Abstract
High-performance liquid chromatography (HPLC) with fluorescence detection was used to study thiol metabolism in legume nodules. Glutathione (GSH) was the major non-protein thiol in all indeterminate nodules examined, as well as in the determinate nodules of cowpea (Vigna unguiculata), whereas homoglutathione (hGSH) predominated in soybean (Glycine max), bean (Phaseolus vulgaris), and mungbean (Vigna radiata) nodules. All nodules had greater thiol concentrations than the leaves and roots of the same plants because of active thiol synthesis in nodule tissue. The correlation between thiol tripeptides and the activities of glutathione synthetase (GSHS) and homoglutathione synthetase (hGSHS) in the nodules of eight legumes, and the contrasting thiol contents and activities in alfalfa (Medicago sativa) leaves (98% hGSH, 100% hGSHS) and nodules (72% GSH, 80% GSHS) indicated that the distribution of GSH and hGSH is determined by specific synthetases. Thiol contents and synthesis decreased with both natural and induced nodule senescence, and were also reduced in the senescent zone of indeterminate nodules. Thiols and GSHS were especially abundant in the meristematic and infected zones of pea (Pisum sativum) nodules. Thiols and γ-glutamylcysteinyl synthetase were also more abundant in the infected zone of bean nodules, but hGSHS was predominant in the cortex. Isolation of full-length cDNA sequences coding for γ-glutamylcysteinyl synthetase from legume nodules revealed that they are highly homologous to those from other higher plants.
The tripeptide glutathione (GSH; γGlu-Cys-Gly) is the major non-protein thiol in most animals, plants, and prokaryotes (Meister and Anderson, 1983; Hausladen and Alscher, 1993; Rennenberg, 1997). In plants, GSH is a versatile antioxidant that can directly scavenge activated oxygen species and participate in the ascorbate-GSH cycle for peroxide removal in the chloroplasts. It is also involved in many other vital functions of plants, including the transport and storage of sulfur, the synthesis of proteins and DNA, tolerance to abiotic and biotic stress, and the detoxification of xenobiotics, air pollutants, and heavy metals (Hausladen and Alscher, 1993; Rennenberg, 1997; May et al., 1998).
The pathway for GSH synthesis is probably shared by all organisms and involves two ATP-dependent steps. In the first reaction, γ-glutamylcysteine (γEC) is formed from Glu and Cys by γ-glutamylcysteinyl synthetase (γECS; EC 6.3.2.2), and in the second reaction Gly is added to the C-terminal site of γEC by GSH synthetase (GSHS; EC 6.3.2.3). In plants γECS and GSHS are present in the chloroplasts and cytosol of leaves (Law and Halliwell, 1986; Klapheck et al., 1987; Hell and Bergmann, 1988, 1990). More recently, the two enzymes have also been found in the roots of maize (Rüegsegger and Brunold, 1993) and of the heavy-metal accumulator Brassica juncea (Schäfer et al., 1998).
Legumes are an interesting plant material with which to study thiol metabolism for various reasons. First, there is an active ascorbate-GSH cycle in the root nodules, which requires a continuous supply of GSH to protect nitrogen fixation against toxic oxygen species (Dalton et al., 1986). Second, the leaves, roots, and seeds of some legumes contain a thiol tripeptide homolog, homoglutathione (hGSH; γGlu-Cys-βAla), instead of or in addition to GSH. The synthesis of hGSH is thought to proceed through γECS and a specific hGSH synthetase (hGSHS; Klapheck, 1988; Macnicol, 1987). Third, GSH is believed to be involved in plant morphogenesis, cell division, control of redox status, and signaling of stress and pathogen attack (Wingate et al., 1988; May et al., 1998). All of these processes, with some modifications (Vasse et al., 1990; Hirsch, 1992; Baron and Zambryski, 1995), are important in nodule formation and functioning, and therefore GSH is likely to be a critical molecule of nodules.
There is scant information about thiol compounds of legume nodules. Thiol tripeptides are known to be at high concentrations in nodules (Dalton et al., 1991; Gogorcena et al., 1995, 1997; Escuredo et al., 1996), but this information is based on an enzymatic assay that does not distinguish between GSH and hGSH (Griffith, 1980). Very recently, Evans et al. (1999) reported that hGSH is more abundant than GSH in soybean nodules. However, they employed an HPLC technique based on the formation and UV detection of dinitrophenyl derivatives from the reaction of 1-fluoro-2,4-dinitrobenzene with the amino groups (Farris and Reed, 1987). The technique is slow since it requires overnight derivatization and lacks the necessary sensitivity and specificity to quantify thiols in small nodule samples or dissected nodule fractions. This is especially true for Cys and γEC, which are present in plant tissues at low concentrations and are also essential for the study of thiol metabolism. Evans et al. (1999) also concluded that natural senescence in soybean nodules is an oxidative stress process. They reported, for example, a decrease in thiol content and increases in catalytic iron, thiol oxidation, and oxidative damage. A few years earlier we reached the same conclusions about stress-induced nodule senescence (Gogorcena et al., 1995, 1997; Escuredo et al., 1996).
The latest paper within this extensive study on stress-induced nodule senescence (Matamoros et al., 1999) reported that thiol contents and thiol synthetase activities of nodules could be conveniently assayed using HPLC with fluorescence detection. In the present study, we have improved this methodology and examined in detail thiol metabolism in legume nodules. Our results show that nodules are a main site of GSH and hGSH synthesis within the plant and provide indirect evidence that thiol compounds play a crucial role in the process of nitrogen fixation.
MATERIALS AND METHODS
Plant and Bacterial Material
The legume-rhizobia symbioses used in this study are indicated in Table I. Nodulated plants were grown in pots containing a 2:1 (v/v) perlite:vermiculite mixture with nitrogen-free nutrient solution under controlled environment conditions (Gogorcena et al., 1997). For senescence studies, the age, growth stage, and treatment of plants are indicated in Tables IV and V. For other experiments, all legumes were between 30 and 35 d old when harvested (except alfalfa, which was between 50 and 54 d old). All plants were at the vigorous vegetative growth stage. Nodules for dissection studies were processed immediately after harvest. All other plant material was flash-frozen in liquid nitrogen and stored at −80°C until extraction.
Table I.
Plant and bacterial material used in this study
| Common Name | Symbiosis |
|---|---|
| Pea | Pisum sativum L. cv Lincoln × Rhizobium leguminosarum biovar viciae NLV8 |
| Broad bean | Vicia faba L. cv Muchamiel × R. leguminosarum biovar viciae NLV8 |
| Alfalfa | Medicago sativa L. cv Aragón × Sinorhizobium meliloti 102F78 |
| Lupine | Lupinus albus L. cv Multolupa × Bradyrhizobium sp. (Lupinus) ISLU16 |
| Soybean | Glycine max Merr. cv Williams × Bradyrhizobium japonicum USDA110 |
| Bean | Phaseolus vulgaris L. cv Contender × R. leguminosarum biovar phaseoli 3622 |
| Mungbean | Vigna radiata Wilczek × Bradyrhizobium sp. (Vigna) 32H1 |
| Cowpea | Vigna unguiculata Walp. cv California # 5 × Bradyrhizobium sp. (Vigna) 32H1 |
Table IV.
Effect of aging and stress-induced senescence on thiol composition and synthesis in pea nodules
| Parameter | 3a | 5b | 7c | 3+Dd |
|---|---|---|---|---|
| Protein (mg g−1 fresh wt) | 22.8 ± 0.9 | 12.2 ± 0.9 | 8.4 ± 0.8 | 14.7 ± 2.4 |
| Shoot fresh wt (g plant−1) | 2.1 ± 0.3 | 5.3 ± 1.2 | 9.6 ± 0.7 | 1.8 ± 0.3 |
| Cys (nmol g−1 fresh wt) | 61.0 ± 4.2 | 39.7 ± 8.4 | 27.5 ± 1.9 | 26.1 ± 2.5 |
| γEC (nmol g−1 fresh wt) | 3.7 ± 0.4 | 0.8 ± 0.6 | 0 | 0 |
| GSH (nmol g−1 fresh wt) | 1,267 ± 37 | 868 ± 91 | 224 ± 52 | 102 ± 24 |
| hGSH (nmol g−1 fresh wt) | 138 ± 11 | 104 ± 14 | 49 ± 6 | 85 ± 16 |
| γECS (nmol min−1 g−1 fresh wt) | 4.2 ± 0.4 | 2.6 ± 0.8 | 1.4 ± 0.1 | 7.5 ± 0.7 |
| GSHS (nmol min−1 g−1 fresh wt) | 17.2 ± 0.6 | 12.4 ± 2.5 | 6.0 ± 1.1 | 8.3 ± 1.6 |
| hGSHS (nmol min−1 g−1 fresh wt) | 10.0 ± 1.0 | 7.1 ± 0.7 | 2.9 ± 0.9 | 4.9 ± 0.4 |
Data are means ± se of three to six samples.
Nodule age, 3 weeks; plant age, 27 d; phenological stage, early vegetative.
Nodule age, 5 weeks; plant age, 41 d; phenological stage, late vegetative.
Nodule age, 7 weeks; plant age, 55 d; phenological stage, late flowering-early fruiting.
Nodule age, 3 weeks; plant age, 27 d (plus 4 d of treatment); treatment, 4 d in continuous darkness; phenological stage, early vegetative.
Table V.
Effect of aging and stress-induced senescence on thiol composition and synthesis in bean nodules
| Parameter | 3a | 5b | 7c | 3+Nd |
|---|---|---|---|---|
| Protein (mg g−1 fresh wt) | 17.2 ± 1.7 | 13.9 ± 0.2 | 6.1 ± 1.7 | 9.6 ± 1.3 |
| Shoot fresh wt (g plant−1) | 5.7 ± 0.4 | 22.7 ± 2.0 | 50.3 ± 4.5 | 5.4 ± 1.1 |
| Cys (nmol g−1 fresh wt) | 42.3 ± 1.8 | 28.7 ± 3.2 | 23.1 ± 2.8 | 17.2 ± 2.6 |
| γEC (nmol g−1 fresh wt) | 1.3 ± 0.8 | 0 | 0 | 0 |
| GSH (nmol g−1 fresh wt) | 180 ± 18 | 142 ± 17 | 126 ± 9 | 74 ± 10 |
| hGSH (nmol g−1 fresh wt) | 350 ± 29 | 204 ± 9 | 225 ± 12 | 39 ± 5 |
| γECS (nmol min−1 g−1 fresh wt) | 4.5 ± 1.1 | 0.7 ± 0.1 | 0.7 ± 0.4 | 1.9 ± 0.9 |
| GSHS (nmol min−1 g−1 fresh wt) | 0.9 ± 0.3 | 0 | 0 | 0.3 ± 0.1 |
| hGSHS (nmol min−1 g−1 fresh wt) | 10.6 ± 0.8 | 7.3 ± 1.4 | 2.0 ± 0.6 | 5.2 ± 0.3 |
Data are means ± se of three to six samples.
Nodule age, 3 weeks; plant age, 25 d; phenological stage, vegetative.
Nodule age, 5 weeks; plant age, 39 d; phenological stage, late flowering-early fruiting.
Nodule age, 7 weeks; plant age, 53 d; phenological stage, fruiting.
Nodule age, 3 weeks; plant age, 25 d (plus 4 d of treatment); treatment, 4 d with nitrate; phenological stage, early vegetative.
Thiol Analysis
Extraction and analysis of thiol compounds were performed by modifying earlier procedures based on the derivatization of thiols with monobromobimane (MBB) and separation of the highly fluorescent adducts by HPLC (Fahey and Newton, 1987; Klapheck, 1988). For senescence and dissection studies, 10 to 20 mg of whole nodules or dissected nodule tissue was used. Although assays could be performed with 10 mg or lower amounts of plant tissue, 50 mg of nodules and 250 mg of leaves or roots were employed when material was not limiting. Volumes of extraction medium and derivatization solution were adjusted accordingly.
Nodules (50 mg) were ground at 0°C in an Eppendorf tube with 500 μL of 200 mm methanesulfonic acid (containing 0.5 mm diethylenetriaminepentaacetic acid). The homogenate was centrifuged at 13,000g for 5 min in the cold, and 200 μL of sample was mixed with 92 μL of 8 mm dithioerythritol (DTE), 400 μL of 200 mm N-[2-hydroxy-ethyl]piperazine-N′-3-propanesulfonic acid (EPPS) buffer, pH 8.0 (containing 5 mm diethylenetriaminepentaacetic acid), and 8 μL of 5 m NaOH. After incubation for 1 h at room temperature, 200 μL of 7 mm MBB (Calbiochem-Novabiochem, San Diego) was added, and the mixture was further incubated for 15 min in the dark. The reaction was stopped by the addition of 350 μL of 20% (v/v) acetic acid. Samples were stored at −80°C for several days before analysis. The samples were centrifuged and filtered, and 10-μL aliquots were injected on the HPLC. The MBB derivatives were resolved on a C18 column (3.9 × 150 mm; 4 μm, Nova-Pak, Waters, Milford, MA), eluted with 15% methanol/0.25% (v/v) acetic acid (pH 3.5) at 1 mL min−1, and detected by fluorescence (model 474 detector, Waters) with excitation at 380 nm and emission at 480 nm. The proportion of GSSG was determined in nodule extracts prepared as before using GSSG reductase and 2-vinylpyridine (Griffith, 1980). For both HPLC and enzymatic thiol determinations, stock solutions of Cys, γEC, GSH, and hGSH were titrated with the Ellman's reagent using an extinction coefficient for 2-nitro-5-thiobenzoate of 13.6 mm−1 cm−1 at 412 nm (Ellman, 1959).
Thiol Synthetase Assays
Extraction and assays of γECS, GSHS, and hGSHS were performed by modification of previous methods (Hell and Bergmann, 1988; Kocsy et al., 1996). Some of these modifications were critical to measure thiol synthetase activities, especially γECS, in nodules. All activities were measured within linear range.
Nodules (50 mg) were ground in an Eppendorf tube with 500 μL of 50 mm Tris-HCl (pH 8.0), 0.2 mm EDTA, 10% glycerol, and 10 mm MgCl2. The homogenate was centrifuged at 13,000g for 10 min and the supernatant was freed from small molecules by repeated dilution and concentration over ultrafiltration membranes (Centricon-10, Amicon, Beverly, MA). The activity of γECS was assayed by HPLC quantification of the synthesized γEC as its MBB derivative. The reaction mixture contained 120 mm HEPES (pH 8.0), 60 mm MgCl2, 6 mm ATP, 6 mm PEP, 6 units of pyruvate kinase, 0.5 mm DTE, 48 mm l-Glu, and 100 μL of extract, in a total volume of 235 μL. The reaction was initiated by the addition of 15 μL of 40 mm l-Cys and terminated after 0 and 60 min at 30°C by transferring an aliquot of 80 μL into derivatization solution. This consisted of 300 μL of 200 mm EPPS buffer, pH 8.0 (containing 5 mm diethylenetriaminepentaacetic acid), and 120 μL of 7 mm MBB. Derivatization was carried out for 15 min at room temperature in the dark and stopped by the addition of 97 μL of 40% (v/v) acetic acid. Samples were stored at −80°C for subsequent HPLC analysis as before. The activities of GSHS and hGSHS were assayed by HPLC quantification of the synthesized GSH or hGSH as their MBB derivatives. The reaction mixture contained 125 mm Tris-HCl (pH 8.5), 50 mm KCl, 25 mm MgCl2, 5 mm ATP, 5 mm PEP, 5 units of pyruvate kinase, 5 mm DTE, 0.5 mm γEC, and 5 mm Gly (GSHS) or βAla (hGSHS), in a total volume of 100 μL. After preincubation at 30°C for 3 min, the reaction was initiated by adding 100 μL of sample and stopped after 0 and 60 min at 30°C by derivatization of aliquots, as described above.
Isolation of Complete cDNA Sequences Encoding γECS from Bean and Pea Nodules
Primers (sense: 5′-GAGCTTAGTGGTGCACC[A/T]CT- TGA-3′ and antisense: 5′-TGCTCAAACCCAAAAGAGT- CAT-3′) were designed to conserved γECS cDNA sequences of tomato, B. juncea, and Arabidopsis. Bean and pea nodule cDNA Lambda ZAP libraries (generously provided by Dr. Carroll Vance, U.S. Department of Agriculture-University of Minnesota, St. Paul) were used as templates for γecs internal sequence amplification. PCR components and concentrations were as follows: 1 μm for sense primer and 0.2 μm for antisense primer, 200 μm for each dNTP, 2.5 mm MgCl2, 0.05% W-1 detergent (GIBCO-BRL, Paisley, UK), and 1.25 units of native Taq DNA polymerase (GIBCO-BRL), in a final volume of 25 μL of the PCR buffer (20 mm Tris-HCl, pH 8.4; 50 mm KCl). Tubes were pre-incubated at 95°C for 3 min to ensure complete denaturation of DNA. Amplification was carried out for 40 cycles at 55°C for 1 min (annealing), 72°C for 1 min (extension), and 95°C for 1 min (denaturation). Additional annealing and extension steps were done at 55°C for 1 min and 72°C for 11 min, respectively. The total volume of the PCR samples was electrophoresed in agarose gels. The PCR products were extracted using a gel-extraction system (Concert Matrix, GIBCO-BRL) and resuspended in 10 μL of sterile water.
For the PCR isolation of the 5′ ends of the γECS cDNAs, the same antisense primer (1 μm) was used with T3 as the sense primer (0.2 μm). For isolation of the 3′ ends, a sense primer (5′-GCTGAGGA[A/G]ATGGGAATTGG-3′) and a T7 antisense primer (both at 0.5 μm) were used. The same PCR program was followed except that the extension steps were at 72°C for 1.5 min and that the annealing step for the amplification of the 3′ end fragments was at 58°C for 1 min. PCR products were gel purified as indicated above.
Aliquots of the resuspended DNA were used to clone each PCR product into the linearized vectors pGEM (Pharmacia Biotech, Piscataway, NJ) or pCR2.1 (Invitrogen, Carlsbad, CA) following the procedures supplied by the manufacturers. The relevant cDNA clones were sequenced in both directions by the dideoxy method (Sanger et al., 1977) using an automated sequencer (PRISM 377, Applied Biosystems, Foster City, CA). Database searches were performed at the National Center for Biotechnology Information by using the BLAST network service. Sequence analyses were done using the Genetics Computer Group (Madison, WI) package.
Dissection Studies
Fresh bean and pea nodules were dissected under the binocular microscope with a sharp surgery blade. Pieces of nodules (10 mg for thiols and γECS; 20 mg for GSHS and hGSHS) were collected in ice-cold Eppendorf tubes (previously weighed to ±0.1 mg) and were extracted as described above. The terminology of nodule anatomy described by Vasse et al. (1990) and Hirsch (1992) was followed. Pea nodules were dissected into meristem plus early symbiotic zone (I plus II), late symbiotic zone (III), and senescent zone (IV). Bean nodules were dissected into cortex and infected zone.
Statistical Analysis
Two to four series of plants were grown at different dates under identical environment conditions. Samples from each series of plants (six to 10 plants per series) were pooled and a similar number of samples were randomly selected from each series. Data of the various series were then pooled for statistical analysis. The number of samples used for calculation of the means and se are stated in each table or figure.
RESULTS
Thiol Metabolism of Nodules Can Be Reliably Studied by HPLC with Fluorescence Detection
Central to this study was the development of a technique for the sensitive and precise determination of thiols and thiol synthetase activities in small amounts of nodule tissue. Samples were incubated with DTE prior to thiol derivatization with MBB at pH 8.0. This was essential to quantify Cys and γEC, since the two thiols tend to rapidly oxidize in the extracts. Preincubation with DTE also reduced the small quantities of the disulfide forms of GSH and hGSH present in the extracts. Tripeptide disulfides were measured using an enzymatic method (Griffith, 1980) and accounted for 3% to 10% of the total glutathione pool, which is consistent with earlier reports showing that most glutathione in plant cells is in the reduced form (for review, see Hausladen and Alscher, 1993).
The measurement of thiol synthetase activities, especially γECS, in whole or fractionated nodules involved more serious difficulties. First, it was essential to deplete extracts of endogenous free thiols, Gly, and βAla, which interfered with the assays. This was accomplished efficiently with ultrafiltration membranes. The process could be completed within 2 h, whereas conventional dialysis required overnight incubation, and gel filtration was not accurate enough with the small volumes (100–250 μL) of extracts used in this study. Second, the concentration of DTE was critical for the synthetase assays. Concentrations in the range of 2 to 5 mm have been reported to be inhibitory for γECS from several plants (Hell and Bergmann, 1990). We found that a low DTE concentration (0.48 mm) was not inhibitory but protective for the enzyme, and that a high Cys concentration (2.4 mm) was optimal to assay for γECS activity. This was because Cys was rapidly oxidized by plant extracts, and lower concentrations of this substrate could become nonsaturating for γECS in the course of the assay.
Additional controls for the assay of γECS activity included boiling of extracts (30 min), omission of ATP or Cys, and preincubation with buthionine sulfoximine (10 mm, 10 min, 30°C), a specific inhibitor of γECS in the presence of ATP (Huang et al., 1988). Additional controls for the assay of GSHS and hGSHS activities included boiling of extracts (30 min) and omission of ATP or γEC. As expected, no activity could be detected under any of those conditions.
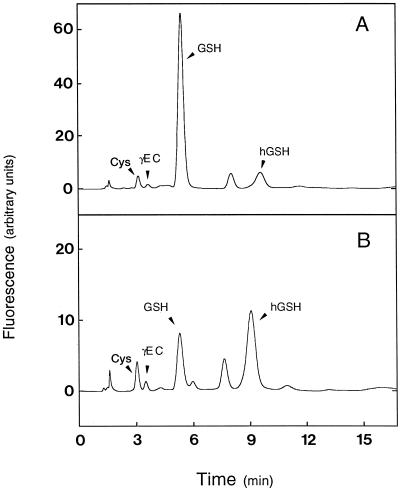
Using the modified technique described in this work, samples of 10 mg or lower were readily analyzed for thiol content and synthetase activities. The method permitted the peak-base separation of Cys, γEC, GSH, and hGSH in a single run with isocratic elution (Fig. 1). The corresponding MBB derivatives showed retention times of approximately 3, 4, 5, and 9 min, and their fluorescence response was linear for at least up to 16 pmol for Cys and γEC and 160 pmol for GSH and hGSH. Samples containing as little as 2 pmol of thiols per 10-μL injection could be accurately measured. Sensitivity can be further enhanced by a factor of two or three by changing the volumes of injection and of the derivatization mixture, but it is at least 50-fold greater than that reported by Farris and Reed (1987).
Figure 1.
Representative HPLC analysis of thiol compounds in legume nodules. A, Soluble extract of pea nodules showing GSH as the predominant thiol. B, Soluble extract of bean nodules showing hGSH as the predominant thiol. Peaks of Cys and γEC are also labeled. Other peaks correspond to MBB or MBB-DTE adducts.
Nodules Are a Major Site of Thiol Synthesis within the Plant
The distribution of non-protein thiols in several legumes of agronomic relevance is indicated in Table II. Four of them (pea, broad bean, alfalfa, and lupine) produce indeterminate nodules (persistent meristems, amide exporters) and the other four (soybean, bean, cowpea, and mungbean) produce determinate nodules (no persistent meristems, ureide exporters). The two types of nodules show important structural and metabolic differences (Hirsch, 1992), and hence a comparison was of considerable interest.
Table II.
Thiol content in legume nodules, roots, and leaves
| Legume | Cys
|
γECa
|
GSH
|
hGSH
|
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| N | R | L | N | L | N | R | L | N | R | L | |
| nmol g−1 fresh wt | |||||||||||
| Pea | 55 ± 5 | 12 ± 2 | 4 ± 3 | 15 ± 4 | 2 ± 2 | 829 ± 71 | 147 ± 15 | 445 ± 18 | 107 ± 10 | 69 ± 11 | 0 |
| Broad bean | 25 ± 1 | 1 ± 1 | 11 ± 0 | 6 ± 0 | 0 | 650 ± 9 | 96 ± 1 | 383 ± 27 | 0 | 0 | 0 |
| Alfalfa | 90 ± 2 | 20 ± 2 | 17 ± 1 | 95 ± 3 | 3 ± 2 | 840 ± 53 | 36 ± 3 | 16 ± 3 | 327 ± 31 | 166 ± 14 | 887 ± 22 |
| Lupine | 12 ± 2 | 0 | 23 ± 2 | 37 ± 5 | 8 ± 3 | 340 ± 33 | 49 ± 3 | 120 ± 32 | 0 | 0 | 0 |
| Soybean | 52 ± 3 | 11 ± 1 | 13 ± 3 | 5 ± 1 | 5 ± 3 | 130 ± 7 | 3 ± 3 | 7 ± 4 | 471 ± 48 | 235 ± 38 | 305 ± 23 |
| Bean | 39 ± 2 | 11 ± 1 | 13 ± 2 | 4 ± 2 | 8 ± 4 | 119 ± 10 | 1 ± 1 | 0 | 322 ± 14 | 70 ± 6 | 198 ± 24 |
| Mungbean | 74 ± 6 | 6 ± 2 | 12 ± 2 | 24 ± 2 | 3 ± 1 | 217 ± 11 | 4 ± 2 | 1 ± 1 | 356 ± 30 | 74 ± 13 | 170 ± 38 |
| Cowpea | 99 ± 15 | 0 | 23 ± 5 | 8 ± 3 | 0 | 587 ± 28 | 35 ± 4 | 270 ± 25 | 7 ± 2 | 37 ± 3 | 0 |
Data are means ± se of four to eight samples. N, Nodules; R, roots; L, leaves.
There was no detectable γEC in roots for any legume species.
Legume nodules contained, on average, 6-fold more thiol tripeptides (GSH plus hGSH) than the roots and 2.2-fold more than the leaves of the same plants. The relative abundance of GSH and hGSH was strikingly dependent on the legume species and plant tissue. All indeterminate nodules examined contained GSH as the major or sole tripeptide. This was also true for the corresponding leaves, with the exception of alfalfa. In this legume, GSH was the most abundant (72%) tripeptide in nodules, but, surprisingly, hGSH predominated by far in leaves (98%) and roots (82%). On the other hand, hGSH was the most abundant tripeptide in the determinate nodules of soybean, bean, and mungbean. These legumes were previously described as containing almost exclusively hGSH in leaves, roots, and seeds (Klapheck, 1988). Our data confirm that hGSH was virtually the only tripeptide (>98%) present in the leaves and roots of those legumes, but also show that their determinate nodules contained substantial amounts (22%–38%) of GSH (Table II). Unexpectedly, the leaves and determinate nodules of cowpea (Vigna unguiculata), a species closely related to mungbean (Vigna radiata), contained almost exclusively GSH. Clearly, the production of hGSH is not a characteristic feature of the tribe Phaseoleae, contrary to some early suggestions (Grill et al., 1986; Klapheck, 1988), nor is it linked to specific structural or metabolic features of determinate nodules, such as ureide production.
The thiol precursors Cys and γEC were more abundant in nodules than in leaves or roots, but were present at much lower levels (<15%) than the total tripeptides in all tissues examined (Table II). The content of Cys was greater than that of γEC for all legume tissues except in alfalfa and lupine nodules. In fact, the dipeptide was not detectable in the roots or leaves of most legumes. Considering a 85% water content, the average concentrations of thiols in nodules can be roughly estimated in the range of 30 to 120 μm for Cys, 7 to 50 μm for γEC, and 0.4 to 1.4 mm for total thiol tripeptides.
The finding that nodules have substantially greater levels of GSH, hGSH, and their thiol precursors than leaves, which are considered a main source of non-protein thiols within the plant (Rennenberg, 1997), is a strong indication that GSH and hGSH are synthesized in nodules. This was confirmed by the determination of all of the enzyme activities required for the synthesis of thiol tripeptides in nodule extracts (Table III). By using optimized methods, γECS activity, traditionally recalcitrant to assay because of the low level and instability of the enzyme, was clearly measurable at rates between 2 and 9 nmol γEC min−1 g−1 fresh weight. Similarly, GSHS was found to be the predominant (pea, alfalfa) or exclusive (broad bean, lupine) thiol tripeptide synthetase in indeterminate nodules, and hGSHS was the predominant (soybean, bean) or exclusive (mungbean) synthetase in determinate nodules, with the exception of cowpea (Table III). Thus, only GSHS was detected in cowpea nodules and, in fact, at greater activity rates than in the other legumes (Table III). This observation is fully consistent with GSH being the only tripeptide present in cowpea nodules (Table II).
Table III.
Enzyme activities involved in GSH and hGSH synthesis in legume nodules
| Legume | γECS | GSHS | hGSHS |
|---|---|---|---|
| nmol min−1 g−1 fresh wt | |||
| Pea | 6.6 ± 1.2 | 11.4 ± 1.2 | 6.9 ± 0.4 |
| Broad bean | 8.3 ± 1.1 | 8.2 ± 0.7 | 0 |
| Alfalfa | 3.3 ± 1.0 | 4.4 ± 0.5 | 1.1 ± 0.3 |
| Lupine | 9.4 ± 1.3 | 14.9 ± 1.6 | 0 |
| Soybean | 1.7 ± 0.2 | 2.6 ± 0.9 | 6.3 ± 0.2 |
| Bean | 4.5 ± 1.3 | 0.7 ± 0.2 | 7.0 ± 1.0 |
| Mungbean | 2.7 ± 0.8 | 0 | 6.0 ± 1.0 |
| Cowpea | 3.0 ± 1.5 | 15.8 ± 1.8 | 0 |
Data are means ± se of four to six samples.
Thiol Content and Synthesis Decrease with Nodule Senescence
To study the effect of natural (aging) and stress-induced nodule senescence on thiol composition and synthesis, samples of indeterminate (pea) and determinate (bean) nodules were harvested at fixed time points during plant ontogeny. In both pea (Table IV) and bean (Table V), there was a steady decline of non-protein thiols with advancing age. As could be anticipated, this was accompanied by the loss of nodule soluble protein and an increase in the shoot fresh weight, marking, respectively, the progression of nodule senescence and the transition from the vegetative stage to the flowering and fruiting stages of plants.
Mature pea nodules (5 weeks) contained between 25% and 45% less protein, Cys, thiol tripeptides, and thiol synthetase activities than young nodules (3 weeks). The reductions in these parameters were between 55% and 82% for old nodules (7 weeks). The dipeptide γEC was only detectable (and at low levels) in young nodules (Table IV). Placement of young pea plants for 4 d in continuous darkness induced nodule senescence, as was clearly evidenced by a 36% decline in the total soluble protein of nodules. Dark treatment led to decreases in thiols and thiol synthetase activities, with the exception of γECS, down to the values recorded for mature and old nodules. It is noteworthy that the dark treatment caused a 92% decline in the content of GSH (the major tripeptide) of young nodules, but only a 38% decrease in hGSH content and a 79% increase in the extractable γECS activity (Table IV).
Similarly, mature bean nodules (5 weeks) had between 20% and 40% less protein, Cys, thiol tripeptides, and hGSHS activity than young nodules (3 weeks), 84% less γECS activity, and no detectable GSHS activity (Table V). In old nodules (7 weeks), the contents of Cys and thiol tripeptides were 30% to 45% lower than those of young nodules, whereas soluble protein was reduced by 65% and γECS and hGSHS activities by 80%. Induction of bean nodule senescence by nitrate, as evidenced by a 44% decline in soluble protein, caused decreases of 50% to 60% in Cys, γECS activity, and hGSHS activity down to or below the values observed in the oldest nodules (Table V). The nitrate-induced declines in the nodule contents of hGSH (the major tripeptide) and GSH were 89% and 59%, respectively, but the age-related decline was approximately 35% for both thiols (Table V).
Thiol Synthesis Is Especially Active in the Meristematic and Infected Zones of Pea Nodules
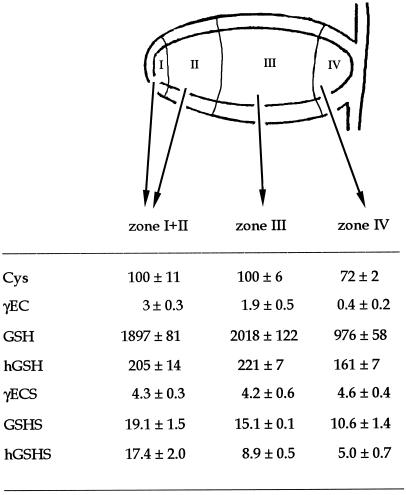
Experiments were designed to further investigate the effect of age at the tissue level and the possible association between thiol synthesis and nitrogen fixation. Indeterminate nodules characteristically show an age gradient from the apical meristem to the senescent basal tissue (Vasse et al., 1990; Hirsch, 1992). Pea nodules were dissected into the meristematic-early symbiotic zone (white, with no detectable leghemoglobin), the late-symbiotic zone (bright red), and the senescent zone (brown-green, indicative of leghemoglobin degradation).
The senescent zone contained 50% less GSH and 25% less Cys and hGSH than the other zones (Fig. 2). However, γECS activity was approximately 4.3 nmol γEC min−1 g−1 fresh weight in all three zones in which nodules were dissected, indicating that this activity was not limiting GSH or hGSH synthesis in the senescent zone. In contrast, there was a progressive decline in GSHS and hGSHS activities with the age of nodule tissue. The senescent zone had 45% less GSHS activity and 71% less hGSHS activity than the meristematic-early symbiotic zone, which may explain at least in part the lower GSH and hGSH contents in the former. The estimated concentrations of Cys (0.12 mm), GSH (2.3 mm), and hGSH (0.25 mm) were similar for both the meristematic-early symbiotic zone and the late symbiotic zone.
Figure 2.
Thiol composition and synthesis in different zones of pea nodules. Data are means ± se of four to eight samples of nodule fractions. Thiol contents are expressed in nanomoles per gram fresh weight and enzyme activities in nanomoles per minute per gram fresh weight.
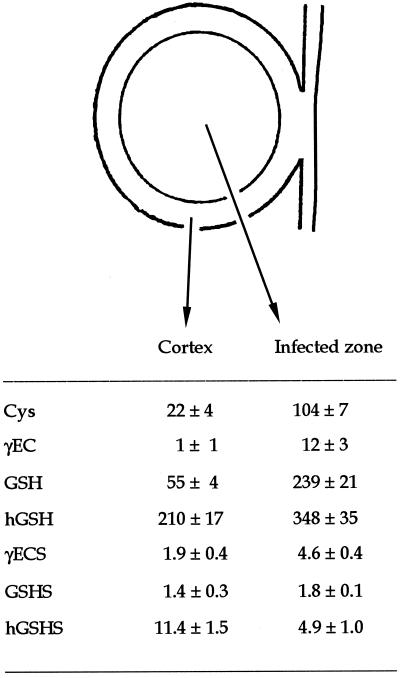
γECS and hGSHS Are More Abundant, Respectively, in the Infected Zone and Cortex of Bean Nodules
In determinate nodules, cell divisions cease early during nodule development and there are no obvious age differences among the various nodule tissues (Hirsch, 1992). In this case, bean nodules were dissected into the cortex and infected zone to investigate whether thiol synthesis was more active in the nitrogen-fixing tissue. The infected zone had between 2- and 5-fold more Cys, GSH, hGSH, and γECS activity than the nodule cortex (Fig. 3). The infected zone also contained 12 nmol γEC g−1 fresh weight, whereas the dipeptide was virtually below detection levels in the cortex. The proportion of thiol tripeptides was different in the two nodule tissues, with a hGSH/GSH ratio of 3.8 in the cortex and 1.5 in the infected zone. The estimated concentrations of thiols in the cortex were 26 μm Cys, <1 μm γEC, 65 μm GSH, and 247 μm hGSH. The corresponding concentrations in the infected zone were 122 μm Cys, 14 μm γEC, 281 μm GSH, and 409 μm hGSH.
Figure 3.
Thiol composition and synthesis in the cortex and infected zone of bean nodules. Data are means ± se of four to eight samples of nodule fractions. Thiol contents are expressed in nanomoles per gram fresh weight and enzyme activities in nanomoles per minute per gram fresh weight.
The relative activities of the thiol tripeptide synthetases were also clearly different in the cortex and infected tissue. Whereas both nodule regions showed similar values of GSHS activity, the nodule cortex had 2.3-fold more hGSHS activity than the infected zone or, in other terms, accounted for 70% of the whole nodule hGSHS activity on a fresh weight basis (Fig. 3). This remarkably high hGSHS activity in the cortex was consistently found in all the dissection experiments conducted in this work.
Complete cDNA Sequences Reveal High Homology among γECS Proteins of Higher Plants
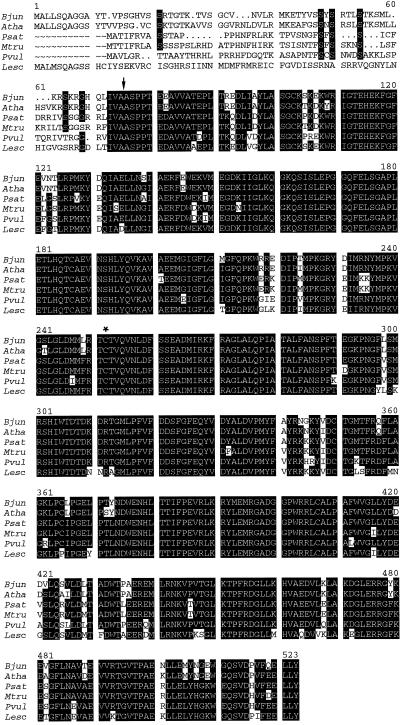
The enzyme γECS is considered to be critical in GSH homeostasis in plants (Rennenberg, 1997; May et al., 1998; Noctor and Foyer, 1998), and therefore we initiated a study of γecs in legume nodules. To this purpose, oligonucleotide primers were designed to conserved γecs sequences of tomato, B. juncea, and Arabidopsis, and used to screen pea and bean nodule cDNA libraries by PCR. Full-length coding sequences of approximately 2 kb were obtained for the γecs of both legumes and showed high identity with other γecs sequences available in the database. Pea and bean cDNA sequences were approximately 80% identical to those of tomato, B. juncea, and Arabidopsis. The nucleotide sequences of pea, bean, and Medicago truncatula showed between 86% and 91% identity.
The ORF for the γECS of pea nodules was predicted to encode a 499-amino acid polypeptide, with an expected molecular mass of 56.6 kD and a pI value of 6.22. The corresponding sequence of γECS of bean nodules was 508 amino acids long, with a predicted mass of 57.6 kD and a pI value of 6.12. The deduced amino acid sequences of γECS from pea and bean nodules shared 85% to 88% identity with those of tomato, B. juncea, and Arabidopsis, and identities reached 88% to 93% when only the sequences of the three legumes were compared (Fig. 4). The sequences included the putative active site Cys residue, which is present in the γECS proteins of Trypanosoma brucei, Caenorhabditis elegans, yeast, and rat (Lueder and Phillips, 1996), in addition to those of higher plants. The sequences of γECS from pea and bean nodules also included a putative transit peptide at the N terminus (Fig. 4), with a conserved cleavage-site motif (Ile-Val-Ala↓Ala) that is predicted to target γECS to the plastids (Gavel and von Heijne, 1990). The position of the cleavage site is consistent with the high variability of the putative signal peptide (51 residues for pea and 60 residues for bean) as opposed to the high identity of the γECS sequences from the Ile-Val-Ala-Ala motif to the C terminus (Fig. 4).
Figure 4.
Deduced amino acid sequences of γECS proteins from higher plants. Abbreviations and accession numbers are as follows: B. juncea (Bjun; accession no. Y10848), Arabidopsis (Atha; accession no. Y09944), P. sativum (Psat; accession no. AF128455), M. truncatula (Mtru; accession no. AF041340), P. vulgaris (Pvul; accession no. AF128454), and L. esculentum (Lesc; accession no. AF017983). Residues in white lettering on a black background are identical in at least four species. The putative cleavage site and active Cys residues are indicated by an arrow and asterisk, respectively.
DISCUSSION
Legume nodules contain higher concentrations of all non-protein thiols than the leaves and roots of the same plants, as well as high activities of all enzymes involved in GSH and hGSH synthesis, which indicates that the thiols are actively synthesized within the nodules. Furthermore, a comparison across legume species showed a close correlation between the major thiol tripeptide and the major synthetase activity present in the nodules, which strongly suggests that the relative abundance of GSH and hGSH is dictated by the distribution of the corresponding synthetases. This conclusion is supported by the strikingly different thiol tripeptide composition of alfalfa leaves (98% hGSH) and nodules (72% GSH). These results are consistent with the finding that GSHS activity is 4-fold higher than hGSHS activity in nodules but not detectable in leaves, and confirm that there are two synthetases, GSHS and hGSHS, in alfalfa nodules but a single enzyme, hGSHS, in alfalfa leaves. Indeed, two distinct thiol synthetases, identified on the basis of their different affinity for Gly and βAla, have been partially purified from pea (GSHS) and mungbean (hGSHS) leaves (Macnicol, 1987), which only contain GSH and hGSH, respectively (Klapheck, 1988; this work).
Very recently, Frendo et al. (1999) reported that the distribution of GSH and hGSH in M. truncatula plants is correlated with the expression of two genes with high homology to Arabidopsis gshs, reinforcing the view that two different enzymes, GSHS and hGSHS, are involved in GSH and hGSH synthesis. Although they did not provide details on how the level of hGSH, which is not commercially available, was estimated, their results indicate that the leaves of M. truncatula only contain GSH, whereas the nodules have both hGSH and GSH. Comparison of this finding with our thiol determination in alfalfa confirms our conclusion that the ability of legumes to synthesize hGSH has no taxonomic value.
An interesting finding in this study is that all nodules examined have substantial amounts of GSH, even in those legumes such as soybean, bean, and mungbean that contain hGSH exclusively in their leaves or roots. Some GSH of nodules might have originated in the bacteroids, which may become partly broken during thiol extraction with methanesulfonic acid. If this is so, the total GSH content of nodules could be even greater after complete breakage of bacteroids and the hGSH/GSH ratio in the plant fraction of soybean, bean, and mung bean nodules would be also higher. In any case, the high concentrations of thiols in nodules relative to leaves and roots strongly suggest that GSH and hGSH play an important role in nitrogen fixation. This hypothesis is reinforced by dissection experiments revealing that thiols are more abundant in the meristematic and infected zones than in the senescent zone of pea nodules, and are more abundant in the infected zone than in the cortex of bean nodules.
The lower content of GSH in the senescent zone of pea nodules, however, cannot be explained only on the basis of reduced synthesis, since γECS activity was similar to that of the meristematic and infected zones, and the declines in Cys and GSHS activity were rather modest. The decline in GSH may have been due for the most part to enhanced degradation, because oxidative reactions, probably linked to the breakdown of heme and formation of green pigments from leghemoglobin (Roponen, 1970), are augmented in the senescent zone. The same process may also explain the decline in the average thiol concentration of whole nodules with advancing age or during stress-induced senescence, where nearly 90% of GSH (pea) and hGSH (bean) was lost.
The relatively high thiol concentrations and synthetase activities in the meristematic and early symbiotic zones of pea nodules may be physiologically relevant. Because active cell division is confined to the persistent meristems of indeterminate nodules, GSH may be involved in the control of cell proliferation in nodules, as proposed for the apical meristem of Arabidopsis roots (Sánchez-Fernández et al., 1997; May et al., 1998). This does not preclude other possible functions of GSH in indeterminate nodules, such as the modulation of gene expression in the early symbiotic zone.
The consistently greater hGSHS activity in the cortex of bean nodules is puzzling but may provide a preliminary clue to elucidate the role of hGSHS and hGSH in determinate nodules. For example, it would be worth investigating whether the function of hGSHS is related to the vascular bundles, which are confined to the nodule cortex. Despite the remarkably high hGSHS activity, however, the cortex of bean nodules contained less hGSH than the infected zone. The control of GSH (and presumably hGSH) synthesis could be exerted by different mechanisms. These include the availability of the thiol precursors Cys and γEC and the amount of γECS enzyme (Rennenberg, 1997; May et al., 1998). Because there is virtually no γEC in the cortex, and Cys content and γECS activity are only 21% and 41%, respectively, of those existing in the infected zone, the lower hGSH content in the cortex is probably due to a limitation of hGSH synthesis rather than to transport of the tripeptide into the infected zone. The relative availability of Gly and βAla for GSHS and hGSHS in the cortex and infected zone, as well as the possible contribution of bacteroids to GSH synthesis, may be also important in determining the different abundance of the thiol tripeptides in both nodule regions.
Another mechanism for the regulation of GSH synthesis is feedback inhibition of γECS by GSH (Rennenberg, 1997; May et al., 1998; Noctor and Foyer, 1998). The estimated concentrations of GSH plus hGSH in the infected zone of pea and bean nodules are, respectively, 2.6 and 0.7 mm, which would be sufficient to inhibit γECS in vitro (Hell and Bergmann, 1990). However, our data do not support the idea that this inhibition occurs in vivo, since the extractable γECS activities are in fact greater in the infected zone of both nodules. The lack of apparent inhibition of γECS by GSH in vivo has been described in other plant systems, but a conclusive explanation has not yet been offered (Rennenberg, 1997; May et al., 1998). Thus, the inhibition of γECS might be overcome by high concentrations of Glu, which competes with GSH (Hell and Bergmann, 1990; Rennenberg, 1997). Another possibility is that the relative concentrations of GSH and γECS differ in the cytosol and organelles of nodules, as occurs in pea leaves, where 72% of γECS activity (Hell and Bergmann, 1990) but only 10% of total GSH (Bielawski and Joy, 1986) are located in the chloroplasts.
The reaction catalyzed by γECS is generally assumed to be the rate-limiting step in GSH synthesis (May et al., 1998; Noctor and Foyer, 1998), and indeed the γECS activities extractable from nodules were in most cases lower than those of the predominant GSHS or hGSHS enzymes. Like γECS from other sources, the nodule enzyme was irreversibly inhibited by buthionine sulfoximine, indicating that the reaction proceeds through the formation of an enzyme-bound γ-glutamyl-phosphate intermediate (Huang et al., 1988; Hell and Bergmann, 1990). Nodule γECS was not inhibited by low (0.48 mm) or high (5 mm) DTE concentrations. The same result was observed in leaves of Arabidopsis and maize (May and Leaver, 1994), while inhibition by DTE was found in tobacco suspension cells and in spinach and pea leaves (Hell and Bergmann, 1990). The lack of inhibition by this thiol reagent suggests that nodule γECS is a monomeric enzyme, unlike γECS from tobacco cells, which dissociates into two equal and inactive subunits in the presence of 5 mm DTE (Hell and Bergmann, 1990).
The derived amino acid sequences of γECS from pea and bean nodules were highly homologous to those of other higher plants and contained the purported active site Cys residue shared by the γECS proteins of species as evolutionary distant as the protozoans, yeasts, plants, and mammals (May and Leaver, 1994; Luedder and Phillips, 1996; May et al., 1998). The sequences also included a putative plastid signal peptide of 50 to 60 amino acids, as suggested by matching to a conserved cleavage-site motif, by the relatively high content of Ser plus Thr and Ala (but also Arg) residues, and by the almost complete absence of acidic residues (von Heijne et al., 1989; Gavel and von Heijne, 1990). The variability of the putative signal peptides is somewhat surprising, as is the finding that the signal peptides of legumes are significantly shorter than those of the other higher plants. The plastid location of legume nodule γECS will need to be verified by subcellular fractionation, N-terminal sequencing of the mature protein, or import in vitro. This is important because predictive algorithms for the subcellular localization of proteins such as PSORT (Nakai and Kanehisa, 1992) suggest compatibility also with a mitochondrial and peroxisomal targeting of γECS.
The instability and low abundance of γECS in plant tissues have hampered the complete purification of the enzyme and the study of mechanisms controlling γECS expression and activity in plants. Because thiol metabolites are actively synthesized and required for nodule functioning, experiments are under way to obtain recombinant γECS proteins and use them to investigate the regulatory mechanisms of thiol synthesis in legume nodules.
ACKNOWLEDGMENTS
We are most grateful to Carroll Vance for the gift of cDNA libraries, Gautam Sarath for the synthesis of hGSH, and Frank Minchin for critically reading the manuscript. We also thank Gloria Rodríguez for growing the plants.
Footnotes
This work was supported by the Dirección General de Enseñanza Superior e Investigación Científica (Ministry of Education and Culture, Spain; grant nos. PB98–0522, 2FD97–1101, and HB98–163). M.A.M., J.F.M., I.I.-O., and M.C.R. were the recipients, respectively, of a predoctoral fellowship from the Gobierno Vasco, a postdoctoral contract from the Ministry of Education and Culture, a postdoctoral fellowship from the European Union (Training and Mobility Program), and a predoctoral fellowship from the Ministry of Education and Culture.
LITERATURE CITED
- Baron C, Zambryski PC. The plant response in pathogenesis, symbiosis, and wounding: variations on a common theme? Annu Rev Genet. 1995;29:107–129. doi: 10.1146/annurev.ge.29.120195.000543. [DOI] [PubMed] [Google Scholar]
- Bielawski W, Joy KW. Reduced and oxidised glutathione and glutathione-reductase activity in tissues of Pisum sativum. Planta. 1986;169:267–272. doi: 10.1007/BF00392324. [DOI] [PubMed] [Google Scholar]
- Dalton DA, Post CJ, Langeberg L. Effects of ambient oxygen and of fixed nitrogen on concentrations of glutathione, ascorbate, and associated enzymes in soybean root nodules. Plant Physiol. 1991;96:812–818. doi: 10.1104/pp.96.3.812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalton DA, Russell SA, Hanus FJ, Pascoe GA, Evans HJ. Enzymatic reactions of ascorbate and glutathione that prevent peroxide damage in soybean root nodules. Proc Natl Acad Sci USA. 1986;83:3811–3815. doi: 10.1073/pnas.83.11.3811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellman GL. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959;82:70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- Escuredo PR, Minchin FR, Gogorcena Y, Iturbe-Ormaetxe I, Klucas RV, Becana M. Involvement of activated oxygen in nitrate-induced senescence of pea root nodules. Plant Physiol. 1996;110:1187–1195. doi: 10.1104/pp.110.4.1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans PJ, Gallesi D, Mathieu C, Hernandez MJ, de Felipe M, Halliwell B, Puppo A. Oxidative stress occurs during soybean nodule senescence. Planta. 1999;208:73–79. [Google Scholar]
- Fahey RC, Newton GL. Determination of low-molecular-weight thiols using monobromobimane fluorescent labeling and high-performance liquid chromatography. Methods Enzymol. 1987;143:85–96. doi: 10.1016/0076-6879(87)43016-4. [DOI] [PubMed] [Google Scholar]
- Farris MW, Reed DJ. High-performance liquid chromatography of thiols and disulfides: dinitrophenol derivatives. Methods Enzymol. 1987;143:101–109. doi: 10.1016/0076-6879(87)43018-8. [DOI] [PubMed] [Google Scholar]
- Frendo P, Gallesi D, Turnbull R, Van de Sype G, Hérouart D, Puppo A. Localisation of glutathione and homoglutathione in Medicago truncatula is correlated to a differential expression of genes involved in their synthesis. Plant J. 1999;17:215–219. [Google Scholar]
- Gavel Y, von Heijne G. A conserved cleavage-site motif in chloroplast transit peptides. FEBS Lett. 1990;261:455–458. doi: 10.1016/0014-5793(90)80614-o. [DOI] [PubMed] [Google Scholar]
- Gogorcena Y, Gordon AJ, Escuredo PR, Minchin FR, Witty JF, Moran JF, Becana M. N2 fixation, carbon metabolism, and oxidative damage in nodules of dark-stressed common bean plants. Plant Physiol. 1997;113:1193–1201. doi: 10.1104/pp.113.4.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogorcena Y, Iturbe-Ormaetxe I, Escuredo PR, Becana M. Antioxidant defenses against activated oxygen in pea nodules subjected to water stress. Plant Physiol. 1995;113:1193–1201. doi: 10.1104/pp.108.2.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith OW. Determination of glutathione and glutathione disulfide using glutathione reductase and 2-vinylpyridine. Anal Biochem. 1980;106:207–212. doi: 10.1016/0003-2697(80)90139-6. [DOI] [PubMed] [Google Scholar]
- Grill E, Gekeler W, Winnacker EL, Zenk HH. Homo-phytochelatins are heavy metal-binding peptides of homo-glutathione containing Fabales. FEBS Lett. 1986;205:47–50. [Google Scholar]
- Hausladen A, Alscher RG. Glutathione. In: Alscher RG, Hess JL, editors. Antioxidants in Higher Plants. Boca Raton, FL: CRC Press; 1993. pp. 1–30. [Google Scholar]
- Hell R, Bergmann L. Glutathione synthetase in tobacco suspension cultures: catalytic properties and localization. Physiol Plant. 1988;72:70–76. [Google Scholar]
- Hell R, Bergmann L. γ-Glutamylcysteine synthetase in higher plants: catalytic properties and subcellular localization. Planta. 1990;180:603–612. doi: 10.1007/BF02411460. [DOI] [PubMed] [Google Scholar]
- Hirsch AM. Developmental biology of legume nodulation. New Phytol. 1992;122:211–237. doi: 10.1111/j.1469-8137.1992.tb04227.x. [DOI] [PubMed] [Google Scholar]
- Huang CS, Moore WR, Meister A. On the active site thiol of γ-glutamylcysteine synthetase: relationships to catalysis, inhibition, and regulation. Proc Natl Acad Sci USA. 1988;85:2464–2468. doi: 10.1073/pnas.85.8.2464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klapheck S. Homoglutathione: isolation, quantification, and occurrence in legumes. Physiol Plant. 1988;74:727–732. [Google Scholar]
- Klapheck S, Latus C, Bergmann L. Localization of glutathione synthetase and distribution of glutathione in leaf cells of Pisum sativum L. J Plant Physiol. 1987;131:123–131. [Google Scholar]
- Kocsy G, Brunner M, Rüegsegger A, Stamp P, Brunold C. Glutathione synthesis in maize genotypes with different sensitivities to chilling. Planta. 1996;198:365–370. [Google Scholar]
- Law MY, Halliwell B. Purification and properties of glutathione synthetase from spinach (Spinacia oleracea) leaves. Plant Sci. 1986;43:185–191. [Google Scholar]
- Lueder DV, Phillips MA. Characterization of Trypanosoma brucei γ-glutamylcysteine synthetase, an essential enzyme in the biosynthesis of trypanothione (diglutathionylspermidine) J Biol Chem. 1996;271:17485–17490. doi: 10.1074/jbc.271.29.17485. [DOI] [PubMed] [Google Scholar]
- Macnicol PK. Homoglutathione and glutathione synthetases of legume seedlings: partial purification and substrate specificity. Plant Sci. 1987;53:229–235. [Google Scholar]
- Matamoros MA, Baird LM, Escuredo PR, Dalton DA, Minchin FR, Iturbe-Ormaetxe I, Rubio MC, Moran JF, Gordon AJ, Becana M. Stress-induced legume root nodule senescence: physiological, biochemical, and structural alterations. Plant Physiol. 1999;121:97–111. doi: 10.1104/pp.121.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May MJ, Leaver CJ. Arabidopsis thaliana γ-glutamylcysteine synthetase is structurally unrelated to mammalian, yeast, and Escherichia coli homologs. Proc Natl Acad Sci USA. 1994;91:10059–10063. doi: 10.1073/pnas.91.21.10059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May MJ, Vernoux T, Leaver C, Van Montagu M, Inzé D. Glutathione homeostasis in plants: implications for environmental sensing and plant development. J Exp Bot. 1998;49:649–667. [Google Scholar]
- Meister A, Anderson ME. Glutathione. Annu Rev Biochem. 1983;52:711–760. doi: 10.1146/annurev.bi.52.070183.003431. [DOI] [PubMed] [Google Scholar]
- Nakai K, Kanehisa M. A knowledge base for predicting protein localization sites in eukaryotic cells. Genomics. 1992;14:897–911. doi: 10.1016/S0888-7543(05)80111-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noctor G, Foyer CH. Ascorbate and glutathione: keeping active oxygen under control. Annu Rev Plant Physiol Plant Mol Biol. 1998;49:249–279. doi: 10.1146/annurev.arplant.49.1.249. [DOI] [PubMed] [Google Scholar]
- Rennenberg H. Molecular approaches to glutathione biosynthesis. In: Cram WJ, DeKok LJ, Stulem I, Brunold C, Rennenberg H, editors. Sulphur Metabolism in Higher Plants. Leiden, The Netherlands: Backhuys Publishers; 1997. pp. 59–70. [Google Scholar]
- Roponen I. The effect of darkness on the leghemoglobin content and amino acid levels in the root nodules of pea plants. Physiol Plant. 1970;23:452–460. [Google Scholar]
- Rüegsegger A, Brunold C. Localization of γ-glutamylcysteine synthetase and glutathione synthetase activity on maize seedlings. Plant Physiol. 1993;101:561–566. doi: 10.1104/pp.101.2.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez-Fernández R, Fricker M, Corben LB, White NS, Sheard N, Leaver CJ, Van Montagu M, Inzé D, May MJ. Cell proliferation and hair root tip growth in the Arabidopsis root are under mechanistically different forms of redox control. Proc Natl Acad Sci USA. 1997;94:2745–2750. doi: 10.1073/pnas.94.6.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F, Nicklen S, Coulson AR. DNA sequencing with chain terminator inhibitors. Proc Natl Acad Sci USA. 1977;83:8073–8076. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schäfer HJ, Haag-Kerwer A, Rausch T. cDNA cloning and expression analysis of genes encoding GSH synthesis in roots of the heavy-metal accumulator Brassica juncea L.: evidence for Cd-induction of a putative mitochondrial γ-glutamylcysteine synthetase isoform. Plant Mol Biol. 1998;37:87–97. doi: 10.1023/a:1005929022061. [DOI] [PubMed] [Google Scholar]
- Vasse J, de Billy F, Camut S, Truchet G. Correlation between ultrastructural differentiation of bacteroids and nitrogen fixation in alfalfa nodules. J Bacteriol. 1990;172:4295–4306. doi: 10.1128/jb.172.8.4295-4306.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Heijne G, Steppuhn J, Herrmann RG. Domain structure of mitochondrial and chloroplast targeting peptides. Eur J Biochem. 1989;180:535–545. doi: 10.1111/j.1432-1033.1989.tb14679.x. [DOI] [PubMed] [Google Scholar]
- Wingate VPM, Lawton MA, Lamb CJ. Glutathione causes a massive and selective induction of plant defense genes. Plant Physiol. 1988;87:206–210. doi: 10.1104/pp.87.1.206. [DOI] [PMC free article] [PubMed] [Google Scholar]