Abstract
It is widely accepted that human immunodeficiency virus type 1 (HIV-1) envelope glycoprotein 120 (gp120) plays an important role in HIV-1-induced neural injury and pathogenesis of HIV-1-associated dementia (HAND). Multiple pathways have been proposed for gp120-induced neurotoxicity, amongst is the activation of N-Methyl-D-Aspartate receptors (NMDARs). It has been shown that gp120 causes neuronal injury or death and gp120 transgenic mice exhibit neurological similarity to that of HAND, all of which can be blocked or attenuated by NMDAR antagonists. Several lines of evidence indicate the subtype and location of activated NMDARs are key determinants of the nature of NMDAR physiology. To examine the subtype and the location of NMDARs affected by gp120, we studied gp120 on subtype NMDAR-mediated EPSCs in the CA1 region of rat hippocampal slices through “blind” whole-cell patch recordings. Our results showed bath application of gp120 increased both NR2A- and NR2B-mediated EPSCs possibly via a presynaptic mechanism, with much stronger effect on NR2B-mediated EPSCs. In contrast, gp120 failed on enhancing AMPA receptor-mediated EPSCs. Ca2+ imaging studies revealed that gp120 potentiated glutamate-induced increase of intracellular Ca2+ concentration in rat hippocampal neuronal cultures which were blocked by a NMDAR antagonist, but not by an AMPA receptor antagonist, indicating gp120 induce Ca2+ influx through NMDARs. Further investigation demonstrated that gp120 increased the EPSCs mediated by extrasynaptic NR2BRs. Taken together, these results demonstrate that gp120 interacts with both NR2A and NR2B subtypes of NMDARs with a predominant action on the extrasynaptic NR2B, which may implicate a role NR2B may play in HIV-1-associated neuropathology.
Keywords: HIV, synaptic transmission, CA1, glutamate receptors, neurodegeneration
Introduction
Brain infection with human immunodeficiency virus type 1 (HIV-1) often provokes neurocognitive impairment termed collectively as HIV-1-associated neurocognitive disorders (HAND) (Antinori et al., 2007). The severity of HAND can vary, from asymptomatic to mild neurocognitive impairment and in its most severe form, a debilitating dementia commonly called HIV-1-associated dementia (HAD) (Antinori et al., 2007; Grant, 2008). Although the introduction of combination antiretroviral therapy (cART) has significantly decreased the incidence of HAD, the milder forms of HAND remains prevalent even in the era of cART (Heaton et al., 2010; Heaton et al., 2011; Alfahad and Nath, 2013). The causes for continuing high rates of HAND in the cART era are uncertain, but multifactorial mechanisms have been proposed including, but not limited to, incomplete viral suppression in the central nervous system (CNS) due to poor CNS penetration of some commonly used antiretroviral drugs, presence of drug-resistant viral stain, possible neurotoxicity of cART and the possibility that even very low levels of viral replication in the CNS could induce neural injury or dysfunction due to a prolonged exposure to neuro-inflammatory responses and neurotoxic viral proteins(Heaton et al., 2011; Jaeger and Nath, 2012; Gates and Cysique, 2016). Among the viral proteins is HIV-1 envelope glycoprotein 120 (gp120) that has been implicated as having neurotoxic effects in the CNS(Zhang et al., 2011; Hoefer et al., 2015).
Gp120 is a viral product with neurotoxic activity in HIV-1-infected brains. Shed off from virions and/or secreted from HIV-1-infected mononuclear phagocytes, gp120 has the potential to diffuse and interact directly with surrounding and distant neural cells possibly through the chemokine receptor CXCR4 (Kaul et al., 2001). Alternatively, it may act indirectly on local and distant neural cells by stimulating uninfected mononuclear phagocytes (brain perivascular macrophages and microglia) to release soluble cellular factors (Nath, 2002). Studies have demonstrated that gp120 induces neuronal apoptotic death in vitro at very low (picomolar) concentrations and its neurotoxic effects were dose-dependent with and without the presence of glial cells (Meucci and Miller, 1996; Kaul and Lipton, 1999). Indeed, the gp120-induced apoptosis has been observed in both human neurons and rat neuronal cultures (Meucci and Miller, 1996; Lannuzel et al., 1997; Acquas et al., 2004). When administered systematically in rats, gp120 produced not only neuronal apoptosis but cognitive deficit as well (Glowa et al., 1992). Moreover, transgenic mice constitutively overexpressing gp120 in astrocytes in the brain display a spectrum of neuronal and glial changes resembling abnormalities in the brains of HIV-1-infected patients with HAND(Toggas et al., 1994; Hoefer et al., 2015). Nevertheless, gp120 has been implicated in HIV-1-associated neurotoxic activity and HAND pathogenesis via multiple mechanisms, prominent among the mechanisms is its interaction with N-Methyl-D-Aspartate (NMDA) receptors (NMDAR)(Kaul et al., 2001; Kaul, 2008).
Functional NMDARs are multimeric assemblies composed of at least one obligatory NR1 subunit (Dingledine et al., 1999; Cull-Candy et al., 2001) and two or more NR2 subunits (Behe et al., 1995). The NR1 subunit is ubiquitously expressed through the CNS and contains the glycine-binding site (Kuryatov et al., 1994). The NR2 subunits (A, B, C and D) contain the glutamate-binding site and their expression is time- and tissue-specific (Monyer et al., 1994). It has been shown that the HIV-1-associated neuronal injury and death can be attenuated or prevented by NMDAR antagonists, suggesting an involvement of NMDAR in HIV-1-associated neurotoxicity (Muller et al., 1996; Kaul et al., 2001; Anderson et al., 2004; O'Donnell et al., 2006; Shin et al., 2012). However, most of previous studies have focused on an indirect mechanism for HIV-1/gp120-assoociated neurotoxicity, that is, HIV-1-infected or gp120-stimulated mononuclear phagocytes release soluble molecules leading to NMDAR activation and resultant neuronal injury (O'Donnell et al., 2006; Yang et al., 2013). Relative fewer studies have investigated direct interactions of gp120 and NMDAR-mediated synaptic transmission. To these ends, we examined effects of gp120 on NMDAR-mediated excitatory postsynaptic currents (EPSCs) recorded in the CA1 neurons of rat hippocampal slices using whole-cell patch recording technique. Our results revealed that gp120 enhanced NMDAR-mediated EPSCs via a presynaptic mechanism.
Materials and methods
Animals
Male Sprague-Dawley rats (15 to 35 d old), purchased from Charles River Laboratories (Wilmington, MA) were used for preparation of hippocampal brain slices. Animals were housed at a constant temperature (22°C) and relative humidity (50%) under a regular light-dark cycle (light on at 7:00 AM and off at 5:00 PM) with free access to food and water. All animal use procedures were strictly reviewed by the Institutional Animal Care and Use Committee (IACUC) of the University of Nebraska Medical Center (IACUC # 00-062-07).
Chemical and biological reagents
Drugs used in this study were HIV-1gp120 IIIB (Immunodiagnostics, Inc. Woburn, MA), T140 was kindly provided by Professor Nobutaka Fujii (Kyoto, Japan). 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX), 2-amino-5-phosphonovalerate (APV), (R)-3-(2-Carboxypiperazin-4-yl) propyl-1-phosphonic acid (R-CPP), ifenprodil, tetrodotoxin (TTX) and picrotoxin were purchased from TOCRIS Bioscience (Ellisville, MO). Picrotoxin was dissolved in dimethyl sulfoxide (DMSO) and the final DMSO concentration in artificial cerebrospinal fluid (ACSF) was less than or equal to 0.1%. TTX, CNQX, APV, R-CPP, and ifenprodil were pre-prepared separately in 1000× stock solutions and stored at -20°C refrigerator, thawed on experimental day just before use and diluted to the test concentrations. Drugs were applied onto brain slices via bath perfusion or added to the culture media. For bath perfusion, the time needed for a drug to reach the chamber was about 1 min. Unless otherwise indicated, all other chemicals were purchased from Sigma-Aldrich (St. Louis, MO).
Hippocampal brain slices and electrophysiology
Rat hippocampal brain slices were prepared as previously described (Xiong et al., 1996). Briefly, animals were anesthetized with isoflurane and decapitated, and brains were quickly removed from the cranial cavity. The brain was quickly removed from cranial cavity and placed into an ice-cold (∼4°C) oxygenated ACSF contained (in mM): NaCl 124.0, KCl 3.0, CaCl2 2.0, MgCl2 2.0, NaH2PO4 1.25, NaHCO3 26.0, glucose 10.0 and TTX 0.1 μM. ACSF was saturated with 95% O2 and 5% CO2 and had a pH of 7.4-7.5. The hippocampi were dissected free, and transverse hippocampal slices (400μM in thickness) were cut using a tissue chopper. The slices were then incubated in oxygenated ACSF at room temperature for at least 1 h before being transferred into a recording chamber. During electrophysiological experiments, a single hippocampal slice was transferred into the recording chamber each time and superfused with ACSF at a constant flow rate of 2.5 ml/min with the use of a peristaltic pump (Rainin Instrument Co., Woburn, MA). The temperature of the perfusion was maintained at 30±1°C with an automatic temperature controller (Warner Instrument Corp., Hamden, CT). To prevent epileptic activities, a surgical cut was made between CA1 and CA3 areas after the slice was transferred to the recording chamber.
Whole-cell patch clamp recordings were made from CA1 neurons using a “blind” method with microelectrodes (5.0-9.0 MΩ) filled with (in mM): K-gluconate 130.0, K-methysulphate 17.5, NaCl 8.0, HEPES 10.0, Mg-ATP 2.0, GTP 0.2, EGTA 0.1, pH 7.25-7.35 (adjusted with KOH), osmolality 292-308 mOsm. The recording microelectrodes were made from borosilicated glass capillaries (WPI, Sarasota, FL) with inner filaments that allow for quick back-filling. In the CA1 pyramidal cell layer, the neuronal cells recorded were voltage-clamed at -70mV and excitatory postsynaptic currents (EPSCs) were evoked by a constant current stimulation (30-120μA in intensity, 20 μsec in duration, 0.05 Hz) of Schaffer collateral fibers using an insulated bipolar tungsten electrode (except for the tip) and amplified by an Axopatch-1D amplifier (Molecular Devices, Union City, CA). To eliminate the possibility that gp120 activates inhibitory interneurons, all experiments were conducted in the presence of 50μM picrotoxin. The NMDAR-mediated EPSCs (EPSCnmdar) were recorded at holding potential of -50 mV in low-Mg2+ (0.5mM), high-Ca2+ (2.5mM) ACSF supplemented with glycine (1μM) and CNQX (10μM), and the isolated EPSCNMDAR was confirmed by AP-V(50μM) reversible blockade. AMPAR-mediated EPSCs (EPSCAMPAR) were recorded at holding potential of -70 mV in the presence of AP-V (50μM) in ACSF, and the isolated EPSCAMPAR was confirmed by CNQX (10μM) reversible blockade. The isolation of NR2A-containing NMDAR (NR2AR)-mediated EPSCs (EPSCNR2AR) or NR2B-containing NMDAR (NR2BR)-mediated EPSCs (EPSCNR2BR) was achieved by using a specific NR2AR antagonist R-CPP (Feng et al., 2005) or a specific NR2BR antagonist ifenprodil (Williams, 1993), respectively. The spontaneous mini EPSCs (mEPSCs) were recorded in gap-free mode with the holding potential of -70mV for recording mEPSCAMPAR or -50mV for recording mEPSCNMDAR. Paired-pulse facilitation (PPF) of EPSCNMDAR was recorded from the CA1 neurons in rat hippocampal slices in low-Mg2+ (0.5mM), high-Ca2+ (2.5mM) ACSF supplemented with glycine (1 μM) and AMPA receptor antagonist CNQX (10μM). The cells recorded were voltage-clamped at -50 mV. The PPF ratio (PPFR) was calculated as the percentage (%) by dividing the peak amplitude of the first EPSCNMDAR from the amplitude of the second EPSCNMDAR.
A 5 to 10 min control recording was made in each experiment once the adjustment of stimulation parameters had been achieved after rupture of the cell membrane. Cells with resting membrane potential more negative than -55 mV were used for the study. Each recording trial consisted of an average of three consecutive sweeps. The evoked signals were filtered at 1 kHz and digitized at 2.5 or 5.0 kHz using a Digidata 1440A interface (Molecular Devices). pCLAMP 10 software (Molecular Devices) was used for data acquisition and analyses. The series resistance was constantly monitored by delivering a hyperpolarizing voltage pulse during recording and the cells with > 20% changes in access resistance were excluded from the analysis.
Primary hippocampal neuronal culture
Hippocampal neuronal cultures were prepared from the embryonic (E18) Sprague-Dawley rats (Charles River Laboratories) using the methods described previously (Blair et al., 2008). Briefly, female rats with 18-19 days of gestation were anesthetized with isoflurane, and embryonic pups were surgically dissected out and decapitated. Hippocampi were harvested under sterile conditions. The hippocampal tissue was enzymatically dissociated in 0.125% trypsin II (Sigma-Aldrich). Hippocampal neural cells were isolated and placed in poly-D-lysine-coated 35 mm plastic culture dishes contained 2 ml of neurobasal medium to a culture cell density of 5×105 cells/ml. The cultures were maintained in neurobasal medium supplemented with B27 (2%, v/v, Invitrogen, San Diego, CA), L-glutamine (0.5mM) and 1% penicillin/streptomycin for at least 7-10 days before being used for experiments.
Calcium imaging
Rat fetal hippocampal neurons were plated on poly-d-lysine-coated 25-mm glass coverslips and cultured in neurobasal medium for 14 days before being used for calcium imaging. On the experimental day, medium was removed and cells were loaded with 7μM Fura-II AM (Molecular Probes, Eugene, OR, USA) in Mg+2-free external solution contained (in mM) 145 NaCl, 5 KCl, 2 CaCl2, 10 HEPES, and 10 glucose, pH 7.4 with NaOH and supplemented with 0.018% pluronic acid (Molecular Probes) and 0.09% dimethyl sulfoxide (DMSO) for 40–50 min at 37°C. Then cells were rinsed 4 times with external solution, mounted into a perfusion chamber, and perfused with the external solution at room temperature. Fields including 10–20 cells were selected, and the change in intracellular calcium level was measured by monitoring the excitation intensity at 340 nm and 380 nm wavelengths using a photometer (Photon Technology International, London, ON, Canada) coupled to an inverted Nikon TMD Diaphot epifluorescent microscope (Nikon, Melville, NY, USA). All measurements were made at room temperature. Each cell in the image was analyzed independently for each time point in the captured sequence. Intracellular calcium of the cell was presented by the ratio of fluorescent signals (F340/F380). During experiments, neurons were continuously perfused with external solution containing 0.1 μM TTX and 1μM glycine at a flow rate of 2 ml/min. Drugs (glutamate, GP120, CNQX, APV) were applied through four parallel tubes connected to the perfusion chamber. The intracellular calcium concentrations was calculated as follows: [Ca2+]i = Kd [(R - Rmin)/(Rmax - R)] (380min/380max), where Rmin and Rmax are the fluorescence ratios in the absence (with 3 mM EGTA) or presence of saturating calcium (3 mM), respectively, and Kd = 224 nM. Excitation control, image acquisition and outline analyses of images were performed using a PTI Deltascan system as described previously (Whitney et al., 2008).
Data Analyses
Data were analyzed and displayed using Clampfit 10 (Molecular Devices), Mini Analysis Program (Synaptosoft Inc, Decatur, GA) and Origin 8.5 (Northampton, MA). All numerical data were expressed as mean ± SEM unless otherwise indicated. Statistical significance was determined using ANOVA for a multi-group data (Figs 3, 7 and 8), or two-tailed Student's t-tests (the rest data). The level of significance was determined at p < 0.05.
Figure 3. Gp120 enhancement of EPSCNMDAR via CXCR4.
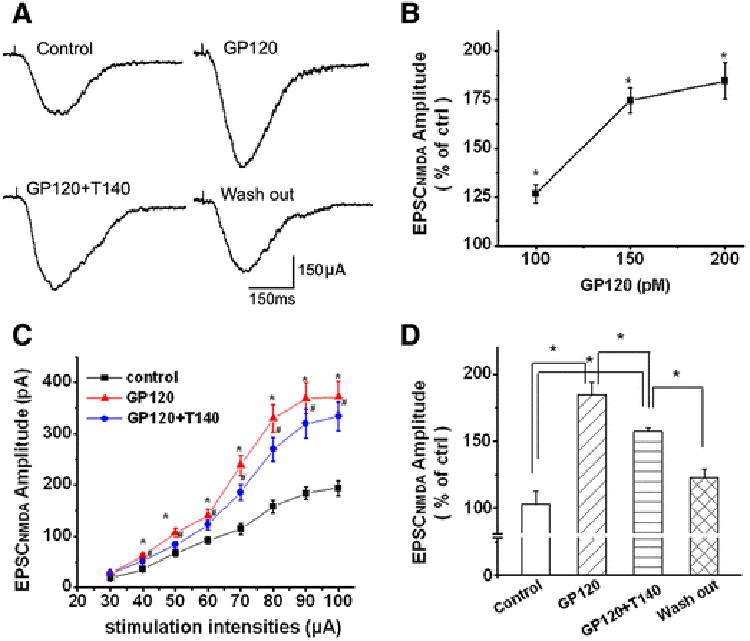
A. Exemplary whole-cell current traces illustrating gp120 (200pM) increase of EPSCNMDAR recorded from a CA1 neuron in a rat hippocampal slice before (Ctrl), during bath perfusion of gp120 (gp120), gp120+T140 (50nM) (gp120+T140), or after washout of gp120+T140 (washout). Note that bath application of gp120 produced an increase of EPSCNMDAR (upper right), addition of T140 to the bath partially inhibited gp120-induced increase of EPSCNMDAR (lower left) and the EPSCNMDAR returned to the control level after washout of gp120 +T140 (lower right). B. A dose-responsive curve showing that gp120 increased EPSCNMDAR in a dose-dependent manner. C. The input/output curve shows that gp120 significantly increased the amplitude of EPSCNMDAR evoked by an electric stimulation on Schaffer collateral fibers with different intensities (30μA∼100μA) and that the gp120-induced increase of the EPSCNMDAR amplitudes were partially inhibited by T140 (n=9, * p<0.05 vs ctrl, #p<0.05 vs gp120. D. Bar graph shows the average of normalized EPSCNMDAR amplitudes measured before (Ctrl), during bath application of gp120 (gp120), during bath application of gp20+T140 (GP120+T140), or after washout of gp120+T140 (washout). Values are the means ± SEM, n=9, *p<0.05 as determined by one-way ANOVA.
Figure 7. Gp120 enhancement of EPSCNR2AR and EPSCNR2BR.
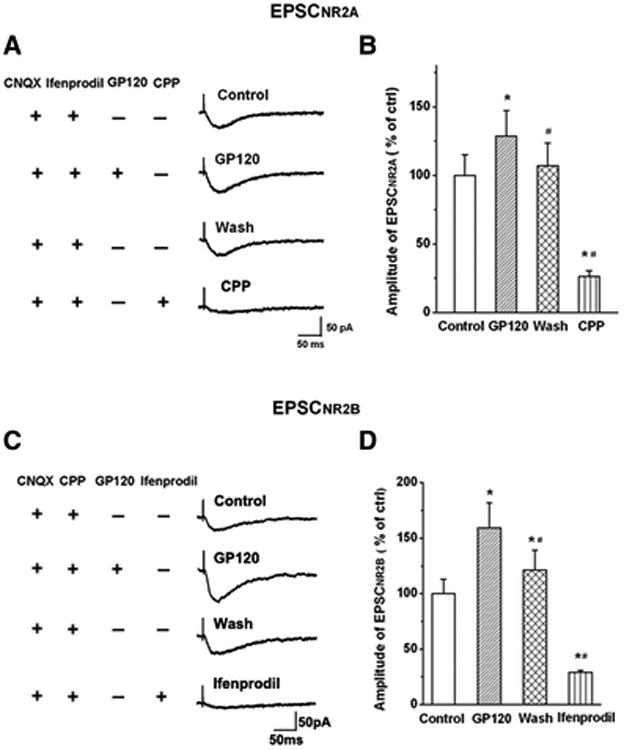
A. EPSCNR2AR was isolated by addition of AMPAR antagonist CNQX (10μM) and NR2BR antagonist ifenprodil (10μM) to the perfusate. Exemplary EPSCNR2AR traces were recorded in a CA1 neuronal cell before (Ctrl), during (gp120), after (washout) bath perfusion of gp120, and subsequent pharmacological confirmation of EPSCNR2AR by a specific NR2AR blocker R-CPP (CPP). Note that bath perfusion of gp120 increased EPSCNR2A and the EPSCNR2A returned to control level after washout of gp120. Addition of R-CPP almost completely blocked EPSCNR2A, demonstrating the pharmacologically isolated EPSCs were EPSCNR2AR. B. EPSCNR2BR was isolated with the addition of AMPAR antagonist CNQX (10μM) and NR2AR antagonist R-CPP (1μM) to the perfusate. Exemplary EPSCNR2BR traces were recorded from another CA1 neuronal cell before (Ctrl), during (gp120), after (washout) bath perfusion of gp120, and pharmacological confirmation of EPSCNR2BR by a specific NR2BR blocker ifenprodil (Ifenprodil). B and D are summary bar graphs showing average amplitudes (% of control) of EPSCnr2ar (B) and EPSCNR2BR (D) recorded under various experimental conditions as indicated, respectively. Note that gp120 enhanced both EPSCNR2AR (128.61±18.64% of control, n=11, M±SD) and EPSCNR2BR (159.25±22.77% of control, n=8, M±SD) with a significant stronger effect (t(17)=0.0046; p<0.01) on enhancing EPSCNR2BR and EPSCNR2AR. * p<0.05 vs control; # p<0.05 vs gp120 group.
Figure 8. gp120 interacts with extrasynaptic NR2BRs (exNR2BRs).
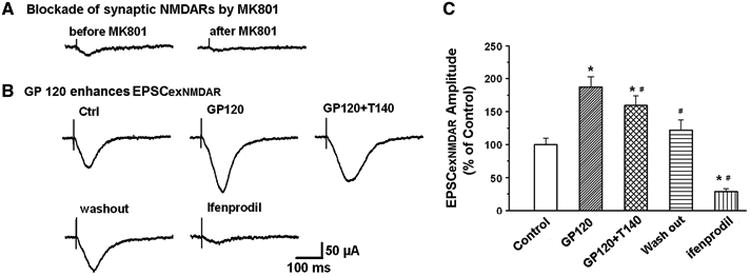
A. Blockade of synaptic EPSCNMDAR by MK801 (70μM, an open NMDAR channel blocker)combined with a 10min low frequency (0.05Hz) stimulation of Schaffer-collateral fiber protocol. B. Exemplary EPSCNMDAR traces recorded in different experimental conditions after synaptic NMDARs were blocked by MK801 as shown in A. Note addition of gp120 enhanced EPSCNMDAR which was most likely mediated via an interaction with extrasynaptic NMDARs. Since NMDARs in the hippocampus are composed mainly of NR2ARs and NR2BRs, the enhancement of EPSCNMDAR by gp120 under the blockade of synaptic NMDARs (predominantly NR2ARSs) suggests that gp120 may act most on exNR2BRs. This suggestion was supported by the experimental results that addition of ifenprodil, a specific NR2BR antagonist, blocked EPSCNMDAR, demonstrating gp120 enhancement of EPSCexNR2BR. The gp120-mediated enhancement of EPSCexNR2BR was attenuated by T140, indicating gp120 interacts with CXCR4. C. Average EPSCexNR2BR recorded under different experimental conditions as indicated in panel B. Note a significant enhancement of EPSCexNR2BR by gp120 and its attenuation by a CXCR4 receptor blocker T140. These results illustrate that gp120 acts on exNR2BRs. n=8, *p<0.05 vs control, #p<0.05 vs gp120.
Results
Enhancement of EPSCs by gp120 in hippocampal slices
To understand whether gp120 alters synaptic transmission, we examined its effects on synaptic transmission by analyzing the EPSCs recorded in the Schaffer-collateral to the CA1 synapses. Bath application of gp120 (200pM) produced a significant increase of EPSCs with average peak EPSC amplitudes of 174.36±6.74% of basal level (n=8, Fig. 1). The differences were statistically significant (p<0.001) in comparison with the average EPSC amplitude recorded in control group, demonstrating gp120 enhancement of EPSCs in hippocampal slices. The gp120-induced increase of EPSCs usually occurred 4–5 min after gp120 reached the recording chamber, peaked at 10-15 min and washed out in 20–25 min. The short latency of onset of gp120 increase of EPSCs suggests a possible direct effect of gp120 on neuronal cells recorded.
Figure 1. Gp120 enhancement of EPSCs in the CA1 region of rat hippocampal slices.
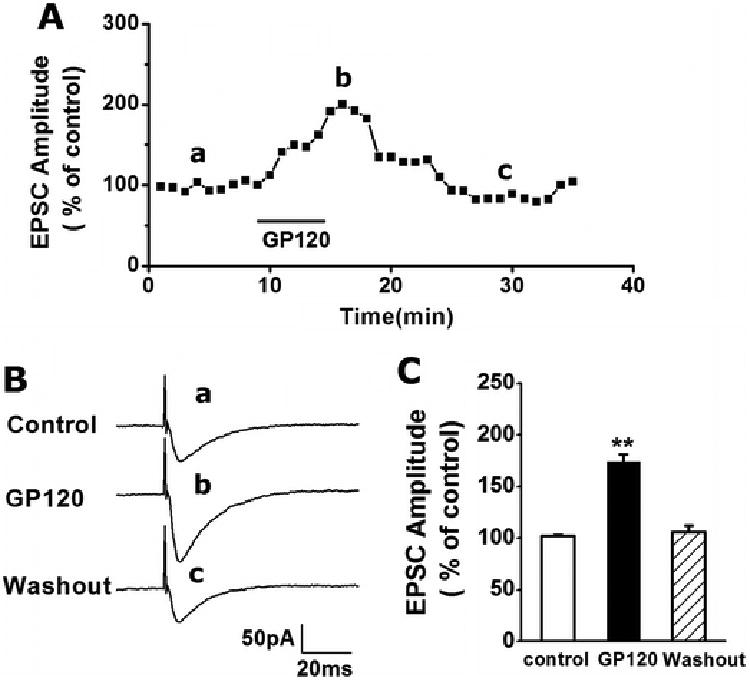
A. A representative time course illustrating bath application of gp120 (200pM), indicated by a horizontal bar, increased the EPSC amplitudes recorded in a CA1 neuron. The graph plots normalized amplitudes of the EPSCs evoked in response to constant current stimulation of Schaffer-collateral fibers (120μA, 20μs, 0.05Hz). Each point in this graph is an average of 3 consecutive EPSCs. B. Representative individual EPSCs taken from different time points as indicated by letters a, b and c. C. Summary data (n=8) showing average EPSC peak amplitudes before (control), during (gp120) and after (washout) bath application of gp120. Note gp120 significantly increased amplitude of EPSCs. ** p < 0.001 vs. Ctrl.
Gp120 enhanced EPSCNMDAR, but not EPSCAMPAR
As the excitatory synaptic transmission in the CA1 region of hippocampus is mainly glutamatergic, composed of AMPAR-mediated EPSCs (EPSCAMPAR) and NMDAR-mediated EPSCs (EPSCNMDAR). We next examined the effects of gp120 on pharmacologically isolated EPSCAMPAR and EPSCNMDAR. Bath application of gp120, at concentrations of 200pM and 400pM, had no significant effect on EPSCAMPAR in the presence of a specific NMDAR antagonist APV (50μM) in the perfusate (Fig. 2). In contrast, it significantly increased EPSCNMDAR in a concentration-dependent manner when CNQX (10μM), a specific AMPAR antagonist, was added to the perfusate (Fig. 3A). At concentrations of 100pM, 150pM and 200pM, gp120 significantly (p<0.05) increased amplitude of EPSCNMDAR to 126.54±4.73% (n=5), 174.59±6.28% (n=6) and 184.46±9.37% of control (n=9), respectively. Among the different concentrations tested, 200pM exhibited a robust enhancement effect on EPSCNMDAR (Fig. 3B). The average normalized EPSCNMDAR amplitudes (% of control) measured before (Ctrl), during bath perfusion gp120 (GP120), gp120+T140(50nM)(GP120+T140), and after washout of GP120+T140 (washout) were 102.79±9.46%, 184.46±9.37%, 157.08±2.90% and 122.48±6.31%, respectively (n=9, p<0.05, Fig. 3D). At 200pM, gp120 also increased input-output (I-O) responses mediated by NMDARs in response to an electrical stimulation of Schaffer collateral fibers (Fig. 3C). The gp120-induced increase of NMDAR-mediated EPSCs and I-O responses was partially blocked by T140 (50nM, n=9, p<0.05), a specific CXCR4 receptor blocker (Figs. 3A, 3C, 3D), suggesting that gp120 interacts with chemokine receptor CXCR4.
Figure 2. GP120 has no significant effect on EPSCAMPAR.
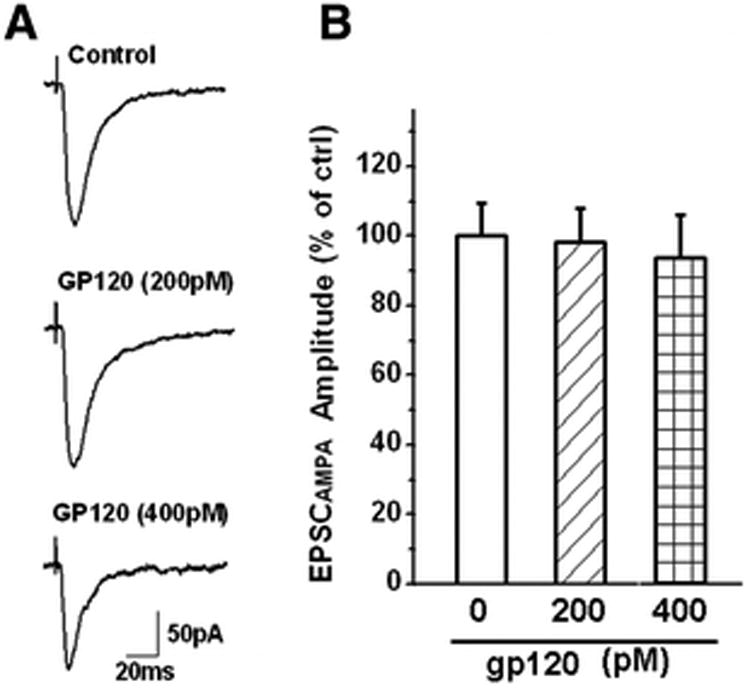
EPSCAMPAR was recorded in the presence of a specific NMDA receptor antagonist APV (50μM) in the perfusate. The recorded EPSCAMPAR can be blocked by addition of a specific AMPAR antagonist CNQX (10 μM) to the bath. A. Representative EPSCAMPAR current traces recorded from a CA1 pyramidal neuron in a rat hippocampal slice showing the amplitudes of EPSCAMPAR were not altered by bath application of gp120 at a concentration of 200pM (n=9), or even at 400pM (n=5). Each trace shown was an average of consecutive 10 evoked EPSCAMPAR. B. A summary bar graph illustrating average EPSCAMPAR amplitudes measured before (Ctrl) and during bath perfusion of gp120 (gp120). Note that gp120 failed to alter the EPSCAMPAR amplitudes at either 200pM (n=9) or 400pM (n=5).
Presynaptic site of gp120 action
To determine the site of action, we analyzed the effects of gp120 (200pM) on spontaneous min EPSCNMDAR (mEPSCNMDAR) and spontaneous mini EPSCAMPAR (mEPSCAMPAR) isolated pharmacologically as described in Figs. 2 and 3. Bath application of gp120 had neither effect on the amplitude or the frequency of mEPSCAMPAR (n=9, p> 0.05, Fig. 4), which was consistent with its effect on EPSCAMPAR as shown in Fig. 2. In contrast, gp120 significantly enhanced the frequency of mEPSCNMDAR without significant change of the amplitude of mEPSCNMDAR. When applied via bath perfusion, gp120 increased the frequency of mEPSCNMDAR from 28.85±5.03events/per min to 58.67±6.13 events/per min. Addition of 50nM T140 to the bath reduced mEPSCNMDAR frequency to 45.45±4.78 event/per min (n=9, p<0.05, Figs 5A, 5B), indicating an involvement of CXCR4 in gp120 enhancement of mEPSCNMDAR. The average amplitudes of mEPSCNMDAR before and during bath perfusion of gp120 were 10.41±1.97 pA and 10.17±1.62pA,respectively. The difference was not statistically significant indicating gp120 had no significant effect on amplitude of mEPSCNMDAR (n=9, p>0.05, Figs. 5A, 5C). As gp120 increased mEPSCNMDAR frequency without significant effect mEPSCNMDAR amplitude, suggesting a presynaptic site of gp120 action. To further analyze the site of gp120 action, we conducted paired-pulse facilitation (PPF) tests in the Schaffer-collateral to the CA1 synapses. The EPSCNMDAR was evoked in response to a pair of electrical stimuli separated by an interval of 150 ms and then the paired-pulse facilitation ratio (PPFR) was calculated. Our data showed that bath application of gp120 significantly increased the PPFR of EPSCNMDAR from 79.64±2.9% to 132.62±1.78% (n=8, p<0.05, Figs. 6A, 6B). These results further demonstrated that gp120 acts on a presynaptic site.
Figure 4. Gp120 failure on alteration of spontaneous mini EPSCAMPAR (mEPSCAMPAR).
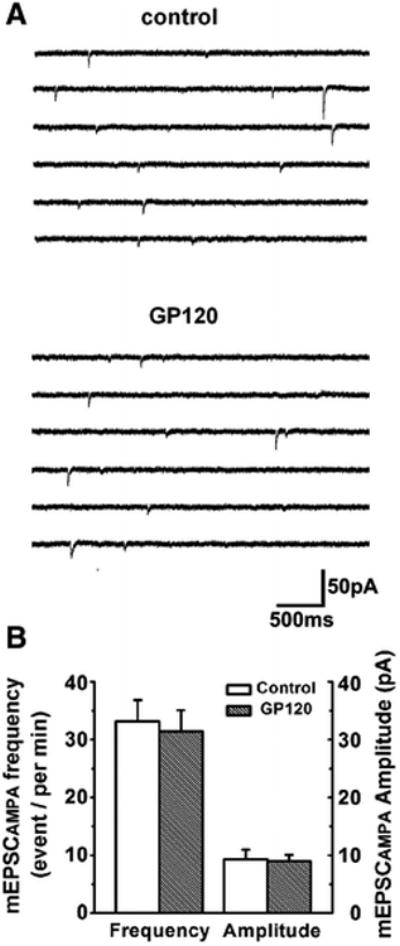
A. Representative traces of mEPSCAMPAR recorded from a CA1 neuron in a rat hippocampal slice in the presence of NMDA receptor antagonist AP-V (50μM) in the perfusate. Addition of gp120 to the perfusate failed to alter mEPSCAMPAR. B. Bar graph showing average event frequency and amplitude of mEPSCAMPAR were not significantly changed after addition og gp120 (200pM) to the perfusate (n=9, p> 0.05 vs control.
Figure 5. Gp120 increase of frequency, but not amplitude, of mEPSCNMDAR.
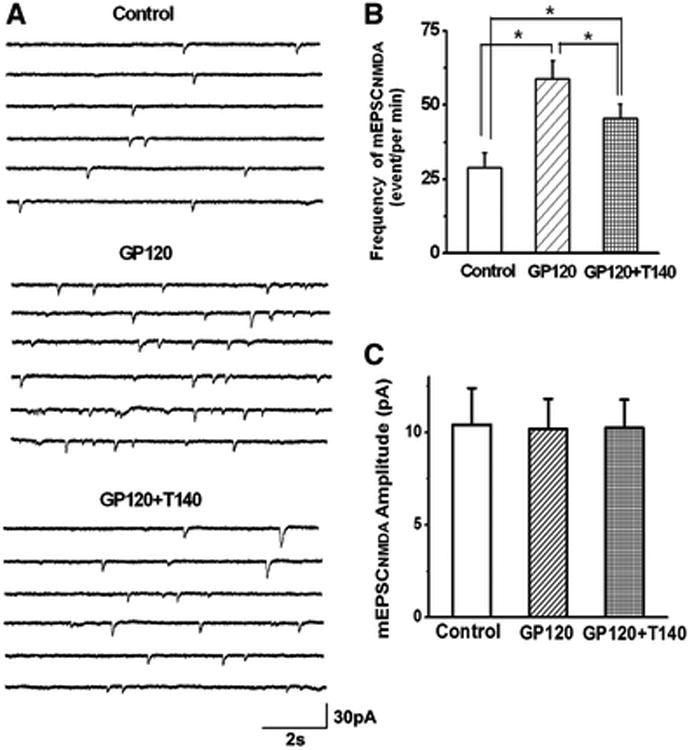
A. Representative traces of mEPSCNMDAR were recorded before (Ctrl), during bath perfusion of 200pM (gp120), and during bath perfusion of gp120 and T140 (gp120+T140). Data showed that the frequency of mEPSCNMDAR was increased by bath application of gp120, which was partially blocked by addition of T140 to the bath. B-C. Bar graph illustrates that gp120 increased the mean frequency of mEPSCNMDAR, but not the mean amplitude, suggesting a presynaptic site for gp120 action. The gp120-associated increase of mean frequency was attenuated by T140, a specific CXCR4 antagonist, indicating gp120 may interacts with chemokine receptor CXCR4. Graphed values are the means ± SEM, n=9, *p<0.05 as determined by one-way ANOVA test.
Figure 6. gp120 increased paired-pulse facilitation (PPF) of EPSCNMDAR.
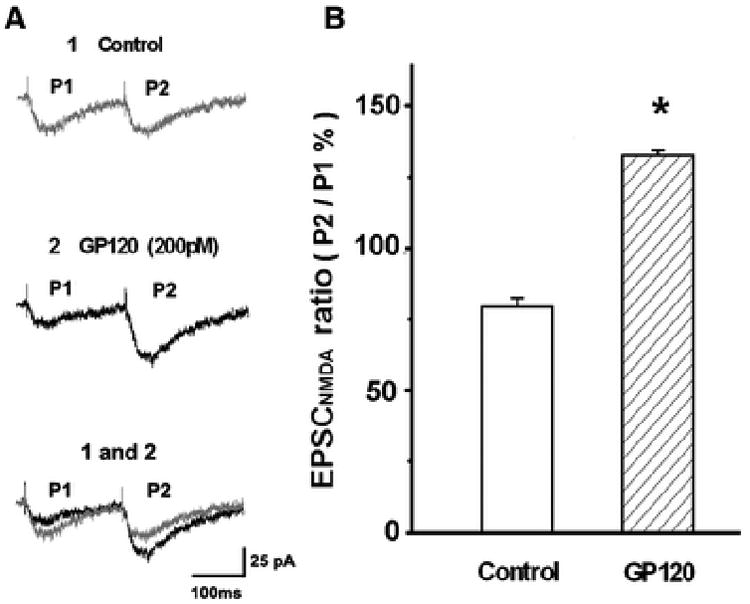
Paired-pulse facilitation of EPSCNMDAR was generated by paired-pulse stimulations with inter-stimulus pulse interval of150ms. The paired-pulse ratio was calculated by dividing the first EPSCNMDAR peak amplitude from the second EPSCNMDAR peak amplitude. A. Representative PPF traces of EPSCNMDAR were recorded from a CA1 neuron in a rat hippocampal slice in the presence of glycine (1μM) and AMPA receptor antagonist CNQX (10μM) before (Ctrl) and during bath perfusion of gp120 (gp120). B. Summarized bar graph illustrating averaged paired-pulse ratio of EPSCNMDAR were significantly elevated by bath application of gp120 (200pM). n=8 * p<0.05 vs Ctrl
GP120 had stronger effects on enhancing EPSCNR2BR than EPSCNR2AR
The major NR2 subtypes of NMDARs in the hippocampus are NR2A and NR2B. To evaluate the contributions of NR2ARs and NR2BRs to gp120-induced increase of EPSCNMDAR, we examined NR2BR antagonist ifenprodil (10μM, which has a high affinity for NR2BRs with an IC50=0.34μM and IC50 =146μM for NR2ARs)(Williams, 1993), and NR2AR antagonist R-CPP (1μM), which has ∼7-fold greater selectivity for NR2ARs compared with NR2BRs (Feng et al., 2005), on their abilities to block gp120-induced increase of EPSCNR2BR and EPSCNR2AR, respectively. In the presence of ifenprodil or R-CPP in the perfusate, bath application of gp120 produced an increase of EPSCNR2AR to 128.61 ± 18.64 % of control (n=11, p<0.05 vs control, Figs 7A, 7B) or EPSCNR2BR to 159.25 ± 22.77 % of control (n=8, p<0.05 vs control, Figs. 7C, 7D), respectively. The difference between the peak amplitudes of EPSCNR2BR and EPSCNR2AR was statistically significant (p<0.05), demonstrating that GP120 had a stronger effect on enhancing EPSCNR2BR than EPSCNR2AR (Fig. 7).
Evidence for GP120 activation of exNR2BR
It has been proposed that the location of NMDARs makes the key difference: survival-promoting signals derive from synaptic NMDARs, which consist of predominant NR2ARs, whereas a cell-death signal comes from extrasynaptic NMDARs, which contain mostly NR2BRs (Tovar and Westbrook, 1999; Hardingham et al., 2002; Liu et al., 2007) To determine if gp120 enhancement of EPSCNR2BR was mediated via extrasynaptic NR2BR (exNR2BR), we blocked synaptic NMDAR with an irreversible, use-dependent NMDAR channel blocker MK801 (70μM) applied to the slices via bath perfusion. During 5-10 min bath perfusion of brain slices with MK801, repeated stimulation (30μA, 1Hz) of Schaffer collateral fibers to evoke glutamate release and activate synaptic NMDAR, creating an environment for MK801 to block these synaptic NMDAR. After MK801 blockade of synaptic NMDAR, we increased stimulation intensities from 30μA to 120μA (0.05Hz) to elicit exNR2BR-mediated EPSC (EPSCexNR2BR) which was blocked by ifenprodil (Figs. 8B, 8C). As shown in Fig. 8, gp120 retained its ability to enhance EPSCexNR2BR to 187.46 ± 15.62 % of control (n=8, p<0.05 vs control, Figs 8B, 8C) after the synaptic NMDAR were blocked by MK801. Gp120 enhancement of EPSCexNR2BR was significantly attenuated by T140 to 159.44 ± 14.68 % of control (n=8, p<0.05 vs control, Figs 8B, 8C), indicating gp120 increased EPSCexNR2BR via CXCR4 receptors.
gp120 increased intracellular Ca2+ concentration [Ca2+]i via NMDARS
Studies have demonstrated that over-activation of NMDA receptors is a primary cause of excitotoxicity because of its high permeability to Ca2+ (Choi, 1988; Lipton, 1994). To determine if gp120 enhancement of EPSCNMDAR is associated with an increase of [Ca2+] i, we examined if bath application gp120 could increase glutamate(Glu)-induced increase of [Ca2+] on primary rat hippocampal neuronal cultures. Our results showed that Glu (100μM) caused an increase of [Ca2+]i determined by the ratio of fluorescent signals (F340/F380), and addition of gp120(200pM) produced a further increase of [Ca2+] i (n=12, Fig 9A, 9B). In the presence of AMPAR blocker CNQX in the perfusate, gp120 remained its ability to enhance Glu-induced increase on [Ca2+] i, indicating such an increase of [Ca2+]i was mediated via NMDARs (n=12, Figs. 9A, 9B). However, gp120 failed to enhance Glu-induced increase of [Ca2+]i in the presence of NMDAR antagonist in the perfusate, suggesting AMPARs were not involved in gp120-associated increase of [Ca2+] i (n=12, Figs. 9A, 9B). These results clearly indicate that gp120 interacts with NMDARs.
Figure 9.
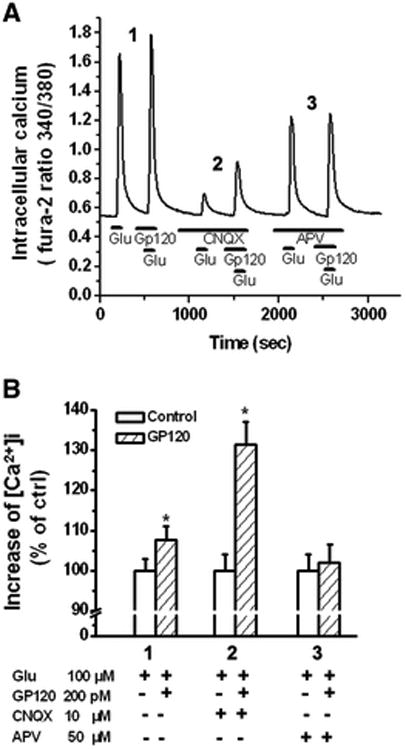
gp120 potentiation of glutamate-induced increase of [Ca2+]I via NMDARs. A. An example of Ca2+ imaging study showing gp120 potentiation of glutamate (Glu)-induced Ca2+ influx via NMDARs in a cultured rat hippocampal neuron. Note that gp120 produced an increase of [Ca2+ ]I in the presence of an AMPAR antagonist CNQX (10μM). The gp120-associated potentiation disappeared when NMDARs were blocked by addition of a specific NMDAR antagonist APV, demonstrating that gp120 interacts with NMDARs. B. Bar graph shows average [Ca2+ ]I in different experimental conditions and illustrates gp120 potentiation of Glu-induced increase of [Ca2+ ]I via NMDARs. Experiments were done in triplicates. n=12, * p < 0.05 vs control.
Discussion
In the present study we demonstrated that bath application of gp120 increased the amplitude of electrically evoked EPSCs in the CA1 region of rat hippocampal slices. We then isolated EPSCAMPAR and EPSCNMDAR pharmacologically and revealed that gp120 enhanced the amplitude of EPSCNMDAR in a dose-dependent manner without significant effects on EPSCAMPAR. Such an enhancement of EPSCNMDAR was significantly attenuated by T140, a specific CXCR4 receptor antagonist, indicating gp120 enhancement of EPSCNMDAR via CXCR4 receptors. Paired-pulse facilitation analysis showed gp120 increase of the facilitation ratio of EPSCNMDAR, suggesting a presynaptic site of gp120 action. This suggestion was supported by another set of experiments that bath application of gp120 increased the occurrence frequency of mEPSCNMDAR without change on the amplitude of mEPSCNMDAR. Further studies unraveled that gp120 increased both EPSCNR2AR and EPSCNR2BR with significant stronger effect on EPSCNR2BR than EPSCNR2AR. Using an experimental protocol to block synaptic NMDARs (Thomas et al., 2006; Yang et al., 2013), we revealed that gp120 significantly increased extra-synaptic EPSCNMDAR which was blocked by a NR2BR antagonist ifenprodil, demonstrating for the first time, to our knowledge, that gp120 acts on extra-synaptic NR2BRs.
HAND is believed to result from the complex interactions of both viral and cellular factors with direct and indirect mechanisms of neurotoxicity. Gp120 is one of viral neurotoxins implicated as an important factor in the pathogenesis of HAND. Gp120 is toxic to neural cells and can promote detrimental alterations on synaptic transmission and plasticity, leading to an impairment of cognitive function (Dreyer and Lipton, 1995; Lannuzel et al., 1997; Dong and Xiong, 2006). Studies have shown that gp120 causes neuronal damage through multiple pathways including direct interactions with neurons via cell surface receptors and ion channels (Lannuzel et al., 1995; Medina et al., 1999) or by stimulating mononuclear phagocytes (brain perivascular macrophages and microglia) release of neurotoxic substances, the so-called indirect mechanism, which in turn act on neuronal cells leading to neuronal damage (Xiong et al., 2003; O'Donnell et al., 2006; Yang et al., 2013). It is widely accepted that the major pathway by which gp120 causes neuronal injury is via activation of NMDARs and resultant raising intracellular calcium concentration (Barks et al., 1995; Kaul, 2008). Indeed, the involvement of NMDARs in HIV-1-associated neurotoxicity has been demonstrated by experiments that NMDAR antagonists, but not AMPAR antagonists, attenuated or prevented gp120-induced neuronal injury or death (Muller et al., 1996; Kaul et al., 2001). The gp120-associated neurotoxicity observed in vitro has been validated in a transgenic mouse model overexpressing gp120 in astrocytes (Toggas et al., 1994; Toggas and Mucke, 1996) and neurodegeneration observed in gp120-transgenic animals was ameliorated by the NMDAR antagonist memantine (Toggas et al., 1996). Besides, gp120 has been shown to trigger NMDAR-mediated cell death in human neurons (Wu et al., 1996; Lannuzel et al., 1997). These results have clearly demonstrated a role for NMDARs in HIV-1-associated neuropathology (Kaul et al., 2001; O'Donnell et al., 2006; Kaul, 2008). However, how gp120 alters NMDAR physiology is not fully understood. We found that gp120 increased EPSCNMDAR, but not EPSCAMPAR, in hippocampal CA1 neurons. We also found that gp120 potentiated glutamate-mediated Ca2+ influx in the presence of specific AMPAR antagonist CNQX, but not in the presence of specific NMDAR antagonist APV, suggesting gp120 potentiates glutamate-induced increase of intracellular Ca2+ concentration via NMDARs. As an overload of intracellular Ca2+ is widely believed to be toxic to neurons, the increase of EPSCNMDAR and intracellular Ca2+ concentration by gp120 may implicate molecular mechanisms for gp120-induced neuronal injury. It is worth pointing out that our Ca2+ imaging experiments were conducted at the room temperature, an experimental condition may slow down cellular signaling and responses. Thus, it may be possible to see a greater increase of intracellular Ca2+ concentration induced by gp120 at a physiological temperature.
The levels of intracellular Ca2+ concentration are, however, not the only determinant of NMDAR-mediated neurotoxicity (Riccio and Ginty, 2002). Several lines of evidence indicate the subtype and location of activated NMDARs are key determinants of the nature of NMDAR signaling and resultant physiological and pathophysiological processes (Hardingham et al., 2002; Liu et al., 2007; Hardingham and Bading, 2010). It has been proposed that in adult cortex NR2ARs may preferentially target synaptic sites, whereas NR2BRs may target to extra-synaptic sites (Rumbaugh and Vicini, 1999; Tovar and Westbrook, 1999; Mohrmann et al., 2000; Townsend et al., 2003). While NR2ARs are believed to be important for normal synaptic transmission, NR2BRs are thought to be associated with pathological neuronal damage (Hardingham et al., 2002; Riccio and Ginty, 2002; Liu et al., 2007). Indeed, activation of NR2BRs has been shown to induce excitotoxicity and neuronal degeneration (Hardingham et al., 2002; Liu et al., 2007) and NR2BR has been implicated in several types of synaptic signaling, modulation of learning and memory processing (Tang et al., 1999), and in a number of neurodegenerative disorders in humans (Loftis and Janowsky, 2003). A previous study has shown that the conditioned media recovered from gp120-stimulated human monocyte-derived macrophages had much stronger effect on enhancing EPSCNR2BR than EPSCNR2AR in the CA1 neurons of rat hippocampal slices and the enhancement of EPSCNR2BR was associated with the conditioned media-induced neuronal injury observed in cultured rat hippocampal neurons (Yang et al., 2013). In this study, we found that gp120 per se enhanced both EPSCNR2AR and EPSCNR2BR with a significant (p<0.01) stronger effect on EPSCNR2BR than EPSCNR2AR. The pattern of gp120 “preferentially” enhancement of EPSCNR2BR suggests a role for NR2BR subtype of NMDARs in gp120-associated neuropathogenesis. Moreover, when synaptic NMDARs, presumably NR2ARs, were blocked by an irreversible, use-dependent open NMDAR channel blocker MK801 with a low frequency stimulation for 10-15 min, a significant increase of extra-synaptic NR2BRs-mediated EPSCNR2BR by gp120 was also observed, further demonstrating that gp120 interacts with NR2BRs. Given activation of extra-synaptic NR2BRs is a cell death signal (Petralia, 2012), the observed gp120 enhancement of extra-synaptic NR2BRs-mediated EPSCNR2BR may implicate an important molecular mechanism for gp120 neurotoxic activity and pathogenesis of HAND.
Interestingly, gp120-mediated increase of EPSCNMDAR was attenuated by both a CXCR4 antagonist and NMDAR antagonists, suggesting that gp120 interacts with both CXCR4s and NMDARs. As CXCR4 is a co-receptor for HIV-1 cellular infection and the binding of gp120 to CXCR4s is required for HIV-1 entry into the target cells. Besides its interaction with CXCR4s, gp120 has also been shown to interact with NMDARs in the brain causing neuronal injury (Pattarini et al., 1998; Dong and Xiong, 2006). Both CXCR4 and NMDARs, in particular the NR2BRs, are expressed at presynaptic terminals (Brasier and Feldman, 2008; Larsen et al., 2011; Reaux-Le Goazigo et al., 2012), it is therefore not surprising to get the results that gp120 interacts with both CXCR4s and NMDARs. However, the signaling pathways that gp120 interacts with the both are not well understood. A recent study by Pittaluga et al demonstrated a functional coupling between CXCR4s and NMDARs in rat hippocampal noradrenergic and glutamatergic nerve terminals (Di Prisco et al., 2016). They proposed that the activation of CXCR4s co-localized with NMDARs in neve terminals triggers a cascade of intracellular signaling which reinforces NMDAR-mediated neurotransmitter release by favoring the phosphorylation of tyrosine residues in the NR2B subtypes of NMDARs (Di Prisco et al., 2016). Whether the aforementioned cross-talk occurred in the present study remains to be determined.
It is worth noticing that gp120 enhanced the paired-pulse facilitation ratio of EPSCNMDAR and increased the occurrence frequency, but not the amplitude, of mEPSCNMDAR, suggesting a presynaptic site of action for gp120. These results are supported by the findings that functional CXCR4 and NMDARs are expressed at presynaptic terminals (Larsen et al., 2011; Petralia, 2012; Reaux-Le Goazigo et al., 2012; Banerjee et al., 2016). On the other hand, gp120 was also found to potentiate glutamate-induced increase of intracellular Ca2+ levels and to enhance extra-synaptic NR2BR-mediated EPSCs, indicating that gp120 acts on postsynaptic NMDARs as well. Thus, these results may constitute a contour that gp120 interacts not only with presynaptic CXCR4 and NM2B receptors, but postsynaptic NMDARs, in particular the extra-synaptic NR2BRs, as well (Fig. 10). The blockade of gp120-mediated enhancement of EPSCNMDA and gp120 alteration of mEPSCNMDAR frequency by CXCR4 antagonist T140 and NR2BR antagonist ifenprodil implies the existence of functional coupling between presynaptic CXCR4 and NR2B receptors as described by Anna Pittaluga and her colleagues(Di Prisco et al., 2016). However, the lack of an effect for gp120 on EPSCAMPA, even at a higher concentration of 400pM, is puzzling. The possible explanation is that gp120 may most likely, by its biological nature, be less potent in activation of APMA receptors or other unknown mechanisms. This explanation appears to be “supported” by an earlier study that gp120 had no effects on enhancing AMPA-induced 3H noradrenaline release in rat hippocampal and cortical noradrenaline nerve endings(Pittaluga and Raiteri, 1994). Thus, the lack of an effect on AMPA receptors may reflect the biological nature of gp120 on APMA receptors. As both CXCR4 and NMDA receptors (in particular NR2B subtype) are not only expressed in presynaptic terminals but are also coupled functionally (Di Prisco et al., 2016), gp120 may activate these two types of receptors, resulting in an increase of [Ca2+]i in presynaptic terminals and resultant quantal release of neurotransmitter glutamate, leading to an enhancement of EPSCNMDAR. Gp120 also acts on postsynaptic NMDARs, contributing further to the enhancement of EPSCNMDAR. Another possibility is that gp120 activates glial cells and the activated glial cells release cytokines, chemokines and amino acids, such as CCL-2, platelet-activating factor and glutamate, leading to an enhancement of EPSCNMDAR in the hippocampal slices (Epstein and Gelbard, 1999; Lu et al., 2007; Zhou et al., 2016). Further experimentation is needed to confirm this possibility.
Figure 10.
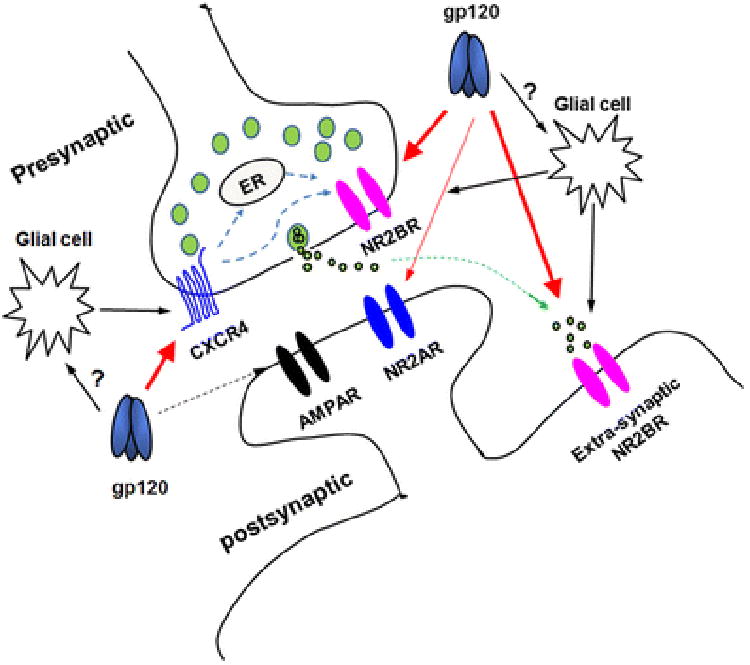
A schematic diagram illustrating the sites of gp120 action in the hippocampal slices. As shown in big red arrows, gp120 acts on presynaptic CXCR4 and on pre- and post-synaptic NR2BRs. It is also possible that gp120 acts on glial cells, resulting in glial cell release of neurotoxic molecules including, but not limited to, cytokines, chemokines, amino acids, etc, which in turn act on CXCR4 and NMDARs (black arrows). Gp120-induced enhancement of EPSCNMDAR can be blocked by a CXCR4 blocker T140, suggesting the existence a functional coupling between CXCR4 and NMDARs as proposed by Anna Pittaluga and her colleagues (Di Prisco et al., 2016).
The biological significance of gp120 enhancement of EPSCNMDAR remains to be elucidated. It is well known that dendrite pruning and neuronal apoptosis are common pathological features seen in HIV-1-infected patients with HAND. Such pathological alterations in dendrites and neurons can be caused by gp120 alone and this viral protein may be directly responsible for HIV-1-associated neurotoxicity. Indeed, ample evidence indicates that gp120 is a potent neurotoxin acting at extremely low concentrations (Lipton, 1998) and its neurotoxic activity has been demonstrated both in vitro and in vivo (Dreyer and Lipton, 1995; Barks et al., 1997; Doble, 1999), as well as in a transgenic animal models that expressed gp120(Toggas et al., 1994). A widely accepted common pathway by which gp120 induces neural cell injury or death is the activation of NMDAR and resultant elevation of [Ca2+]I, leading to dendritic and neuronal injury. Therefore, the enhancement of EPSCNMDAR may reflect a scenario in HIV-1-infected brain, especially in the era of cART, that gp120, even at low levels, enhances excitatory synaptic transmission mediated by glutamate, a major excitatory neurotransmitter for majority of the excitatory synaptic transmission in the brain. Such an enhancement of NMDAR-mediated synaptic activity, if lasts for a long period of time such as scenario of chronic HIV-1 infection, may lead to an over-activation of NMDARs and increase of [Ca2+]i, eventually resulting in dendritic and neuronal injury and pathogenesis of HAND.
Acknowledgments
This work was supported by NIH grant R01 NS063878 (HX) and the National Natural Science Foundation of China 81660213 (YZ).
Footnotes
Compliance with Ethical Standards: All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. All procedures performed in studies involving animals were in accordance with the ethical standards of University of Nebraska Medical Center, Omaha, NE, USA.
Competing interests: The authors declare that they have no competing interests.
References
- Acquas E, Bachis A, Nosheny RL, Cernak I, Mocchetti I. Human immunodeficiency virus type 1 protein gp120 causes neuronal cell death in the rat brain by activating caspases. Neurotox Res. 2004;5:605–615. doi: 10.1007/BF03033180. [DOI] [PubMed] [Google Scholar]
- Alfahad TB, Nath A. Update on HIV-associated neurocognitive disorders. Curr Neurol Neurosci Rep. 2013;13:387. doi: 10.1007/s11910-013-0387-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson ER, Gendelman HE, Xiong H. Memantine protects hippocampal neuronal function in murine human immunodeficiency virus type 1 encephalitis. J Neurosci. 2004;24:7194–7198. doi: 10.1523/JNEUROSCI.1933-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antinori A, et al. Updated research nosology for HIV-associated neurocognitive disorders. Neurology. 2007;69:1789–1799. doi: 10.1212/01.WNL.0000287431.88658.8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee A, Larsen RS, Philpot BD, Paulsen O. Roles of Presynaptic NMDA Receptors in Neurotransmission and Plasticity. Trends Neurosci. 2016;39:26–39. doi: 10.1016/j.tins.2015.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barks JD, Sun R, Malinak C, Silverstein FS. gp120, an HIV-1 protein, increases susceptibility to hypoglycemic and ischemic brain injury in perinatal rats. Exp Neurol. 1995;132:123–133. doi: 10.1016/0014-4886(95)90066-7. [DOI] [PubMed] [Google Scholar]
- Barks JD, Liu XH, Sun R, Silverstein FS. gp120, a human immunodeficiency virus-1 coat protein, augments excitotoxic hippocampal injury in perinatal rats. Neuroscience. 1997;76:397–409. doi: 10.1016/s0306-4522(96)00373-9. [DOI] [PubMed] [Google Scholar]
- Behe P, Stern P, Wyllie DJ, Nassar M, Schoepfer R, Colquhoun D. Determination of NMDA NR1 subunit copy number in recombinant NMDA receptors. Proc R Soc Lond B Biol Sci. 1995;262:205–213. doi: 10.1098/rspb.1995.0197. [DOI] [PubMed] [Google Scholar]
- Blair RE, Sombati S, Churn SB, Delorenzo RJ. Epileptogenesis causes an N-methyl-d-aspartate receptor/Ca2+-dependent decrease in Ca2+/calmodulin-dependent protein kinase II activity in a hippocampal neuronal culture model of spontaneous recurrent epileptiform discharges. Eur J Pharmacol. 2008;588:64–71. doi: 10.1016/j.ejphar.2008.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brasier DJ, Feldman DE. Synapse-specific expression of functional presynaptic NMDA receptors in rat somatosensory cortex. J Neurosci. 2008;28:2199–2211. doi: 10.1523/JNEUROSCI.3915-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi DW. Glutamate neurotoxicity and diseases of the nervous system. Neuron. 1988;1:623–634. doi: 10.1016/0896-6273(88)90162-6. [DOI] [PubMed] [Google Scholar]
- Cull-Candy S, Brickley S, Farrant M. NMDA receptor subunits: diversity, development and disease. Curr Opin Neurobiol. 2001;11:327–335. doi: 10.1016/s0959-4388(00)00215-4. [DOI] [PubMed] [Google Scholar]
- Di Prisco S, Olivero G, Merega E, Bonfiglio T, Marchi M, Pittaluga A. CXCR4 and NMDA Receptors Are Functionally Coupled in Rat Hippocampal Noradrenergic and Glutamatergic Nerve Endings. J Neuroimmune Pharmacol. 2016 doi: 10.1007/s11481-016-9677-6. [DOI] [PubMed] [Google Scholar]
- Dingledine R, Borges K, Bowie D, Traynelis SF. The glutamate receptor ion channels. Pharmacol Rev. 1999;51:7–61. [PubMed] [Google Scholar]
- Doble A. The role of excitotoxicity in neurodegenerative disease: implications for therapy. Pharmacol Ther. 1999;81:163–221. doi: 10.1016/s0163-7258(98)00042-4. [DOI] [PubMed] [Google Scholar]
- Dong J, Xiong H. Human immunodeficiency virus type 1 gp120 inhibits long-term potentiation via chemokine receptor CXCR4 in rat hippocampal slices. J Neurosci Res. 2006;83:489–496. doi: 10.1002/jnr.20745. [DOI] [PubMed] [Google Scholar]
- Dreyer EB, Lipton SA. The coat protein gp120 of HIV-1 inhibits astrocyte uptake of excitatory amino acids via macrophage arachidonic acid. Eur J Neurosci. 1995;7:2502–2507. doi: 10.1111/j.1460-9568.1995.tb01048.x. [DOI] [PubMed] [Google Scholar]
- Epstein LG, Gelbard HA. HIV-1-induced neuronal injury in the developing brain. J Leukoc Biol. 1999;65:453–457. doi: 10.1002/jlb.65.4.453. [DOI] [PubMed] [Google Scholar]
- Feng B, Morley RM, Jane DE, Monaghan DT. The effect of competitive antagonist chain length on NMDA receptor subunit selectivity. Neuropharmacology. 2005;48:354–359. doi: 10.1016/j.neuropharm.2004.11.004. [DOI] [PubMed] [Google Scholar]
- Gates TM, Cysique LA. The Chronicity of HIV Infection Should Drive the Research Strategy of NeuroHIV Treatment Studies: A Critical Review. CNS Drugs. 2016;30:53–69. doi: 10.1007/s40263-015-0302-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glowa JR, Panlilio LV, Brenneman DE, Gozes I, Fridkin M, Hill JM. Learning impairment following intracerebral administration of the HIV envelope protein gp120 or a VIP antagonist. Brain Res. 1992;570:49–53. doi: 10.1016/0006-8993(92)90562-n. [DOI] [PubMed] [Google Scholar]
- Grant I. Neurocognitive disturbances in HIV. Int Rev Psychiatry. 2008;20:33–47. doi: 10.1080/09540260701877894. [DOI] [PubMed] [Google Scholar]
- Hardingham GE, Bading H. Synaptic versus extrasynaptic NMDA receptor signalling: implications for neurodegenerative disorders. Nat Rev Neurosci. 2010;11:682–696. doi: 10.1038/nrn2911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardingham GE, Fukunaga Y, Bading H. Extrasynaptic NMDARs oppose synaptic NMDARs by triggering CREB shut-off and cell death pathways. Nat Neurosci. 2002;5:405–414. doi: 10.1038/nn835. [DOI] [PubMed] [Google Scholar]
- Heaton RK, et al. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology. 2010;75:2087–2096. doi: 10.1212/WNL.0b013e318200d727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK, et al. HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: differences in rates, nature, and predictors. J Neurovirol. 2011;17:3–16. doi: 10.1007/s13365-010-0006-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoefer MM, Sanchez AB, Maung R, de Rozieres CM, Catalan IC, Dowling CC, Thaney VE, Pina-Crespo J, Zhang D, Roberts AJ, Kaul M. Combination of methamphetamine and HIV-1 gp120 causes distinct long-term alterations of behavior, gene expression, and injury in the central nervous system. Exp Neurol. 2015;263:221–234. doi: 10.1016/j.expneurol.2014.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaeger LB, Nath A. Modeling HIV-associated neurocognitive disorders in mice: new approaches in the changing face of HIV neuropathogenesis. Dis Model Mech. 2012;5:313–322. doi: 10.1242/dmm.008763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaul M. HIV's double strike at the brain: neuronal toxicity and compromised neurogenesis. Front Biosci. 2008;13:2484–2494. doi: 10.2741/2860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaul M, Lipton SA. Chemokines and activated macrophages in HIV gp120-induced neuronal apoptosis. Proc Natl Acad Sci U S A. 1999;96:8212–8216. doi: 10.1073/pnas.96.14.8212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaul M, Garden GA, Lipton SA. Pathways to neuronal injury and apoptosis in HIV-associated dementia. Nature. 2001;410:988–994. doi: 10.1038/35073667. [DOI] [PubMed] [Google Scholar]
- Kuryatov A, Laube B, Betz H, Kuhse J. Mutational analysis of the glycine-binding site of the NMDA receptor: structural similarity with bacterial amino acid-binding proteins. Neuron. 1994;12:1291–1300. doi: 10.1016/0896-6273(94)90445-6. [DOI] [PubMed] [Google Scholar]
- Lannuzel A, Lledo PM, Lamghitnia HO, Vincent JD, Tardieu M. HIV-1 envelope proteins gp120 and gp160 potentiate NMDA-induced [Ca2+]i increase, alter [Ca2+]i homeostasis and induce neurotoxicity in human embryonic neurons. Eur J Neurosci. 1995;7:2285–2293. doi: 10.1111/j.1460-9568.1995.tb00649.x. [DOI] [PubMed] [Google Scholar]
- Lannuzel A, Barnier JV, Hery C, Huynh VT, Guibert B, Gray F, Vincent JD, Tardieu M. Human immunodeficiency virus type 1 and its coat protein gp120 induce apoptosis and activate JNK and ERK mitogen-activated protein kinases in human neurons. Ann Neurol. 1997;42:847–856. doi: 10.1002/ana.410420605. [DOI] [PubMed] [Google Scholar]
- Larsen RS, Corlew RJ, Henson MA, Roberts AC, Mishina M, Watanabe M, Lipton SA, Nakanishi N, Perez-Otano I, Weinberg RJ, Philpot BD. NR3A-containing NMDARs promote neurotransmitter release and spike timing-dependent plasticity. Nat Neurosci. 2011;14:338–344. doi: 10.1038/nn.2750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipton SA. AIDS-related dementia and calcium homeostasis. Ann N Y Acad Sci. 1994;747:205–224. doi: 10.1111/j.1749-6632.1994.tb44411.x. [DOI] [PubMed] [Google Scholar]
- Lipton SA. Neuronal injury associated with HIV-1: approaches to treatment. Annu Rev Pharmacol Toxicol. 1998;38:159–177. doi: 10.1146/annurev.pharmtox.38.1.159. [DOI] [PubMed] [Google Scholar]
- Liu Y, Wong TP, Aarts M, Rooyakkers A, Liu L, Lai TW, Wu DC, Lu J, Tymianski M, Craig AM, Wang YT. NMDA receptor subunits have differential roles in mediating excitotoxic neuronal death both in vitro and in vivo. J Neurosci. 2007;27:2846–2857. doi: 10.1523/JNEUROSCI.0116-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loftis JM, Janowsky A. The N-methyl-D-aspartate receptor subunit NR2B: localization, functional properties, regulation, and clinical implications. Pharmacol Ther. 2003;97:55–85. doi: 10.1016/s0163-7258(02)00302-9. [DOI] [PubMed] [Google Scholar]
- Lu SM, Tong N, Gelbard HA. The phospholipid mediator platelet-activating factor mediates striatal synaptic facilitation. J Neuroimmune Pharmacol. 2007;2:194–201. doi: 10.1007/s11481-007-9064-4. [DOI] [PubMed] [Google Scholar]
- Medina I, Ghose S, Ben-Ari Y. Mobilization of intracellular calcium stores participates in the rise of [Ca2+]i and the toxic actions of the HIV coat protein GP120. Eur J Neurosci. 1999;11:1167–1178. doi: 10.1046/j.1460-9568.1999.00550.x. [DOI] [PubMed] [Google Scholar]
- Meucci O, Miller RJ. gp120-induced neurotoxicity in hippocampal pyramidal neuron cultures: protective action of TGF-beta1. J Neurosci. 1996;16:4080–4088. doi: 10.1523/JNEUROSCI.16-13-04080.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohrmann R, Hatt H, Gottmann K. Developmental regulation of subunit composition of extrasynaptic NMDA receptors in neocortical neurones. Neuroreport. 2000;11:1203–1208. doi: 10.1097/00001756-200004270-00012. [DOI] [PubMed] [Google Scholar]
- Monyer H, Burnashev N, Laurie DJ, Sakmann B, Seeburg PH. Developmental and regional expression in the rat brain and functional properties of four NMDA receptors. Neuron. 1994;12:529–540. doi: 10.1016/0896-6273(94)90210-0. [DOI] [PubMed] [Google Scholar]
- Muller WE, Pergande G, Ushijima H, Schleger C, Kelve M, Perovic S. Neurotoxicity in rat cortical cells caused by N-methyl-D-aspartate (NMDA) and gp120 of HIV-1: induction and pharmacological intervention. Prog Mol Subcell Biol. 1996;16:44–57. doi: 10.1007/978-3-642-79850-4_3. [DOI] [PubMed] [Google Scholar]
- Nath A. Human immunodeficiency virus (HIV) proteins in neuropathogenesis of HIV dementia. J Infect Dis. 2002;186(2):S193–198. doi: 10.1086/344528. [DOI] [PubMed] [Google Scholar]
- O'Donnell LA, Agrawal A, Jordan-Sciutto KL, Dichter MA, Lynch DR, Kolson DL. Human immunodeficiency virus (HIV)-induced neurotoxicity: roles for the NMDA receptor subtypes. J Neurosci. 2006;26:981–990. doi: 10.1523/JNEUROSCI.4617-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattarini R, Pittaluga A, Raiteri M. The human immunodeficiency virus-1 envelope protein gp120 binds through its V3 sequence to the glycine site of N-methyl-D-aspartate receptors mediating noradrenaline release in the hippocampus. Neuroscience. 1998;87:147–157. doi: 10.1016/s0306-4522(98)00125-0. [DOI] [PubMed] [Google Scholar]
- Petralia RS. Distribution of extrasynaptic NMDA receptors on neurons. ScientificWorldJournal. 2012;2012:267120. doi: 10.1100/2012/267120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittaluga A, Raiteri M. HIV-1 envelope protein gp120 potentiates NMDA-evoked noradrenaline release by a direct action at rat hippocampal and cortical noradrenergic nerve endings. Eur J Neurosci. 1994;6:1743–1749. doi: 10.1111/j.1460-9568.1994.tb00566.x. [DOI] [PubMed] [Google Scholar]
- Reaux-Le Goazigo A, Rivat C, Kitabgi P, Pohl M, Melik Parsadaniantz S. Cellular and subcellular localization of CXCL12 and CXCR4 in rat nociceptive structures: physiological relevance. Eur J Neurosci. 2012;36:2619–2631. doi: 10.1111/j.1460-9568.2012.08179.x. [DOI] [PubMed] [Google Scholar]
- Riccio A, Ginty DD. What a privilege to reside at the synapse: NMDA receptor signaling to CREB. Nat Neurosci. 2002;5:389–390. doi: 10.1038/nn0502-389. [DOI] [PubMed] [Google Scholar]
- Rumbaugh G, Vicini S. Distinct synaptic and extrasynaptic NMDA receptors in developing cerebellar granule neurons. J Neurosci. 1999;19:10603–10610. doi: 10.1523/JNEUROSCI.19-24-10603.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin AH, Kim HJ, Thayer SA. Subtype selective NMDA receptor antagonists induce recovery of synapses lost following exposure to HIV-1 Tat. Br J Pharmacol. 2012;166:1002–1017. doi: 10.1111/j.1476-5381.2011.01805.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang YP, Shimizu E, Dube GR, Rampon C, Kerchner GA, Zhuo M, Liu G, Tsien JZ. Genetic enhancement of learning and memory in mice. Nature. 1999;401:63–69. doi: 10.1038/43432. [DOI] [PubMed] [Google Scholar]
- Thomas CG, Miller AJ, Westbrook GL. Synaptic and extrasynaptic NMDA receptor NR2 subunits in cultured hippocampal neurons. J Neurophysiol. 2006;95:1727–1734. doi: 10.1152/jn.00771.2005. [DOI] [PubMed] [Google Scholar]
- Toggas SM, Mucke L. Transgenic models in the study of AIDS dementia complex. Curr Top Microbiol Immunol. 1996;206:223–241. doi: 10.1007/978-3-642-85208-4_12. [DOI] [PubMed] [Google Scholar]
- Toggas SM, Masliah E, Mucke L. Prevention of HIV-1 gp120-induced neuronal damage in the central nervous system of transgenic mice by the NMDA receptor antagonist memantine. Brain Res. 1996;706:303–307. doi: 10.1016/0006-8993(95)01197-8. [DOI] [PubMed] [Google Scholar]
- Toggas SM, Masliah E, Rockenstein EM, Rall GF, Abraham CR, Mucke L. Central nervous system damage produced by expression of the HIV-1 coat protein gp120 in transgenic mice. Nature. 1994;367:188–193. doi: 10.1038/367188a0. [DOI] [PubMed] [Google Scholar]
- Tovar KR, Westbrook GL. The incorporation of NMDA receptors with a distinct subunit composition at nascent hippocampal synapses in vitro. J Neurosci. 1999;19:4180–4188. doi: 10.1523/JNEUROSCI.19-10-04180.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend M, Yoshii A, Mishina M, Constantine-Paton M. Developmental loss of miniature N-methyl-D-aspartate receptor currents in NR2A knockout mice. Proc Natl Acad Sci U S A. 2003;100:1340–1345. doi: 10.1073/pnas.0335786100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitney NP, Peng H, Erdmann NB, Tian C, Monaghan DT, Zheng JC. Calcium-permeable AMPA receptors containing Q/R-unedited GluR2 direct human neural progenitor cell differentiation to neurons. FASEB J. 2008;22:2888–2900. doi: 10.1096/fj.07-104661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams K. Ifenprodil discriminates subtypes of the N-methyl-D-aspartate receptor: selectivity and mechanisms at recombinant heteromeric receptors. Mol Pharmacol. 1993;44:851–859. [PubMed] [Google Scholar]
- Wu P, Price P, Du B, Hatch WC, Terwilliger EF. Direct cytotoxicity of HIV-1 envelope protein gp120 on human NT neurons. Neuroreport. 1996;7:1045–1049. doi: 10.1097/00001756-199604100-00018. [DOI] [PubMed] [Google Scholar]
- Xiong H, Baskys A, Wojtowicz JM. Brain-derived peptides inhibit synaptic transmission via presynaptic GABAB receptors in CA1 area of rat hippocampal slices. Brain Res. 1996;737:188–194. doi: 10.1016/0006-8993(96)00731-7. [DOI] [PubMed] [Google Scholar]
- Xiong H, McCabe L, Skifter D, Monaghan DT, Gendelman HE. Activation of NR1a/NR2B receptors by monocyte-derived macrophage secretory products: implications for human immunodeficiency virus type one-associated dementia. Neurosci Lett. 2003;341:246–250. doi: 10.1016/s0304-3940(03)00194-0. [DOI] [PubMed] [Google Scholar]
- Yang J, Hu D, Xia J, Liu J, Zhang G, Gendelman HE, Boukli NM, Xiong H. Enhancement of NMDA receptor-mediated excitatory postsynaptic currents by gp120-treated macrophages: implications for HIV-1-associated neuropathology. J Neuroimmune Pharmacol. 2013;8:921–933. doi: 10.1007/s11481-013-9468-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Liu J, Katafiasz B, Fox H, Xiong H. HIV-1 gp120-induced axonal injury detected by accumulation of beta-amyloid precursor protein in adult rat corpus callosum. J Neuroimmune Pharmacol. 2011;6:650–657. doi: 10.1007/s11481-011-9259-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Tang H, Xiong H. Chemokine CCL2 enhances NMDA receptor-mediated excitatory postsynaptic current in rat hippocampal slices-a potential mechanism for HIV-1-associated neuropathy? J Neuroimmune Pharmacol. 2016;11:306–315. doi: 10.1007/s11481-016-9660-2. [DOI] [PMC free article] [PubMed] [Google Scholar]


