Abstract
Background
Chronic active antibody mediated rejection (c-aABMR) is a major cause of long-term kidney allograft loss. It is hypothesized that frequent sub-therapeutic exposure to immunosuppressive drugs, in particular tacrolimus (Tac), is a risk factor for the development of c-aABMR. The intra-patient variability (IPV) in Tac exposure may serve as a substitute biomarker for underexposure and/or non-adherence. In this study, the association between Tac IPV and the development of c-aABMR was investigated.
Methods
We retrospectively included 59 patients diagnosed with c-aABMR and compared them to 189 control patients matched for age, year of transplantation and type of kidney donor. The Tac IPV was calculated from pre-dose tacrolimus concentrations measured over a 3 year period preceding the diagnosis of c-aABMR. The mean Tac predose concentrations (C0), Tac IPV, renal allograft function and graft survival were compared between the groups.
Results
Tac IPV was 24.4% for the cases versus 23.6% for the controls (p = 0.47). The mean Tac C0 was comparable for the cases (5.8 ng/mL) and control patients (6.1 ng/mL, p = 0.08). Only in the c-aABMR group a significant decline in both mean Tac C0 and allograft function over the timespan of 3 years was observed (p = 0.03 and p<0.001). Additionally, in the group of c-aABMR patients a high IPV was associated with inferior graft survival (p = 0.03).
Conclusions
A high Tac IPV per se does not predispose to the development of c-aABMR but is associated with inferior graft survival once c-aABMR is diagnosed.
Introduction
Despite a significant improvement in short-term kidney allograft survival in the past decade, long-term kidney allograft survival has remained relatively unchanged[1]. Chronic active antibody mediated rejection (c-aABMR) contributes substantially to these disappointing long-term transplantation outcomes[2, 3]. c-aABMR is believed to be the result of recurrent endothelial activation by pre-existing or de novo anti-HLA antibodies leading to numerous pathological abnormalities[4, 5]. It has been hypothesized that the development of c-aABMR is partially attributable to poor adherence or inadequate maintenance immunosuppression[3, 6].
In most centers, tacrolimus (Tac) is the cornerstone of the maintenance immunosuppressive regimen after renal transplantation[7]. Tac is a drug that requires frequent predose concentration monitoring to maintain therapeutic exposure[8]. It has a narrow therapeutic window and displays considerable intra-patient variability (IPV). The Tac IPV is defined as the fluctuation in Tac concentrations within an individual patient over a certain period of time[9]. These fluctuations in Tac exposure may result in periodic excessive or insufficient exposure, possibly leading to (nephro)toxicity or acute rejection. Many studies have reported an association between a high Tac IPV and inferior graft outcomes[10]. Patients with more variability in Tac exposure were more likely to develop donor-specific anti-HLA antibodies, lose their grafts and develop chronic histological lesions[3, 11–17]. In these studies, allograft failure was often defined as a composite endpoint which included the diagnosis of c-aABMR[3, 6, 11, 12, 15, 18]. However, the association between Tac IPV and the development of c-aABMR has never been analyzed separately. In this study, the association between Tac IPV and the risk of c-aABMR was investigated.
Subjects and methods
Study population
For this retrospective case-control study, all kidney transplant recipients transplanted in our center between 2000 and 2013 were eligible. The main inclusion criteria was the use of Tac as maintenance immunosuppression for both cases and controls. The year 2000 was chosen because then Tac became the CNI of choice in our center.
The standard immunosuppressive regimen did not include induction therapy before the year 2006–2007. Thereafter, patients received induction therapy with basiliximab and were set on a triple immunosuppressive regimen consisting of tacrolimus, mycophenolate mofetil and prednisolone after transplantation. In the first year after transplantation prednisolone was slowly tapered to 0 after 3 months. As per local protocol, the administration of Tac was slowly tapered after the first 6 months and Tac C0 were aimed at levels between 5–7 ng/ml. There were no other inclusion criteria regarding the use of other (maintenance) immunosuppressive drugs. This retrospective study was reviewed and approved by the Institutional Ethics Committee from the Erasmus MC, Rotterdam, The Netherlands. Due to the retrospective nature of the study no informed consent was needed.
Patients diagnosed with histologically-proven (suspicious) c-aABMR were defined as cases. The cases were selected from the pathology database at our center and were diagnosed after a for-cause biopsy. The diagnosis of c-aABMR was made at time of biopsy by an experienced renal pathologist based on the current Banff classification[19–21]. Patients were excluded if insufficient data was available (see data collection).
As a control group we selected kidney transplant recipients that showed no evidence of c-aABMR. All patients in the control group have had no clinical suspicion of c-aABMR until point of inclusion. Additionally, if present, all previous biopsy results have been screened for signs of (suspicious) c-aABMR. Controls were matched for age, year of transplantation, type of kidney donor (deceased versus living) and a minimal graft survival which resembled the cases’ time to c-aABMR diagnosis. The matching process was performed by coding all patients, both cases and controls, for the aforementioned factors. By means of an algorithm patients were found to be a positive match if all 4 factors were alike. The matches were selected from the transplantation database of our center. For every single c-aABMR case all possible matches were included.
Data collection
Demographic and baseline transplantation characteristics were collected for all patients. These characteristics were retrieved from the local transplantation database and complete for more than 90%. In addition, data on absolute (i.e. nondose-corrected) tacrolimus whole-blood, pre-dose concentrations were collected. Only Tac C0 measured after month 6 after transplantation were included, to allow for stabilization of drug dosing[11, 12]. Furthermore, only Tac C0 sampled during outpatient clinic visits were included to minimize bias from measurements made during hospitalization.
Tac C0 were collected over a period of 3 years. A minimum of 8 out-patient clinical measurements of Tac C0 over a minimal time period of 2 years was necessary per individual for an adequate calculation of Tac IPV.
The mean Tac C0 exposure over the 3 years was calculated as well as the Tac C0 exposure per year. The Tac IPV of the cases was calculated in the 3 year time period prior to c-aABMR diagnosis (endpoint, t0). The IPV of the controls was calculated over a similar time period, dependent on the matched cases’ time to endpoint (t0). The Tac IPV of interest for each patient was calculated as follows:
Formula 1. Formula used for calculating the intra-patient variability often also referred to as mean absolute deviation (MAD)[9, 11]. Xmean is the mean Tac C0 of all available samples in the 3 years prior to c-aABMR diagnosis. X1 represents the first available Tac C0 measurement, X2 the second…, and so on.
Furthermore, estimated glomerular filtration rate (eGFR, MDRD) measurements were collected at routine out-patient clinic controls[22]. Data was collected at the time of endpoint (t0) and 1 (t-1), 2 (t-2), and 3 (t-3) years prior to the histological diagnosis of c-aABMR (or matched controls endpoint). Data on allograft function was complete up to 90%.
Outcome
The association between Tac IPV and the development of c-aABMR was the primary outcome of interest. Secondary outcomes were allograft function, the mean Tac C0 exposure and its change over time for both the cases and controls. Additionally, the change in mean Tac C0 exposure per year and its relation to allograft function was assessed. Potential confounders such as age, sex, time to diagnosis and type of transplantation were analyzed as possible contributing factors to high Tac IPV in patients with c-aABMR.
Statistical analysis
The baseline characteristics of both cases and controls are reported using summary statistics and frequency tables for continuous and categorical variables. Differences between cases and controls were analyzed by the Chi-square test for categorical variables and with independent and paired sample t-test test for continuous variables, as appropriate. Additionally, several clinical characteristics such as age, gender, type of transplantation, mismatch and time to c-aABMR diagnosis were analyzed for association with Tac IPV in the c-aABMR cases. The groups were divided based on the characteristic and IPV percentages were compared by means of independent sample t-test. Linear regression was used to examine the correlation between allograft function and mean Tac C0. Overall graft survival was assessed by Kaplan-Meier survival analysis with log-rank statistics for difference. The software IBM SPSS statistics 21 was used to perform the statistical analysis. Variables were considered statistically significant with a two-tailed P-value of <0.05.
Results
Patient characteristics
Two hundred and forty-eight renal transplant patients were identified for this study and included for analysis. Fifty-nine patients were considered cases and the remaining 189 were matched controls. The characteristics of cases and controls are displayed in Table 1. The median time from kidney transplantation to c-aABMR diagnosis (t0, endpoint) was 6.1 (IQR 3.5–8.3) years. The follow-up for both cases and controls was 3 years. Matching was achieved successfully as there were no differences between cases and controls with regard to age at transplantation, donor type and year of transplantation. More importantly, no statistically significant differences were found in mean eGFR (48 ml/min/1.73m2 cases vs. 50 ml/min/1.73m2 controls, p = 0.55) and mean Tac C0 (6.1 ng/ml vs. 6.1 ng/ml, p = 0.98) at starting point (t-3) which was 3 years prior to c-aABMR diagnosis or matched controls endpoint. There was however a significant difference in gender with significantly more male patients in the c-aABMR cases.
Table 1. Demographic and clinical characteristics.
| Cases (n = 59) | Controls (n = 189) | p-value | ||
|---|---|---|---|---|
| Age, years, mean (sd) | 49 (±13) | 52 (±13) | 0.23 | |
| Gender, n (%) | Female | 21 (36) | 39 (21) | 0.02 |
| Male | 38 (64) | 150 (79) | ||
| Donor type, n (%) | Deceased donor | 19 (32) | 41 (22) | 0.10 |
| Living donor | 40 (68) | 148 (78) | ||
| Donor age, years, mean (sd) | 51 (±12) | 51 (±13) | 0.71 | |
| Previous Transplantation, n (%) | Yes | 12 (20) | 24 (13) | 0.15 |
| No | 47 (80) | 165 (87) | ||
| Time of Transplantation, year | 2000–2012 | 2000–2012 | ||
| Time to diagnosis, years, median (IQR) | 6.1 (3.5–8.3) | - | ||
| Total HLA mismatch, median (IQR) | 3 (2–5) | 3 (3–5) | 0.70 | |
| PRAa current, mean (range) | 1.5 (0–8) | 3.2 (0–96) | 0.44 | |
| PRA peak, mean (range) | 9.5 (0–62) | 6.6 (0–80) | 0.49 | |
| Previous BPARb, n (%) | Yes | 15 (25) | 36 (19) | 0.30 |
| No | 44 (75) | 153 (81) | ||
| eGFR at t-3, ml/min/1.73m2, mean (sd) | 48 (±12) | 50 (±15) | 0.55 | |
| Trough-level at t-3, ng/mL, mean (sd) | 6.1 (±1.9) | 6.1 (±1.7) | 0.98 | |
| C0 measurements, n, mean (range) | 17 (8–33) | 15 (8–44) | 0.01 | |
| Primary kidney disease, n (%) | Diabetic | 8 (13.6) | 36 (19) | |
| Nephropathy | ||||
| Hypertensive | 10 (16.9) | 44 (23.3) | ||
| Nephropathy | ||||
| IgA Nephropathy | 3 (5.1) | 12 (6.3) | ||
| Polycystic Kidney | 10 (16.9) | 24 (12.7) | ||
| Disease | ||||
| FSGSc | 3 (5.1) | 8 (4.2) | ||
| Obstructive | 1 (1.7) | 7 (3.7) | ||
| Nephropathy | ||||
| Unknown | 3 (5.1) | 11 (5.8) | ||
| Other | 21 (35.6) | 47 (25) |
a PRA, Panel Reactive Antibody;
b BPAR, biopsy proven acute rejection (incl. borderline changes);
c FSGS, Focal Segmental Glomerulosclerosis
Tacrolimus intra-patient variability and mean tacrolimus predose concentrations
For the calculation of Tac IPV on average 17 (8–33) Tac C0 measurements were available for the cases and 15 (8–44) measurements for the controls. The number of available Tac C0 measurements was significantly higher for the cases (p = 0.01). This slight but significant difference could be explained by the increased number of out-patient clinic visits (related to impairment of renal function or the appearance of proteinuria) prior to the for-cause biopsy, in the cases.
The mean Tac IPV was 24.4% (range: 12.0%-48.3%) in the cases and 23.6% (range: 9.7%-46.3%) in the controls and showed no statistically significant difference (p = 0.47). We were unable to identify any specific clinical characteristics, such as age, gender, type of transplantation (living or deceased) and time to c-aABMR diagnosis to be associated with a significant difference in IPV (Table 2).
Table 2. Clinical characteristics associated with IPV in c-aABMR cases.
| Clinical characteristic | Tac IPV, %, mean (sd) | p-value | |
|---|---|---|---|
| Age | <50yrs (n = 29) >50yrs (n = 30) |
22.8% (±7.4) 25.9% (±7.4) |
0.12 |
| Gender | Male (n = 38) Female (n = 21) |
24.9% (±7.7) 23.5% (±7.2) |
0.51 |
| Type of transplantation | PM (n = 19) Living (n = 40) |
24.4% (±5.9) 24.4% (±8.2) |
0.99 |
| Mismatch | Mismatch<3 (n = 31) Mismatch>3 (n = 26) |
24.1% (±7.1) 24.8% (±8.3) |
0.72 |
| Time to c-aABMR | <2250 days (n = 30) >2250 days (n = 29) |
25.4% (±7.3) 23.3% (±7.7) |
0.28 |
Similarly, there was no significant difference in mean Tac C0 over the total 3 year period (t-3-t0). The cases had a mean Tac C0 of 5.8 ng/mL [range: 3.3 ng/mL-8.5 ng/mL] versus a mean Tac C0 of 6.1 ng/mL [range: 3.6 ng/mL-10.0 ng/mL] for the controls (p = 0.08).
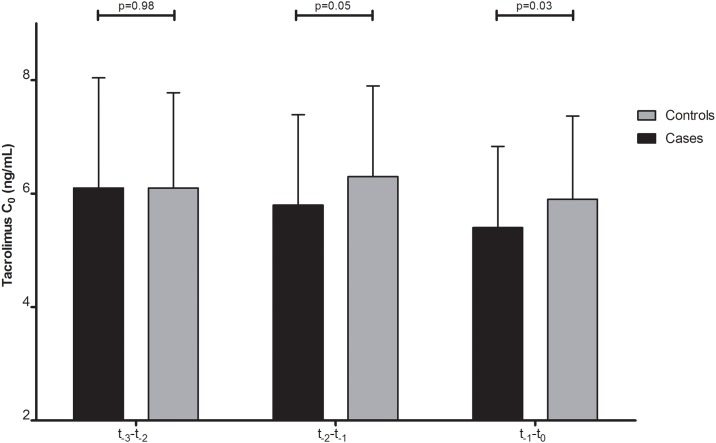
There was however a significantly declining trend noted in mean Tac C0 during the 3 years preceding the diagnosis of c-aABMR among cases but not controls (Fig 1) (p = 0.02 for cases versus p = 0.18 for controls). When mean Tac C0 was analyzed per year, patients who developed c-aABMR had a mean Tac C0 of 6.1 ng/mL between t-3-t-2, which fell to 5.8 (t-2-t-1) and 5.4 ng/mL (t-1-t-0) in the years thereafter. Controls had a similar mean Tac C0 to the cases between t-3-t-2 (6.1 ng/mL; p = 0.98) which in the years thereafter showed no decline (6.3 ng/mL (t-2-t-1) and 5.9 ng/mL (t-1-t-0)). The difference in mean Tac C0 in the year prior to endpoint (t-1-t-0) between the cases and controls was significant at a P-value of 0.03.
Fig 1. The tacrolimus (Tac) predose concentrations (C0) spread over the 3 years prior to c-aABMR diagnosis/matched controls endpoints (t0) for both c-aABMR patients (cases) and controls.
Tac C0 (t-3-t-2) was 6.1 (±1.94) ng/mL for both cases and controls. Tac C0 (t-2-t-1) was 5.8 (±1.59) ng/mL for the cases and 6.3 (±1.60) ng/mL for the controls. Tac C0 (t-1-t-0) was 5.4 (±1.43) ng/mL for the cases and 5.9 (±1.47) ng/mL for the controls.
Allograft function
Cases and controls showed a similar allograft function at start of analysis (t-3) of 48 ml/min/1.73m2 and 50 ml/min/1.73m2, respectively (p = 0.55). In the following years the cases had a substantial decline in allograft function. The average eGFR of the cases deteriorated from 48 ml/min/1.73m2 3 years before the diagnosis of c-aABMR to 45 (t-2), 42 (t-1), and 32 ml/min/1.73m2 (t0; Fig 2). The controls on the other hand had a stable decline in allograft function of only 1 ml/min/1.73m2 per year. The average eGFR of the controls decreased from 50 (t-3) to 49 (t-2), to 48 (t-1), and to 47 ml/min/1.73m2 (t0; Fig 2). The allograft function of cases and controls did not differ significantly in the first 2 years of follow up (p = 0.55 at t-3 and p = 0.07 at t-2). However, in the 2 years thereafter the cases had a significantly inferior allograft function compared to the controls (p = 0.005 at t-1 and p<0.001 at t0).
Fig 2. Allograft function of both patients with c-aABMR (cases) and controls.
*t-3, p = 0.55; **t0, p<0.001.
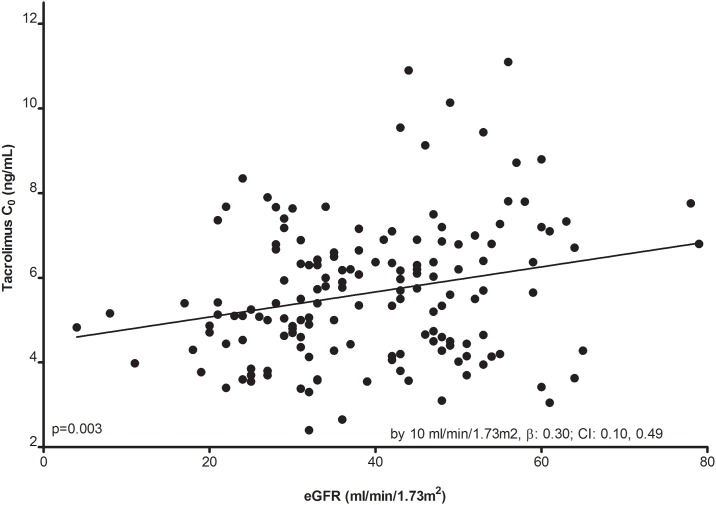
Linear regression analysis showed a positive association between increasing Tac C0 and a better allograft function (by 10 ml/min/1.73m2, β: 0.30; CI: 0.10, 0.49; P = 0.003, Fig 3). This correlation was not present for the controls (p = 0.49).
Fig 3. Association between tacrolimus (Tac) predose concentrations (C0) and allograft function for the c-aABMR patients (cases).
Allograft survival
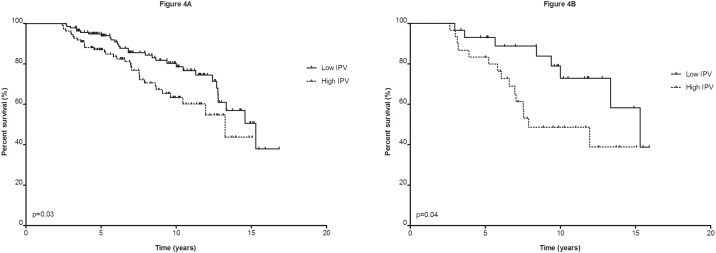
To assess the association of intra-patient variability and Tac C0 with overall graft survival, the patients were divided dichotomously based on the cohort’s mean IPV and C0. The average IPV of the low IPV group (≤24%) was 18.5% and 30.4% for the high IPV group (>24%). Notably, a high IPV was significantly associated with inferior graft survival (13.3 years for ≤24% IPV vs. 11.2 for >24% IPV; log rank, p = 0.03)(Fig 4A). However, this association was only present for the cases. The cases with a low IPV showed an average survival of 13.1 years compared to only 9.9 years in cases with a high IPV (log rank, p = 0.04; Fig 4B). The controls had a survival of 13.4 years (≤24% IPV) versus 11.6 years (>24%)(log rank, p = 0.31).
Fig 4. Allograft survival.
(A) allograft survival based on ≤24% tacrolimus (Tac) intra-patient variability (IPV) vs. >24% Tac IPV of cases and controls combined, (B) Allograft survival of c-aABMR cases based on ≤24% Tac IPV vs. >24%.
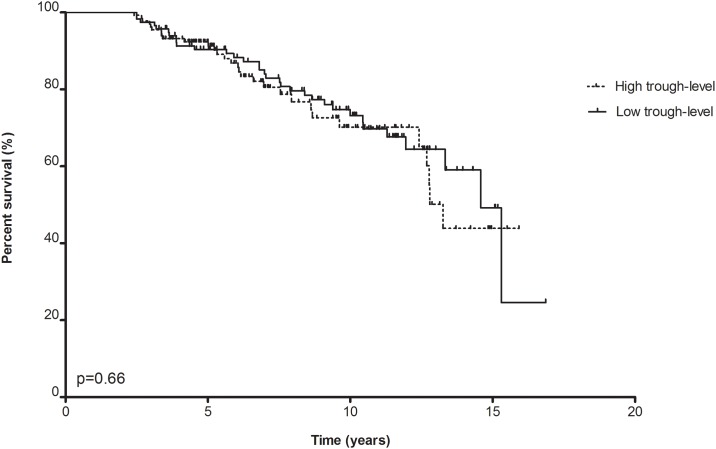
A similar analysis based on below or above average Tac C0 did not show any differences for either the case or control group (12.7 years for ≤5.9 ng/mL vs. 12.2 years; log rank, p = 0.66)(Fig 5).
Fig 5. Allograft survival based on tacrolimus (Tac) predose concentrations (C0) ≤5.9 ng/mL vs. >5.9 ng/mL of c-aABMR patients (cases) and controls combined.
Discussion
In this retrospective case-control study we tested the hypothesis that a high IPV of tacrolimus predose concentrations is associated with a higher risk for developing chronic-active antibody mediated rejection of the kidney allograft. However, we found that in cases with c-aABMR the IPV for tacrolimus in the years prior to the diagnoses was similar to the IPV of matched controls.
Fluctuations in Tac C0 are very common in the first weeks or months after transplantation due to numerous causes such as drug-drug interactions, tapering of corticosteroid doses and/or changes in gastro-intestinal motility. Typically however, after a couple of weeks or months patients reach a more stable situation as the inter-occasion variability decreases. The differences in Tac C0 tend to get smaller over time. Fluctuations in Tac C0 in the maintenance phase after transplantation may therefor represent suboptimal patient-adherence. The calculated intra-patient variability of Tac C0 is considered a surrogate marker for non-adherence[9, 10, 23].
There are however several methods for the calculation of the fluctuation in Tac C0. One of which is the variance (σ2). The variance is used for the quantification of the intrapatient variability by showing the data distribution around the mean[13, 15]. Another useful method to determine the Tac IPV is by calculating the coefficient of variation (CV) [13, 24–28]. The CV assesses the degree of variation of Tac C0 measurements. We however, have chosen to use the mean absolute deviation (MAD) as a statistical measure for Tac IPV. Although the calculation of Tac IPV by means of MAD or CV are quite similar, the MAD is less susceptible to outliers. This is because the CV uses the squared deviation from the mean, whereas the MAD uses the absolute deviation[9, 11].
However, as stated above, the time period over which Tac C0 are collected, is of greater importance and should be taken into account when interpreting Tac IPV data. And even though a great variation has been reported in literature, on average Tac IPV is between 15% and 30% which is in line with our findings[11, 13, 24–28].
Borra et al. were the first to establish an association between high intra-patient variability in Tac C0 and the increased risk of developing graft loss[11]. Similar results have been published since[12–15, 29–31]. However, no evidence has been provided for a link between suboptimal Tac patient-adherence in the years after transplantation and the risk for late antibody mediated rejection of the renal allograft.
The current studies tend to rely on broad composite end-points consisting of a range of diagnoses such as late acute rejection, transplant glomerulopathy, graft failure and/or death with function[11–15]. It can be expected that considerably different results may be obtained once the composite endpoint is modified, as previously shown by the extension study of Borra et al[12]2. They demonstrated a substantially smaller effect size of Tac IPV on long-term allograft outcomes after adjustment of the composite endpoint. Furthermore a majority of these studies focus on IPV over a brief time span in the first year after transplantation while chronic humoral rejection is usually diagnosed at an average of 4–6 years after transplantation.
The remainder of studies on this subject define non-adherence as self-reported drug-noncompliance, clinical suspicions by the attending physician and/or repeated nonattendance at clinic visits or laboratory testing[3, 14]. This information is usually not present in most medical records except when the graft outcome was unfavorable which may create a biased interpretation of the results.
In this study, IPV in cases and matched controls was similar and could therefore not support the hypothesis that a high IPV is associated with an increased risk for the development of chronic active antibody mediated rejection. These findings are in line with previous results published by Halloran et al. where nonadherence was not significantly associated with late chronic active antibody mediated rejection[32]. Similarly, Vanhove et al. observed that high IPV of Tac predicted chronic allograft damage such as tubular atrophy and severe fibrosis but not inflammation or transplant glomerulopathy lesions[16].
Although cases and controls in our study had similar Tac C0 and renal function at starting point, the cases showed a significantly declining trend in both Tac C0 and renal function compared to the controls. The controls had a mean Tac C0 which varied around 6 ng/mL whereas the cases showed a gradual decline reaching Tac C0 of 5.4 ng/mL at endpoint. A similar development was visible in renal function where the controls showed an average decline in renal function of 1 ml/min/1.73m2 per year while the deterioration in graft function of the cases gradually increased to 10 ml/min/1.73m2 in the year prior to c-aABMR diagnosis. The decline in Tac C0 was significantly correlated to the decline in renal allograft function for the cases. Most likely, these observations reflect the physicians inclination to taper Tac dosage after witnessing a decline in allograft function, in an attempt to avoid presumed tacrolimus-related nephrotoxicity. Unfortunately, the entries made in the medical records were not sufficiently informative to support this conclusion with data.
Of interest is the significant association of an inferior graft survival for the c-aABMR patients with a high IPV versus those with a low IPV. This association was not found for the controls. This intriguing finding is in line with previous research suggesting poorer graft survival for patients with high IPV’s[11–15]. The explanation can only be speculative but it suggests that not the risk for development of c-aABMR is IPV-related but rather the subsequent severity of the ongoing humoral rejection. Adequate maintenance of Tac C0 seems to provide a better control of the chronic antibody mediated rejection which carries in general a poor prognosis for graft survival.
Our study does have several limitations. First, the causes of IPV cannot be determined due to the retrospective nature of the study. Even though multiple factors are known to contribute to a high IPV, nonadherence is considered a dominant cause[10, 16]. Secondly, external validation might not be possible while it is a single center study and may not reflect the transplant population of other centers and their immunosuppressive regimen. However, the Tac C0 were in range with what is considered common practice (6.1 ng/mL and 5.8 ng/mL).
In conclusion, we demonstrated that a high intra-patient variability of tacrolimus long-term after kidney transplantation is not associated with the occurrence of chronic antibody mediated rejection but unfavorably affects graft survival in patients with c-aABMR.
Supporting information
(SAV)
Acknowledgments
The authors are grateful to dr. N. Shukur and Mrs. J. Kal-van Gestel for their valuable contributions. The authors also wish to thank the staff of the department of Nephrology and Transplantation (Erasmus MC).
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The authors received no specific funding for this work.
References
- 1.Lamb KE, Lodhi S, Meier-Kriesche HU. Long-term renal allograft survival in the United States: a critical reappraisal. Am J Transplant. 2011;11(3):450–62. doi: 10.1111/j.1600-6143.2010.03283.x . [DOI] [PubMed] [Google Scholar]
- 2.Gourishankar S, Leduc R, Connett J, Cecka JM, Cosio F, Fieberg A, et al. Pathological and clinical characterization of the ‘troubled transplant’: data from the DeKAF study. Am J Transplant. 2010;10(2):324–30. doi: 10.1111/j.1600-6143.2009.02954.x . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sellares J, de Freitas DG, Mengel M, Reeve J, Einecke G, Sis B, et al. Understanding the causes of kidney transplant failure: the dominant role of antibody-mediated rejection and nonadherence. Am J Transplant. 2012;12(2):388–99. doi: 10.1111/j.1600-6143.2011.03840.x . [DOI] [PubMed] [Google Scholar]
- 4.Cosio FG, Gloor JM, Sethi S, Stegall MD. Transplant glomerulopathy. Am J Transplant. 2008;8(3):492–6. doi: 10.1111/j.1600-6143.2007.02104.x . [DOI] [PubMed] [Google Scholar]
- 5.Gloor JM, Sethi S, Stegall MD, Park WD, Moore SB, DeGoey S, et al. Transplant glomerulopathy: subclinical incidence and association with alloantibody. Am J Transplant. 2007;7(9):2124–32. doi: 10.1111/j.1600-6143.2007.01895.x . [DOI] [PubMed] [Google Scholar]
- 6.El-Zoghby ZM, Stegall MD, Lager DJ, Kremers WK, Amer H, Gloor JM, et al. Identifying specific causes of kidney allograft loss. Am J Transplant. 2009;9(3):527–35. doi: 10.1111/j.1600-6143.2008.02519.x . [DOI] [PubMed] [Google Scholar]
- 7.Matas AJ, Smith JM, Skeans MA, Lamb KE, Gustafson SK, Samana CJ, et al. OPTN/SRTR 2011 Annual Data Report: kidney. Am J Transplant. 2013;13 Suppl 1:11–46. doi: 10.1111/ajt.12019 . [DOI] [PubMed] [Google Scholar]
- 8.Wallemacq P, Armstrong VW, Brunet M, Haufroid V, Holt DW, Johnston A, et al. Opportunities to optimize tacrolimus therapy in solid organ transplantation: report of the European consensus conference. Ther Drug Monit. 2009;31(2):139–52. doi: 10.1097/FTD.0b013e318198d092 . [DOI] [PubMed] [Google Scholar]
- 9.Shuker N, van Gelder T, Hesselink DA. Intra-patient variability in tacrolimus exposure: causes, consequences for clinical management. Transplant Rev (Orlando). 2015;29(2):78–84. doi: 10.1016/j.trre.2015.01.002 . [DOI] [PubMed] [Google Scholar]
- 10.Lieber SR, Volk ML. Non-adherence and graft failure in adult liver transplant recipients. Dig Dis Sci. 2013;58(3):824–34. doi: 10.1007/s10620-012-2412-0 . [DOI] [PubMed] [Google Scholar]
- 11.Borra LC, Roodnat JI, Kal JA, Mathot RA, Weimar W, van Gelder T. High within-patient variability in the clearance of tacrolimus is a risk factor for poor long-term outcome after kidney transplantation. Nephrol Dial Transplant. 2010;25(8):2757–63. doi: 10.1093/ndt/gfq096 . [DOI] [PubMed] [Google Scholar]
- 12.Shuker N, Shuker L, van Rosmalen J, Roodnat JI, Borra LC, Weimar W, et al. A high intrapatient variability in tacrolimus exposure is associated with poor long-term outcome of kidney transplantation. Transpl Int. 2016;29(11):1158–67. doi: 10.1111/tri.12798 . [DOI] [PubMed] [Google Scholar]
- 13.Hsiau M, Fernandez HE, Gjertson D, Ettenger RB, Tsai EW. Monitoring nonadherence and acute rejection with variation in blood immunosuppressant levels in pediatric renal transplantation. Transplantation. 2011;92(8):918–22. doi: 10.1097/TP.0b013e31822dc34f . [DOI] [PubMed] [Google Scholar]
- 14.Pollock-Barziv SM, Finkelstein Y, Manlhiot C, Dipchand AI, Hebert D, Ng VL, et al. Variability in tacrolimus blood levels increases the risk of late rejection and graft loss after solid organ transplantation in older children. Pediatr Transplant. 2010;14(8):968–75. doi: 10.1111/j.1399-3046.2010.01409.x . [DOI] [PubMed] [Google Scholar]
- 15.Sapir-Pichhadze R, Wang Y, Famure O, Li Y, Kim SJ. Time-dependent variability in tacrolimus trough blood levels is a risk factor for late kidney transplant failure. Kidney Int. 2014;85(6):1404–11. doi: 10.1038/ki.2013.465 . [DOI] [PubMed] [Google Scholar]
- 16.Vanhove T, Vermeulen T, Annaert P, Lerut E, Kuypers DRJ. High Intrapatient Variability of Tacrolimus Concentrations Predicts Accelerated Progression of Chronic Histologic Lesions in Renal Recipients. Am J Transplant. 2016;16(10):2954–63. doi: 10.1111/ajt.13803 . [DOI] [PubMed] [Google Scholar]
- 17.Rodrigo E, Segundo DS, Fernández-Fresnedo G, López-Hoyos M, Benito A, Ruiz JC, et al. Within-Patient Variability in Tacrolimus Blood Levels Predicts Kidney Graft Loss and Donor-Specific Antibody Development. Transplantation. 2016;100(11):2479–85. doi: 10.1097/TP.0000000000001040 [DOI] [PubMed] [Google Scholar]
- 18.O’Regan JA, Canney M, Connaughton DM, O’Kelly P, Williams Y, Collier G, et al. Tacrolimus trough-level variability predicts long-term allograft survival following kidney transplantation. J Nephrol. 2016;29(2):269–76. doi: 10.1007/s40620-015-0230-0 . [DOI] [PubMed] [Google Scholar]
- 19.Loupy A, Haas M, Solez K, Racusen L, Glotz D, Seron D, et al. The Banff 2015 Kidney Meeting Report: Current Challenges in Rejection Classification and Prospects for Adopting Molecular Pathology. Am J Transplant. 2017;17(1):28–41. doi: 10.1111/ajt.14107 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haas M, Sis B, Racusen LC, Solez K, Glotz D, Colvin RB, et al. Banff 2013 meeting report: inclusion of c4d-negative antibody-mediated rejection and antibody-associated arterial lesions. Am J Transplant. 2014;14(2):272–83. doi: 10.1111/ajt.12590 . [DOI] [PubMed] [Google Scholar]
- 21.Mengel M, Sis B, Haas M, Colvin RB, Halloran PF, Racusen LC, et al. Banff 2011 Meeting report: new concepts in antibody-mediated rejection. Am J Transplant. 2012;12(3):563–70. Epub 2012/02/04. doi: 10.1111/j.1600-6143.2011.03926.x . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130(6):461–70. . [DOI] [PubMed] [Google Scholar]
- 23.Shemesh E, Fine RN. Is calculating the standard deviation of tacrolimus blood levels the new gold standard for evaluating non-adherence to medications in transplant recipients? Pediatr Transplant. 2010;14(8):940–3. doi: 10.1111/j.1399-3046.2010.01396.x . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Prytula AA, Bouts AH, Mathot RA, van Gelder T, Croes LK, Hop W, et al. Intra-patient variability in tacrolimus trough concentrations and renal function decline in pediatric renal transplant recipients. Pediatr Transplant. 2012;16(6):613–8. doi: 10.1111/j.1399-3046.2012.01727.x . [DOI] [PubMed] [Google Scholar]
- 25.Stifft F, Stolk LM, Undre N, van Hooff JP, Christiaans MH. Lower Variability in 24-Hour Exposure During Once-Daily Compared to Twice-Daily Tacrolimus Formulation in Kidney Transplantation. Transplantation. 2013. . [DOI] [PubMed] [Google Scholar]
- 26.van Hooff J, Van der Walt I, Kallmeyer J, Miller D, Dawood S, Moosa MR, et al. Pharmacokinetics in stable kidney transplant recipients after conversion from twice-daily to once-daily tacrolimus formulations. Ther Drug Monit. 2012;34(1):46–52. doi: 10.1097/FTD.0b013e318244a7fd . [DOI] [PubMed] [Google Scholar]
- 27.Wu MJ, Cheng CY, Chen CH, Wu WP, Cheng CH, Yu DM, et al. Lower variability of tacrolimus trough concentration after conversion from prograf to advagraf in stable kidney transplant recipients. Transplantation. 2011;92(6):648–52. doi: 10.1097/TP.0b013e3182292426 . [DOI] [PubMed] [Google Scholar]
- 28.Wehland M, Bauer S, Brakemeier S, Burgwinkel P, Glander P, Kreutz R, et al. Differential impact of the CYP3A5*1 and CYP3A5*3 alleles on pre-dose concentrations of two tacrolimus formulations. Pharmacogenet Genomics. 2011;21(4):179–84. doi: 10.1097/FPC.0b013e32833ea085 . [DOI] [PubMed] [Google Scholar]
- 29.Gallagher HM, Sarwar G, Tse T, Sladden TM, Hii E, Yerkovich ST, et al. Erratic tacrolimus exposure, assessed using the standard deviation of trough blood levels, predicts chronic lung allograft dysfunction and survival. J Heart Lung Transplant. 2015;34(11):1442–8. doi: 10.1016/j.healun.2015.05.028 . [DOI] [PubMed] [Google Scholar]
- 30.Taber DJ, Su Z, Fleming JN, McGillicuddy JW, Posadas-Salas MA, Treiber FA, et al. Tacrolimus Trough Concentration Variability and Disparities in African American Kidney Transplantation. Transplantation. 2017. doi: 10.1097/TP.0000000000001840 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Whalen HR, Glen JA, Harkins V, Stevens KK, Jardine AG, Geddes CC, et al. High Intrapatient Tacrolimus Variability Is Associated With Worse Outcomes in Renal Transplantation Using a Low-Dose Tacrolimus Immunosuppressive Regime. Transplantation. 2017;101(2):430–6. doi: 10.1097/TP.0000000000001129 . [DOI] [PubMed] [Google Scholar]
- 32.Halloran PF, Merino Lopez M, Barreto Pereira A. Identifying Subphenotypes of Antibody-Mediated Rejection in Kidney Transplants. Am J Transplant. 2016;16(3):908–20. doi: 10.1111/ajt.13551 . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(SAV)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.