Abstract
OBJECTIVES
To examine the specificity of dementia coding in large populations.
DESIGN
Retrospective cohort and chart review study of dementia diagnosis.
SETTING
U.S. Department of Veterans Affairs (VA) New England healthcare system.
PARTICIPANTS
Veterans aged 50 and older given outpatient visit codes for dementia between January 1, 2000, and December 31, 2009.
MEASUREMENTS
The frequency of the code “dementia not otherwise specified (DNOS)” as a first and final diagnosis was determined. DNOS use was examined according to provider type and geographic location. The medical records of 100 individuals with unspecified dementia were reviewed to determine their underlying diagnoses and describe their examination.
RESULTS
Twenty-two thousand fifty veterans diagnosed with dementia were identified over 10 years of follow-up. One-third of all cases had no specific dementia code (n = 6,659). DNOS was the most commonly used code as a first dementia diagnosis (42.5%) and was second only to Alzheimer’s type dementia (35.8%) as a final diagnosis. Individuals who saw geriatricians and neurologists were most likely to have a specific dementia diagnosis, and DNOS use was lowest in centers with the most dementia specialists. Only 12% of primary care physicians performed cognitive testing the first time they used the DNOS code, compared with 98% of specialists. Nearly half of individuals with a persistent diagnosis of DNOS met criteria for a specific dementia.
CONCLUSION
Substantial overuse was found of non-specific dementia codes in the VA New England healthcare system, leading to an underestimation of the prevalence of Alzheimer’s disease and other dementias. System-based changes in dementia coding and greater access to dementia specialists may help improve diagnostic specificity.
Keywords: Alzheimer’s disease, coding, dementia, diagnosis, veterans
Currently, 5.4 million Americans suffer from Alzheimer’s disease, and by 2050, its prevalence is expected to triple,1 but the actual size and shape of this epidemic is difficult to grasp, because the disease is so often unrecognized. Even when dementia is diagnosed, providers may assign a nonspecific diagnostic International Classification of Diseases, Ninth Revision (ICD-9) code such as 294.8 (Dementia not otherwise specified (DNOS)). Distinguishing between specific dementias is critical clinically because treatment and prognosis may differ substantially based on the underlying diagnosis. Accurate coding for dementia is also important, because ICD codes are used to estimate disease incidence and prevalence, estimate the cost of care, obtain reimbursement, and conduct epidemiological research.2 Although a number of high-quality population-based studies have generated accurate data about the prevalence of specific dementias in the United States,3,4 few have focused on the prevalence of unspecified dementia. To better understand the specificity of dementia coding, the use of outpatient dementia visit codes in Integrated Service Network 1 (VISN-1), the New England region of the U.S. Department of Veterans Affairs healthcare system (VAHCS), was examined over a 10-year period.
When a VISN-1 clinician types in the keyword “dementia” to code a visit in the electronic health record, a drop-down list with 71 choices appears. The first three choices are dementia of the Alzheimer’s type (DAT), late onset (290.0), dementia not otherwise specified (294.8) and vascular dementia (290.4). Alzheimer’s disease (331.0) is not included. DNOS is a code generally reserved for use before the dementia examination is complete or if a clear diagnosis cannot be made after the examination. Thus, although it may be common as a first diagnosis, it should be an infrequent final diagnosis. The goal of this study was to describe the pattern of DNOS use in VISN-1 and to examine the medical records of individuals with a diagnosis of DNOS to better understand the lack of diagnostic specificity.
METHODS
VISN-1 Database Analysis
All veterans in VISN-1 who were given outpatient visit codes for dementia between January 1, 2000, and December 31, 2009, were identified using the National VA Patient Care database. The electronic medical record was used to review individual charts. Approval and a waiver of informed consent was obtained from the institutional review board of the Boston VAHCS.
Veterans were included if they had one or more of the following ICD-9 dementia codes: DAT (331.0, 290.0, 290.13, 290.2x, 290.3), vascular dementia (290.4x), frontotemporal dementia (331.1, 331.11), dementia with Lewy bodies (331.82), dementia of Parkinson’s disease (332.0 + 294.8), and other dementias: Huntington’s disease (333.4), alcohol related (291.1, 291.2), normal-pressure hydrocephalus (331.3+ a dementia code), and DNOS (294.8).
The frequency of DNOS and the other dementia categories as initial and final diagnoses was determined. Initial diagnosis was the first dementia code assigned. Final diagnoses was defined as patients with code(s) for only one specific dementia type and were considered to have that dementia, patients with codes for more than one specific dementia type were considered to have mixed dementia, and patients with codes for DNOS only had a final diagnosis of DNOS. Coding patterns of primary care physicians (PCPs), neuropsychologists, psychiatrists, geriatricians, and neurologists were then compared. Which provider gave the first dementia diagnosis was determined, and then the distribution of initial diagnostic codes was examined according to provider type.
Chart Review Study
One hundred patients from the Boston VAHCS whose only dementia diagnosis was DNOS were randomly selected for chart review. A medical student trained in data extraction (DB) and a geriatrician specializing in dementia care (JAD) reviewed each chart independently according to a predefined protocol. Any disagreement was clarified by consensus. Information on basic demographic characteristics, differential diagnosis, cognitive testing, neuroimaging, and dementia treatment was recorded. Reviewers determined whether participants met Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) criteria for dementia.5 Diagnoses were further classified into subtypes if possible according to DSM-IV and National Institute of Neurological and Communicative Disorders and Stroke and Alzheimer’s Disease and Related Disorders Association criteria for Alzheimer’s disease,6 DSM-IV and National Institute of Neurological Disorders and Stroke and Association Internationale pour la Recherché et l’Enseignement en Neurosciences criteria for vascular dementia,7 and accepted criteria for dementia with Lewy bodies8 and frontotemporal dementia.9 Reviewers recorded whether they agreed with the use of DNOS and the reason they thought the providers chose to use an unspecified code. Statistical analysis was primarily descriptive. Means were used for continuous variables and proportions for categorical variables. All analyses were performed using SAS version 9.0 (SAS Institute, Inc., Cary, NC).
RESULTS
VISN-1 Database Analysis
Over the 10-year follow-up period, 22,050 veterans diagnosed with dementia in New England were identified; 15,391 (69.8%) had codes for specific dementias and 6,659 (30.2%) for DNOS only. The frequency of dementia codes is presented in Table 1. DNOS was the most common first diagnosis (42.5%), followed by DAT (33.7%) and vascular dementia (13.9%). DAT was the most common final dementia diagnosis (35.8%), followed by DNOS (30.2%) and vascular dementia (13.1%). DNOS was the initial code for 35.6% of cases with dementia with Lewy bodies, 20.0% with vascular dementia, 19.2% with DAT, and 18.3% with mixed dementias.
Table 1.
Frequency of Dementia Code Usage as First and Final Dementia Diagnosis in the Veterans Affairs New England Healthcare System
| Code Used as First Dementia Diagnosis |
Code Used as Final Dementia Diagnosis |
|
|---|---|---|
|
|
|
|
| Dementia Type | N (%) | |
| Dementia not otherwise specified | 9,368 (42.5) | 6,659 (30.2) |
|
| ||
| Alzheimer’s type dementia | 7,443 (33.7) | 7,881 (35.8) |
|
| ||
| Vascular dementia | 3,063 (13.9) | 2,894 (13.1) |
|
| ||
| Dementia of Parkinson’s | 571 (2.6) | 571 (2.6) |
|
| ||
| Frontotemporal dementia | 441 (2.0) | 403 (1.8) |
|
| ||
| Lewy body dementia | 136 (0.6) | 188 (0.9) |
|
| ||
| Other dementiasa | 1,028 (4.7) | 1,023 (4.6) |
|
| ||
| Mixed dementiab | 2,431 (11.0) | |
|
| ||
| Total | 22,050 (100) | 22,050 (100) |
Includes alcohol-related dementias, normal-pressure hydrocephalus, and Huntington’s disease.
More than one specific dementia diagnosis given.
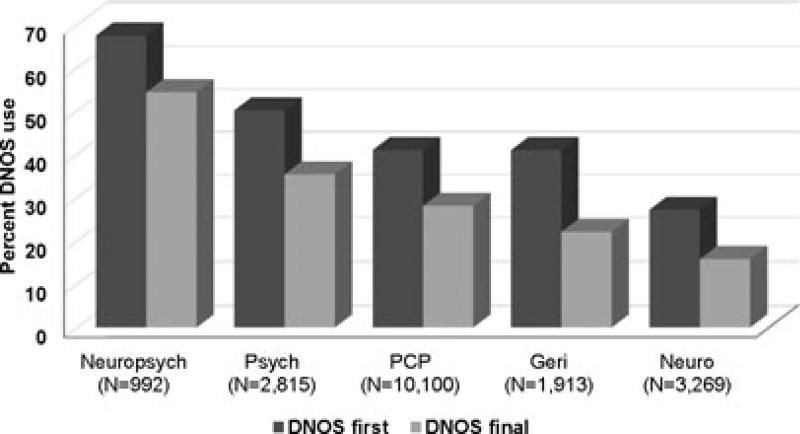
Neuropsychologists were most likely to use DNOS as a first diagnosis (67.5% of dementia diagnoses made), followed by mental health providers (50.2%) and PCPs and geriatricians (41.0% each). In comparison, neurologists used DNOS as an initial diagnosis in only 27.2% of patients (Figure 1). Individuals who saw geriatricians and neurologists were most likely to obtain a specific final diagnosis. There was wide regional variation in the use of DNOS codes. In Togus, Maine, DNOS was the initial code in 58.6% of all dementia cases and the final code in 42.7%. In contrast, DNOS accounted for only 28.9% of initial diagnoses and 19.9% of final diagnoses in Bedford, Massachusetts.
Figure 1.
Dementia not otherwise specified (DNOS) codes as a percentage of all initial and final dementia diagnoses by provider to give first dementia diagnosis. Neuropsych = neuropsychologist; Psych = psychiatrist; PCP = primary care physician; Geri = geriatrician; Neuro = neurologist.
Chart Review Study
The 100 participants with DNOS selected for chart review had a mean age of 80.2 ± 8.4. There was evidence of ongoing care from a non-VA provider in 60% of charts and from a non-VA neurologist in 32%. Sixty-three percent of individuals were current or past smokers, and one-third had used alcohol heavily.
PCP Use of DNOS
The veteran’s VA PCP gave the first DNOS code in 74 of 100 individuals evaluated. A non-VA physician previously diagnosed the majority of individuals (75.7%) with dementia. There was documentation of cognitive testing by PCPs in only 12% of charts at the time of the first DNOS code and only 2% on follow-up visits. PCPs identified a specific type of suspected dementia in their notes 30% of the time, and this was usually a diagnosis that an outside provider had previously given the individual. A VA dementia specialist referred half of the participants for evaluation. When evaluating individuals with no previous diagnosis of dementia (n = 18), PCPs performed cognitive testing in 17%, gave a differential diagnosis in 17%, and referred 66.7% to a specialist.
Reviewers agreed that PCPs’ use of DNOS as an initial code was reasonable in 64 cases (85.1%), mostly because of the absence of any documentation with which to make a specific diagnosis; 6.8% could have been given specific diagnoses based on information in the PCP’s notes, and 8.1% did not meet criteria for dementia at all. The most common reasons reviewers identified for the lack of a specific diagnosis were that a non-VA provider had previously diagnosed the individual with dementia and the PCP did not perform a confirmatory examination (49%); the PCP was waiting for additional examination (10%); and the participant refused further examination at the VA (10%).
Specialist Use of DNOS
Sixty-three of the patients reviewed saw a dementia specialist. A VA PCP had previously given 37 (59%) of these a DNOS diagnosis. Specialists included 42 neurologists (66.7%), 12 geriatricians (19.0%), seven psychiatrists (11.1%), and two neuropsychologists (3.2%). Cognitive testing was documented in 98.4% of specialists’ notes on the initial encounter and in 67% of charts on subsequent specialist visits; 69% had undergone a Mini-Mental State Examination (MMSE) or the equivalent, and 24.2% had detailed neuropsychological testing. Neuroimaging was available to the specialist at the first visit in 34 (54.8%), and specialists ordered imaging for 17 (28.3%). Specialists mentioned a specific dementia diagnosis in 57.1% of notes. DAT was the most frequent (19.1%), followed by vascular dementia (7.9%) and mixed DAT and vascular (7.9%). Reviewers agreed that 42.4% of the patients given a diagnosis of DNOS by specialists met criteria for a specific dementia diagnosis. The following reasons that specialists did not use a specific code were identified: mixed dementia was suspected (17.4%); waiting for additional testing or outside records (15.9%); no apparent reason, because adequate information to specify was in the chart (14.3%); and insufficient examination by the specialist to make a diagnosis (12.7%).
Underlying Diagnoses on Follow-Up
On review of all follow-up notes related to the dementia diagnosis, reviewers found that a specific diagnosis could have been given some time during follow-up in 48% of cases (Table 2). DAT was the most common underlying diagnosis, followed by vascular and mixed dementias.
Table 2.
Reviewer Diagnosis of 100 Charts Coded for Dementia Not Otherwise Specified Only
| Diagnosis | N (%) |
|---|---|
| No specific diagnosis | 52 (52) |
| Alzheimer’s type | 16 (16) |
| Vascular | 9 (9) |
| Mixed Alzheimer’s and vascular | 8 (8) |
| Dementia of Parkinson’s disease | 3 (3) |
| Mild cognitive impairment | 3 (3) |
| Cognitive disorder not otherwise specified | 2 (2) |
| Mental illness | 2 (2) |
| Alcohol related | 1 (1) |
| Normal pressure hydrocephalus | 1 (1) |
| Progressive supranuclear palsy | 1 (1) |
| Traumatic brain injury | 1 (1) |
| Late cognitive effects of stroke | 1 (1) |
DISCUSSION
More than one-third of individuals with dementia in the VA New England HCS are never given a diagnostic code for a specific dementia subtype. Veterans who saw geriatricians and neurologists were more likely to have a specific diagnosis, and neurologists were far less likely than other providers to code DNOS initially. VA centers with the most dementia care specialists had lower rates of DNOS use than other centers. On chart review, nearly half of veterans with a persistent diagnosis of DNOS met diagnostic criteria for a specific dementia. Few PCPs documented any evidence of cognitive testing, whereas dementia specialists almost always performed testing. Despite identifying a specific dementia in their note, specialists frequently continued to use DNOS. The majority of veterans with no specific dementia diagnosis were treated with dementia medications. Together, these findings show that specific dementia codes are substantially underused in VISN-1 and suggest that system-based changes might improve dementia diagnosis.
Population-based incidence studies estimate that DAT accounts for 63% to 76% of all dementia cases in the United States.3,10,11 In a subset of the Health and Retirement Study, DAT accounted for 69.9% of prevalent dementia and vascular dementia for 17.4%.4 All other dementia types, including “dementia of undetermined etiology” accounted for only 12.7% of cases. In contrast, 36% of the dementia cases in the current study were unspecified, and only 36% had DAT as a final diagnosis. In a chart review study of 410 individuals that dementia specialists diagnosed in the Houston VA Medical Cetner, DNOS accounted for 46.8% of all diagnoses.12 In a national VAHCS study, the prevalence of DAT was 44.6%, followed by other dementia (40.8%) and vascular dementia (11.9%).13 DNOS largely accounted for other dementia. A validation study of dementia diagnoses in the Danish Hospital Registers found an even higher prevalence of unspecified dementia codes.14 Of 197 randomly selected individuals with ICD-10 codes for dementia, 55.3% had a final diagnosis of DNOS. On expert chart review, 33% of these patients met criteria for specific dementias.
Substantial variation was found in dementia coding patterns according to provider type and region, suggesting that the specificity of dementia diagnoses is correlated with the availability of neurologists and geriatricians. In a non-VA study using a national database to identify individuals with DAT, the type of physician first seen strongly correlated with the likelihood of ever obtaining a specific diagnosis.15 Approximately 30% of individuals who saw a family practitioner first, 48% who saw a psychiatrist first, and 63% who saw a neurologist first were given a diagnosis of DAT at the initial consultation. Neuropsychologists had the highest rates of nonspecific code use in the current study, perhaps because they focus exclusively on cognitive testing and may not have access to other clinical information with which to make a definitive diagnosis. Boston and Bedford, Massachusetts, had the lowest rates of nonspecific code use. These VISN-1 sites are part of a VA Geriatric Research, Education and Clinical Center (GRECC), a specialized center for the care of elderly veterans; this GRECC has a specific focus on dementia and is staffed by neurologists and geriatricians. For most of the study period, the Boston VAHCS required specialist approval for dementia medications, fostering referral to these specialized dementia clinics. The data suggest that that strategy may be a successful method to improve the quality of dementia diagnosis and coding.
Nevertheless, chart review revealed that specialists overused nonspecific codes as well. Information with which to make a specific diagnosis was present in one-third of the specialists’ initial notes, yet they persisted in using DNOS. In about half of these, mixed dementia was suspected. Rather than coding for the primary process or using two codes, specialists chose not to specify. The program in electronic medical record that clinicians use to assign codes may explain this “diagnostic inertia.” Previously used codes are automatically listed and can be easily “clicked,” whereas use of a new code requires a few additional steps. Overall, nearly half of veterans evaluated could have been given a specific dementia diagnosis at some point during follow-up. In 8% of cases, specialists found that veterans referred to them with a diagnosis of DNOS did not have dementia. This underlines the importance of a thorough medical, psychiatric, and psychosocial evaluation for dementia, because mislabeling someone with this diagnosis can emotionally devastate them and their caregivers and deprive them of treatment for the true cause of their cognitive impairment.
The overuse of DNOS by PCPs was associated with a striking lack of examination for dementia on chart review. Few PCPs documented a differential diagnosis for the cognitive impairment or performed any cognitive testing. This may be in large part because outside providers previously examined and made the majority of the diagnoses the individuals had. Many veterans enter the VAHCS seeking coverage for expensive medications, including drugs for dementia, but documentation of a differential diagnosis or cognitive testing was only marginally higher in the 25% presenting to PCPs with a new cognitive complaint. This may reflect the attitude of PCPs that a specialist can best make a dementia diagnosis.16,17 Research has shown that, using a good history and standard cognitive tests, PCPs can diagnose dementia accurately more than 90% of the time,18,19 but PCPs consistently identify a variety of obstacles to dementia diagnosis in the primary care setting, including insufficient training20,21 and lack of time.17 These obstacles contribute not only to undercoding, but also to a much more important problem—missed and delayed dementia diagnosis.22 In one study of a large internal medicine practice, more than 75% of individuals with moderate to severe cognitive impairment were undiagnosed.23 The effect of educational initiatives to promote earlier recognition of dementia in primary care have been disappointing, and the most hopeful interventions may be those that address system-level obstacles.22
A number of caveats and limitations should be considered in the interpretation of these results. The VA is a federally funded healthcare system, and thus coding practices may be significantly different from those of other systems that are influenced by considerations of reimbursement. This study is limited to veterans, who are almost exclusively male and tend to have higher rates of physical and mental illness than a general population, limiting its generalizability. Veterans have higher rates of vascular disease, head trauma, alcohol abuse, and other factors that may contribute to cognitive impairment and make diagnosis of dementia more difficult. At least 50% of veterans with dementia receive care simultaneously from VA and non-VA providers.24 This may influence diagnosis and coding patterns, but it is difficult to identify such individuals accurately. Although the chart review yielded detailed and valuable information about physician practice patterns, the population was drawn exclusively from the Boston VAMC and may not be representative of all of VISN-1. In addition, chart review relies only on what is documented in the medical record and thus might underestimate the true prevalence of cognitive testing by PCPs. This work also has a number of strengths. Although many studies have examined the diagnosis and care of specific dementias in large healthcare systems, few have focused on the large proportion of individuals with nonspecific diagnoses. The large regional VISN-1 database allowed variations in dementia coding to be examined according to provider type and practice location.
CONCLUSION
In summary, a substantial overuse of nonspecific dementia codes was found in the New England region of the VAHCS, leading to an underestimation of the true prevalence of DAT and other dementias in this population. Nonspecific coding seems to reflect a lack of differential diagnosis and examination, particularly on the part of PCPs. These findings suggest two important system-level changes that might improve the accuracy of dementia coding and diagnosis in the VAHCS. First, computerized coding systems should be revised to foster accurate and specific coding and help providers avoid vague diagnoses. Second, automatic referral of individuals with newly identified cognitive impairment to dementia specialists would substantially increase the frequency of adequate dementia examination and diagnosis. Innovative models of care should be explored, including those that could provide access to providers with expertise in dementia diagnosis and dementia care management within the primary care setting.25 Of all U.S. healthcare systems, the VA is perhaps most amenable to such systemic changes. More research is needed to better understand the accuracy of specific dementia codes in the VAHCS and the prevalence of nonspecific code use in non-VA environments.
Acknowledgments
We would like to acknowledge Dr. Susan Cooley for her thoughtful reading of the manuscript and her comments.
Sponsor’s Role: The funders had no role in the design or conduct of the study; collection, management, analysis, or interpretation of the data; or preparation, review, or approval of the manuscript.
Funding: This research was funded by a VA Career Development award (JAD), and an American Federation for Aging Research MSTAR grant (DB). The Massachusetts Veteran’s Research and Information Center is supported by the VA Cooperative Studies Program.
Footnotes
This paper was presented in abstract form at the American Geriatrics Society Meeting in Washington, District of Columbia, May 2011.
Conflict of Interest: The authors report no disclosures or conflict of interest associated with this publication.
Author Contributions: Study conception and design: DB, NWK, JAD. Acquisition of data: EL, JMG. Analysis and interpretation of data: DB, NWK, EL, JMG, JAD. Drafting manuscript: JAD. Critical revision: DB, NWK, EL, JMG, JAD. Statistical analysis: JAD. Obtaining funding: JAD, EL, JMG. Supervision: NWK, JMG, JAD.
References
- 1.Thies W, Bleiler L. Alzheimer’s disease facts and figures. Alzheimers Dement. 2011;7:208–244. doi: 10.1016/j.jalz.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 2.Fillit H, Geldmacher DS, Welter RT, et al. Optimizing coding and reimbursement to improve management of Alzheimer’s disease and related dementias. J Am Geriatr Soc. 2002;50:1871–1878. doi: 10.1046/j.1532-5415.2002.50519.x. [DOI] [PubMed] [Google Scholar]
- 3.Hebert LE, Scherr PA, Beckett LA, et al. Age-specific incidence of Alzheimer’s disease in a community population. JAMA. 1995;273:1354–1359. [PubMed] [Google Scholar]
- 4.Plassman BL, Langa KM, Fisher GG, et al. Prevalence of dementia in the United States: The Aging, Demographics, and Memory Study. Neuroepidemiology. 2007;29:125–132. doi: 10.1159/000109998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Diagnostic and Statistical Manual of Mental Disorders. 4. Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- 6.Dubois B, Feldman HH, Jacova C, et al. Research criteria for the diagnosis of Alzheimer’s disease: Revising the NINCDS-ADRDA criteria. Lancet Neurol. 2007;6:734–746. doi: 10.1016/S1474-4422(07)70178-3. [DOI] [PubMed] [Google Scholar]
- 7.Roman GC, Tatemichi TK, Erkinjuntti T, et al. Vascular dementia: Diagnostic criteria for research studies. Report of the NINDS-AIREN International Workshop. Neurology. 1993;43:250–260. doi: 10.1212/wnl.43.2.250. [DOI] [PubMed] [Google Scholar]
- 8.McKeith IG, Dickson DW, Lowe J, et al. Diagnosis and management of dementia with Lewy bodies: Third report of the DLB Consortium. Neurology. 2005;65:1863–1872. doi: 10.1212/01.wnl.0000187889.17253.b1. [DOI] [PubMed] [Google Scholar]
- 9.The Lund and Manchester Groups. Clinical and neuropathological criteria for frontotemporal dementia. The Lund and Manchester Groups. J Neurol Neurosurg Psychiatry. 1994;57:416–418. doi: 10.1136/jnnp.57.4.416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bachman DL, Wolf PA, Linn RT, et al. Incidence of dementia and probable Alzheimer’s disease in a general population: The Framingham Study. Neurology. 1993;43:515–519. doi: 10.1212/wnl.43.3_part_1.515. [DOI] [PubMed] [Google Scholar]
- 11.Rocca WA, Cha RH, Waring SC, et al. Incidence of dementia and Alzheimer’s disease: A reanalysis of data from Rochester, Minnesota, 1975–1984. Am J Epidemiol. 1998;148:51–62. doi: 10.1093/oxfordjournals.aje.a009560. [DOI] [PubMed] [Google Scholar]
- 12.Kalkonde YV, Pinto-Patarroyo GP, Goldman T, et al. Differences between clinical subspecialties in the outpatient evaluation and treatment of dementia in an academic medical center. Dement Geriatr Cogn Disord. 2010;29:28–36. doi: 10.1159/000254701. [DOI] [PubMed] [Google Scholar]
- 13.Krishnan LL, Petersen NJ, Snow AL, et al. Prevalence of dementia among Veterans Affairs medical care system users. Dement Geriatr Cogn Disord. 2005;20:245–253. doi: 10.1159/000087345. [DOI] [PubMed] [Google Scholar]
- 14.Phung TK, Andersen BB, Hogh P, et al. Validity of dementia diagnoses in the Danish hospital registers. Dement Geriatr Cogn Disord. 2007;24:220–228. doi: 10.1159/000107084. [DOI] [PubMed] [Google Scholar]
- 15.Knopman D, Donohue JA, Gutterman EM. Patterns of care in the early stages of Alzheimer’s disease: Impediments to timely diagnosis. J Am Geriatr Soc. 2000;48:300–304. doi: 10.1111/j.1532-5415.2000.tb02650.x. [DOI] [PubMed] [Google Scholar]
- 16.Iliffe S, Manthorpe J, Eden A. Sooner or later? Issues in the early diagnosis of dementia in general practice: A qualitative study. Fam Pract. 2003;20:376–381. doi: 10.1093/fampra/cmg407. [DOI] [PubMed] [Google Scholar]
- 17.Turner S, Iliffe S, Downs M, et al. General practitioners’ knowledge, confidence and attitudes in the diagnosis and management of dementia. Age Ageing. 2004;33:461–467. doi: 10.1093/ageing/afh140. [DOI] [PubMed] [Google Scholar]
- 18.Larson EB, Edwards JK, O’Meara E, et al. Neuropathologic diagnostic outcomes from a cohort of outpatients with suspected dementia. J Gerontol A Biol Sci Med Sci. 1996;51A:M313–M318. doi: 10.1093/gerona/51a.6.m313. [DOI] [PubMed] [Google Scholar]
- 19.Rasmusson DX, Brandt J, Steele C, et al. Accuracy of clinical diagnosis of Alzheimer disease and clinical features of patients with non-Alzheimer disease neuropathology. Alzheimer Dis Assoc Disord. 1996;10:180–188. doi: 10.1097/00002093-199601040-00002. [DOI] [PubMed] [Google Scholar]
- 20.Baloch S, Moss SB, Nair R, et al. Practice patterns in the evaluation and management of dementia by primary care residents, primary care physicians, and geriatricians. Proc (Bayl Univ Med Cent) 2010;23:121–125. doi: 10.1080/08998280.2010.11928598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Iliffe S, Austin T, Wilcock J, et al. Design and implementation of a computer decision support system for the diagnosis and management of dementia syndromes in primary care. Methods Inf Med. 2002;41:98–104. [PubMed] [Google Scholar]
- 22.Bradford A, Kunik ME, Schulz P, et al. Missed and delayed diagnosis of dementia in primary care: Prevalence and contributing factors. Alzheimer Dis Assoc Disord. 2009;23:306–314. doi: 10.1097/WAD.0b013e3181a6bebc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Callahan CM, Hendrie HC, Tierney WM. Documentation and evaluation of cognitive impairment in elderly primary care patients. Ann Intern Med. 1995;122:422–429. doi: 10.7326/0003-4819-122-6-199503150-00004. [DOI] [PubMed] [Google Scholar]
- 24.Zhu CW, Penrod JD, Ross JS, et al. Use of Medicare and Department of Veterans Affairs health care by veterans with dementia: A longitudinal analysis. J Am Geriatr Soc. 2009;57:1908–1914. doi: 10.1111/j.1532-5415.2009.02405.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Judge KS, Bass DM, Snow AL, et al. Partners in dementia care: A care coordination intervention for individuals with dementia and their family caregivers. Gerontologist. 2011;51:261–272. doi: 10.1093/geront/gnq097. [DOI] [PubMed] [Google Scholar]