apo A-IV) is another important satiating signal. Apo A-IV is synthesized by intestinal enterocytes during the absorption of lipids, and as triglycerides are packaged into chylomicrons, apo A-IV attaches to the chylomicrons as they enter the lymph on their way to the circulation [40–42]. Once within the blood, apo A-IV is released into the plasma where it interacts with the pancreas, liver, and other target organs to improve glycemia by increasing insulin secretion [43] and decreasing gluconeogenesis [44]. It also contributes to limiting food intake [45–47], in part by enhancing the vagally-mediated CCK signal to the brain [48–50]. Although apo A-IV does not cross the BBB [51], we have found that apo A-IV is synthesized in areas of the hypothalamus that influence food intake and metabolism [52–54]. Thus, administration of apo A-IV either systemically (acting via the vagus nerves) or directly into the CSF reduces food intake [45–47].
Many metabolically important signals are able to stimulate the brain via the blood. In order to be influential, circulating signals reaching the brain via the arterial system must either be lipid soluble and pass relatively freely through the BBB (e.g., steroid hormones such as estrogen and corticosterone), interact with receptors in the brain’s circumventricular organs which have a relaxed BBB (e.g., amylin acting in the area postrema to influence food intake and body weight [55]), or else be transported through the BBB into the brain’s interstitial fluid to gain access to receptors on neurons and glial cells. The latter is the method by which many nutrients (e.g., glucose, amino acids) and peptide hormones (e.g., insulin, leptin, urocortin, cytokines) that influence energy homeostasis enter the brain [56–58]. For most of these molecules, there are specific receptors on the luminal surface of the endothelial cells that comprise part of the BBB [58,59]. As an example, BBB endothelial cells express insulin receptors and a commonly accepted model is that when circulating insulin binds to the receptor on the endothelial cell, the combination is taken into the cell and transported across it to the abluminal side where the insulin is subsequently released into the interstitial fluid [19,30,32].
As discussed above, when insulin is administered into the CSF of awake, behaving animals and humans, they act as if they are carrying more fat than actually exists in the body and they consequently eat less food and lose weight. Conversely, when the normal insulin signal within the brain is experimentally reduced, whether by genetically knocking out or silencing brain insulin receptors [60,61] or else pharmacologically disrupting insulin signaling locally in the hypothalamus [62], animals behave as if they are underweight and increase their food intake. Thus, insulin provides a catabolic tone within the brain, and when that tone is experimentally manipulated higher or lower, energy intake and body fat are changed.
While approaches such as these are important for uncovering how insulin acts once within the brain, the findings may have little to do with actual physiology. We have therefore been focusing our attention on the normal pathway by which insulin enters the brain; i.e., its insulin receptor-mediated transport through the BBB. Many years ago we found that the amount of insulin that appears in the CSF is only a small fraction of what is found in the blood, and further that the percentage of blood insulin present in the CSF is reduced in obesity [7,63], and similar findings were reported for humans [21,64]. This is important because it means that the insulin resistance that characterizes most peripheral tissues in the obese state includes the BBB transport system as well. We also found that the brain itself is relatively insulin resistant in the obese state; i.e., relative to lean controls, neither genetically [11] nor dietary-induced obese rats [65] reduce their food intake when administered insulin directly into the 3rd ventricle of the brain. We more recently found that the reduced insulin transport through the BBB that occurs in dietary-obese animals can be reversed by weight loss; i.e., the percentage of plasma insulin in the CSF is higher and the brain itself is also more sensitive to insulin [66].
4. Insulin detemir
Insulin promotes the uptake of glucose and nutrients into most tissues throughout the body, including muscle and adipose tissue and is an important anabolic hormone. Diabetic patients who self-administer exogenous insulin to manage their blood glucose levels therefore often experience weight gain as an undesirable side-effect as their adipose tissue mass increases [67]. Conversely, within the brain and as discussed above, insulin is catabolic. Thus, an improved treatment strategy for diabetes would be to develop a formulation of insulin that lowers circulating glucose appropriately while at the same time increasing insulin’s penetration into the brain, thereby potentially compensating for any peripheral anabolic action. Glargine and detemir are both long-acting formulations of insulin designed to have a relatively long functional half-life in the blood and thereby help improve the maintenance of glycemia. While both cause comparable reductions and maintenance of blood glucose, interestingly, diabetics who use the detemir formulation are able to effectively manage blood glucose without experiencing undesired weight gain relative to normal human insulin or insulin glargine [67,68]. We and others have also observed this in rodent models [69,70]. More recently we found that insulin detemir prevents weight gain due in part to an enhanced anorectic action in the CNS relative to other insulin formulations. Specifically, we found that whereas insulin detemir is transported into the brain to the same capacity of regular human insulin, it has a more prolonged anorectic action [69], suggesting that the prevention of weight gain observed clinically in diabetic patients taking insulin detemir is likely a result of an enhanced CNS suppression of food intake brought about by detemir’s longer functional half-life in the brain.
5. CCK and insulin transport into the CNS
Whereas detemir insulin is effective at prolonging the insulin signal in the brain, we more recently asked whether there are ways to increase the normal passage of insulin through the BBB. Many years ago we found that when animals are fasted, less insulin enters the CSF than when they are fed [6]. This was consistent with the concept that a fasted individual, who needs to consume more food than normal when it becomes available, would benefit from having a reduced ‘brake’ in the brain in the form of insulin. The important point, however, is that the findings suggest that some factor associated with feeding might enhance the transport of insulin into the brain. Several years ago Cano and colleagues [71,72] reported that the transport of the adiposity hormone leptin into the brain is enhanced by CCK. Since insulin and leptin both reduce food intake when administered into the CSF [73–75], we asked whether CCK might influence insulin transport as well.
We first found that brain capillary endothelial cells that comprise the BBB express both insulin and CCK receptors, suggesting that an interaction could occur [19,76]. When we administered insulin peripherally to rats in the presence or absence of the administration of CCK, twice as much insulin appeared in the CSF in the presence of CCK in spite of no change of plasma insulin [76]. These findings indicate that CCK may partly exert its satiating effects by increasing the transport of insulin into the CNS. Thus, when CCK levels are increased during meals, insulin is anticipated to enter the CSF in greater amounts. This is consistent with our previous findings that the transport of insulin into the CNS occurs most rapidly following meals [6]. We also expect that situations in which the circulating levels of gut hormones such as CCK are elevated, such as after some successful bariatric surgeries [77,78], will lead to weight loss, in part, by enhancing insulin transport into the CNS.
6. Estrogen and insulin transport into the CNS
Estrogen has a complex role when it comes to influencing body weight and potentially interacting with insulin in this regard. It is well established that females of many mammalian species weigh less than males, and that if they are ovariectomized, greatly reducing estrogen levels, they overeat and gain weight; and that administering estrogen to them in a manner that simulates normal secretory patterns, causes body weight to return to normal [79]. Based on this, estrogen would be classified as a catabolic hormone. However, we have found that central insulin is far more efficacious at reducing food intake and body weight of male rats than of female rats, and that estrogen is responsible for blunting insulin’s central catabolic action [24,80]. This sexual dimorphism has also been reported for humans receiving insulin into the CSF via the intranasal route [25]. Based on this interaction, estrogen would seem to be anabolic in the brain, or at least to oppose insulin’s catabolic action. We therefore hypothesized that estrogen must have a complex interaction with insulin in terms of influencing energy balance, and we asked whether estrogen might alter insulin passage through the BBB.
It is well-known that estrogen strengthens the effects of other signals that reduce food intake and body weight, including CCK [81–83], apolipoprotein A4 [84], and leptin [24], among many others. Further, E2 increases systemic insulin sensitivity in human and rodent models [85–87] and plays functional roles at the BBB by promoting the integrity of the BBB in response to ischemia [88,89] and inflammation [90]. We therefore isolated microvessels of the BBB and found that endothelial cells, besides co-expressing both insulin and CCK receptors, also co-express estrogen receptor α (ERα). We next asked whether estrogen enhances insulin transport into the CNS of rats [76]. Surprisingly, although chronic estrogen treatment significantly improved peripheral insulin sensitivity and prevented body weight gain during the prevention of dietary obesity, it did not increase the appearance of insulin in the CSF. If anything, estrogen administration was associated with reduced insulin transport into the CNS relative to weight-matched controls, which had effective insulin transport [19]. Estrogen also had no effect on insulin transport in chow-fed controls [19].
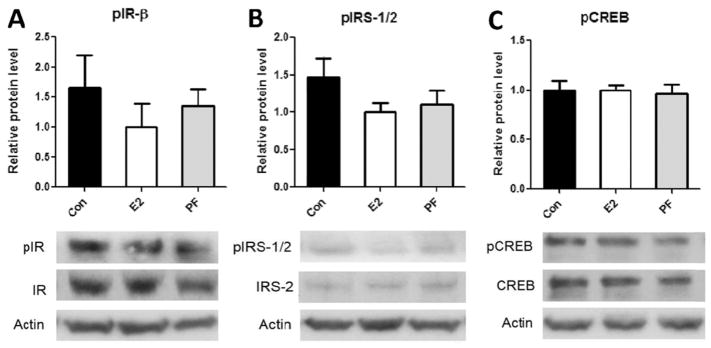
While these findings were unexpected, estrogen may still increase the activation of insulin signaling pathways within the brain via mechanisms that are independent of insulin transport, or are even perhaps completely independent of insulin action. We therefore asked whether E2-treatment increases hypothalamic insulin signaling in rats that underwent prevention of HFD-DIO (Fig. 1). Although E2 substantially improved insulin sensitivity and reduced body weight (as reported [19]), it did not improve the activation of proximal or distal insulin signaling pathways in the mediobasal hypothalamus (MBH) (Fig. 1). Consistently, pair-fed rats that were weight-matched to the body weight of the E2-treated group did not exhibit improved hypothalamic insulin signaling. In summary, these findings indicate that although E2 is effective at preventing the metabolic syndrome during maintenance on a HFD, it does not exert its effects by increasing insulin signaling in the MBH.
Fig. 1.
Estradiol does not increase the activation of insulin signaling in the mediobasal hypothalamus of DIO rats. Male long-evans rats underwent a 1-month prevention of HFD-DIO via cyclic E2 treatment. The MBH were then micropunched, as described [19]. Protein expression and phosphorylation were assessed via Western blotting for phosphorylated (p) and total insulin receptor (IR) (A), insulin receptor substrates 1 and 2 (IRS-1/2) (B), and cyclic AMP response-element binding protein (C) (a distal protein in the insulin signaling cascade). Upper panels display the levels of phosphorylated proteins as quantified by Image J, while lower panels display representative Western blots. No significant differences were observed among groups for any proteins, as assessed with one-way ANOVA, n = 6–7, mean ± SEM.
7. Effect of E2 on BBB-expression of genes relevant to metabolism
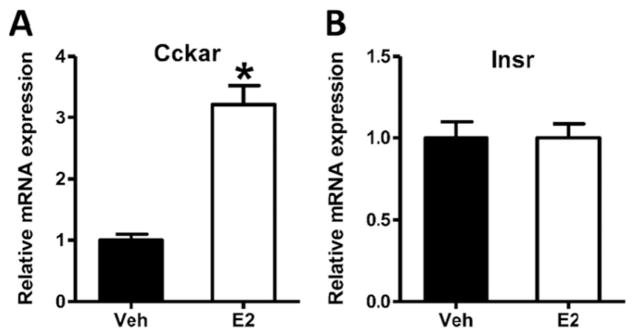
Although we have not found evidence that E2 enhances the transport of insulin or leptin into the brain, we recently observed that E2 increases the expression of the CCK-1R mRNA at the BBB of OVX rats by approximately 3 times that of vehicle-injected controls (Fig. 2A). In comparison, the level of insulin receptor mRNA was unaffected by E2 treatment under the same conditions (Fig. 2B). This finding is intriguing, because it has been previously demonstrated that E2 increases the satiating potency of CCK [83,91,92]. Therefore, an interaction of E2 with CCK signaling at the BBB may partly underlie this effect.
Fig. 2.
Estradiol increases the expression of CCK-1 receptor mRNA (Cckar) in brain microvessels of OVX rats. Female Long-Evans rats were ovariectomized (OVX) and allowed to recover for 2 weeks. OVX rats were then injected subcutaneously with estradiol-3-benzoate (E2, 10 μg/kg, 2 μL, Sigma) or vehicle (sesame oil, 2 μL, Sigma), for two consecutive days. Throughout this time, food was removed in order to prevent the impact of differential food intake following E2 treatment. Rats were then sacrificed and brain microvessels were isolated and analyzed via qPCR for expression of CCK-1R mRNA (A) and Insulin receptor mRNA (Insr, B). Data are presented as mean ± SEM and were analyzed with the Student’s t-test, n = 8, P < 0.05 vs vehicle controls. All procedures were approved by the Institutional Animal Care and Use Committee and were conducted in an AALAC-accredited facility.
8. Summary
Randall Sakai made substantial contributions in his lifetime and he has bestowed a powerful scientific vision onto his trainees and colleagues, including the use of CSF to study behavioral endocrinology in general and ingestive behavior in particular. This approach has resulted in many important findings by Randall’s and our labs. Findings highlighted in this report stem from the mutual interests of Randall’s and our approaches and offer novel perspectives about how energy balance and food intake behavior is integrated at the levels of the BBB and brain. The finding that the insulin analog, detemir has prolonged action may offer beneficial effects not only to obese diabetics, but also to for individuals with neurological disorders in which brain insulin signaling is impaired, such as Alzheimer’s disease [93,94]. Another interesting avenue will be to better understand the mechanism by which CCK and other satiation signals enhance the transport of signals such as insulin into the CNS, a mechanism that could be used to improve the delivery of drugs through the BBB. Our finding that estrogen ameliorates symptoms of the metabolic syndrome independently of improving insulin transport into the CNS reveals the complexity of central insulin signaling; under some conditions, insulin may be ineffective at entering the brain despite appearing insulin sensitive in peripheral tissues. As we continue to understand how the transport of molecules through the BBB is influenced by hormones under normal conditions and in metabolic diseases, this will open the door to new therapeutics that can safely ameliorate obesity and its co-morbidities. Studying the transport of molecules into the CNS via their appearance in the CSF will continue to be an invaluable approach towards this end.
HIGHLIGHTS.
Transport of molecules into the brain can be readily assessed using cerebrospinal fluid.
Insulin can be structurally modified to increase its anorectic action in the brain.
Insulin transport into the CNS is affected by hormones.
The blood-brain barrier may be an important target for ameliorating metabolic diseases.
Acknowledgments
This work was supported by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) awards DK017844, DK92779, DK95440, DK103557 and DK059803.
References
- 1.Weisinger RS, Woods SC. Formalin-like sodium appetite and thirst elicited by a conditioned stimulus in rats. J Comp Physiol Psychol. 1972;80:413–421. doi: 10.1037/h0032984. (Available from: http://www.ncbi.nlm.nih.gov/pubmed/5071896) [DOI] [PubMed] [Google Scholar]
- 2.Weisinger RS, Woods SC. Aldosterone-elicited sodium appetite. Endocrinology. 1972;89:538–544. doi: 10.1210/endo-89-2-538. (Available from: http://www.ncbi.nlm.nih.gov/pubmed/5558002. [DOI] [PubMed] [Google Scholar]
- 3.Frankmann SP, Sakai RR, Simpson JB. Sodium appetite and cerebrospinal fluid sodium concentration during hypovolemia. Appetite. 1987;9:57–64. doi: 10.1016/0195-6663(87)90053-5. (Available from: http://www.ncbi.nlm.nih.gov/pubmed/3662494. [DOI] [PubMed] [Google Scholar]
- 4.Stein LJ, Dorsa DM, Baskin DG, Figlewicz DP, Porte D, Woods SC. Reduced effect of experimental peripheral hyperinsulinemia to elevate cerebrospinal fluid insulin concentrations of obese Zucker rats. Endocrinology. 1987;121:1611–1615. doi: 10.1210/endo-121-5-1611. (Available from: http://www.ncbi.nlm.nih.gov/pubmed/3311715) [DOI] [PubMed] [Google Scholar]
- 5.Steffens AB, Scheurink AJ, Porte D, Woods SC, Porte D, Jr, Woods SC. Penetration of peripheral glucose and insulin into cerebrospinal fluid in rats. Am J Physiol. 1988;255:R200–R204. doi: 10.1152/ajpregu.1988.255.2.R200. (Available from: http://www.ncbi.nlm.nih.gov/pubmed/3044143) [DOI] [PubMed] [Google Scholar]
- 6.Strubbe JH, Porte D, Woods SC. Insulin responses and glucose levels in plasma and cerebrospinal fluid during fasting and refeeding in the rat. Physiol Behav. 1988;44:205–208. doi: 10.1016/0031-9384(88)90139-4. (Available from: http://www.sciencedirect.com/science/article/pii/0031938488901394) [DOI] [PubMed] [Google Scholar]
- 7.Israel PA, Park CR, Schwartz MW, Green PK, Sipols AJ, Woods SC, et al. Effect of diet-induced obesity and experimental hyperinsulinemia on insulin uptake into CSF of the rat. Brain Res Bull. 1993;30:571–575. doi: 10.1016/0361-9230(93)90084-o. (Available from: http://www.sciencedirect.com/science/article/pii/036192309390084O) [DOI] [PubMed] [Google Scholar]
- 8.Sakai RR, He PF, Yang XD, Ma LY, Guo YF, Reilly JJ, et al. Intracerebroventricular administration of AT1 receptor antisense oligonucleotides inhibits the behavioral actions of angiotensin II. J Neurochem. 1994;62:2053–2056. doi: 10.1046/j.1471-4159.1994.62052053.x. (Available from: http://www.ncbi.nlm.nih.gov/pubmed/8158154. [DOI] [PubMed] [Google Scholar]
- 9.Sakai RR, Ma LY, He PF, Fluharty SJ. Intracerebroventricular administration of angiotensin type 1 (AT1) receptor antisense oligonucleotides attenuate thirst in the rat. Regul Pept. 1995;59:183–192. doi: 10.1016/0167-0115(95)00111-n. (Available from: http://www.ncbi.nlm.nih.gov/pubmed/8584753. [DOI] [PubMed] [Google Scholar]
- 10.Woods SC, Lotter EC, McKay LD, Porte D., Jr Chronic intracerebroventricular infusion of insulin reduces food intake and body weight of baboons. Nature. 1979;282:503–505. doi: 10.1038/282503a0. (Available from: http://www.nature.com/nature/journal/v282/n5738/abs/282503a0.html) [DOI] [PubMed] [Google Scholar]
- 11.Ikeda H, West DB, Pustek JJ, Figlewicz DP, Greenwood MRC, Porte D, Jr, et al. Intraventricular insulin reduces food intake and body weight of lean but not obese Zucker rats. Appetite. 1986;7:381–386. doi: 10.1016/s0195-6663(86)80006-x. (Available from: http://www.ncbi.nlm.nih.gov/pubmed/3539015) [DOI] [PubMed] [Google Scholar]
- 12.Krause EG, Melhorn SJ, Davis JF, Scott KA, Ma LY, de Kloet AD, et al. Angiotensin type 1 receptors in the subfornical organ mediate the drinking and hypothalamic-pituitary-adrenal response to systemic isoproterenol. Endocrinology. 2008;149:6416–6424. doi: 10.1210/en.2008-0477. (Available from: http://www.ncbi.nlm.nih.gov/pubmed/18687780) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Kloet AD, Krause EG, Kim D-H, Sakai RR, Seeley RJ, Woods SC. The effect of angiotensin-converting enzyme inhibition using captopril on energy balance and glucose homeostasis. Endocrinology. 2009;150:4114–4123. doi: 10.1210/en.2009-0065. (Available from: http://www.ncbi.nlm.nih.gov/pubmed/19497971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Krause EG, de Kloet AD, Flak JN, Smeltzer MD, Solomon MB, Evanson NK, et al. Hydration state controls stress responsiveness and social behavior. J Neurosci. 2011;31:5470–5476. doi: 10.1523/JNEUROSCI.6078-10.2011. (Available from: http://www.ncbi.nlm.nih.gov/pubmed/21471383) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Krause EG, de Kloet AD, Scott KA, Flak JN, Jones K, Smeltzer MD, et al. Blood-borne angiotensin II acts in the brain to influence behavioral and endocrine responses to psychogenic stress. J Neurosci. 2011;31:15009–15015. doi: 10.1523/JNEUROSCI.0892-11.2011. (Available from: http://www.ncbi.nlm.nih.gov/pubmed/22016534) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Kloet AD, Krause EG, Scott KA, Foster MT, Herman JP, Sakai RR, et al. Central angiotensin II has catabolic action at white and brown adipose tissue. Am J Physiol Endocrinol Metab. 2011;301:E1081–E1091. doi: 10.1152/ajpendo.00307.2011. (Available from: http://www.ncbi.nlm.nih.gov/pubmed/21862725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu M, Shen L, Begg DP, D’alessio DA, Woods SC. Insulin increases central apolipoprotein E levels as revealed by an improved technique for collection of cerebrospinal fluid from rats. J Neurosci Methods. 2012;209:106–112. doi: 10.1016/j.jneumeth.2012.05.034. (Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3402581&tool=pmcentrez&rendertype=abstract) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu M, Kuhel DG, Shen L, Hui DY, Woods SC. Apolipoprotein E does not cross the blood-cerebrospinal fluid barrier, as revealed by an improved technique for sampling CSF from mice. Am J Phys Regul Integr Comp Phys. 2012;303:R903–R908. doi: 10.1152/ajpregu.00219.2012. (Available from: http://ajpregu.physiology.org/content/303/9/R903) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.May AA, Bedel ND, Shen L, Woods SC, Liu M. Estrogen and insulin transport through the blood-brain barrier. Physiol Behav. 2016;163:312–321. doi: 10.1016/j.physbeh.2016.05.019. (Available from: http://www.sciencedirect.com/science/article/pii/S0031938416302517) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Woods SC, Porte D. Effect of intracisternal insulin on plasma glucose and insulin in the dog. Diabetes. 1975;24:905–909. doi: 10.2337/diab.24.10.905. (Available from: http://www.ncbi.nlm.nih.gov/pubmed/1100459) [DOI] [PubMed] [Google Scholar]
- 21.Owen OE, Reichard GA, Boden G, Shuman C. Comparative measurements of glucose, beta-hydroxybutyrate, acetoacetate and insulin in blood and cerebrospinal fluid during starvation. Metabolism. 1974;23:7–14. doi: 10.1016/0026-0495(74)90098-5. (Available from: http://www.ncbi.nlm.nih.gov/pubmed/4808514) [DOI] [PubMed] [Google Scholar]
- 22.Rafaelsen OJ. Action of insulin on glucose uptake of rat brain slices and isolated rat cerebellum. J Neurochem. 1961;7:45–51. doi: 10.1111/j.1471-4159.1961.tb13496.x. (Available from: http://doi.wiley.com/10.1111/j.1471-4159.1961.tb13496.x) [DOI] [PubMed] [Google Scholar]
- 23.Brown LM, Clegg DJ, Benoit SC, Woods SC. Intraventricular insulin and leptin reduce food intake and body weight in C57BL/6J mice. Physiol Behav. 2006;89:687–691. doi: 10.1016/j.physbeh.2006.08.008. (Available from: http://www.ncbi.nlm.nih.gov/pubmed/16979194. [DOI] [PubMed] [Google Scholar]
- 24.Clegg DJ, Brown LM, Woods SC, Benoit SC. Gonadal hormones determine sensitivity to central leptin and insulin. Diabetes. 2006;55:978–987. doi: 10.2337/diabetes.55.04.06.db05-1339. (Available from: http://diabetes.diabetesjournals.org/content/55/4/978) [DOI] [PubMed] [Google Scholar]
- 25.Hallschmid M, Benedict C, Schultes B, Fehm HL, Born J, Kern W. Intranasal insulin reduces body fat in men but not in women. Diabetes. 2004;53:3024–3029. doi: 10.2337/diabetes.53.11.3024. (Available from: http://diabetes.diabetesjournals.org/content/53/11/3024) [DOI] [PubMed] [Google Scholar]
- 26.Woods SC, Seeley RJ, Porte DJ, Schwartz MW. Signals that regulate food intake and energy homeostasis. Science. 1998;280:1378–1383. doi: 10.1126/science.280.5368.1378. (Available from: <Go to ISI>://WOS:000073883400035) [DOI] [PubMed] [Google Scholar]
- 27.Schwartz MW, Woods SC, Porte D, Jr, Seeley RJ, Baskin DG. Central nervous system control of food intake. Nature. 2000;404(6778):661–671. doi: 10.1038/35007534. (Available from: http://www.nature.com/nature/journal/v404/n6778/full/404661a0.html) [DOI] [PubMed] [Google Scholar]
- 28.Porte D, Baskin DG, Schwartz MW. Leptin and insulin action in the central nervous system. Nutr Rev. 2002;60:85–87. S20-9-84. doi: 10.1301/002966402320634797. (Available from: http://www.ncbi.nlm.nih.gov/pubmed/12403080) [DOI] [PubMed] [Google Scholar]
- 29.Rushing PA, Hagan MM, Seeley RJ, Lutz TA, Woods SC. Amylin: a novel action in the brain to reduce body weight. Endocrinology. 2000 Feb;141(2):850–850.33.2000;141:853. doi: 10.1210/endo.141.2.7378. [DOI] [PubMed] [Google Scholar]
- 30.Begg DP, Woods SC. The central insulin system and energy balance. Handb Exp Pharmacol. 2012;209:111–129. doi: 10.1007/978-3-642-24716-3_5. (Available from: http://www.ncbi.nlm.nih.gov/pubmed/22249812) [DOI] [PubMed] [Google Scholar]
- 31.Woods SC, Seeley RJ. Adiposity signals and the control of energy homeostasis. Nutrition. 2000;16:894–902. doi: 10.1016/s0899-9007(00)00454-8. (Available from: http://www.ncbi.nlm.nih.gov/pubmed/11054594) [DOI] [PubMed] [Google Scholar]
- 32.Banks WA, Owen JB, Erickson MA. Insulin in the brain: there and back again. Pharmacol Ther. 2012;136:82–93. doi: 10.1016/j.pharmthera.2012.07.006. (Available from: http://www.ncbi.nlm.nih.gov/pubmed/22820012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.WHO. Obesity and overweight. Fact Sheet Number 311. [Internet] 2015;2015 (Available from: http://www.who.int/mediacentre/factsheets/fs311/en/) [Google Scholar]
- 34.Morton GJ, Meek TH, Schwartz MW. Neurobiology of food intake in health and disease. Nat Rev Neurosci. 2014;15:367–378. doi: 10.1038/nrn3745. (Available from: http://www.ncbi.nlm.nih.gov/pubmed/24840801) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bray GA. Afferent signals regulating food intake. Proc Nutr Soc. 2000;59:373–384. doi: 10.1017/s0029665100000422. [DOI] [PubMed] [Google Scholar]
- 36.Schwartz GJ, Moran TH. CCK elicits and modulates vagal afferent activity arising from gastric and duodenal sites. Ann N Y Acad Sci. 1994;713:121–128. doi: 10.1111/j.1749-6632.1994.tb44058.x. (Available from: http://www.ncbi.nlm.nih.gov/pubmed/8185153) [DOI] [PubMed] [Google Scholar]
- 37.Moran TH, Baldessarini AR, Salorio CF, Lowery T, Schwartz GJ. Vagal afferent and efferent contributions to the inhibition of food intake by cholecystokinin. Am J Phys. 1997;272:R1245–R1251. doi: 10.1152/ajpregu.1997.272.4.R1245. (Available from: http://www.ncbi.nlm.nih.gov/pubmed/9140026) [DOI] [PubMed] [Google Scholar]
- 38.Steinert RE, Beglinger C, Langhans W. Intestinal GLP-1 and satiation: from man to rodents and back. Int J Obes. 2016;40:198–205. doi: 10.1038/ijo.2015.172. (Available from: http://www.ncbi.nlm.nih.gov/pubmed/26315842) [DOI] [PubMed] [Google Scholar]
- 39.Vahl TP, Tauchi M, Durler TS, Elfers EE, Fernandes TM, Bitner RD, et al. Glucagon-like peptide-1 (GLP-1) receptors expressed on nerve terminals in the portal vein mediate the effects of endogenous GLP-1 on glucose tolerance in rats. Endocrinology. 2007;148:4965–4973. doi: 10.1210/en.2006-0153. (Available from: http://www.ncbi.nlm.nih.gov/pubmed/17584962) [DOI] [PubMed] [Google Scholar]
- 40.Kalogeris TJ, Fukagawa K, Tsuchiya T, Qin X, Tso P. Intestinal synthesis and lymphatic secretion of apolipoprotein A-IV after cessation of duodenal fat infusion: mediation by bile. Biochim Biophys Acta. 1999;1436:451–466. doi: 10.1016/s0005-2760(98)00152-0. [DOI] [PubMed] [Google Scholar]
- 41.Hayashi H, Nutting DF, Fujimoto K, Cardelli JA, Black D, Tso P. Transport of lipid and apolipoproteins A-I and A-IV in intestinal lymph of the rat. J Lipid Res. 1990;31:1613–1625. [PubMed] [Google Scholar]
- 42.Tso P. Intestinal lipid absorption and apolipoprotein A-IV production. Appetite. 1999;32:421. doi: 10.1006/appe.1999.0239. [DOI] [PubMed] [Google Scholar]
- 43.Wang F, Kohan AB, Kindel TL, Corbin KL, Nunemaker CS, Obici S, et al. Apolipoprotein A-IV improves glucose homeostasis by enhancing insulin secretion. Proc Natl Acad Sci U S A. 2012;109:9641–9646. doi: 10.1073/pnas.1201433109. (Available from: http://www.ncbi.nlm.nih.gov/pubmed/22619326) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li X, Xu M, Wang F, Kohan AB, Haas MK, Yang Q, et al. Apolipoprotein A-IV reduces hepatic gluconeogenesis through nuclear receptor NR1D1. J Biol Chem. 2014;289:2396–2404. doi: 10.1074/jbc.M113.511766. (Available from: http://www.ncbi.nlm.nih.gov/pubmed/24311788) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tso P, Liu M, Kalogeris TJ. The role of apolipoprotein A-IV in food intake regulation. J Nutr. 1999;129:1503–1506. doi: 10.1093/jn/129.8.1503. [DOI] [PubMed] [Google Scholar]
- 46.Tso P, Liu M, John KT, Thomson AB. The role of apolipoprotein a-iv in the regulation of food intake. Annu Rev Nutr. 2001;21:231–254. doi: 10.1146/annurev.nutr.21.1.231. [DOI] [PubMed] [Google Scholar]
- 47.Fujimoto K, Machidori H, Iwakiri R, Yamamoto K, Fujisaki J, Sakata T, et al. Effect of intravenous administration of apolipoprotein A-IV on patterns of feeding, drinking and ambulatory activity of rats. Brain Res. 1993;608:233–237. doi: 10.1016/0006-8993(93)91463-3. [DOI] [PubMed] [Google Scholar]
- 48.Yoshimichi G, Lo CC, Tamashiro KLK, Ma L, Lee DM, Begg DP, et al. Effect of peripheral administration of cholecystokinin on food intake in apolipoprotein AIV knockout mice. Am J Physiol Gastrointest Liver Physiol. 2012;302:G1336–G1342. doi: 10.1152/ajpgi.00325.2010. (Available from: http://www.ncbi.nlm.nih.gov/pubmed/22461023) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lo CM, Zhang DM, Pearson K, Ma L, Sun W, Sakai RR, et al. Interaction of apolipoprotein AIV with cholecystokinin on the control of food intake. Am J Phys Regul Integr Comp Phys. 2007;293:R1490–R1494. doi: 10.1152/ajpregu.00329.2007. [DOI] [PubMed] [Google Scholar]
- 50.Lo CM, Xu M, Yang Q, Zheng S, Carey KM, Tubb MR, et al. Effect of intraperitoneal and intravenous administration of cholecystokinin-8 and apolipoprotein AIV on intestinal lymphatic CCK-8 and apo AIV concentration. Am J Phys Regul Integr Comp Phys. 2009;296:R43–R50. doi: 10.1152/ajpregu.90410.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shen L, Pearson KJ, Xiong Y, Lo C-M, Tso P, Woods SC, et al. Characterization of apolipoprotein A-IV in brain areas involved in energy homeostasis. Physiol Behav. 2008;95:161–167. doi: 10.1016/j.physbeh.2008.05.022. (Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2574991&tool=pmcentrez&rendertype=abstract) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liu M, Shen L, Doi T, Woods SC, Seeley RJ, Tso P. Neuropeptide Y and lipid increase apolipoprotein AIV gene expression in rat hypothalamus. Brain Res. 2003;971:232–238. doi: 10.1016/s0006-8993(03)02402-8. [DOI] [PubMed] [Google Scholar]
- 53.Shen L, Tso P, Woods SC, Sakai RR, Davidson WS, Liu M. Hypothalamic apolipoprotein A-IV is regulated by leptin. Endocrinology. 2007;148:2681–2689. doi: 10.1210/en.2006-1596. (Available from: http://www.ncbi.nlm.nih.gov/pubmed/17363460) [DOI] [PubMed] [Google Scholar]
- 54.Tso P, Sun W, Liu M. Gastrointestinal satiety signals IV. Apolipoprotein A-IV. Am J Physiol Gastrointest Liver Physiol. 2004;286:G885–G890. doi: 10.1152/ajpgi.00511.2003. [DOI] [PubMed] [Google Scholar]
- 55.Lutz TA, Meyer U. Amylin at the interface between metabolic and neurodegenerative disorders. Front Neurosci. 2015;9:216–228. doi: 10.3389/fnins.2015.00216. (Available from: http://www.ncbi.nlm.nih.gov/pubmed/26136651) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pan W, Kastin AJ. TNFalpha transport across the blood-brain barrier is abolished in receptor knockout mice. Exp Neurol. 2002;174:193–200. doi: 10.1006/exnr.2002.7871. (Available from: http://www.sciencedirect.com/science/article/pii/S0014488602978714) [DOI] [PubMed] [Google Scholar]
- 57.Banks WA. Brain meets body: the blood-brain barrier as an endocrine interface. Endocrinology. 2012;153:4111–4119. doi: 10.1210/en.2012-1435. (Available from: http://www.ncbi.nlm.nih.gov/pubmed/22778219) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Banks WA. Role of the blood-brain barrier in the evolution of feeding and cognition. Ann N Y Acad Sci. 2012;1264:13–19. doi: 10.1111/j.1749-6632.2012.06568.x. (Available from: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3464352/) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Begg DP, Woods SC. The endocrinology of food intake. Nat Rev Endocrinol. 2013;9:584–597. doi: 10.1038/nrendo.2013.136. (Available from: http://www.ncbi.nlm.nih.gov/pubmed/23877425) [DOI] [PubMed] [Google Scholar]
- 60.Bruning JC, Gautam D, Burks DJ, Gillette J, Schubert M, Orban PC, et al. Role of brain insulin receptor in control of body weight and reproduction. Science. 2000;289:2122–2125. doi: 10.1126/science.289.5487.2122. (Available from: http://www.ncbi.nlm.nih.gov/pubmed/11000114) [DOI] [PubMed] [Google Scholar]
- 61.Obici S, Feng Z, Karkanias G, Baskin DG, Rossetti L. Decreasing hypothalamic insulin receptors causes hyperphagia and insulin resistance in rats. Nat Neurosci. 2002;5:566–572. doi: 10.1038/nn0602-861. (Available from: http://dx.doi.org/10.1038/nn0602-861) [DOI] [PubMed] [Google Scholar]
- 62.Obici S, Feng Z, Tan J, Liu L, Karkanias G, Rossetti L. Central melanocortin receptors regulate insulin action. J Clin Invest. 2001;108:1079–1085. doi: 10.1172/JCI12954. (Available from: http://www.jci.org/articles/view/12954) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Stein LJ, Dorsa DM, Baskin DG, Figlewicz DP, Ikeda H, Frankmann SP, et al. Immunoreactive insulin levels are elevated in the cerebrospinal fluid of genetically obese Zucker rats. Endocrinology. 1983;113:2295–2301. doi: 10.1210/endo-113-6-2299. [DOI] [PubMed] [Google Scholar]
- 64.Kern W, Benedict C, Schultes B, Plohr F, Moser A, Born J, et al. Low cerebrospinal fluid insulin levels in obese humans. Diabetologia. 2006;49:2790–2792. doi: 10.1007/s00125-006-0409-y. (Available from: http://www.ncbi.nlm.nih.gov/pubmed/16951936) [DOI] [PubMed] [Google Scholar]
- 65.Clegg DJ, Benoit SC, Reed JA, Woods SC, Dunn-Meynell A, Levin BE. Reduced anorexic effects of insulin in obesity-prone rats fed a moderate-fat diet. Am J Phys Regul Integr Comp Phys. 2005;288:R981–R986. doi: 10.1152/ajpregu.00675.2004. (Available from: http://www.ncbi.nlm.nih.gov/pubmed/15604298) [DOI] [PubMed] [Google Scholar]
- 66.Begg DP, Mul JD, Liu M, Reedy BM, D’Alessio DA, Seeley RJ, et al. Reversal of diet-induced obesity increases insulin transport into cerebrospinal fluid and restores sensitivity to the anorexic action of central insulin in male rats. Endocrinology. 2013;154:1047–1054. doi: 10.1210/en.2012-1929. (Available from: http://endo.endojournals.org/content/154/3/1047.long) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Raslová K, Tamer SC, Clauson P, Karl D. Insulin detemir results in less weight gain than NPH insulin when used in basal-bolus therapy for type 2 diabetes mellitus, and this advantage increases with baseline body mass index. Clin Drug Investig. 2007;27:279–285. doi: 10.2165/00044011-200727040-00007. (Available from: http://www.ncbi.nlm.nih.gov/pubmed/17358100) [DOI] [PubMed] [Google Scholar]
- 68.Zachariah S, Sheldon B, Shojaee-Moradie F, Jackson NC, Backhouse K, Johnsen S, et al. Insulin detemir reduces weight gain as a result of reduced food intake in patients with type 1 diabetes. Diabetes Care. 2011;34:1487–1491. doi: 10.2337/dc11-0098. (Available from: http://www.ncbi.nlm.nih.gov/pubmed/21593292) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Begg DP, May AA, Mul JD, Liu M, D’Alessio DA, Seeley RJ, et al. Insulin detemir is tansported from blood to cerebrospinal fluid and has prolonged central anorectic action relative to NPH insulin. Diabetes. 2015;64:2457–2466. doi: 10.2337/db14-1364. (Available from: http://www.ncbi.nlm.nih.gov/pubmed/25667307) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Russell-Jones D, Danne T, Hermansen K, Niswender K, Robertson N, Thalange K, et al. Weight-sparing effect of insulin detemir: a consequence of central nervous system-mediated reduced energy intake? Diabetes Obes Metab. 2015;17:919–927. doi: 10.1111/dom.12493. (Available from: http://doi.wiley.com/10.1111/dom.12493) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cano V, Ezquerra L, Ramos MP, Ruiz-Gayo M. Regulation of leptin distribution between plasma and cerebrospinal fluid by cholecystokinin receptors. Br J Pharmacol. 2003;140:647–652. doi: 10.1038/sj.bjp.0705477. (Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=1574067&tool=pmcentrez&rendertype=abstract) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cano V, Merino B, Ezquerra L, Somoza B, Ruiz-Gayo M. A cholecystokinin-1 receptor agonist (CCK-8) mediates increased permeability of brain barriers to leptin. Br J Pharmacol. 2008;154:1009–1015. doi: 10.1038/bjp.2008.149. (Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2451056&tool=pmcentrez&rendertype=abstract) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Air EL, Benoit SC, Blake Smith KA, Clegg DJ, Woods SC. Acute third ventricular administration of insulin decreases food intake in two paradigms. Pharm Biol. 2002;72:423–429. doi: 10.1016/s0091-3057(01)00780-8. (Available from: http://www.ncbi.nlm.nih.gov/pubmed/11900815) [DOI] [PubMed] [Google Scholar]
- 74.Air EL, Benoit SC, Clegg DJ, Seeley RJ, Woods SC. Insulin and leptin combine additively to reduce food intake and body weight in rats. Endocrinology. 2002;143:2449–2452. doi: 10.1210/endo.143.6.8948. [DOI] [PubMed] [Google Scholar]
- 75.Seeley RJ, van Dijk G, Campfield LA, Smith FJ, Burn P, Nelligan JA, et al. Intra-ventricular leptin reduces food intake and body weight of lean rats but not obese Zucker rats. Horm Metab Res. 1996;28:664–668. (Available from: http://www.ncbi.nlm.nih.gov/pubmed/9013738) [Google Scholar]
- 76.May AA, Liu M, Woods SC, Begg DP. CCK increases the transport of insulin into the brain. Physiol Behav. 2016;165:392–397. doi: 10.1016/j.physbeh.2016.08.025. (Available from: http://www.ncbi.nlm.nih.gov/pubmed/27570192) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Berthoud HR. The vagus nerve, food intake and obesity. Regul Pept. 2008;149:15–25. doi: 10.1016/j.regpep.2007.08.024. (Available from: http://www.ncbi.nlm.nih.gov/pubmed/18482776) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ochner CN, Gibson C, Shanik M, Goel V, Geliebter A. Changes in neurohormonal gut peptides following bariatric surgery. Int J Obes. 2011;35:153–166. doi: 10.1038/ijo.2010.132. (Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3632050&tool=pmcentrez&rendertype=abstract) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Shen L, Liu Y, Wang DQH, Tso P, Woods SC, Liu M. Estradiol stimulates apolipoprotein A-IV gene expression in the nucleus of the solitary tract through estrogen receptor-α. Endocrinology. 2014;155:3882–3890. doi: 10.1210/en.2014-1239. (Available from: http://www.ncbi.nlm.nih.gov/pubmed/25051443) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Clegg DJ, Riedy CA, Smith KAB, Benoit SC, Woods SC. Differential sensitivity to central leptin and insulin in male and female rats. Diabetes. 2003;52:682–687. doi: 10.2337/diabetes.52.3.682. (Available from: http://www.ncbi.nlm.nih.gov/pubmed/12606509) [DOI] [PubMed] [Google Scholar]
- 81.Asarian L, Geary N. Estradiol enhances cholecystokinin-dependent lipid-induced satiation and activates estrogen receptor-alpha-expressing cells in the nucleus tractus solitarius of ovariectomized rats. Endocrinology. 2007;148:5656–5666. doi: 10.1210/en.2007-0341. (Available from: http://www.ncbi.nlm.nih.gov/pubmed/17823256) [DOI] [PubMed] [Google Scholar]
- 82.Eckel LA, Geary N. Endogenous cholecystokinin’s satiating action increases during estrus in female rats. Peptides. 1999;20:451–456. doi: 10.1016/s0196-9781(99)00025-x. (Available from: http://www.ncbi.nlm.nih.gov/pubmed/10458514) [DOI] [PubMed] [Google Scholar]
- 83.Geary N, Trace D, McEwen B, Smith GP. Cyclic estradiol replacement increases the satiety effect of CCK-8 in ovariectomized rats. Physiol Behav. 1994;56:281–289. doi: 10.1016/0031-9384(94)90196-1. (Available from: http://www.sciencedirect.com/science/article/pii/0031938494901961) [DOI] [PubMed] [Google Scholar]
- 84.Shen L, Wang DQH, Lo CM, Tso P, Davidson WS, Woods SC, et al. Estradiol increases the anorectic effect of central apolipoprotein A-IV. Endocrinology. 2010;151:3163–3168. doi: 10.1210/en.2010-0203. (Available from: http://www.ncbi.nlm.nih.gov/pubmed/20484461) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Alonso A, Fernandez R, Moreno M, Ordonez P, Gonzalez-Pardo H, Conejo NM, et al. Positive effects of 17β-estradiol on insulin sensitivity in aged ovariectomized female rats. J Gerontol A Biol Sci Med Sci. 2006;61:419–426. doi: 10.1093/gerona/61.5.419. (Available from: http://biomedgerontology.oxfordjournals.org/content/61/5/419.long#ref-10) [DOI] [PubMed] [Google Scholar]
- 86.Andersson B, Mattsson LA, Hahn L, Mårin P, Lapidus L, Holm G, et al. Estrogen replacement therapy decreases hyperandrogenicity and improves glucose homeostasis and plasma lipids in postmenopausal women with noninsulin-dependent diabetes mellitus. J Clin Endocrinol Metab. 1997;82:638–643. doi: 10.1210/jcem.82.2.3746. (Available from: http://press.endocrine.org/doi/full/10.1210/jcem.82.2.3746) [DOI] [PubMed] [Google Scholar]
- 87.Riant E, Waget A, Cogo H, Arnal J-F, Burcelin R, Gourdy P. Estrogens protect against high-fat diet-induced insulin resistance and glucose intolerance in mice. Endocrinology. 2009;150:2109–2117. doi: 10.1210/en.2008-0971. (Available from: http://press.endocrine.org/doi/full/10.1210/en.2008-0971) [DOI] [PubMed] [Google Scholar]
- 88.Li M, Zhang Z, Sun W, Koehler RC, Huang J. 17β-estradiol attenuates breakdown of blood-brain barrier and hemorrhagic transformation induced by tissue plasminogen activator in cerebral ischemia. Neurobiol Dis. 2011;44:277–283. doi: 10.1016/j.nbd.2011.07.004. (Available from: http://www.ncbi.nlm.nih.gov/pubmed/21816222) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Liu R, Wen Y, Perez E, Wang X, Day AL, Simpkins JW, et al. 17β-Estradiol attenuates blood-brain barrier disruption induced by cerebral ischemia-reperfusion injury in female rats. Brain Res. 2005;1060:55–61. doi: 10.1016/j.brainres.2005.08.048. ([cited 2016 Jul 7];1060:55–61. Available from: http://www.ncbi.nlm.nih.gov/pubmed/16212944) [DOI] [PubMed] [Google Scholar]
- 90.de Velasco EGR, Santizo R, Feinstein DL, Adamsom P, Greenwood J, Koenig HM, et al. Estrogen inhibits NFκB-dependent inflammation in brain endothelium without interfering with IκB degradation. Neuroreport. 2002;13:1469–1472. doi: 10.1097/00001756-200208070-00024. (Available from: https://uic.pure.elsevier.com/en/publications/estrogen-inhibits-nfκb-dependent-inflammation-in-brain-endotheliu) [DOI] [PubMed] [Google Scholar]
- 91.Asarian L, Geary N. Cyclic estradiol treatment phasically potentiates endogenous cholecystokinin’s satiating action in ovariectomized rats. Peptides. 1999;20:445–450. doi: 10.1016/s0196-9781(99)00024-8. (Available from: http://www.ncbi.nlm.nih.gov/pubmed/10458513) [DOI] [PubMed] [Google Scholar]
- 92.Butera PC, Xiong M, Davis RJ, Platania SP. Central implants of dilute estradiol enhance the satiety effect of CCK-8. Behav Neurosci. 1996;110:823–830. doi: 10.1037//0735-7044.110.4.823. (Available from: http://www.ncbi.nlm.nih.gov/pubmed/8864272. [DOI] [PubMed] [Google Scholar]
- 93.Schubert M, Gautam D, Surjo D, Ueki K, Baudler S, Schubert D, et al. Role for neuronal insulin resistance in neurodegenerative diseases. Proc Natl Acad Sci U S A. 2004;101:3100–3105. doi: 10.1073/pnas.0308724101. (Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=365750&tool=pmcentrez&rendertype=abstract\nhttp://www.ncbi.nlm.nih.gov/pubmed/14981233\nhttp://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=PMC365750) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Cholerton B, Baker LD, Craft S. Insulin resistance and pathological brain ageing. Diabet Med. 2011;28:1463–1475. doi: 10.1111/j.1464-5491.2011.03464.x. (Available from: http://www.ncbi.nlm.nih.gov/pubmed/21974744) [DOI] [PubMed] [Google Scholar]