Abstract
Over 20 years ago, sphingosine-1-phosphate (S1P) was discovered to be a bioactive signaling molecule. Subsequent studies later identified two related kinases, sphingosine kinase 1 and 2, which are responsible for the phosphorylation of sphingosine to S1P. Many stimuli increase sphingosine kinase activity and S1P production and secretion. Outside the cell, S1P can bind to and activate five S1P-specific G protein-coupled receptors (S1PR1–5) to regulate many important cellular and physiological processes in an autocrine or paracrine manner. S1P is found in high concentrations in the blood where it functions to control vascular integrity and trafficking of lymphocytes. Obesity increases blood S1P levels in humans and mice. With the world wide increase in obesity linked to consumption of high-fat, high-sugar diets, S1P is emerging as an accomplice in liver pathobiology, including acute liver failure, metabolic syndrome, control of blood lipid and glucose homeostasis, nonalcoholic fatty liver disease, and liver fibrosis. Here, we review recent research on the importance of sphingosine kinases, S1P, and S1PRs in liver pathobiology, with a focus on exciting insights for new therapeutic modalities that target S1P signaling axes for a variety of liver diseases.
Keywords: Metabolic syndrome, nonalcoholic fatty liver disease, sphingosine-1-phosphate, acute liver failure, steatosis
Introduction
Sphingosine, which is produced by hydrolysis of the sphingolipid core ceramide, is phosphorylated to the pluripotent bioactive signaling molecule sphingosine-1-phosphate (S1P) by sphingosine kinase 1 and 2 (SphK1 and SphK2). In addition to its role as a signaling molecule, production of S1P is required for the complete degradation of sphingoid bases and serves to reduce the level of ceramide, another signaling sphingolipid. Many cellular stimuli and hormones induce phosphorylation and activation of cytosolic SphK1 and its translocation to the plasma membrane where its substrate sphingosine resides. These topics have been reviewed extensively (Hannun and Obeid 2008, Maceyka et al. 2012, Maceyka and Spiegel 2014).
Newly produced S1P can be exported out of the cell by ATP-binding cassette (ABC) transporters or by major facilitator superfamily member spinster 2 (Spns2) (reviewed in Nishi et al. 2014, Takabe and Spiegel 2014) where it then can bind and activate a family of five S1P-specific G protein-coupled receptors (S1PR1–5), whose activation initiates a diverse range of cellular responses (Pyne and Pyne 2010, Maceyka and Spiegel 2014). Recently, several direct intracellular targets of S1P have been identified. For example, SphK1-produced S1P binds to and activates TNF receptor-associated factor 2 (TRAF2) that serves as a platform for recruitment and stimulation of IκB kinase, important for the activation of the transcription factor NF-κB (Alvarez et al. 2010). In contrast to SphK1, SphK2 is typically found in intracellular compartments, including the endoplasmic reticulum, mitochondria, and nucleus (Maceyka and Spiegel 2014). Nuclear produced S1P has an important role in regulation of gene expression by acting as an endogenous inhibitor of histone deacetylases (Hait et al. 2009).
S1P is present in high levels in the blood and has emerged as a key mediator of numerous physiological and pathophysiological responses, such as cell growth and survival, differentiation, migration, vascular integrity, lymphocyte trafficking, and immune responses, to name a few (Pyne and Pyne 2010, Orr Gandy and Obeid 2013, Maceyka and Spiegel 2014). The liver is a vital organ in the body that is critical for many functions, including detoxification of various metabolites, and in carbohydrate, protein, amino acid, and lipid metabolism. Because of its multidimensional functions, liver disease can endanger survival of the whole organism. There has been a marked increase in the rate of obesity and obesity-related diseases linked to the Western diet, and S1P is emerging as an important player in liver pathobiology. Here, we discuss the emerging role of the SphK/S1P/S1PR axis in the functions and pathobiology of the liver including acute liver failure (ALF), metabolic syndrome, control of blood lipid and glucose homeostasis, nonalcoholic fatty liver disease (NAFLD), and liver fibrosis (Figure 1 and Table 1).
Figure 1.
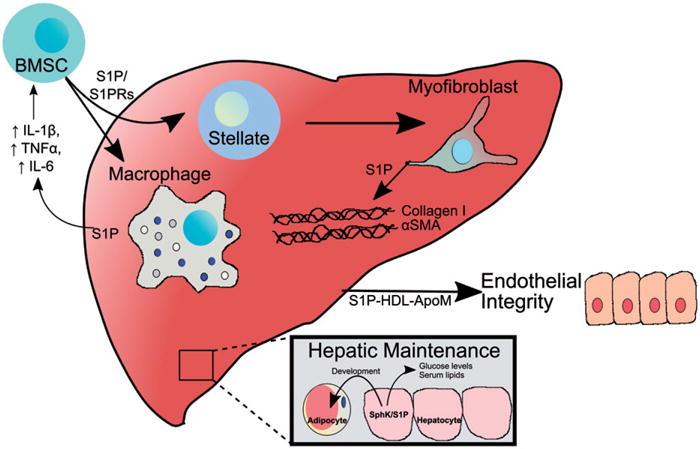
S1P signaling in the liver is involved in multiple physiological and pathophysiological processes. This figure depicts several processes regulated by S1P described in the text. S1P promotes recruitment of bone marrow mesenchymal stem cells (BMSC) through activation of S1PRs. In the liver, these cells differentiate into hepatic stellate cells. When quiescent stellate cells are activated due to liver injury, they transdifferentiate to myofibroblasts which express α-smooth muscle actin and secrete collagen I to form the fibrotic extracellular matrix in a S1P-dependent manner. The liver also secretes S1P-containing apoM and S1P/apoM in HDL promotes endothelial barrier integrity. S1P also regulates hepatic maintenance of serum lipids, glucose levels, and adipocyte development. S1P released from the liver in response to injury promotes recruitment of a variety of immune cells, including liver-resident macrophages known as Kupffer cells leading to hepatic inflammation (see color version of this figure at www.tandfonline.com/ibmg).
Table 1.
Liver pathophysiology associated with the sphingosine-1-phosphate axis.
| Pathophysiology | Effects of sphingosine-1-phosphate |
|---|---|
| Glucose tolerance |
Systemic glucose • ↑ S1P levels associated with ↑ glucose (Fox et al. 2011, Kowalski et al. 2013) Liver • ↑ Ceramide leads to ↑ Akt signaling and ↑ glucose tolerance (Osawa et al. 2011) • ↑ SphK2 leads to ↑ hepatic glucose tolerance (Lee et al. 2015) • ↑ S1P associates with ↑ insulin resistance (Fayyaz et al. 2014) Pancreas • ↑ glucose associated with ↑ S1P and ↑ insulin secretion (Cantrell Stanford et al. 2012) |
| Nonalcoholic fatty liver disease (NAFLD) |
Liver • S1P caused ↑ in NF-κB, IL-1ß, IL-6, and TNF-α induced inflammation (Wang et al. 2013) • ↓ Loss of SphK1 associated with ↓ weight and ↓ lipid accumulation (Geng et al. 2015, Chen et al. 2016a) • ↑ in palmitate causes ↑ lipid and triglyceride accumulation (Chen et al. 2016a) • ↑ SphK2 expression improved weight, lipid levels, and ↑ glucose tolerance (Lee et al. 2015, Nagahashi et al. 2015) • ↓ SphK2 correlated with ↑ hepatosteatosis along with systemic cholesterol and triglycerides (Nagahashi et al. 2015) • ↑ ER stress leads to ↑ SphK2 and ↑ S1P with improved glucose tolerance and ↓ hepatosteatosis (Lee et al. 2015) |
| Obesity |
Circulation • ↑ S1P levels correlated with ↑ body fat percentage and BMI (Kowalski et al. 2013) Adipocytes • ↓ S1PR2/S1P signaling associated with ↓ adipose tissue differentiation (Jeong et al. 2015) and ↓ lipid accumulation (Moon et al. 2014) |
| Hepatic fibrosis and liver injury |
Fibrogenesis • ↑ S1P inhibits growth of human hepatic fibroblasts (Davaille et al. 2000). • ↑ S1P induces apoptosis of human hepatic myofibroblasts in receptor independent manner (Davaille et al. 2002) • ↑ S1P signals through Edg receptor to also activate Akt, ERK1/2 for survival in human hepatic fibroblasts (Davaille et al. 2002). • ↓ S1P plasma concentration associated with progression of CCl4 induced liver fibrosis (Ikeda et al. 2010) • ↑ S1P leads to Col a1 (I) and Col a1 (III) fibrosis development (Xiu et al. 2015) • ↑ S1P enhanced human hepatic myofibroblasts (Li et al. 2011). • SphK1 involved with hepatic fibrosis angiogenesis (Yang et al. 2013) Migration • S1PR3 signaling brings myofibroblasts to liver (Li et al. 2009) • S1PR2/3 activate Akt for bone marrow derived macrophage/monocyte migration (Yang et al. 2015) Liver injury • S1PR2 has inhibitory role in liver regeneration after acute liver injury (Ikeda et al. 2009) |
Hepatic production of apoM regulates S1P plasma levels
The liver is an important organ for regulating S1P levels in the blood, which in turn play a significant role in maintaining vascular and epithelial barriers, vascular tone and inflammation. Plasma S1P is mainly contributed by erythrocytes (Pappu et al. 2007), platelets, endothelial cells, and liver (Yatomi et al. 1997, Hanel et al. 2007, Bode et al. 2010, Jonnalagadda et al. 2014). Erythrocytes, which are an important source of plasma S1P, can phosphorylate sphingosine to generate as well as store S1P but the mechanism of their release of S1P is not well understood. Platelets also store S1P and release it upon activation (Jonnalagadda et al. 2014). In human plasma, the concentrations of S1P are around 1 lM and approximately 60% of S1P is associated with HDL and 40% with albumin (Aoki et al. 2005, Yatomi 2008, Christoffersen et al. 2011). Hepatocytes produce apolipoprotein M (apoM), a component of approximately 5% of the HDL particles that are the major carrier of plasma S1P (Frej et al. 2015, Ruiz et al. 2017). Indeed, circulating S1P levels were shown to directly correlate with apoM levels in mice and humans (Christoffersen et al. 2011, Kurano et al. 2013, Liu et al. 2014, Frej et al. 2015). Crystal structure of the apoM-S1P complex and molecular dynamics studies indicate that apoM binds S1P in an amphipathic pocket of its lipocalin domain with high affinity and suggest that S1P is not spontaneously released from apoM but requires binding to other molecules such as the S1PRs (Christoffersen et al. 2011, Zhang et al. 2016). Binding of S1P to apoM was linked to the rate of secretion of apoM from hepatocytes as mutations which resulted in its signal sequence cleavage reduced the amount of S1P associated with HDL, decreased HDL particle size, and reduced circulating S1P in vivo (Liu et al. 2015). Intriguingly, LDL receptor overexpression also reduced circulating S1P and apoM levels and the rate of clearance of exogenous S1P–apoM complexes in an apoE-dependent manner (Kurano et al. 2015), suggesting multiple levels of regulation of circulating S1P levels by the liver.
Many lines of evidence have linked circulating S1P to the maintenance of endothelial barrier function (Christensen et al. 2016, Yanagida and Hla 2017). Current evidence suggests the ApoM–S1P–HDL complex secreted by the liver is the endogenous mediator of endothelial barrier integrity. HDL-delivered S1P activated S1PR1 on endothelial cells in vitro while HDL from apoM−/− mice, which have significantly reduced circulating S1P, did not (Christoffersen et al. 2011). ApoM/HDL induced S1PR1 internalization, activation of MAPK and Akt, and increased endothelial adherens junction formation. Other studies using selective agonists and antagonists have also linked vascular barrier integrity to S1PR1 but not to other S1PRs (Ruiz et al. 2017). Subsequently, it was shown that apoM−/− mice have increased basal lung vasculature leakage that was reversed with the S1PR1 agonist SEW2871. ApoM-delivered S1P also reduced edema in a subcutaneous inflammation model in a S1PR1-dependent fashion (Christensen et al. 2016). Plasma concentrations of apoM decrease during sepsis and in systemic inflammatory response syndrome in both mice and humans, and this reduction has been linked to cytokine-induced decreased hepatocyte production of apoM mRNA and protein (Feingold et al. 2008, Kumaraswamy et al. 2012). However, in many such studies, S1P levels were not directly measured. Similarly, it was shown that levels of S1P and apoM inversely correlated with vascular leakage in humans infected with dengue (Michels et al. 2015). Moreover, the ability of ApoM/HDL to act as a biased agonist on S1PR1 inhibits vascular inflammation, which may be related to the cardiovascular protective functions of HDL (Galvani et al. 2015). Together, these results indicate that circulating apoM–S1P produced by the liver is important for the maintenance of endothelial barrier integrity.
Role of S1P in acute liver failure
Though more rare than chronic liver diseases, ALF has high mortality rates. ALF has a variety of causes, including viral infection, toxins, and ischemia, and is mediated by hepatocyte dysfunction and/or apoptosis. Recent studies have implicated S1P in certain ALF models. Curiously, although in most studies S1P seems to contribute to liver damage, in others, it seems to have a protective role. For example, S1P is important for recruitment and activation of liver-damaging immune cells, and yet several in vitro studies indicate that S1P protects hepatocytes from apoptotic insults (Osawa et al. 2005, Liu et al. 2013, Nowatari et al. 2015).
Inflammation-induced liver injury
Hyperactivation of the immune system is a common pathological inducer of acute organ damage, including the liver, and mouse models have indicated that various liver-resident immune cells such as macrophages, natural killer T cells, and dendritic cells play important roles. Given the importance of S1P in regulation of trafficking and activation of a variety of immune cells, it is not surprising that S1P signaling is involved in induction of inflammation-mediated liver damage. In viral-induced ALF in rabbits, expression of SphK1, S1P levels, and S1PR1 are all increased in the liver, and correlated with TNF-α and IL-6 levels and disease severity (Crespo et al. 2016). In agreement, treatment with the S1PR1 functional antagonists FTY720 or KRP203, both of which induce lymphopenia, was protective in a concanavalin-A-mediated, T cell-dependent liver damage model (Kaneko et al. 2006). Similarly, in lipopolysaccharide (LPS)-induced liver failure, S1P accumulated and S1PR1 expression was upregulated in the liver (Rosenberg et al. 2016). In a different ALF model, treatment with LPS plus D-galactosamine (D-Gal) induced macrophage-dependent hepatocyte cell death and liver failure. LPS/D-Gal increased serum S1P levels and upregulated SphK1 expression in peripheral blood monocytes as well as in Kupffer cells, liver resident macrophages (Lei et al. 2015b). Treatment with a pan SphK inhibitor, N,N-dimethylsphingosine, reduced mortality and was associated with reduced liver inflammation and cell death, reduced serum markers of liver failure, AST and ALT, and serum levels of the cytokines TNF-α, IL-1, and IL-6. Further work implicated PKCδ in mediating the effects of SphK, as inhibition of SphK reduced the activation of PKCδ, and inhibition of PKCδ with Rottlerin also protected from LPS/D-Gal-induced ALF (Lei et al. 2015a). Another group extended these findings, demonstrating that LPS/D-Gal-induced ALF could be ameliorated with a more specific SphK1 inhibitor (Tian et al. 2016). Intriguingly, they showed that hepatic SphK1, S1PR1, and S1PR3 levels were all elevated in ALF, and that inhibitors of any one of these proteins reduced serum levels of the pro-inflammatory cytokines IL-6 and TNF-α. However, only inhibition of SphK1, but not S1PR1 or S1PR3, protected the liver by significantly reducing hepatic hemorrhage and caspase-3 activation as well as serum AST and ALT (Tian et al. 2016). An explanation for the discrepancy is that S1P can signal either through both S1PR1 and S1PR3 to induce ALF, or that SphK1 may promote ALF directly in the liver in a cytokine-independent manner. Another study also reported that LPS/D-Gal upregulated hepatic and PBMC SphK1 and that inhibition of the complement factor C5a receptor (C5aR), which is upregulated in liver and PBMCs during ALF, reduced SphK1 expression, serum TNF-α, IL-6, and IL-1β, liver damage, and mortality (Lei et al. 2016).
Ischemia/reperfusion-induced liver injury
Acute liver damage can also be induced by ischemia and reperfusion (I/R), as is often seen after liver transplantation. One group showed that activation of S1PR1, but not S1PR2 or S1PR3, protected the liver from I/R damage induced by restriction of blood flow in situ, reducing apoptosis and cytokine secretion and increasing vascular integrity in a MAPK- and Akt-dependent manner (Park et al. 2010). However, others demonstrated that S1P was increased in both I/R and transplanted livers. Treatment with a weak SphK2 inhibitor reduced S1P levels and improved survival and liver function (Shi et al. 2012). The SphK2 inhibitor also reduced activation of NF-κB and production of TNF-α and IL-1, which was accompanied by a marked reduction in the infiltration of monocytes, neutrophils, and CD4 + T cells. These results suggest that the ischemic liver produces S1P that initiates secretion of cytokines and recruitment of inflammatory cells leading to organ damage and failure. Different effects of S1P noted above may be related to whether S1P is increased in the circulation (Park et al. 2010) or generated in the liver itself (Shi et al. 2012). It also cannot be excluded that the SphK2 inhibitor has additional targets. These studies highlight the need for analysis of S1P levels both in circulation and in the organ itself during I/R as well as thorough determination of effects of inhibitors and specificity of S1PR agonists/antagonists used.
S1P is an important mediator of liver fibrosis
Chronic liver injury is a serious problem due to the associated apoptosis, inflammation and fibrosis. The latter is the most concerning aspect as it leads to cirrhosis, liver failure and ultimately requires liver transplantation (Bataller and Brenner 2005). Fibrosis is induced when quiescent hepatic stellate cells are induced to transdifferentiate to myofibroblasts, the cells responsible for laying down the fibrotic extracellular matrix (Pellicoro et al. 2014). It was suggested that the myofibroblasts, which express α-smooth muscle actin and secrete collagen I to form the fibrotic extracellular matrix, are dependent on the S1P axis (Sato et al. 2016). However, liver samples taken during resection for hepatocellular carcinoma showed no detectable differences in S1P levels between normal and fibrotic liver (Sato et al. 2016). Because SPNS2, a transporter of S1P, seemed to be elevated, it was suggested that S1P is transported out of hepatocytes and signals through S1PR2 in an autocrine loop that promotes fibrosis (Sato et al. 2016).
In contrast, another study found that S1P levels measured by HPLC were elevated in fibrotic human livers, as well as myofibroblast expression of S1PR1 and S1PR3, but not S1PR2 (Li et al. 2011). Migration of human myofibroblasts in vitro was S1PR1/3-dependent but independent of S1PR2 (Li et al. 2011). In subsequent studies, this group used a carbon tetrachloride-induced model of liver fibrosis in mice, and demonstrated that S1PR3 promoted recruitment of bone marrow mesenchymal stem cells to the liver that then differentiated into myofibroblasts. Moreover, prevention of the upregulation of S1PR3 via the RNA binding protein HuR reduced injury-induced migration of bone marrow mesenchymal stem cells to the liver (Chang et al. 2017). Furthermore, it has been suggested that S1P is synthesized in hepatocytes in response to palmitate and released into the extracellular environment leading to activation of hepatic stellate cells by binding to S1PR3 (Al Fadel et al. 2016). In contrast, others reported that the S1PR2 antagonist JTE-013 blocked liver fibrosis (Wang et al. 2015). Similar results were observed in a bile duct ligation-induced model of liver fibrosis in which knockout of S1PR2 was protective (Wang et al. 2017). Disruption of the hepatocyte–endothelium interaction in the injured liver frequently results in impaired regeneration and fibrosis. A recent study has shown that activation of endothelial S1PR1 by S1P bound to HDL enhances regeneration and inhibits fibrosis in the liver (Ding et al. 2016). Together, these studies suggest that there are multiple roles for S1P signaling through different S1PRs at various stages of liver fibrosis, which at times may be opposing. For example, while S1P signaling may increase fibrosis through recruitment and activation of hepatic stellate cells, S1PR1 signaling in the endothelium promotes proper neo-vascularization of damaged liver and prevention of further liver damage (Ding et al. 2016). Further studies are needed to determine at which stages S1P plays a role in fibrosis or inflammation and whether altered S1P metabolism in fibrotic liver could be a therapeutic target.
S1P signaling in viral hepatitis
Hepatitis B virus (HBV) and hepatitis C virus (HCV) induce liver fibrosis in chronically infected patients and are two of the most common hepatic infections in the world (Rehermann and Nascimbeni 2005, Perz et al. 2006). Both viruses have an outer membrane derived from cellular membranes, and like most membrane viruses, sphingolipids are a crucial constituent of these viruses (Sakamoto et al. 2005, Grammatikos et al. 2015, Schneider-Schaulies and Schneider-Schaulies 2015). Indeed, myriocin, an inhibitor of SPT, the first and committed step in de novo sphingolipid biosynthesis, suppressed replication of HCV in cultured cells and in animals (Umehara et al. 2006). Yet, not much is known about the role of S1P or SphKs in HBV and HCV infections. Chronic infection with HBV contributes to development of HCC. The HBV X protein, a multifunctional regulatory protein that plays an important role in this process, upregulates SphK1 through the transcription factor AP2α, promoting proliferation of hepatoma cells (Lu et al. 2015). However, in contrast to SphK1 that promotes viral replication, SphK2 inhibits it (Yamane et al. 2014). These opposing effects were linked to differential effects on lipid peroxidation, with SphK2 promoted-lipid peroxidation reducing viral yields (Yamane et al. 2014). In HCV patients, serum S1P levels were reduced relative to HBV patients as measured by mass spectrometry (Grammatikos et al. 2015). Moreover, increased serum levels of the SphK substrates sphingosine and sphinganine correlated with liver fibrosis progression and poor treatment outcome in HCV but not in HBV infection (Grammatikos et al. 2015). This study suggests that sphingosine and sphinganine may be biomarkers for the progression of HCV infections and further studies are needed to evaluate their usefulness for noninvasive prediction of liver fibrosis.
Role of S1P signaling in fatty liver disease
NAFLD is a prevailing health concern in the USA and is a spectrum disorder affecting the liver due primarily to overconsumption of fat-enriched food. Initially, accumulation of lipids leads to a relatively benign state of steatosis. However, over time, accumulation of lipids induces liver inflammation, termed steatohepatitis (NASH), characterized by increased immune cell infiltration followed by fibrosis. Further disease exacerbation leads to liver cirrhosis and ultimately hepatocellular carcinoma. Moreover, during disease progression, other metabolic issues typically arise, including insulin resistance and elevated triglycerides (Cohen and Chun 2011, Satapathy and Sanyal 2015).
High-fat diets (HFDs) are typically characterized by elevated levels of palmitate, the primary precursor of ceramide and other sphingolipids. Indeed, palmitate has been shown to induce sphingolipid synthesis in hepatocytes (Watt et al. 2012) and numerous reports have documented the relationship between a HFD, increased ceramide levels, and NAFLD. Elegant reviews have highlighted the numerous metabolic complications mediated by elevated ceramide that are associated with NAFLD (Chavez and Summers 2012, Pagadala et al. 2012). It has been suggested that insulin resistance in obesity is associated with increases of pro-inflammatory cytokines TNF-α and IL-6 that can induce ceramide production in the liver. Ceramide, in turn, activates PP2A and attenuates Akt signaling, inducing apoptosis, causing hepatic insulin resistance (Chavez and Summers 2012). Ceramide also contributes to the development of NAFLD and progression to NASH (Pagadala et al. 2012). However, much less is known of the role of the ceramide metabolites sphingosine and S1P in NAFLD and metabolic syndrome.
Lipidomic analyzes of tissues from obese mice that were fed for 16–52 weeks on a HFD (42% kcal fat) provided some insights into how HFD affects sphingosine and S1P levels. As expected, these mice had elevated liver levels of palmitate-containing sphingolipids as well as sphingosine and S1P compared to normal chow fed mice. Moreover, these mice also had elevated levels of sphinganine and sphinganine-1-phosphate, suggesting that the increased dietary fat was inducing de novo sphingolipid synthesis (Sanyal and Pacana 2015). Consistent with these results, adding palmitic acid to primary mouse hepatocytes increased SphK1 expression, raised S1P levels, and increased lipid accumulation. Consistent with this, it was observed that SphK1 expression is increased in livers from humans with NAFLD and also in mice with high saturated fat feeding, which recapitulated the human disease (Geng et al. 2015). From these initial findings, it seems likely that SphK1 and formation of S1P play a role in regulation of lipid storage. Additional in vitro studies support the notion that SphK1 is a pro-steatosis gene. For example, SphK1 expression is closely linked to expression of the lipid storage gene PPARc (Ross et al. 2013, Geng et al. 2015). Further support came from the demonstration that weight gain and triglyceride levels in mice fed a 21% kcal fat diet enriched with fructose for 24 weeks were lower in SphK1 knockout mice compared to wild type mice. Importantly, hepatic pathology was less severe in the SphK1−/− mice as determined by the NAFLD Activity Score (Chen et al. 2016a). However, the role of SphK1 in NAFLD is not limited to steatosis (Figure 2). Mouse and rat models of NAFLD have shown that SphK1 is important in hepatic inflammation and progression to steatohepatitis (Wang et al. 2013, Geng et al. 2015). After 18 weeks on a high saturated fat diet, there were elevated numbers of macrophages recruited to the liver that correlated with increased inflammatory cytokines such as TNF-α and MCP1 (Geng et al. 2015). Moreover, although SphK1 knockout mice gained weight to a similar extent as their wild type littermates, they had reduced liver lipid accumulation, cytokine signaling, and macrophage accumulation. In vitro, the saturated fat palmitate induced hepatocyte expression of SphK1, TNF-α, and MCP1, and this effect could be blocked by knockdown of either SphK1 or S1PR1. These data suggest that saturated fats upregulate SphK1 and S1P that signals through S1PRs to promote inflammatory signaling in the liver (Geng et al. 2015). This may have clinical relevance as patients with nonalcoholic steatohepatitis have elevated liver levels of SphK1. Elevation of liver SphK1 and S1PRs was also observed in a rat model of steatohepatitis induced by a high fructose diet (Wang et al. 2013). Similarly, in a rat model of high fat-induced NAFLD, inhibition of sphingolipid synthesis with myriocin decreased ceramide and sphinganine and concomitantly reduced signs of NAFLD, including reduced liver enzymes (Kurek et al. 2014). Myriocin-treated animals on the high fat diet also had reduced liver fat accumulation and reduced immune cell infiltration (Chavez and Summers 2012).
Figure 2.
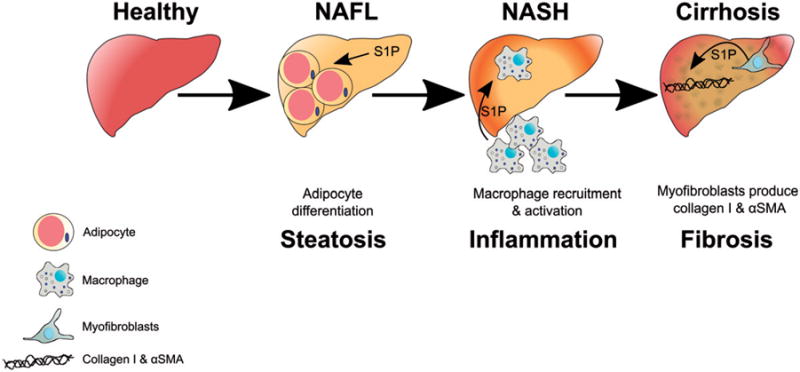
S1P regulates progression of nonalcoholic fatty liver disease (NAFLD). This figure illustrates potential roles of S1P in the progression of NAFLD from nonalcoholic fatty liver (NAFL) to nonalcoholic steatohepatitis (NASH) and finally to cirrhosis. Initially S1P increases adipocyte differentiation and accumulation of lipids in the liver. S1P produced in the liver increases pro-inflammatory cytokines such as IL-1β, TNFα, and IL-6, which recruit and activate macrophages to the liver. Lastly, S1P stimulates myofibroblasts to secrete collagen I and α smooth muscle actin (αSMA) (see color version of this figure at www.tandfonline.com/ibmg).
Deposition of fat and free fatty acids in the liver can cause lipotoxicity involved in the development and progression of fatty liver diseases. SphK1 expression suppressed ER stress-mediated pro-apoptotic pathways and prevented IRE1α activation and JNK phosphorylation (Qi et al. 2015). Thus, this study provides further evidence for a role of SphK1 in hepatocyte survival and uncovers a novel mechanism of protection against ER stress-mediated cell death (Qi et al. 2015). SphK2 has been less well studied but also appears to have a role in NAFLD. SphK2 was upregulated in murine liver after administration of the ER-stress inducing agent tunicamycin (Lee et al. 2015). Lentiviral overexpression of SphK2 in liver of mice led to increased phosphorylation of Akt in liver, muscle and adipose tissue. In addition, mice overexpressing SphK2 demonstrated improved glucose tolerance while on a 60% kcal fat diet compared to wild type mice. Moreover, fatty acid oxidation was increased in these mice that were accompanied by elevated S1P levels (Lee et al. 2015). Similarly, it was found that in contrast to SphK1 knockout mice, SphK2 knockout mice rapidly developed steatosis on a HFD (Nagahashi et al. 2015). The increased liver fat accumulation was linked in this study to the role of SphK2 in promoting transcription by producing S1P in the nucleus that inhibited HDAC activity, by showing that SphK2 knockout mice had reduced expression of a number of genes involved in hepatic lipid metabolism. Intriguingly, it was also found that S1PR2 knockout mice had a similar phenotype as the SphK2 knockouts that was suggested to be due to the inability of the S1PR2 mice to upregulate SphK2 in response to a HFD (Nagahashi et al. 2015).
Additional insight into the roles SphKs and S1P in NAFLD has come from studies using FTY720, a prodrug that is phosphorylated mainly by SphK2 to produce FTY720-phosphate that is a S1P mimetic that is a ligand of all of the S1PRs except S1PR2 (Brinkmann et al. 2010). FTY720-phosphate also acts as a functional antagonist for S1PR1 as it induces its degradation. Many studies have demonstrated that this effect on S1PR1 interferes with and ultimately mitigates immune responses (Brinkmann et al. 2010). In a murine model of NAFLD, administration of FTY720 reduced liver/body weight ratio along with decreased hepatic inflammation, fibrosis and hepatocyte ballooning (Mauer et al. 2017). Perhaps most importantly, FTY720 treatment was able to reverse NAFLD symptoms even after they had already been initiated, suggesting that FTY720 may be a useful therapeutic for these liver disorders.
Role of liver S1P signaling in metabolic syndrome
Though it has many causes, NAFLD is commonly associated with metabolic syndrome, the name given to a collection of symptoms, including obesity, insulin resistance, dyslipidemia, and hypertension, that predispose individuals to type 2 diabetes, cancer, and cardiovascular diseases. Metabolic syndrome is a serious health issue, affecting nearly 1 in 4 US adults (Beltran-Sanchez et al. 2013), and involves multiple organs, including the pancreas, skeletal muscle, adipocytes, and the immune system. Because the role of S1P in these various organs and metabolic diseases has been recently reviewed (Brice and Cowart 2011, Chen et al. 2016b), this section will focus specifically on the role of S1P signaling in the liver in metabolic syndrome. The liver plays a very important function in maintaining systemic glucose levels by producing glucose during periods of glucose deprivation (Saltiel and Kahn 2001). Adenoviral delivery of SphK1 to the liver greatly reduced blood glucose levels and dyslipidemia in the KK/Ay mouse model of type 2 diabetes (Ma et al. 2007). The protective effect of SphK1 was linked to its enhancement of insulin-stimulated kinases Akt and GSK3β. Although hepatic SphK1 was downregulated in mice fed a high-fat high-sucrose diet, overexpression of SphK1 in liver reduced triglyceride content in mice fed a low but not HFD and had no effect on glucose tolerance (Kowalski et al. 2015). Conversely, in hepatocytes, palmitate was shown to induce S1P production that opposed insulin signaling (Fayyaz et al. 2014). In addition, S1P was elevated in the livers of New Zealand obese mice, and S1PR2 inhibition reversed the insulin resistance in these mice. Interestingly, glucose stimulated SphK2 and increased S1P in pancreatic islets that correlated with increased glucose-stimulated insulin secretion (Cantrell Stanford et al. 2012). Moreover, treatment of mice with a SphK inhibitor impaired glucose disposal due to decreased plasma insulin levels, suggesting that S1P may be involved in glucose stimulated insulin secretion from beta cells (Cantrell Stanford et al. 2012). Hepatic overexpression of SphK2 in mice fed a HFD also increased S1P, and also decreased hepatic accumulation of lipid droplets by a SphK2-dependent increase in fatty acid oxidation in liver. In addition, glucose intolerance and insulin resistance were markedly reduced by improved hepatic insulin signaling through SphK2 upregulation (Lee et al. 2015). Because the ER stress-mediated UPR pathways upregulate SphK2 expression, it was suggested that this ameliorates hepatic steatosis and insulin resistance in mice (Lee et al. 2015).
Another area of research that has recently received much attention is related to the effects of adiponectin, which controls glucose and lipid homeostasis, on sphingolipid metabolism. A series of studies have shown that binding of adiponectin to its receptors AdipoR1 and AdipoR2, led to enhanced deacylation of ceramide to sphingosine and increased conversion to S1P. This conversion promoted survival of functional beta cells, allowing for sufficient insulin production to meet insulin demands. Alleviation of ceramide accumulation in the liver also led to improvements in hepatic insulin action (Holland et al. 2011, 2013).
Concluding remarks
S1P, the enzymes that control its levels through synthesis and degradation, and the multiple receptors that respond to it have important roles in numerous physiological hepatic functions and are critically involved in hepatic pathologies, particularly NAFLD and HCC. Thus, to mitigate their pathological contributions, development of targeted pharmacological interventions is being actively pursued. However, utilization of these approaches will require significant preliminary studies as S1P has pleiotropic effects depending on the receptor targeted or even the cell types in which S1PRs are expressed. Given the critical roles for S1P reviewed here, it is hoped that further pharmacological studies will not only provide useful therapeutics but will also aid in elucidating the mechanisms for the actions of S1P in different hepatic pathologies.
Acknowledgments
Funding
This work was supported by National Institutes of Health grant No. R01GM043880 to S.S. T.R. was supported by T32 training grant in Digestive Disease and Liver Disease 5T32DK007150–39.
Footnotes
Disclosure statement
The authors declare that they have no conflicts of interest with the contents of this article.
References
- Al Fadel F, et al. Involvement of sphingosine 1-phosphate in palmitate-induced non-alcoholic fatty liver disease. Cell cellular physiology and biochemistry: international journal of experimental cellular physiology, biochemistry, and pharmacology. 2016;40:1637–1645. doi: 10.1159/000453213. [DOI] [PubMed] [Google Scholar]
- Alvarez SE, et al. Sphingosine-1-phosphate is a missing cofactor for the e3 ubiquitin ligase TRAF2. Nature. 2010;465:1084–1088. doi: 10.1038/nature09128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoki S, et al. Sphingosine 1-phosphate-related metabolism in the blood vessel. Journal of biochemistry. 2005;138:47–55. doi: 10.1093/jb/mvi100. [DOI] [PubMed] [Google Scholar]
- Bataller R, Brenner DA. Liver fibrosis. Journal of clinical investigation. 2005;115:209–218. doi: 10.1172/JCI24282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beltran-Sanchez H, et al. Prevalence and trends of metabolic syndrome in the adult U.S. population, 1999–2010. Journal of the American College of Cardiology. 2013;62:697–703. doi: 10.1016/j.jacc.2013.05.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bode C, et al. Erythrocytes serve as a reservoir for cellular and extracellular sphingosine 1-phosphate. Journal of cellular biochemistry. 2010;109:1232–1243. doi: 10.1002/jcb.22507. [DOI] [PubMed] [Google Scholar]
- Brice SE, Cowart LA. Sphingolipid metabolism and analysis in metabolic disease. Advances in experimental medicine and biology. 2011;721:1–17. doi: 10.1007/978-1-4614-0650-1_1. [DOI] [PubMed] [Google Scholar]
- Brinkmann V, et al. Fingolimod (fty720): discovery and development of an oral drug to treat multiple sclerosis. Nature reviews drug discovery. 2010;9:883–897. doi: 10.1038/nrd3248. [DOI] [PubMed] [Google Scholar]
- Cantrell Stanford J, et al. Sphingosine 1-phosphate (S1P) regulates glucose-stimulated insulin secretion in pancreatic beta cells. The journal of biological chemistry. 2012;287:13457–13464. doi: 10.1074/jbc.M111.268185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang N, et al. Hur mediates motility of human bone marrow-derived mesenchymal stem cells triggered by sphingosine 1-phosphate in liver fibrosis. Journal of molecular medicine. 2017;95:69–82. doi: 10.1007/s00109-016-1460-x. [DOI] [PubMed] [Google Scholar]
- Chavez JA, Summers SA. A ceramide-centric view of insulin resistance. Cell metabolism. 2012;15:585–594. doi: 10.1016/j.cmet.2012.04.002. [DOI] [PubMed] [Google Scholar]
- Chen J, et al. Deletion of sphingosine kinase 1 ameliorates hepatic steatosis in diet-induced obese mice: role of PPARc. Biochimica et biophysica acta. 2016a;1861:138–147. doi: 10.1016/j.bbalip.2015.11.006. [DOI] [PubMed] [Google Scholar]
- Chen W, et al. Sphingosine 1-phosphate in metabolic syndrome (review) International journal of molecular medicine. 2016b;38:1030–1038. doi: 10.3892/ijmm.2016.2731. [DOI] [PubMed] [Google Scholar]
- Christensen PM, et al. Impaired endothelial barrier function in apolipoprotein M-deficient mice is dependent on sphingosine-1-phosphate receptor 1. The FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2016;30:2351–2359. doi: 10.1096/fj.201500064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christoffersen C, et al. Endothelium-protective sphingosine-1-phosphate provided by HDL-associated apolipoprotein m. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:9613–9618. doi: 10.1073/pnas.1103187108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen JA, Chun J. Mechanisms of fingolimod's efficacy and adverse effects in multiple sclerosis. Annals of neurology. 2011;69:759–777. doi: 10.1002/ana.22426. [DOI] [PubMed] [Google Scholar]
- Crespo I, et al. Melatonin inhibits the sphingosine kinase 1/sphingosine-1-phosphate signaling pathway in rabbits with fulminant hepatitis of viral origin. Journal of pineal research. 2016;61:168–176. doi: 10.1111/jpi.12335. [DOI] [PubMed] [Google Scholar]
- Davaille J, et al. Antiproliferative properties of sphingosine 1-phosphate in human hepatic myofibroblasts. A cyclooxygenase-2 mediated pathway. The journal of biological chemistry. 2000;275:34628–34633. doi: 10.1074/jbc.M006393200. [DOI] [PubMed] [Google Scholar]
- Davaille J, et al. Sphingosine 1-phosphate triggers both apoptotic and survival signals for human hepatic myofibroblasts. The journal of biological chemistry. 2002;277:37323–37330. doi: 10.1074/jbc.M202798200. [DOI] [PubMed] [Google Scholar]
- Ding BS, et al. HDL activation of endothelial sphingosine-1-phosphate receptor-1 (S1P1) promotes regeneration and suppresses fibrosis in the liver. JCI insight. 2016;1:e87058. doi: 10.1172/jci.insight.87058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fayyaz S, et al. Involvement of sphingosine 1-phosphate in palmitate-induced insulin resistance of hepatocytes via the S1P2 receptor subtype. Diabetologia. 2014;57:373–382. doi: 10.1007/s00125-013-3123-6. [DOI] [PubMed] [Google Scholar]
- Feingold KR, et al. Infection and inflammation decrease apolipoprotein M expression. Atherosclerosis. 2008;199:19–26. doi: 10.1016/j.atherosclerosis.2007.10.007. [DOI] [PubMed] [Google Scholar]
- Fox TE, et al. Circulating sphingolipid biomarkers in models of type 1 diabetes. Journal of lipid research. 2011;52:509–517. doi: 10.1194/jlr.M010595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frej C, et al. Quantification of sphingosine 1-phosphate by validated LC–MS/MS method revealing strong correlation with apolipoprotein m in plasma but not in serum due to platelet activation during blood coagulation. Analytical and bioanalytical chemistry. 2015;407:8533–8542. doi: 10.1007/s00216-015-9008-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvani S, et al. HDL-bound sphingosine 1-phosphate acts as a biased agonist for the endothelial cell receptor S1P1 to limit vascular inflammation. Science signalling. 2015;8:ra79. doi: 10.1126/scisignal.aaa2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng T, et al. SPHK1 mediates hepatic inflammation in a mouse model of NASH induced by high saturated fat feeding and initiates proinflammatory signaling in hepatocytes. Journal of lipid research. 2015;56:2359–2371. doi: 10.1194/jlr.M063511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grammatikos G, et al. Variations in serum sphingolipid levels associate with liver fibrosis progression and poor treatment outcome in hepatitis C virus but not hepatitis B virus infection. Hepatology. 2015;61:812–822. doi: 10.1002/hep.27587. [DOI] [PubMed] [Google Scholar]
- Hait NC, et al. Regulation of histone acetylation in the nucleus by sphingosine-1-phosphate. Science. 2009;325:1254–1257. doi: 10.1126/science.1176709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanel P, et al. Erythrocytes store and release sphingosine 1-phosphate in blood. The FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2007;21:1202–1209. doi: 10.1096/fj.06-7433com. [DOI] [PubMed] [Google Scholar]
- Hannun YA, Obeid LM. Principles of bioactive lipid signalling: lessons from sphingolipids. Nature reviews molecular cell biology. 2008;9:139–150. doi: 10.1038/nrm2329. [DOI] [PubMed] [Google Scholar]
- Holland WL, et al. Receptor-mediated activation of ceramidase activity initiates the pleiotropic actions of adiponectin. Nature medicine. 2011;17:55–63. doi: 10.1038/nm.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland WL, et al. An fgf21-adiponectin-ceramide axis controls energy expenditure and insulin action in mice. Cell metabolism. 2013;17:790–797. doi: 10.1016/j.cmet.2013.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda H, et al. Sphingosine 1-phosphate regulates regeneration and fibrosis after liver injury via sphingosine 1-phosphate receptor 2. Journal of lipid research. 2009;50:556–564. doi: 10.1194/jlr.M800496-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda H, et al. Plasma concentration of bioactive lipid mediator sphingosine 1-phosphate is reduced in patients with chronic hepatitis C. Clinica chimica acta; international journal of clinical chemistry. 2010;411:765–770. doi: 10.1016/j.cca.2010.02.063. [DOI] [PubMed] [Google Scholar]
- Jeong JK, et al. Modulation of the expression of sphingosine 1-phosphate 2 receptors regulates the differentiation of pre-adipocytes. Molecular medicine reports. 2015;12:7496–7502. doi: 10.3892/mmr.2015.4388. [DOI] [PubMed] [Google Scholar]
- Jonnalagadda D, et al. Granule-mediated release of sphingosine-1-phosphate by activated platelets. Biochimica et biophysica acta. 2014;1841:1581–1589. doi: 10.1016/j.bbalip.2014.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko T, et al. Sphingosine-1-phosphate receptor agonists suppress concanavalin a-induced hepatic injury in mice. Biochemical and biophysical research communications. 2006;345:85–92. doi: 10.1016/j.bbrc.2006.04.067. [DOI] [PubMed] [Google Scholar]
- Kowalski GM, et al. Plasma sphingosine-1-phosphate is elevated in obesity. PLoS one. 2013;8:e72449. doi: 10.1371/journal.pone.0072449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowalski GM, et al. Overexpression of sphingosine kinase 1 in liver reduces triglyceride content in mice fed a low but not high-fat diet. Biochimica et biophysica acta. 2015;1851:210–219. doi: 10.1016/j.bbalip.2014.12.002. [DOI] [PubMed] [Google Scholar]
- Kumaraswamy SB, et al. Decreased plasma concentrations of apolipoprotein m in sepsis and systemic inflammatory response syndromes. Critical care. 2012;16:R60. doi: 10.1186/cc11305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurano M, et al. Liver involvement in sphingosine 1-phosphate dynamism revealed by adenoviral hepatic overexpression of apolipoprotein M. Atherosclerosis. 2013;229:102–109. doi: 10.1016/j.atherosclerosis.2013.04.024. [DOI] [PubMed] [Google Scholar]
- Kurano M, et al. LDL receptor and apoE are involved in the clearance of apoM-associated sphingosine 1-phosphate. The journal of biological chemistry. 2015;290:2477–2488. doi: 10.1074/jbc.M114.596445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurek K, et al. Inhibition of ceramide de novo synthesis reduces liver lipid accumulation in rats with nonalcoholic fatty liver disease. Liver international: official journal of the liver. 2014;34:1074–1083. doi: 10.1111/liv.12331. [DOI] [PubMed] [Google Scholar]
- Lee SY, et al. Activation of sphingosine kinase 2 by endoplasmic reticulum stress ameliorates hepatic steatosis and insulin resistance in mice. Hepatology. 2015;62:135–146. doi: 10.1002/hep.27804. [DOI] [PubMed] [Google Scholar]
- Lei YC, et al. Sphingosine kinase 1 dependent protein kinase c-delta activation plays an important role in acute liver failure in mice. World journal of gastroenterology. 2015a;21:13438–13446. doi: 10.3748/wjg.v21.i48.13438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei YC, et al. Inhibition of sphingosine kinase 1 ameliorates acute liver failure by reducing high-mobility group box 1 cytoplasmic translocation in liver cells. World journal of gastroenterology. 2015b;21:13055–13063. doi: 10.3748/wjg.v21.i46.13055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei YC, et al. C5a/c5aR pathway is essential for up-regulating SPHK1 expression through p38-mapk activation in acute liver failure. World journal of gastroenterology. 2016;22:10148–10157. doi: 10.3748/wjg.v22.i46.10148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, et al. Homing of bone marrow mesenchymal stem cells mediated by sphingosine 1-phosphate contributes to liver fibrosis. Journal of hepatology. 2009;50:1174–1183. doi: 10.1016/j.jhep.2009.01.028. [DOI] [PubMed] [Google Scholar]
- Li C, et al. Sphingosine 1-phosphate (S1P)/S1P receptors are involved in human liver fibrosis by action on hepatic myofibroblasts motility. Journal of hepatology. 2011;54:1205–1213. doi: 10.1016/j.jhep.2010.08.028. [DOI] [PubMed] [Google Scholar]
- Liu Y, et al. Hepatopoietin Cn reduces ethanol-induced hepatoxicity via sphingosine kinase 1 and sphingosine 1-phosphate receptors. The journal of pathology. 2013;230:365–376. doi: 10.1002/path.4194. [DOI] [PubMed] [Google Scholar]
- Liu M, et al. Hepatic apolipoprotein M (apoM) overexpression stimulates formation of larger apoM/sphingosine 1-phosphate-enriched plasma high density lipoprotein. The journal of biological chemistry. 2014;289:2801–2814. doi: 10.1074/jbc.M113.499913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M, et al. Uncleaved apoM signal peptide is required for formation of large apoM/sphingosine 1-phosphate (S1P)-enriched HDL particles. The journal of biological chemistry. 2015;290:7861–7870. doi: 10.1074/jbc.M114.631101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu ZP, et al. Hepatitis B virus x protein promotes human hepatoma cell growth via upregulation of transcription factor ap2alpha and sphingosine kinase 1. Acta pharmacologica sinica. 2015;36:1228–1236. doi: 10.1038/aps.2015.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma MM, et al. Sphingosine kinase 1 participates in insulin signalling and regulates glucose metabolism and homeostasis in kk/ay diabetic mice. Diabetologia. 2007;50:891–900. doi: 10.1007/s00125-006-0589-5. [DOI] [PubMed] [Google Scholar]
- Maceyka M, et al. Sphingosine-1-phosphate signaling and its role in disease. Trends in cell biology. 2012;22:50–60. doi: 10.1016/j.tcb.2011.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maceyka M, Spiegel S. Sphingolipid metabolites in inflammatory disease. Nature. 2014;510:58–67. doi: 10.1038/nature13475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauer AS, et al. Inhibition of sphingosine 1-phosphate signaling ameliorates murine nonalcoholic steatohepatitis. American journal of physiology Gastrointestinal and liver physiology. 2017;312:G300–G313. doi: 10.1152/ajpgi.00222.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michels M, et al. Decreased plasma levels of the endothelial protective sphingosine-1-phosphate are associated with dengue-induced plasma leakage. The journal of infection. 2015;71:480–487. doi: 10.1016/j.jinf.2015.06.014. [DOI] [PubMed] [Google Scholar]
- Moon MH, et al. Sphingosine-1-phosphate inhibits the adipogenic differentiation of 3t3-l1 preadipocytes. International journal of molecular medicine. 2014;34:1153–1158. doi: 10.3892/ijmm.2014.1856. [DOI] [PubMed] [Google Scholar]
- Nagahashi M, et al. Conjugated bile acid activated S1P receptor 2 is a key regulator of sphingosine kinase 2 and hepatic gene expression. Hepatology. 2015;61:1216–1226. doi: 10.1002/hep.27592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishi T, et al. Molecular and physiological functions of sphingosine 1-phosphate transporters. Biochimica et biophysica acta. 2014;841:759–765. doi: 10.1016/j.bbalip.2013.07.012. [DOI] [PubMed] [Google Scholar]
- Nowatari T, et al. Sphingosine 1-phosphate has anti-apoptotic effect on liver sinusoidal endothelial cells and proliferative effect on hepatocytes in a paracrine manner in human. Hepatology research: the official journal of the Japan Society of Hepatology. 2015;45:1136–1145. doi: 10.1111/hepr.12446. [DOI] [PubMed] [Google Scholar]
- Orr Gandy KA, Obeid LM. Targeting the sphingosine kinase/sphingosine 1-phosphate pathway in disease: review of sphingosine kinase inhibitors. Biochimica et biophysica acta. 2013;1831:157–166. doi: 10.1016/j.bbalip.2012.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osawa Y, et al. Roles for c16-ceramide and sphingosine 1-phosphate in regulating hepatocyte apoptosis in response to tumor necrosis factor-alpha. The journal of biological chemistry. 2005;280:27879–27887. doi: 10.1074/jbc.M503002200. [DOI] [PubMed] [Google Scholar]
- Osawa Y, et al. Acid sphingomyelinase regulates glucose and lipid metabolism in hepatocytes through Akt activation and amp-activated protein kinase suppression. The FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2011;25:1133–1144. doi: 10.1096/fj.10-168351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagadala M, et al. Role of ceramides in nonalcoholic fatty liver disease. Trends in endocrinology and metabolism: TEM. 2012;23:365–371. doi: 10.1016/j.tem.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pappu R, et al. Promotion of lymphocyte egress into blood and lymph by distinct sources of sphingosine-1-phosphate. Science. 2007;316:295–298. doi: 10.1126/science.1139221. [DOI] [PubMed] [Google Scholar]
- Park SW, et al. Sphinganine-1-phosphate attenuates both hepatic and renal injury induced by hepatic ischemia and reperfusion in mice. Shock. 2010;33:31–42. doi: 10.1097/SHK.0b013e3181c02c1f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellicoro A, et al. Liver fibrosis and repair: immune regulation of wound healing in a solid organ. Nature reviews Immunology. 2014;14:181–194. doi: 10.1038/nri3623. [DOI] [PubMed] [Google Scholar]
- Perz JF, et al. The contributions of hepatitis B virus and hepatitis C virus infections to cirrhosis and primary liver cancer worldwide. Journal of hepatology. 2006;45:529–538. doi: 10.1016/j.jhep.2006.05.013. [DOI] [PubMed] [Google Scholar]
- Pyne NJ, Pyne S. Sphingosine 1-phosphate and cancer. Nature reviews Cancer. 2010;10:489–503. doi: 10.1038/nrc2875. [DOI] [PubMed] [Google Scholar]
- Qi Y, et al. Sphingosine kinase 1 protects hepatocytes from lipotoxicity via down-regulation of IRE1α protein expression. The journal of biological chemistry. 2015;290:23282–23290. doi: 10.1074/jbc.M115.677542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehermann B, Nascimbeni M. Immunology of hepatitis B virus and hepatitis C virus infection. Nature reviews Immunology. 2005;5:215–229. doi: 10.1038/nri1573. [DOI] [PubMed] [Google Scholar]
- Rosenberg AJ, et al. Design, synthesis, and in vitro and in vivo evaluation of an (18)f-labeled sphingosine 1-phosphate receptor 1 (S1P1) pet tracer. Journal of medicinal chemistry. 2016;59:6201–6220. doi: 10.1021/acs.jmedchem.6b00390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross JS, et al. Sphingosine kinase 1 is regulated by peroxisome proliferator-activated receptor alpha in response to free fatty acids and is essential for skeletal muscle interleukin-6 production and signaling in diet-induced obesity. The journal of biological chemistry. 2013;288:22193–22206. doi: 10.1074/jbc.M113.477786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz M, et al. High-density lipoprotein-associated apolipoprotein M limits endothelial inflammation by delivering sphingosine-1-phosphate to the sphingosine-1-phosphate receptor 1. Arteriosclerosis, thrombosis, and vascular biology. 2017;37:118–129. doi: 10.1161/ATVBAHA.116.308435. [DOI] [PubMed] [Google Scholar]
- Sakamoto H, et al. Host sphingolipid biosynthesis as a target for hepatitis C virus therapy. Nature chemical biology. 2005;1:333–337. doi: 10.1038/nchembio742. [DOI] [PubMed] [Google Scholar]
- Saltiel AR, Kahn CR. Insulin signalling and the regulation of glucose and lipid metabolism. Nature. 2001;414:799–806. doi: 10.1038/414799a. [DOI] [PubMed] [Google Scholar]
- Sanyal AJ, Pacana T. A lipidomic readout of disease progression in a diet-induced mouse model of nonalcoholic fatty liver disease. Transactions of the American Clinical and Climatological Association. 2015;126:271–288. [PMC free article] [PubMed] [Google Scholar]
- Satapathy SK, Sanyal AJ. Epidemiology and natural history of nonalcoholic fatty liver disease. Seminars in liver disease. 2015;35:221–235. doi: 10.1055/s-0035-1562943. [DOI] [PubMed] [Google Scholar]
- Sato M, et al. Sphingosine kinase-1, S1P transporter spinster homolog 2 and S1P2 mRNA expressions are increased in liver with advanced fibrosis in human. Scientific reports. 2016;6:32119. doi: 10.1038/srep32119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider-Schaulies J, Schneider-Schaulies S. Sphingolipids in viral infection. Biological chemistry. 2015;396:585–595. doi: 10.1515/hsz-2014-0273. [DOI] [PubMed] [Google Scholar]
- Shi Y, et al. Sphingosine kinase-2 inhibition improves mitochondrial function and survival after hepatic ischemia-reperfusion. Journal of hepatology. 2012;56:137–145. doi: 10.1016/j.jhep.2011.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takabe K, Spiegel S. Export of sphingosine-1-phosphate and cancer progression. Journal of lipid research. 2014;9:1839–1846. doi: 10.1194/jlr.R046656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian T, et al. Sphingosine kinase 1 inhibition improves lipopolysaccharide/d-galactosamine-induced acute liver failure by inhibiting mitogen-activated protein kinases pathway. United European gastroenterology journal. 2016;4:677–685. doi: 10.1177/2050640616637968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umehara T, et al. Serine palmitoyltransferase inhibitor suppresses HCV replication in a mouse model. Biochemical and biophysical research communications. 2006;346:67–73. doi: 10.1016/j.bbrc.2006.05.085. [DOI] [PubMed] [Google Scholar]
- Wang X, et al. Morin reduces hepatic inflammation-associated lipid accumulation in high fructose-fed rats via inhibiting sphingosine kinase 1/sphingosine 1-phosphate signaling pathway. Biochemical pharmacology. 2013;86:1791–1804. doi: 10.1016/j.bcp.2013.10.005. [DOI] [PubMed] [Google Scholar]
- Wang R, et al. Exosome adherence and internalization by hepatic stellate cells triggers sphingosine 1-phosphate-dependent migration. Journal of biological chemistry. 2015;290:30684–30696. doi: 10.1074/jbc.M115.671735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, et al. The role of S1PR2 in bile acid-induced cholangiocyte proliferation and cholestasis-induced liver injury in mice. Hepatology. 2017;65:2005–2018. doi: 10.1002/hep.29076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watt MJ, et al. Regulation of plasma ceramide levels with fatty acid oversupply: evidence that the liver detects and secretes de novo synthesised ceramide. Diabetologia. 2012;55:2741–2746. doi: 10.1007/s00125-012-2649-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiu L, et al. Intracellular sphingosine 1-phosphate contributes to collagen expression of hepatic myofibroblasts in human liver fibrosis independent of its receptors. The American journal of pathology. 2015;185:387–398. doi: 10.1016/j.ajpath.2014.09.023. [DOI] [PubMed] [Google Scholar]
- Yamane D, et al. Regulation of the hepatitis C virus RNA replicase by endogenous lipid peroxidation. Nature medicine. 2014;20:927–935. doi: 10.1038/nm.3610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagida K, Hla T. Vascular and immunobiology of the circulatory sphingosine 1-phosphate gradient. Annual review of physiology. 2017;79:67–91. doi: 10.1146/annurev-physiol-021014-071635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, et al. Sphingosine kinase/sphingosine 1-phosphate (S1P)/S1P receptor axis is involved in liver fibrosis-associated angiogenesis. Journal of hepatology. 2013;59:114–123. doi: 10.1016/j.jhep.2013.02.021. [DOI] [PubMed] [Google Scholar]
- Yang L, et al. Sphingosine 1-phosphate receptor 2 and 3 mediate bone marrow-derived monocyte/macrophage motility in cholestatic liver injury in mice. Scientific reports. 2015;5:13423. doi: 10.1038/srep13423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yatomi Y. Plasma sphingosine 1-phosphate metabolism and analysis. Biochimica et biophysica acta. 2008;1780:606–611. doi: 10.1016/j.bbagen.2007.10.006. [DOI] [PubMed] [Google Scholar]
- Yatomi Y, et al. Sphingosine 1-phosphate induces platelet activation through an extracellular action and shares a platelet surface receptor with lysophosphatidic acid. The Journal of biological chemistry. 1997;272:5291–5297. doi: 10.1074/jbc.272.8.5291. [DOI] [PubMed] [Google Scholar]
- Zhang H, et al. Binding characteristics of sphingosine-1-phosphate to apoM hints to assisted release mechanism via the apoM calyx-opening. Scientific reports. 2016;6:30655. doi: 10.1038/srep30655. [DOI] [PMC free article] [PubMed] [Google Scholar]


