Abstract
Purpose
A frequent complaint by older adults is difficulty communicating in challenging acoustic environments. The purpose of this work was to review and summarize information about how speech perception in complex listening situations changes across the adult age range.
Method
This article provides a review of age-related changes in speech understanding in complex listening environments and summarizes results from several studies conducted in our laboratory.
Results
Both degree of high frequency hearing loss and cognitive test performance limit individuals' ability to understand speech in difficult listening situations as they age. The performance of middle-aged adults is similar to that of younger adults in the presence of noise maskers, but they experience substantially more difficulty when the masker is 1 or 2 competing speech messages. For the most part, middle-aged participants in studies conducted in our laboratory reported as much self-perceived hearing problems as did older adult participants.
Conclusions
Research supports the multifactorial nature of listening in real-world environments. Current audiologic assessment practices are often insufficient to identify the true speech understanding struggles that individuals experience in these situations. This points to the importance of giving weight to patients' self-reported difficulties.
Presentation Video
This research forum contains papers from the 2016 Research Symposium at the ASHA Convention held in Philadelphia, PA.
The primary complaint of most adults with hearing impairment is difficulty understanding speech in noisy situations (Committee on Hearing Bioacoustics and Biomechanics, 1988; Pichora-Fuller, 1997). The most problematic situations reported by patients tend to be those that involve more than one intelligible talker. This is consistent with research demonstrating that older adults perform poorer than younger adults on laboratory-based tasks in which there are multiple speech sources, particularly when the competing speech is comprehensible (Helfer, Chevalier, & Freyman, 2010; Tun, O'Kane, & Wingfield, 2002).
Why do these competing speech situations become more challenging as individuals age? The ability to understand a message in the presence of competing speech involves multiple levels of both peripheral and higher level processing, from segregating the message of interest from nonessential background signals to focusing and maintaining attention on the target stream, to decoding, understanding, and encoding the information into memory. Problems at any of these levels can lead to misunderstanding.
An important contributor to these listening difficulties in adverse situations is the peripheral hearing loss that typically accompanies aging. High-frequency hearing loss attenuates and distorts the incoming acoustic signal and is the primary cause of problems understanding speech in noise (Arbogast, Mason, & Kidd, 2005; Hällgren, Larsby, Lyxell, & Arlinger, 2005; Larsby, Hällgren, Lyxell, & Arlinger, 2005; Marrone, Mason, & Kidd, 2008). However, other intrinsic variables also undoubtedly influence the way that older individuals understand speech in complex acoustic environments. For example, older adults may have deficits in sound segregation (Alain, Arnott, Hevenor, Graham, & Grady, 2001; Grube, von Cramon, & Rübsamen, 2003; Summers & Leek, 1998; Vongpaisal & Pichora-Fuller, 2007), at least in part because of reduced sensitivity to temporal fine structure cues that carry fundamental frequency information used to separate sounds sources (Füllgrabe, Moore, & Stone, 2015; Neher, Lunner, Hopkins, & Moore, 2012). When the background noise consists of one or two voices, the masking complex contains spectrotemporal fluctuations. Younger adults can use these instances of reduced masker energy to glimpse portions of the target speech message within the masking complex. However, age and/or age-related hearing loss appears to reduce the ability to take advantage of these fluctuations (Dubno, Horwitz, & Ahlstrom, 2002, 2003; George, Festen, & Houtgast, 2006; Summers & Molis, 2004; Takahashi & Bacon, 1992). There is also evidence that older adults are less able to use indexical information, such as the differences between voices (Helfer & Freyman, 2008; Johnsrude et al., 2013; Naveh-Benjamin & Craik, 1996; Pilotti & Beyer, 2002; Rossi-Katz & Arehart, 2009; Yonan & Sommers, 2000), which is likely important when trying to identify and attend to one message in the presence of competing talkers.
When multiple talkers are present simultaneously in real-world acoustic environments, a particularly salient cue in segregating individual speech streams is the physical point of origin of each voice within that environment (due to the fact that every person occupies his or her own location in space). An essential consideration when examining listening in such competing speech situations is the extent to which the listener can take advantage of spatial separation between the target and masking speech. Spatial release from masking (the difference between speech understanding with target and masking speech co-located and with target and masker spatially separated) appears to decline in older adults (Arbogast et al., 2005; Duquesnoy, 1983; Festen & Plomp, 1990; Gallun, Diedesch, Kampel, & Jakien, 2013; Marrone et al., 2008; Murphy, Daneman, & Schneider, 2006). This is not to say that older adults do not benefit at all from binaural information; rather, the amount of benefit is significantly reduced when evaluated in comparison with younger adults.
The majority of the information described above regarding why the ability to understand speech in adverse environments declines with age can be explained primarily by changes within the auditory system. However, understanding messages in adverse listening environments also requires cognitive mediation. One particularly useful framework that has been conceived to describe the role of cognitive processing in speech perception is the Ease of Language Understanding (ELU) model (Rönnberg, 2003; Rönnberg, Rudner, Foo, & Lunner, 2008; Rönnberg et al., 2013). The ELU model posits that incoming multimodal speech information is rapidly bound into a unified phonological representation at a sublexical level. If this phonological stream matches a corresponding lexical representation in the listener's long-term memory, word understanding is automatic, bypassing the need for significant top-down processing and working memory resources. In this ideal situation, the incoming speech signal is of a high-enough quality to match an adequate number of phonological attributes of a representation within the listener's mental lexicon. The ELU model postulates that this ideal language processing occurs in an implicit, bottom-up manner, without the need for cognitive processing. However, if the incoming phonological stream does not immediately match a representation within the listener's long-term semantic memory, he or she must use explicit top-down cognitive processing in order to successfully understand the message.
Examples of when these top-down processing skills (as measured by tasks of working memory capacity) significantly account for individual variance include situations in which the target speech is masked (Akeroyd, 2008; Conway, Cowan, & Bunting, 2001; Gatehouse, Naylor, & Elberling, 2003; Lunner, 2003; Rudner & Lunner, 2014; Sörqvist & Rönnberg, 2012), when the listener has an impaired auditory system, or when signal processing within a hearing instrument has altered the incoming acoustic stream (Arehart, Souza, Baca, & Kates, 2013; Foo, Rudner, Rönnberg, & Lunner, 2007; Rönnberg et al., 2013; Rönnberg et al., 2008; Souza, Arehart, Shen, Anderson, & Kates, 2015). Mismatch and resultant cognitive resource recruitment are ultimately determined by the fidelity of both the input phonological stream and the phonological representation in an individual's semantic long-term memory.
The ELU model asserts that measures of working memory, especially reading span (Daneman & Carpenter, 1980; Rönnberg et al., 2008) and size-comparison span (SICSPAN; Sörqvist, Ljungberg, & Ljung, 2010), are indicative of an individual's ability to understand language successfully, especially in challenging environments. Suboptimal working memory (especially in older individuals) is associated with reduced speech recognition ability in adverse listening situations (Akeroyd, 2008; Anderson, White-Schwoch, Parbery-Clark, & Kraus, 2013; Desjardins & Doherty, 2013; Humes, Lee, & Coughlin, 2006; Koelewijn, Zekveld, Festen, Rönnberg, & Kramer, 2012; Lee & Humes, 2012). There is also evidence that age-related changes in processing speed are related to a decline in speech perception performance in noise for older adults (Tun et al., 2002; Tun & Wingfield, 1999; Woods, Kalluri, Pentony, & Nooraei, 2013).
It should be noted that not all abilities and functions decline with age. For example, vocabulary increases throughout the adult life span (Verhaeghen, 2003), and better vocabulary has been found to be positively associated with speech understanding in adverse listening conditions (Schneider, Avivi-Reich, & Daneman, 2016). Moreover, older adults appear to be particularly adept at using some forms of top-down processing, such as taking advantage of sentence context (Pichora-Fuller, Schneider, & Daneman, 1995). There is physiological evidence of this type of compensatory processing, as older adults' brain functioning demonstrates less hemispheric asymmetry and greater frontal processing compared to those of the younger adults (Cabeza, 2002; Getzmann & Falkenstein, 2011; Getzmann, Lewald, & Falkenstein, 2014).
In the research discussed above, speech perception was measured using objective tasks in laboratory settings. Perhaps more relevant is the extent to which individuals believe that they experience difficulty in understanding speech outside of the lab.
Self-report measures of listening problems in the varied acoustical conditions of everyday life are extremely valuable in that they have the potential to tap into an individual's subjective awareness of speech processing and the relative amount of cognitive energy that he or she is putting forth to understand a message. Within the framework of the ELU it is proposed that, as phonological mismatch is induced (because of background noise, degraded auditory processing, sensorineural hearing loss, etc.) and explicit processing resources are recruited to facilitate speech understanding, the listener will perceive this increase in cognitive demand as requiring more effort than when the listening situation is ideal and necessitates few top-down resources (Ng, Rudner, Lunner, Pedersen, & Rönnberg, 2013; Rudner, Lunner, Behrens, Thorén, & Rönnberg, 2012; Zekveld, Kramer, & Festen, 2010). Greater perceived effort as reported by the listener can be an indicator of cognitive resource engagement during speech perception, even when speech understanding is successful. It has been suggested that individuals with higher working memory capacities (an indicator of cognitive capacity) experience lower perceived listening effort in adverse conditions (Rudner et al., 2012).
Our research group is particularly interested in defining the problems people experience in real-life, acoustically adverse listening situations. Our lab-based objective measures give a snapshot of performance in a small subset of listening conditions that are somewhat representative of those encountered in our participants' daily lives. We believe that it is essential to also determine how individuals believe they function in complex listening situations outside of the lab. The Speech, Spatial and Qualities of Hearing Scale Questionnaire (SSQ; Gatehouse & Noble, 2004) is a particularly useful tool that allows researchers and clinicians to tap into self-perceived problems in these situations. Research using this questionnaire has shown that performance on speech perception tasks in complex listening environments is significantly correlated with self-perceived difficulty as measured by the SSQ (Brungart, Cohen, Cord, Zion, & Kalluri, 2014; Glyde, Cameron, Dillon, Hickson, & Seeto, 2013). Glyde et al. (2013) assessed spatial processing ability with the Listening in Spatialized Noise–Sentences Test (Cameron & Dillon, 2007) across a wide range of ages and hearing abilities. The authors compensated for elevated auditory thresholds by restoring audibility for each participant with hearing loss. They found that the SSQ score was significantly correlated with performance on the Listening in Spatialized Noise–Sentences Test task, although the relationship was no longer significant once hearing impairment was included in the analyses, suggesting that hearing loss was the driving factor. Glyde et al. (2013) also found that even for participants with thresholds between 0 dB HL and 20 dB HL, there was evidence of spatial hearing performance beginning to decline with increasing hearing threshold.
Other results support the idea that a significant relationship exists between SSQ scores and spatial hearing, even when hearing loss is not significantly correlated with spatial ability. Brungart et al. (2014) found that although the mean SSQ score was not significantly associated with the better ear average thresholds, overall variations in spatial hearing abilities were correlated with subjective hearing as assessed by the speech subscale of the SSQ (Brungart et al., 2014).
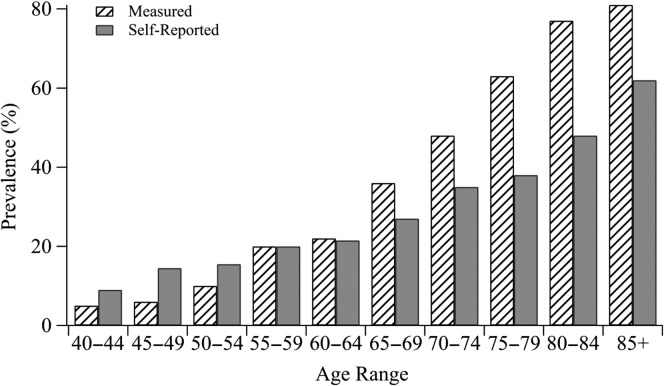
The relationship between self-perceived and measured hearing loss appears to change as individuals age. Figure 1 shows data adapted from Bainbridge and Wallhagen (2014) that compares self-reported hearing ability from the National Health Interview Survey to measured audiometric thresholds gathered from the National Health and Nutrition Examination Survey (Bainbridge & Wallhagen, 2014). This work contrasted the four-frequency average pure-tone thresholds (at 0.5 kHz, 1.0 kHz, 2.0 kHz, and 4.0 kHz) in men and women (averaged together here) at various ages to the percentage of people who self-report having “a little hearing trouble or worse.” It can be observed that the prevalence of self-reported hearing problems in adults from 40 to 55 years of age is greater than the prevalence of objectively measured hearing loss. This trend reverses for individuals over 60 years of age. Banh, Singh, and Pichora-Fuller (2012) also found that older adults with normal hearing thresholds through 4.0 kHz had significantly lower scores on the SSQ (i.e., less perceived difficulty) as compared with those of younger adults with normal hearing (Banh et al., 2012), supporting the idea that at least some older adults may underestimate hearing problems. Moreover, Bainbridge and Wallhagen's (2014) finding that a proportion of middle-aged individuals who have minimal hearing loss (in terms of pure-tone thresholds) feels that they experience hearing problems is consistent with the results of Glyde et al. (2013), which demonstrated that performance can decline on complex listening tasks even when thresholds are within normal limits.
Figure 1.
Prevalence of self-reported hearing difficulty to measured hearing difficulty in adults from 40 years to 85+ years of age. Figure adapted from Bainbridge & Wallhagen, 2014. Self-report data were taken from the National Health Interview Survey, and measured audiometric data are pure-tone audiometric thresholds averaged at 0.5 kHz, 1.0 kHz, 2.0 kHz, and 4.0 kHz gathered from the National Health and Nutrition Examination Survey. Reproduced with permission of Annual Review of Public Health, Volume © by Annual Reviews, http://www.annualreviews.org
In summary, research on age-related changes in speech perception confirms the problems that audiologists hear from their clients: Speech understanding in complex listening situations is effortful and challenging for many middle-aged and older adults. In the sections below, we summarize results from several studies conducted in our lab that were designed to identify the types of listening situations that are particularly difficult as people age and how degree of hearing loss and selected cognitive abilities help explain individual variability in these situations. Also discussed is how the prevalence and nature of self-perceived hearing problems (as measured by selected questions from the SSQ) in participants from our studies change with age.
Speech Perception Across the Adult Age Range: Lab-Based Measures
Over the past few years, our lab has investigated the associations among hearing loss, cognition, and speech perception (Helfer & Freyman, 2014, 2016; Helfer & Jesse, 2015; Helfer, Merchant, & Freyman, 2016). While the specific focus of these four studies varied, each one considered speech perception across three age groups (younger adults age 18–28 years, middle-aged adults age 40–59 years, and older adults age 60+ years) and included a variety of cognitive measures, as well as assessments of self-reported hearing problems. Despite variation in the specific parameters of the speech perception task for each of the four studies (discussed in more detail below), interesting patterns emerged with respect to how individuals in the different adult age groups cope with different types of maskers.
The focus of Helfer and Freyman (2014) was on investigating how selected cognitive abilities and degree of hearing loss influence speech perception in the presence of different types of maskers. The speech perception task required participants to repeat back target sentences in the presence of one or two similar masking sentences, one or two segments from the Rainbow Passage (Fairbanks, 1960), or steady-state noise (SSN). All stimuli came from a single loudspeaker in front of the participant. Target stimuli were sentences from the TVM (Theo-Victor-Michael) sentence corpus (Helfer & Freyman, 2009). This corpus is composed of sentences that start with the cue name Theo, Victor, or Michael and take the form “Cue name discussed the ___ and the ___ today,” where underlines represent one- or two-syllable key words used for scoring. All three groups were evaluated at the same signal-to-noise ratios (SNRs): −3, 0, and +3 dB. Cognitive tasks used in this study included the SICSPAN (Sorqvist et al., 2010) to assess working memory, a Stroop task (Jesse & Janse, 2012) to measure inhibitory ability, and the Connections Test (Salthouse et al., 2000), a version of the trail-making task, which quantifies processing speed and executive functioning. Results of this study showed that middle-aged and older adults were particularly vulnerable to the interfering effects of a single competing talker. Measured cognitive abilities and degree of high-frequency hearing loss were related to speech understanding among the middle-aged and older participants. Interestingly, within this sample of middle-aged and older adults, degree of high-frequency hearing loss, but not age, was significantly associated with cognitive task performance: The greater the threshold elevation, the poorer the performance on the SICSPAN and the Connections task, even though these tests were administered visually.
In another study (Helfer & Jesse, 2015), we examined how lexical characteristics (neighborhood density and word frequency) of both target and masking speech influenced performance. Target and masking speech (pairs of sentences from the TVM corpus) were presented at several SNRs from a single loudspeaker in front of the participant. Participants completed a battery of cognitive tasks similar to the one described above. As anticipated, older adults' performance was poorer than that of younger adults, and target words that were from sparse lexical neighborhoods or that were encountered frequently were easier to understand than words that were less familiar or that came from dense lexical neighborhoods. Of interest was that lexical characteristics of words in the masking sentence also influenced performance. When words in the masker were lexically easy to understand, they interfered to a greater extent with correct target word recognition, but only for younger adults listening at a 3-dB poorer SNR than the other participants. Moreover, when words from the masker were high in frequency of occurrence, older adults were prone to reporting these words erroneously as the target words. Also of note was that speech recognition ability of the middle-aged participants was even poorer than that of the younger participants run at the more adverse SNR. Regression analyses indicated that the greater the high-frequency hearing loss, the more lexical frequency of words in both the target and the masker influenced performance. Cognitive abilities also explained individual differences in overall recognition of words in the target sentence and in susceptibility to lexical characteristics of these words.
We recently explored the benefit of repetition (a commonly used conversational repair strategy) in complex listening environments as a function of age (Helfer & Freyman, 2016). Target TVM sentences were presented from a loudspeaker in front of the participant in the presence of a competing stimulus, which was either another TVM sentence, SSN, or single-channel modulated envelope noise. In this study, the competing stimuli were presented from both the front loudspeaker and a loudspeaker located 60° to the right of the participant with a 4-ms time delay favoring the right loudspeaker. This resulted in the participant perceiving the masker stimulus as being spatially separated from the target, due to the precedence effect. On some trials, the target sentence and its accompanying masker were presented again during the next trial in order to assess the effect of repetition. Although the amount of repetition benefit was modulated by type of masker (with the least benefit obtained when the masker was competing speech), all three groups were assisted by approximately the same amount from repetition.
The final study discussed here investigated the effect of target and masker alignment on competing speech perception (Helfer et al., 2016). In each of the previously discussed studies (and in much of the research literature), target and competing speech maskers are highly aligned in time, with the first word of the target and masking sentences starting at the same time, resulting in much of the sentence structure overlapping (particularly when target and masking stimuli are from the same corpus). In real-world situations, this extreme alignment between to-be-attended and to-be-ignored speech rarely occurs. The purpose of this study was to compare speech recognition in highly aligned conditions versus nonaligned conditions. Participants in Helfer et al. (2016) repeated back target TVM sentences (presented from a front loudspeaker) in the presence of a competing masker composed of two TVM sentences. In aligned conditions, the target and masking sentences all began at the same time and at the beginning of a sentence. In nonaligned conditions, a random point was selected in each masking sentence, and the sentence was started at this point, with the beginning of the sentence appended to the end. The two masking sentences either were both presented from the same side (60° right or left) or were presented in a symmetrical fashion with one masking sentence from the right and another from the left. Participants also completed a battery of cognitive tasks similar to those described above.
Results of this study suggest that presenting target and masking speech in a temporally aligned fashion exaggerates age-related differences in performance. Older adults experienced a greater performance decrement than younger adults when comparing the perception of temporally aligned and nonaligned stimuli, at least when maskers surrounded the target speech (speech understanding scores for all listener groups were near ceiling when the maskers were to the side of the target, regardless of alignment condition). Interestingly, while age and cognitive skills were the most important predictors of performance in the aligned condition, amount of high-frequency hearing loss was the dominant factor in accounting for performance in nonaligned trials. The pattern of results was consistent with aligned conditions leading to more informational masking.
In order to qualitatively explore our results across studies, we aggregated the speech perception data from these four articles. Percent-correct speech recognition scores from each of these studies were averaged, within masker type, across SNRs. Figure 2 displays the outcome of this aggregation by age group and type of competing stimulus (SSN, single-channel envelope modulated noise, or competing speech). It is apparent that the difference in performance between older and younger participants is largest when the masker is competing speech. Also notable is that middle-aged individuals perform similarly to younger participants in the presence of a masker that is nonspeech noise but have substantially more difficulty when the masker is one or two understandable speech messages.
Figure 2.
Percent correct speech recognition, averaged across studies, by listener group and masker condition (SSN = steady-state noise; SEM = speech envelope modulated noise; and competing speech).
This pattern of results suggests that, as individuals age, they are particularly susceptible to background competition that is understandable speech. There are at least two potential reasons for this finding. First, aging and/or age-related hearing loss appears to bring about a decrease in the ability to use brief instances of reduced energy in a fluctuating masker (Dubno et al., 2002, 2003; George et al., 2006; Summers & Molis, 2004; Takahashi & Bacon, 1992). However, as Figure 2 indicates, the difference in performance among groups is greater for speech maskers than for fluctuating noise maskers, suggesting that this explanation cannot account entirely for the obtained results. Another potential contributor to this pattern is that as individuals age, they become more vulnerable to interference from the lexical content of competing speech.
Speech Perception Across the Adult Age Range: Self-Perceived Hearing Problems
We obtained a measure of self-reported hearing difficulty in all of our studies via selected questions from the SSQ (Gatehouse & Noble, 2004). Responses to these questions allowed us to tap into self-perceived speech understanding problems that our participants experience in complex listening environments outside of the lab and to compare the extent of these self-reported problems to results of our lab-based speech understanding measures. The Appendix lists the specific questions used in our studies.
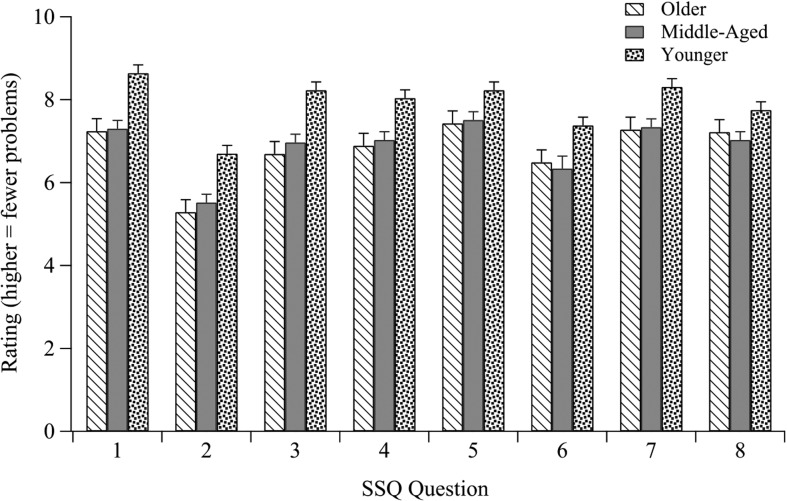
Responses to the selected SSQ questions aggregated across the four studies described above are shown in Figure 3. Although our older participants had, as a group, substantially higher pure-tone thresholds than our middle-aged participants, there was little difference between these two groups on the SSQ ratings. Also obvious in Figure 3 is that middle-aged adults report having more problems than younger adults in the types of situations queried in the SSQ. This supports the experience of many clinical audiologists, who find that it is not uncommon for middle-aged individuals to report that they experience hearing problems in difficult listening situations.
Figure 3.
Average SSQ ratings by group averaged across four studies. Higher ratings indicate fewer problems related to the question topic (see the Appendix for the content of each question). SSQ = Speech, Spatial and Qualities of Hearing Scale Questionnaire.
Discussion and Conclusion
Our examination of the aggregate data from these four studies concurs with previous work demonstrating the differential effect of masker type on speech understanding performance of adults. As individuals age, they have increasing difficulty coping in situations where there is background noise. These problems seem to emerge earlier when the background competition consists of one or two competing talkers. Although our middle-aged participants performed comparably with younger adults in conditions with nonspeech noise maskers (SSN and single-channel envelope modulated noise), they were negatively impacted by understandable competing speech. Moreover, middle-aged individuals consistently reported more self-perceived hearing problems outside of the lab (as indicated by SSQ responses) as compared with younger adults. Taken as a whole, results across labs support the idea that individuals in middle age are especially susceptible to listening conditions with informational masking present, with further declines as people continue to age (Goossens, Vercammen, Wouters, & van Wieringen, 2017; Helfer & Freyman, 2014, 2016; Helfer & Jesse, 2015).
In accordance with the ELU, peripheral and suprathreshold auditory encoding degradations that occur as a result of aging affect the fidelity of incoming phonological representations, leading to the need for greater cognitive resources to segregate and attend to target speech information while inhibiting competing speech information. These deficits seem to begin in middle age even before significant pure-tone threshold elevation emerges on the audiogram. It is possible that subtle age-related changes are significant enough to cause greater difficulty in listening situations inducing cognitive load (i.e., situations with informational masking), while not being deleterious enough to cause similar problems when the competing sounds only produce energetic masking. Age-related declines in temporal processing are well documented in the research literature and may be fundamental contributors to suprathreshold auditory distortions. For example, older individuals have reduced sensitivity to temporal fine structure cues (Füllgrabe et al., 2015; Neher et al., 2012). They are also less sensitive than younger adults to information in the temporal envelope (Fitzgibbons & Gordon-Salant, 1996; He, Mills, Ahlstrom, & Dubno, 2008). Notable is that at least some aspects of temporal processing appear to decline by middle age (Grose, Hall, & Buss, 2006; Grose & Mamo, 2010; Humes, Kewley-Port, Fogerty, & Kinney, 2010; Ozmeral, Eddins, Frisian, & Eddins, 2016). It is also possible that cochlear synaptopathy may begin to impact the resolution of auditory representations in middle age. The additive nature of these auditory processing issues may lead to functional deficits that are realized more significantly in situations requiring the recruitment of greater cognitive resources (i.e., when the auditory scene contains more than one understandable message).
Clinical Implications
The information presented in this article emphasizes the need for audiologists to keep in mind the limitations of current assessment protocols. Pure-tone audiometry and speech recognition in quiet likely give us little relevant information regarding how our patients function in challenging real-world listening environments, which often contain multiple talkers and adverse acoustics. Clinical tests of speech understanding in noise (like the HINT test [Hearting in Noise Test; Nilsson, Soli, & Sullivan, 1994] and QuickSIN test [Quick Speech-in-Noise Test; Killion, Niquette, Gudmundsen, Revit, & Banerjee, 2004]) yield some information about how individuals function but are not routinely performed and are still limited in what they tell us.
Because our current clinical tests are not sufficient to identify real-life problems experienced by our patients, it is imperative that audiologists give significant weight to subjective reports of listening difficulty. We need to believe our patients (and validate, not minimize, their complaints) when they tell us that they are having trouble communicating, even if our clinical tests indicate “normal” functioning. This seems to be especially vital when working with middle-aged adults. The use of a hearing handicap scale (like the SSQ: Gatehouse & Noble, 2004 or the Hearing Handicap Inventory for the Elderly: Ventry & Weinstein, 1982) can help to open up communication about areas of difficulty.
When counseling our patients and their communication partners, it is important to emphasize strategies that help to differentiate the target speech message from the background speech maskers. These strategies include using clear speech and visual speech cues (e.g., lipreading), as well as environmental manipulations that decrease background competition. In addition, research from our lab suggests that middle-aged and older adults benefit from repetition of a message to the same extent as younger listeners (Helfer & Freyman, 2016). This supports the importance of promoting aural rehabilitation activities that include conversational repair strategies and environmental management.
Acknowledgments
Research reported in this publication was supported by the National Institute on Deafness and Other Communication Disorders of the National Institutes of Health under Award Number R01 012057. We thank Richard Freyman, Angela Costanzi, Sarah Laakso, Kim Adamson-Bashaw, and Michael Rogers for their assistance with the experiments discussed in this article. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Appendix
Speech, Spatial and Qualities of Hearing Scale Questionnaire (Gatehouse & Noble, 2004) questions used in experiments described in the present article
You are talking with one person, and there is a TV on in the same room. Without turning the TV down, can you follow what the person you're talking to says?
You are listening to someone talking to you, while at the same time trying to follow the news on TV. Can you follow what both people are saying?
You are in conversation with one person in a room where there are many other people talking. Can you follow what the person you are talking to is saying?
You are in a group of about five people in a busy restaurant. You can see everyone else in the group. Can you follow the conversation?
You are with a group and the conversation switches from one person to another. Can you easily follow the conversation without missing the start of what each new speaker is saying?
Can you easily ignore other sounds when trying to listen to something?
Do you have to put in a lot of effort to hear what is being said in conversations with others?
-
Do you have to concentrate very much when listening to someone or something?
Note. Copyright © British Society of Audiology; International Society of Audiology; Nordic Audiological Society, reprinted by permission of Taylor and Francis Ltd, http://www.tandfonline.com, on behalf of British Society of Audiology; International Society of Audiology; Nordic Audiological Society.
Funding Statement
Research reported in this publication was supported by the National Institute on Deafness and Other Communication Disorders of the National Institutes of Health under Award Number R01 012057.
References
- Akeroyd M. A. (2008). Are individual differences in speech reception related to individual differences in cognitive ability? A survey of twenty experimental studies with normal and hearing-impaired adults. International Journal of Audiology, 47(Suppl. 2), S53–S71. https://doi.org/10.1080/14992020802301142 [DOI] [PubMed] [Google Scholar]
- Alain C., Arnott S. R., Hevenor S., Graham S., & Grady C. L. (2001). “What” and “where” in the human auditory system. Proceedings of the National Academy of Sciences of the United States of America, 98(21), 12301–12306. https://doi.org/10.1073/pnas.211209098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson S., White-Schwoch T., Parbery-Clark A., & Kraus N. (2013). A dynamic auditory-cognitive system supports speech-in-noise perception in older adults. Hearing Research, 300, 18–32. https://doi.org/10.1016/j.heares.2013.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arbogast T. L., Mason C. R., & Kidd G. (2005). The effect of spatial separation on informational masking of speech in normal-hearing and hearing-impaired listeners. The Journal of the Acoustical Society of America, 117(4), 2169–2180. https://doi.org/10.112/1.1861598 [DOI] [PubMed] [Google Scholar]
- Arehart K. H., Souza P., Baca R., & Kates J. M. (2013). Working memory, age, and hearing loss: Susceptibility to hearing aid distortion. Ear and Hearing, 34(3), 251–260. https://doi.org/10.1097/AUD.0b013e318271aa5e [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bainbridge K. E., & Wallhagen M. I. (2014). Hearing loss in an aging American population: Extent, impact, and management. Annual Review of Public Health, 35, 139–152. https://doi.org/10.1146/annurev-publhealth-032013-182510 [DOI] [PubMed] [Google Scholar]
- Banh J., Singh G., & Pichora-Fuller M. K. (2012). Age affects responses on the Speech, Spatial and Qualities of Hearing Scale (SSQ) by adults with minimal audiometric loss. Journal of the American Academy of Audiology, 23(2), 81–91. https://doi.org/10.3766/jaaa.23.2.2 [DOI] [PubMed] [Google Scholar]
- Brungart D. S., Cohen J., Cord M., Zion D., & Kalluri S. (2014). Assessment of auditory spatial awareness in complex listening environments. The Journal of the Acoustical Society of America, 136(4), 1808–1820. https://doi.org/10.1121/1.4893932 [DOI] [PubMed] [Google Scholar]
- Cabeza R. (2002). Hemispheric asymmetry reduction in older adults: The HAROLD model. Psychology and Aging, 17(1), 85–100. [DOI] [PubMed] [Google Scholar]
- Cameron S., & Dillon H. (2007). Development of the Listening in Spatialized Noise–Sentences Test (LISN–S). Ear and Hearing, 28(2), 196–211. [DOI] [PubMed] [Google Scholar]
- Committee on Hearing Bioacoustics and Biomechanics. (1988). Speech understanding and aging. The Journal of the Acoustical Society of America, 83(3), 859–895. [PubMed] [Google Scholar]
- Conway A. R., Cowan N., & Bunting M. F. (2001). The cocktail party phenomenon revisited: The importance of working memory capacity. Psychonomic Bulletin and Review, 8(2), 331–335. [DOI] [PubMed] [Google Scholar]
- Daneman M., & Carpenter P. A. (1980). Individual differences in working memory and reading. Journal of Verbal Learning and Verbal Behavior, 19(4), 450–466. [Google Scholar]
- Desjardins J. L., & Doherty K. A. (2013). Age-related changes in listening effort for various types of masker noises. Ear and Hearing, 34(3), 261–272. https://doi.org/10.1097/AUD.0b013e31826d0ba4 [DOI] [PubMed] [Google Scholar]
- Dubno J. R., Horwitz A. R., & Ahlstrom J. B. (2002). Benefit of modulated maskers for speech recognition by younger and older adults with normal hearing. The Journal of the Acoustical Society of America, 111(6), 2897–2907. [DOI] [PubMed] [Google Scholar]
- Dubno J. R., Horwitz A. R., & Ahlstrom J. B. (2003). Recovery from prior stimulation: Masking of speech by interrupted noise for younger and older adults with normal hearing. The Journal of the Acoustical Society of America, 113(4), 2084–2094. [DOI] [PubMed] [Google Scholar]
- Duquesnoy A. J. (1983). Effect of a single interfering noise or speech source upon the binaural sentence intelligibility of aged persons. The Journal of the Acoustical Society of America, 74(3), 739–743. [DOI] [PubMed] [Google Scholar]
- Fairbanks G. (1960). Voice and articulation drillbook, 2nd ed. (pp. 127). New York, NY: Harper and Row. [Google Scholar]
- Festen J. M., & Plomp R. (1990). Effects of fluctuating noise and interfering speech on the speech-reception threshold for impaired and normal hearing. The Journal of the Acoustical Society of America, 88(4), 1725–1736. [DOI] [PubMed] [Google Scholar]
- Fitzgibbons P. J., & Gordon-Salant S. (1996). Auditory temporal processing in elderly listeners. Journal of the American Academy of Audiology, 7, 183–189. [PubMed] [Google Scholar]
- Foo C., Rudner M., Rönnberg J., & Lunner T. (2007). Recognition of speech in noise with new hearing instrument compression release settings requires explicit cognitive storage and processing capacity. Journal of the American Academy of Audiology, 18(7), 618–631. https://doi.org/10.3766/jaaa.18.7.8 [DOI] [PubMed] [Google Scholar]
- Füllgrabe C., Moore B. C., & Stone M. A. (2015). Age-group differences in speech identification despite matched audiometrically normal hearing: Contributions from auditory temporal processing and cognition. Frontiers in Aging Neuroscience, 6, 347 https://doi.org/10.3389/fnagi.2014.00347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallun F. J., Diedesch A. C., Kampel S. D., & Jakien K. M. (2013). Independent impacts of age and hearing loss on spatial release in a complex auditory environment. Frontiers in Neuroscience, 7, 252 https://doi.org/10.3389/fnins.2013.00252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatehouse S., Naylor G., & Elberling C. (2003). Benefits from hearing aids in relation to the interaction between the user and the environment. International Journal of Audiology, 42(Suppl. 1), 77–85. [DOI] [PubMed] [Google Scholar]
- Gatehouse S., & Noble W. (2004). The Speech, Spatial and Qualities of Hearing Scale (SSQ). International Journal of Audiology, 43(2), 85–99. https://doi.org/10.1080/14992020400050014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- George E. L., Festen J. M., & Houtgast T. (2006). Factors affecting masking release for speech in modulated noise for normal-hearing and hearing-impaired listeners. The Journal of the Acoustical Society of America, 120(4), 2295–2311. [DOI] [PubMed] [Google Scholar]
- Getzmann S., & Falkenstein M. (2011). Understanding of spoken language under challenging listening conditions in younger and older listeners: A combined behavioral and electrophysiological study. Brain Research, 1415, 8–22. https://doi.org/10.1016/j.brainres.2011.08.001 [DOI] [PubMed] [Google Scholar]
- Getzmann S., Lewald J., & Falkenstein M. (2014) Using auditory pre-information to solve the cocktail-party problem: Electrophysiological evidence for age-specific differences. Frontiers in Neuroscience, 8, 413 https://doi.org/10.3389/fnins.2014.00413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glyde H., Cameron S., Dillon H., Hickson L., & Seeto M. (2013). The effects of hearing impairment and aging on spatial processing. Ear and Hearing, 34, 15–28. https://doi.org/10.1097/AUD.0b013e3182617f94 [DOI] [PubMed] [Google Scholar]
- Goossens T., Vercammen C., Wouters J., & van Wieringen A. (2017). Masked speech perception across the adult lifespan: Impact of age and hearing impairment. Hearing Research, 344, 109–124. https://doi.org/10.1016/j.heares.2016.11.004 [DOI] [PubMed] [Google Scholar]
- Grose J. H., Hall J. W., & Buss E. (2006). Temporal processing deficits in the pre-senescent auditory system. The Journal of the Acoustical Society of America, 119, 2305–2315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grose J. H., & Mamo S. K. (2010). Processing of temporal fine structure as a function of age. Ear and Hearing, 31, 755–760. https://doi.org/10.1097/AUD.0b013e3181e627e7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grube M., von Cramon D. Y., & Rübsamen R. (2003). Inharmonicity detection. Effects of age and contralateral distractor sounds. Experimental Brain Research, 153(4), 637–642. [DOI] [PubMed] [Google Scholar]
- Hällgren M., Larsby B., Lyxell B., & Arlinger S. (2005). Speech understanding in quiet and noise, with and without hearing aids. International Journal of Audiology, 44(10), 574–583. https://doi.org/10.1080/14992020500190011 [DOI] [PubMed] [Google Scholar]
- He N. J., Mills J. H., Ahlstrom J. B., & Dubno J. R. (2008). Age-related differences in the temporal modulation transfer function with pure-tone carriers. The Journal of the Acoustical Society of America, 124(6), 3841–3849. https://doi.org/10.1121/1.2998779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helfer K. S., Chevalier J., & Freyman R. L. (2010). Aging, spatial cues, and single- versus dual-task performance in competing speech perception. The Journal of the Acoustical Society of America, 128(6), 3625–3633. https://doi.org/10.1121/1.3502462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helfer K. S., & Freyman R. L. (2008). Aging and speech-on-speech masking. Ear and Hearing, 29(1), 87–98. https://doi.org/10.1097/AUD.0b013e31815d638b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helfer K. S., & Freyman R. L. (2009). Lexical and indexical cues in masking by competing speech. The Journal of the Acoustical Society of America, 125(1), 447–456. https://doi.org/10.1121/1.3035837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helfer K. S., & Freyman R. L. (2014). Stimulus and listener factors affecting age-related changes in competing speech perception. The Journal of the Acoustical Society of America, 136(2), 748–759. https://doi.org/10.1121/1.4887463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helfer K. S., & Freyman R. L. (2016). Age equivalence in the benefit of repetition for speech understanding. The Journal of the Acoustical Society of America, 140(5), EL371 https://doi.org/10.1121/1.4966586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helfer K. S., & Jesse A. (2015). Lexical influences on competing speech perception in younger, middle-aged, and older adults. The Journal of the Acoustical Society of America, 138(1), 363–376. https://doi.org/10.1121/1.4923155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helfer K. S., Merchant G. R., & Freyman R. L. (2016). Aging and the effect of target-masker alignment. The Journal of the Acoustical Society of America, 140(5), 3844 https://doi.org/10.1121/1.4967297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humes L. E., Kewley-Port D., Fogerty D., & Kinney D. (2010). Measures of hearing threshold and temporal processing across the adult lifespan. Hearing Research, 264, 30–40. https://doi.org/10.1016/j.heares.2009.09.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humes L. E., Lee J. H., & Coughlin M. P. (2006). Auditory measures of selective and divided attention in young and older adults using single-talker competition. The Journal of the Acoustical Society of America, 120(5), 2926–2937. [DOI] [PubMed] [Google Scholar]
- Jesse A., & Janse E. (2012). Audiovisual benefit for recognition of speech presented with single-talker noise in older listeners. Language and Cognitive Processes, 27(7–8), 1167–1191. https://doi.org/10.1080/01690965.2011.620335 [Google Scholar]
- Johnsrude I. S., Mackey A., Hakyemez H., Alexander E., Trang H. P., & Carlyon R. P. (2013). Swinging at a cocktail party: Voice familiarity aids speech perception in the presence of a competing voice. Psychological Science, 24(10), 1995–2004. https://doi.org/10.1177/0956797613482467 [DOI] [PubMed] [Google Scholar]
- Killion M. C., Niquette P. A., Gudmundsen G. I., Revit L. J., & Banerjee S. (2004). Development of a quick speechin-noise test for measuring signal-to-noise ratio loss in normal-hearing and hearing-impaired listeners. Journal of the Acoustical Society of America, 116(4), 2395–2405. [DOI] [PubMed] [Google Scholar]
- Koelewijn T., Zekveld A. A., Festen J. M., Rönnberg J., & Kramer S. E. (2012). Processing load induced by informational masking is related to linguistic abilities. International Journal of Otolaryngology, 2012, 86573 https://doi.org/10.1155/2012/865731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsby B., Hällgren M., Lyxell B., & Arlinger S. (2005). Cognitive performance and perceived effort in speech processing tasks: Effects of different noise backgrounds in normal-hearing and hearing-impaired subjects. International Journal of Audiology, 44(3), 131–143. https://doi.org/10.1080/14992020500057244 [DOI] [PubMed] [Google Scholar]
- Lee J. H., & Humes L. E. (2012). Effect of fundamental-frequency and sentence-onset differences on speech-identification performance of young and older adults in a competing-talker background. The Journal of the Acoustical Society of America, 132(3), 1700–1717. https://doi.org/10.1121/1.4740482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunner T. (2003). Cognitive function in relation to hearing aid use. International Journal of Audiology, 42(Suppl. 1), S49–S58. https://doi.org/10.3109/14992020309074624 [DOI] [PubMed] [Google Scholar]
- Marrone N., Mason C. R., & Kidd G. (2008). The effects of hearing loss and age on the benefit of spatial separation between multiple talkers in reverberant rooms. The Journal of the Acoustical Society of America, 124(5), 3064–3075. https://doi.org/10.1121/1.2980441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy D. R., Daneman M., & Schneider B. A. (2006). Why do older adults have difficulty following conversations? Psychology and Aging, 21(1), 49–61. [DOI] [PubMed] [Google Scholar]
- Naveh-Benjamin M., & Craik F. M. (1996). Effects of perceptual and conceptual processing on memory for words and voice: Different patterns for young and old. The Quarterly Journal of Experimental Psychology: Human Experimental Psychology, 49A(3), 780–796. https://doi.org/10.1080/027249896392595 [DOI] [PubMed] [Google Scholar]
- Neher T., Lunner T., Hopkins K., & Moore B. C. (2012). Binaural temporal fine structure sensitivity, cognitive function, and spatial speech recognition of hearing-impaired listeners (L). The Journal of the Acoustical Society of America, 131(4), 2561–2564. https://doi.org/10.1121/1.3689850 [DOI] [PubMed] [Google Scholar]
- Ng E. H. N., Rudner M., Lunner T., Pedersen M. S., & Rönnberg J. (2013). Effects of noise and working memory capacity on memory processing of speech for hearing-aid users. International Journal of Audiology, 52(7), 433–441. https://doi.org/10.3109/14992027.2013.776181 [DOI] [PubMed] [Google Scholar]
- Nilsson M., Soli S. D., & Sullivan J. A. (1994). Development of the Hearing in Noise Test for the measurement of speech reception thresholds in quiet and in noise. Journal of the Acoustical Society of America, 95(2), 1085–1099. [DOI] [PubMed] [Google Scholar]
- Ozmeral E. J., Eddins A. C., Frisian D. R., & Eddins D. A. (2016). Large cross-sectional study of presbycusis reveals rapid progressive decline in auditory temporal acuity. Neurobiology of Aging, 43, 72–78. https://doi.org/10.1016/j.neurobiolaging.2015.12.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pichora-Fuller M. K. (1997). Language comprehension in older listeners. Journal of Speech-Language Pathology and Audiology, 21(2), 125–142. [Google Scholar]
- Pichora-Fuller M. K., Schneider B. A., & Daneman M. (1995). How young and old adults listen to and remember speech in noise. The Journal of the Acoustical Society of America, 97(1), 593–608. https://doi.org/10.1121/1.412282 [DOI] [PubMed] [Google Scholar]
- Pilotti M., & Beyer T. (2002). Perceptual and lexical components of auditory repetition priming in young and older adults. Memory and Cognition, 30(2), 226–236. https://doi.org/10.3758/BF03195283 [DOI] [PubMed] [Google Scholar]
- Rönnberg J. (2003). Cognition in the hearing impaired and deaf as a bridge between signal and dialogue: A framework and a model. International Journal of Audiology, 42, S68–S76. [DOI] [PubMed] [Google Scholar]
- Rönnberg J., Lunner T., Zekveld A., Sörqvist P., Danielsson H., Lyxell B., … Rudner M. (2013). The ease of language understanding (ELU) model: Theoretical, empirical, and clinical advances. Frontiers in Systems Neuroscience, 7, 31 https://doi.org/10.3389/fnsys.2013.00031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rönnberg J., Rudner M., Foo C., & Lunner T. (2008). Cognition counts: A working memory system for ease of language understanding (ELU). International Journal of Audiology, 47, S99–S105. https://doi.org/10.1080/14992020802301167 [DOI] [PubMed] [Google Scholar]
- Rossi-Katz J., & Arehart K. H. (2009). Message and talker identification in older adults: Effects of task, distinctiveness of the talkers' voices, and meaningfulness of the competing message. Journal of Speech, Language, and Hearing Research, 52(2), 435–453. https://doi.org/10.1044/1092-4388(2008/07-0243) [DOI] [PubMed] [Google Scholar]
- Rudner M., & Lunner T. (2014). Cognitive spare capacity and speech communication: A narrative overview. BioMed Research International, 2014, 869726 https://doi.org/10.1155/2014/869726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudner M., Lunner T., Behrens T., Thorén E. S., & Rönnberg J. (2012). Working memory capacity may influence perceived effort during aided speech recognition in noise. Journal of the American Academy of Audiology, 23(8), 577–589. https://doi.org/10.3766/jaaa.23.7.7 [DOI] [PubMed] [Google Scholar]
- Salthouse T. A., Toth J., Daniels K., Parks C., Pak R., Wolbrette M., & Hocking K. J. (2000). Effects of aging on efficiency of task switching in a variant of the trail making test. Neuropsychology, 14(1), 102. [PubMed] [Google Scholar]
- Schneider B. A., Avivi-Reich M., & Daneman M. (2016). How spoken language comprehension is achieved by older listeners in difficult listening situations. Experimental Aging Research, 42(1), 40–63. https://doi.org/10.1080/0361073X.2016.1108749 [DOI] [PubMed] [Google Scholar]
- Sörqvist P., Ljungberg J. K., & Ljung R. (2010). A sub-process view of working memory capacity: Evidence from effects of speech on prose memory. Memory, 18(3), 310–326. https://doi.org/10.1080/09658211003601530 [DOI] [PubMed] [Google Scholar]
- Sörqvist P., & Ronnberg J. (2012). Episodic long-term memory of spoken discourse masked by speech: What is the role for working memory capacity? Journal of Speech, Language, and Hearing Research, 55(1), 210–218. https://doi.org/10.1044/1092-4388(2011/10-0353) [DOI] [PubMed] [Google Scholar]
- Souza P. E., Arehart K. H., Shen J., Anderson M., & Kates J. M. (2015). Working memory and intelligibility of hearing-aid processed speech. Frontiers in Psychology, 6, 526 https://doi.org/10.3389/fpsyg.2015.00526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers V., & Leek M. R. (1998). F0 processing and the separation of competing speech signals by listeners with normal hearing and with hearing loss. Journal of Speech, Language, and Hearing Research, 41(6), 1294–1306. https://doi.org/10.1044/jslhr.4106.1294 [DOI] [PubMed] [Google Scholar]
- Summers V., & Molis M. R. (2004). Speech recognition in fluctuating and continuous maskers: Effects of hearing loss and presentation level. Journal of Speech, Language, and Hearing Research, 47(2), 245–256. https://doi.org/10.1044/1092-4388(2004/020) [DOI] [PubMed] [Google Scholar]
- Takahashi G. A., & Bacon S. P. (1992). Modulation detection, modulation masking, and speech understanding in noise in the elderly. Journal of Speech and Hearing Research, 35(6), 1410–1421. [DOI] [PubMed] [Google Scholar]
- Tun P. A., O'Kane G., & Wingfield A. (2002). Distraction by competing speech in young and older adult listeners. Psychology and Aging, 17(3), 453–467. https://doi.org/10.1037/0882-7974.17.3.453 [DOI] [PubMed] [Google Scholar]
- Tun P. A., & Wingfield A. (1999). One voice too many: Adult age differences in language processing with different types of distracting sounds. The Journals of Gerontology Series B: Psychological Sciences and Social Sciences, 54(5), P317–P327. [DOI] [PubMed] [Google Scholar]
- Ventry I. M., & Weinstein B. E. (1982). The hearing handicap inventory for the elderly: A new tool. Ear and Hearing, 3(3), 128–134. [DOI] [PubMed] [Google Scholar]
- Verhaeghen P. (2003). Aging and vocabulary score: A meta-analysis. Psychology and Aging, 18(2), 332–339. https://doi.org/10.1037/0882-7974.18.2.332 [DOI] [PubMed] [Google Scholar]
- Vongpaisal T., & Pichora-Fuller M. K. (2007). Effect of age on F0 difference limen and concurrent vowel identification. Journal of Speech, Language, and Hearing Research, 50(5), 1139–1156. https://doi.org/10.1044/1092-4388(2007/079) [DOI] [PubMed] [Google Scholar]
- Woods W. S., Kalluri S., Pentony S., & Nooraei N. (2013). Predicting the effect of hearing loss and audibility on amplified speech reception in a multi-talker listening scenario. The Journal of the Acoustical Society of America, 133(6), 4268–4278. https://doi.org/10.1121/1.4803859 [DOI] [PubMed] [Google Scholar]
- Yonan C. A., & Sommers M. S. (2000). The effects of talker familiarity on spoken word identification in younger and older listeners. Psychology and Aging, 15(1), 88–99. https://doi.org/10.1037/0882-7974.15.1.88 [DOI] [PubMed] [Google Scholar]
- Zekveld A. A., Kramer S. E., & Festen J. M. (2010). Pupil response as an indication of effortful listening: The influence of sentence intelligibility. Ear and Hearing, 31(4), 480–490. https://doi.org/10.1097/AUD.0b013e3181d4f251 [DOI] [PubMed] [Google Scholar]