Abstract
Purpose
This research note describes an adapted experimental methodology to administer an exogenous agent to the larynx and upper airway of awake animals. The exogenous agent could be a perturbation. In the current study, the agent was isotonic saline. Isotonic saline was selected because it is safe, of similar composition to extracellular fluid, and used in voice studies. The described approach allowed large animals such as pigs to be comfortably restrained without chemical sedation or anesthesia for extended periods while receiving the agent.
Method
Six Sinclair pigs were successfully trained with positive reinforcement to voluntarily enter and then be restrained in a Panepinto Sling. Once restrained, the pigs accepted a nose cone that delivered nebulized isotonic saline. This procedure was repeated 3 times per day for 20 days. At the end of the study, the larynx and airway tissues were excised and examined using histology and transmission electron microscopy.
Results
Pathology related to the procedure (i.e., nebulized inhaled isotonic saline or stress) was not identified in any examined tissues.
Conclusions
This methodology allowed for repeated application of exogenous agents to awake, unstressed animals. This method can be used repeatedly in the laboratory to test various therapeutics for safety, toxicity, and dosage. Future studies will specifically manipulate the type of agent to further our understanding of laryngeal pathobiology.
Approximately one third of adults experience voice disorders in their lifetime (Roy et al., 2004; Roy, Merrill, Gray, & Smith, 2005). The high prevalence of voice disorders has the potential to negatively impact job performance, personal relationships, and overall quality of life.
Most voice disorders have a chronic etiology. Although phonotrauma is the leading causative factor in inducing voice problems (Colton & Casper, 2005), exposure to pollutants and acid reflux can alter vocal fold tissue and increase susceptibility to phonotraumatic lesions (Jetté, Dill-McFarland, Hanshew, Suen, & Thibeault, 2016; Koufman, 1991). It is important to investigate changes to tissue from these myriad etiologies to fully understand the voice problem. To study the pathophysiology of voice disorders, laryngeal tissues from animal models are exposed to various agents to mimic human exposures. Animal laryngeal tissue is used because it cannot be easily or noninvasively accessed from human beings. For example, recent studies have examined the effects of phonotrauma and acid reflux using in vivo animal models (Durkes & Sivasankar, 2016; Novaleski, Kimball, Mizuta, & Rousseau, 2016). However, these studies typically involved acute exposures, sedation, or animal species with different vocal fold biology than human vocal folds. To simulate laryngeal diseases with a chronic etiology, animals must be exposed to repeated exposures over time.
Porcine Model for the Study of Laryngeal Physiology and Disease
Our laboratory set out to establish a replicable methodology where an animal species that can accurately mimic the human larynx, such as the pig, could be exposed to laryngeal agents repeatedly while awake (i.e., unanesthetized). Determining the appropriate animal model is based on analogous anatomy, physiology, and acoustics to the human larynx. The porcine larynx offers the greatest structural, cellular, immunologic, and neuroanatomical similarity to human vocal folds than any other characterized animal model (Barker, Haverson, Stokes, Birchall, & Bailey, 2005; Gorti, Birchall, Karin, Macchiarini, & Bailey, 1999; Jiang, Raviv, & Hanson, 2001; Knight, McDonald, & Birchall, 2005). These biological similarities are likely the same traits that underlie laryngeal diseases in humans, and thus, findings from the pig species will hopefully translate to improved understanding of human voice disorders.
Considerations in Using Animal Models to Study Laryngeal Tissue
The aim of this study was to develop a methodology for the application of exogenous agents to the upper airway in unanesthetized animals. Sedation and anesthesia have an increased risk of complication if repeated several times a day or even weekly. Anesthetic agents can have unintended effects on animal physiology that may confound animal studies (Muir & Hubbell, 2014). Fasting before anesthesia can prove problematic if the animal needs to be anesthetized multiple days in a row. There needs to be time for the animal to recover from anesthesia, eat, digest, and then fast again before the next round of anesthesia. For these reasons, we set out to develop a methodology to repeatedly challenge awake and restrained animals via an inhalation route. The proposed technique can also be used for other large animals, such as dog and sheep. The advantage of some of these large animals in research studies is abundance of tissue for detailed analysis. There is also an emergence of disease models of the pig for airway research (Gould, Iglesias, Ohlemacher, & German, 2017; Rogers et al., 2008).
Limitations of Existing Methods
Typical restraint methods utilized on pigs in an agricultural setting include snout tying and hog tying (Swindle & Smith, 2013). These methodologies are stressful and potentially painful and can endanger the animal and handler if the pig becomes aggressive as a result of the repeated restraint technique. These restraint techniques also block the availability of the snout for normal breathing necessary for inhalation studies. Moreover, pigs vocalize or “squeal” when handled in a way that is perceived as threatening—that is, restraint for intramuscular injection. The intensive vocalization causes acute trauma to the vocal folds that is visualized endoscopically as posterior edema and vocal fold erythema (A. Durkes, personal observation). Unfortunately, these two gross manifestations of vocal fold trauma are also common endoscopic findings in patients with acute phonotrauma complicating an endoscopic examination. Fortunately, pigs are easily trained and can be acclimated to a sling apparatus such as the Panepinto Sling (Panepinto & Associates; Panepinto, Phillips, Norden, Pryor, & Cox, 1983). Acclimating and training pigs to the sling (Figure 1) is easily accomplished through positive reinforcements such as food and touch. In addition, pigs are social animals and not only prefer to be among their own species, but many also seek out human contact and visibly enjoy socializing in a laboratory setting. This socialization works well when training and utilizing the restraint sling because the pigs quickly associate being restrained in the sling with positive human attention.
Figure 1.
Photograph of the restraint sling apparatus. Each pig is acclimated to the sling and trained to breathe normally through a standard nose cone.
Aims of the Current Study
To test the effectiveness of our methodology, we exposed pigs to nebulized isotonic saline three times per day for 20 days. Nebulized isotonic saline is commonly used as a placebo in trials involving nebulized therapies and is also routinely used to promote mucus clearance of the airways (Eng et al., 1996; Goldman, Teale, & Muers, 1992; Jenkins, Heaton, Fulton, & Moxham, 1987; Sutton et al., 1988). Isotonic saline shows promise for voice problems associated with Sjogren's syndrome and inhaling dry air (Tanner et al., 2016; Tanner, Roy, Merrill, & Elstad, 2007). It is also the gold standard in testing new treatments and technologies. For these reasons, we chose isotonic saline as the nebulized agent. As isotonic saline has the same composition as extracellular fluid, its application alone was not expected to irritate the airway. After repeated exposure over 20 days, we euthanized the animals and examined the structures of the upper airway and vocal fold tissue to confirm that morphology and ultrastructure were unchanged as a result of the methodology. We examined the airways including nares, larynx, trachea, and lung for histologic evidence of pathology. We specifically looked at the epithelium ultrastructurally because the epithelium is the primary target of inhaled agents, and changes in these parameters suggest processes that could lead to voice problems.
Materials and Method
Animal Procedure
Six female adult (35–50 kg) Sinclair minipigs (Sus scrofa) were utilized in this study. The Purdue Animal Care and Use Committee approved the animal use protocol (#1112000344). All pigs were trained to voluntarily enter a restraint sling (Panepinto; Figure 1) and be subsequently lifted off of the ground while a nose cone was placed over the snout. Training methods utilized positive reinforcement to modify the animal's behavior. Positive reinforcement was a combination of pats, scratches, rubs, or an edible treat. Upon loading into the sling, the animals breathed through the nose cone. Next, 3 ml of neutral pH isotonic saline was aerosolized in the nebulizer (Pari LC Sprint nebulizer) and delivered to the nose cone. This process was repeated three times per day, 5 days per week for 4 weeks, for 60 aerosolized challenges to each pig. After 4 weeks, the animals were humanely sacrificed immediately after the final challenge with intravenous Beuthanasia-D Special (Schering Plough Animal Health Corp., Union, NJ). The larynx was immediately removed for processing. All animals received full autopsies to rule out any confounding diseases.
Histology
A 6-mm punch biopsy of the true vocal fold, nasal mucosa, and lung of each animal was fixed in 10% neutral buffered formalin, processed, and embedded in paraffin blocks. For each tissue examined, 5-μm sections were obtained and stained with hematoxylin and eosin. Microscopic examination was performed by a board-certified veterinary pathologist, and interpretation was based on standard histopathological morphology.
Transmission Electron Microscopy
Vocal fold epithelium samples from all animals were immediately fixed in 4% paraformaldehyde in 0.1-M sodium phosphate buffer and then transferred to 2.5% glutaraldehyde in 0.1-M sodium cacodylate buffer. Next, samples were placed in buffered 1% osmium tetroxide containing 0.8% potassium ferricyanide, en bloc stained in aqueous 1% uranyl acetate, dehydrated with a series of ethanol, transferred into propylene oxide, and embedded in Embed-812 resin. A heavy metal tracer, lanthanum nitrate (1%), was added at each subsequent stage of electron microscopy processing to outline the intracellular space for increased accuracy in measurement (Leydon, Imaizumi, Yang, Thibeault, & Fried, 2014). Ultrathin sections were prepared on a Reichert–Jung Ultracut E ultramicrotome. Sections were stained with 2% uranyl acetate and lead citrate. A FEI Tecnai G2 20 electron microscope equipped with a LaB6 source and operating at 100 kV was used to examine and photograph each sample. Ultrastructural features of the epithelium were examined for abnormalities.
Results and Discussion
Here, we described a novel approach focusing on repeated delivery of aerosolized agents to an alert, comfortable animal, devoid of chemical restraint (i.e., anesthesia). Anesthesia often requires recovery time that can greatly impact how often agents can be administered to the larynx. In addition, anesthesia can impact the biological functions of circulatory and nervous system as well as cellular homeostasis (Beilin et al., 1996; Nakao et al., 2001). To model human physiology optimally, iatrogenic manipulation needs to be minimized. A study designed as described in this research note could investigate repeated in vivo challenges or therapeutic delivery on vocal fold structure and function, thus furthering our understanding of voice disorders in comparative animal models with translation to humans.
The novelty of our approach lies in repeated challenges on an alert, comfortable, nonsedated animal. Experiments designed with repeated daily challenges are often limited by either the risky long anesthesia times or the time required to recover between anesthetic events. Our approach eliminates that complication. In addition, dysphagia and voice disorders associated with a neurological component are best investigated in unanesthetized animals (Suzuki & Sasaki, 1977; Wright, Miller, & Corsello, 1990). Anesthesia depresses the autonomic nervous system, thereby impacting the relevance of a study evaluating a disease with a nervous system component (Unoki et al., 2009). By curtailing sedation, a more physiologically relevant and scientifically robust study can be conducted.
In this study, all animals survived without complications after 60 deliveries of isotonic saline to the airway. All pigs voluntarily loaded in the restraint sling and tolerated the nose cone to administer 3 ml of nebulized solution. The pigs remained calm while slung and often reluctantly unloaded, seemingly wanting to stay in the sling for longer periods. Signs of stress such as reluctance to eat, panting, or aggressive behavior were not observed in any of the pigs during the restraint or during normal housing times (Hicks, McGlone, Whisnant, Kattesh, & Norman, 1998). One animal developed a cough during the 4-week study; however, gross autopsy findings were unremarkable in the larynx and lungs of all six animals.
Histology and Ultrastructure Findings
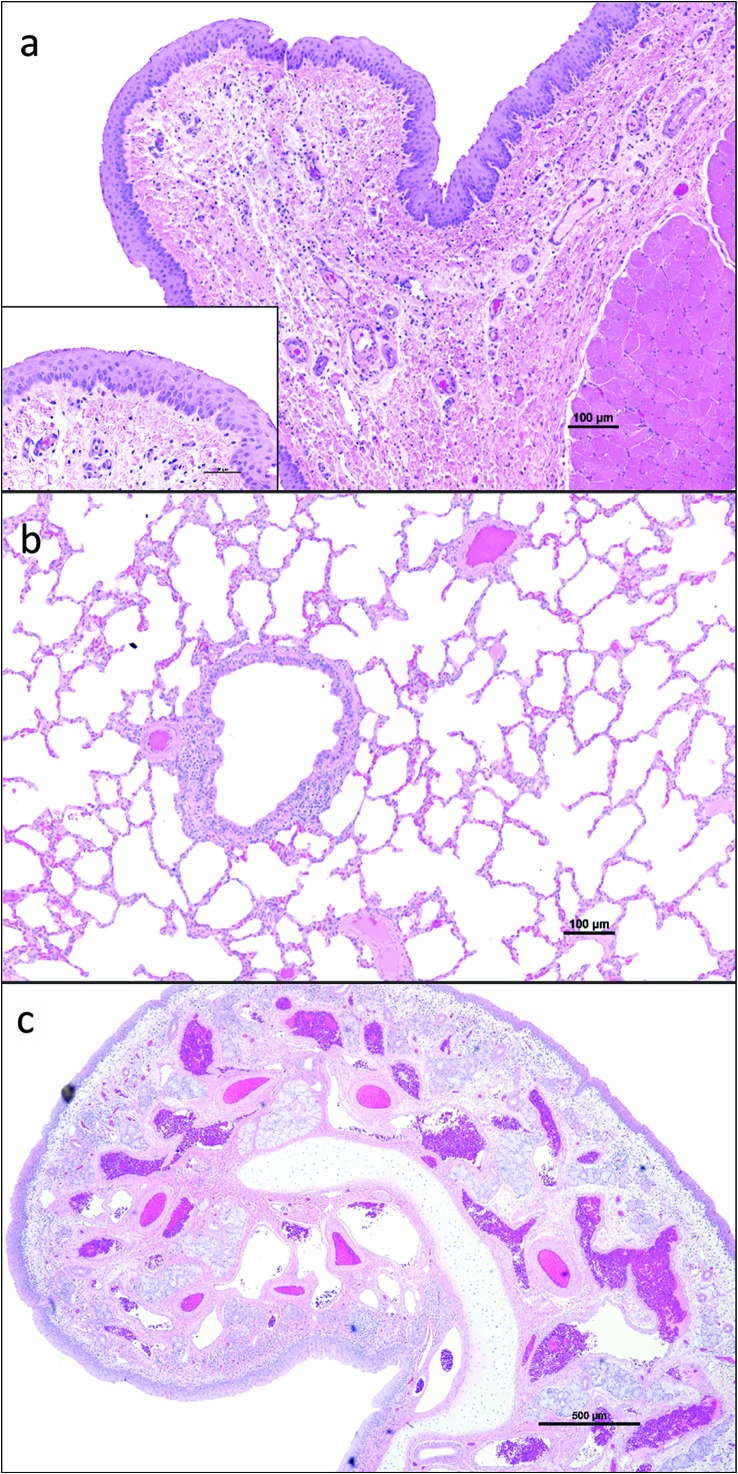
Vocal fold, nasal, and lung histology were similar in all animals and comparable with previous studies conducted in our laboratory. Representative findings are shown in Figure 2. Briefly, the stratified squamous epithelium of the true vocal fold was approximately five cell layers thick and morphologically normal. The lamina propria was made up of loose collagenous matrix with lymphocytes and fewer plasma cells scattered randomly throughout the superficial layers. Collagen fibers were comparably denser (fiber thickness as well as increased numbers of fibers) in the deeper portions of the lamina propria compared with the more superficial layers.
Figure 2.
Representative photomicrographs of true vocal fold (a), lung (b), and nasal conchae (c) stained with hemotxylin and eosin. Insets are higher magnification of the same tissue.
Nasal conchae were lined by a ciliated, pseudostratified columnar epithelium with an underlying lamina propria that contained numerous aggregates of lymphocytes and plasma cells. There were no mitotic figures in the epithelium to suggest hyperplasia. The amount of immune cells scattered throughout the nasal conchae's lamina propria was similar in all animals and within normal limits for pigs. The submucosal glands were unremarkable. No histological differences were noted in the lungs. We examined tissues believed to be specifically affected by an inhaled agent to determine if there were any adverse effects of the methodology employed. The mild-to-moderate scattering of lymphocytes in the lamina propria of pig nasal mucosa and larynx is reportedly normal as the nares of the snout and laryngopharynx are the first lines of defense against inhaled pathogens (Pabst, 1996).
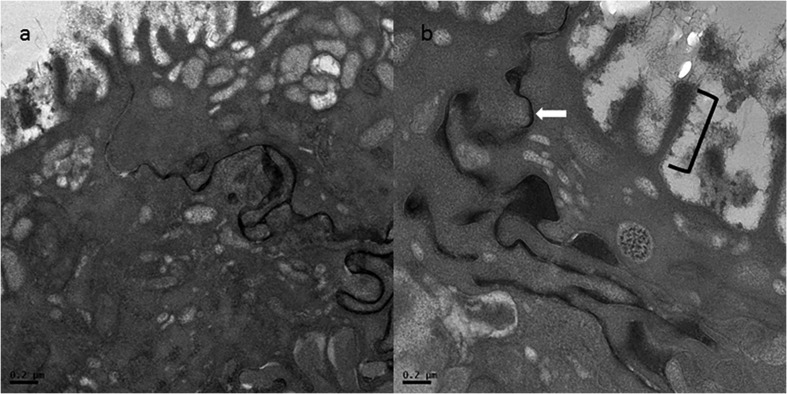
This study also evaluated the ultrastructure of true vocal fold epithelium. Previous reports suggest that the ultrastructure of the true vocal fold epithelium is altered with voice use and reflux disease (Franchi et al., 2007; Johnston et al., 2003; Novaleski et al., 2016). Qualitative evaluation of the overall ultrastructure of the true vocal fold epithelium was unremarkable (Figure 3).
Figure 3.
Transmission electron photomicrograph of vocal fold epithelium at (a) 2550× and (b) 7000× magnification. Microridges (black brackets) and intercellular space (white arrows) are highlighted. The intercellular space is outlined by lanthanum nitrate, an electron-dense extracellular component of the fixative.
Isotonic Saline as Testing Agent
Isotonic saline was chosen as the exogenous agent to be aerosolized in this study. Isotonic saline is used as a therapeutic for individuals with voice problems secondary to inhaling desiccated air or Sjogren's syndrome (Tanner et al., 2010; Tanner, Roy, & Merrill, 2013). Isotonic saline preserves vocal fold hydration homeostasis by maintaining the transepithelial ionic balance (Sivasankar & Fisher, 2007) and is routinely used in ex vivo phonation studies to keep the vocal fold hydrated at baseline (Meyer, Kvit, Devine, & Jiang, 2015; Meyer, McAvoy, & Jiang, 2013). Isotonic saline is also the gold standard for testing and validating new techniques that can be applied to understand laryngeal pathophysiology (Jungheim et al., 2016). Isotonic saline does not adversely affect tissue biomechanics and has also been used in studies of laryngeal mucus (Döllinger, Gröhn, Berry, Eysholdt, & Luegmair, 2014). We chose isotonic saline for its safety and nontoxic profile to laryngeal tissue to test the experimental methodology described here.
Limitations of the Current Study
There are some limitations that need to be considered pertaining to this animal model and experimental methodology. Pigs have convoluted nasal turbinates, and it is possible that an inhaled agent may liquefy in the warm airway and be potentially swallowed before contacting the larynx. This complication would be true of most laboratory species that have relatively long noses including mice, rats, dogs, and sheep. The porcine model was intentionally selected for this investigation because of similarities between pig and human vocal folds. Another potential limitation that is common to all animals is their limited phonatory capability. However, one research group concluded that from a structural perspective, the pig is a superior, large animal model compared with the more commonly used dog (Jiang et al., 2001). The pig and human larynx had similar cricothyroid muscle size, similar rotational mobility at the cricothyroid joint, similar cartilaginous framework, and similar thickness and stiffness of the vocal fold. In addition, the fundamental frequency, a correlate of voice pitch, and range of phonation in pigs are closest to those of humans. The low cost of domestic pigs and access to fresh excised tissue from the slaughterhouse also make this animal model desirable from an experimental standpoint.
Conclusions
In conclusion, we have described a novel experimental methodology that capitalizes on the pig's comparative similarities to the human larynx and trainable nature. We successfully repeatedly administered an exogenous agent via aerosol while the animal remained awake. The animal displayed no behavioral signs of stress. The success of this methodology could be applied to study the effects of controlled dosages of pollutants, allergens, infectious agents, or pathogens.
Acknowledgments
This work was supported by the National Institutes of Health (Grant RO1DC011759 awarded to M. Preeti Sivasankar). This study was performed in accordance with the PHS Policy on Humane Care and Use of Laboratory Animals, the NIH Guide for the Care and Use of Laboratory Animals, and the Animal Welfare Act (7 U.S.C. et seq.); the animal use protocol was approved by the Institutional Animal Care and Use Committee of Purdue University. The authors acknowledge Jessica Roller with her daily work with the animals.
Funding Statement
This work was supported by the National Institutes of Health (Grant RO1DC011759 awarded to M. Preeti Sivasankar).
References
- Barker E., Haverson K., Stokes C., Birchall M., & Bailey M. (2005). The larynx as an immunological organ: Immunological architecture in the pig as a large animal model. Clinical and Experimental Immunology, 143, 6–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beilin B., Shavit Y., Hart J., Mordashov B., Cohn S., Notti I., & Bessler H. (1996). Effects of anesthesia based on large versus small doses of fentanyl on natural killer cell cytotoxicity in the perioperative period. Anesthesia and Analgesia, 82(3), 492–497. [DOI] [PubMed] [Google Scholar]
- Colton R., & Casper J. (2005). Understanding voice problems: A physiological perspective for diagnosis and treatment (3rd ed.). Baltimore, MD: Lippincott Williams & Wilkins. [Google Scholar]
- Döllinger M., Gröhn F., Berry D., Eysholdt U., & Luegmair G. (2014). Preliminary results on the influence of engineered artificial mucus layer on phonation. Journal of Speech, Language, and Hearing Research, 57(2), S637–S647. [DOI] [PubMed] [Google Scholar]
- Durkes A., & Sivasankar M. (2016). In vivo investigation of acidified pepsin exposure to porcine vocal fold epithelia. The Laryngoscope, 126(1), E12–E17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eng P., Morton J., Douglass J., Riedler J., Wilson J., & Robertson C. (1996). Short-term efficacy of ultrasonically nebulized hypertonic saline in cystic fibrosis. Pediatric Pulmonology, 21(2), 77–83. [DOI] [PubMed] [Google Scholar]
- Franchi A., Brogelli B., Massi D., Santucci M., De Campora E., & Gallo O. (2007). Dilation of intercellular spaces is associated with laryngopharyngeal reflux: An ultrastructural morphometic analysis of laryngeal epithelium. European Archives of Oto-Rhino-Laryngology, 264, 907–911. [DOI] [PubMed] [Google Scholar]
- Goldman J., Teale C., & Muers M. (1992). Simplifying the assessment of patients with chronic airflow limitation for home nebulizer therapy. Respiratory Medicine, 86(1), 33–38. [DOI] [PubMed] [Google Scholar]
- Gorti G., Birchall M., Karin H., Macchiarini P., & Bailey M. (1999). A preclinical model for laryngeal transplantation: Anatomy and mucosal immunology of the porcine larynx. Transplantation, 68(11), 1638–1642. [DOI] [PubMed] [Google Scholar]
- Gould F., Iglesias B., Ohlemacher J., & German R. (2017). Pre-pharyngeal swallow effects of recurrent laryngeal nerve lesion on bolus shape and airway protection in an infant pig model. Dysphagia, 32(3), 362–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicks T., McGlone J., Whisnant C., Kattesh H., & Norman R. (1998). Behavioral, endocrine, immune, and performance measures for pigs exposed to acute stress. Journal of Animal Science, 76(2), 474–483. [DOI] [PubMed] [Google Scholar]
- Jenkins S., Heaton R., Fulton T., & Moxham J. (1987). Comparison of domiciliary nebulized salbutamol and salbutamol from a metered-dose inhaler in stable chronic airflow limitation. Chest, 91(6), 804–807. [DOI] [PubMed] [Google Scholar]
- Jetté M., Dill-McFarland K., Hanshew A., Suen G., & Thibeault S. (2016). The human laryngeal microbiome: Effects of cigarette smoke and reflux. Scientific Reports, 6, 35882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang J., Raviv J., & Hanson D. (2001). Comparison of the phonation-related structures among pig, dog, white-tailed deer, and human larynges. Annals of Otology, Rhinology & Laryngology, 110(12), 1120–1125. [DOI] [PubMed] [Google Scholar]
- Johnston N., Bulmer D., Gill G., Panetti M., Ross P., Pearson J., … Koufman J. (2003). Cell biology of laryngeal epithelial defenses in health and disease: Further studies. Annals of Otology, Rhinology & Laryngology, 112(6), 481–491. [DOI] [PubMed] [Google Scholar]
- Jungheim M., Donner S., Bleeker S., Ripken T., Krueger A., & Ptok M. (2016). Effect of saline inhalation on vocal fold epithelial morphology evaluated by optical coherence tomography. The Laryngoscope, 126, E332–E336. [DOI] [PubMed] [Google Scholar]
- Knight M., McDonald S., & Birchall M. (2005). Intrinsic muscles and distribution of the recurrent laryngeal nerve in the pig larynx. European Archives of Oto-Rhino-Laryngology, 262(5), 281–285. [DOI] [PubMed] [Google Scholar]
- Koufman J. (1991). The otolaryngologic manifestations of gastroesophageal reflux disease (GERD): A clinical investigation of 225 patients using ambulatory 24-hour pH monitoring and an experimental investigation of the role of acid and pepsin in the development of laryngeal injury. The Laryngoscope, 101(4, Pt. 2, Suppl. 53), 1–78. [DOI] [PubMed] [Google Scholar]
- Leydon C., Imaizumi M., Yang D., Thibeault S., & Fried M. (2014). Structural and functional vocal fold epithelial integrity following injury. The Laryngoscope, 124(12), 2764–2769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer J., Kvit A., Devine E., & Jiang J. (2015). Permeability of canine vocal fold lamina propria. The Laryngoscope, 125(4), 941–945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer J., McAvoy K., & Jiang J. (2013). Rehydration capacities and rates for various porcine tissues after dehydration. PLoS One, 8, e72573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muir W. III, & Hubbell J. (2014). Handbook of veterinary anesthesia. St. Louis, MO: Elsevier Health Sciences. [Google Scholar]
- Nakao Y., Itoh Y., Kuang T., Cook M., Jehle J., & Sokoloff L. (2001). Effects of anesthesia on functional activation of cerebral blood flow and metabolism. Proceedings of the National Academy of Sciences, 98(13), 7593–7598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novaleski C., Kimball E., Mizuta M., & Rousseau B. (2016). Acute exposure to vibration is an apoptosis-inducing stimulus in the vocal fold epithelium. Tissue and Cell, 48(5), 407–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pabst R. (1996). The respiratory immune system of pigs. Veterinary Immunology and Immunopathology, 54(1–4), 191–195. [DOI] [PubMed] [Google Scholar]
- Panepinto L., Phillips R., Norden S., Pryor P., & Cox R. (1983). A comfortable, minimum stress method of restraint for Yucatan miniature swine. Laboratory Animal Science, 33(1), 95–97. [PubMed] [Google Scholar]
- Rogers C., Stoltz D., Meyerholz D., Ostedgaard L., Rokhlina T., Taft P., … Itani O. (2008). Disruption of the CFTR gene produces a model of cystic fibrosis in newborn pigs. Science, 321(5897), 1837–1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy N., Merrill R., Gray S., & Smith E. (2005). Voice disorders in the general population: Prevalence, risk factors, and occupational impact. The Laryngoscope, 115(11), 1988–1995. [DOI] [PubMed] [Google Scholar]
- Roy N., Merrill R., Thibeault S., Parsa R., Gray S., & Smith E. (2004). Prevalence of voice disorders in teachers and the general population. Journal of Speech, Language, and Hearing Research, 47, 281–293. [DOI] [PubMed] [Google Scholar]
- Sivasankar M., & Fisher K. (2007). Vocal fold epithelial response to luminal osmotic perturbation. Journal of Speech, Language, and Hearing Research, 50(4), 886–898. [DOI] [PubMed] [Google Scholar]
- Sutton P., Gemmell H., Innes N., Davidson J., Smith F., Legge J., & Friend J. (1988). Use of nebulised saline and nebulised terbutaline as an adjunct to chest physiotherapy. Thorax, 43(1), 57–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki M., & Sasaki C. (1977). Laryngeal spasm: A neurophysiologic redefinition. Annals of Otology, Rhinology & Laryngology, 86(2), 150–157. [DOI] [PubMed] [Google Scholar]
- Swindle M., & Smith A. (2013). Best practices for performing experimental surgery in swine. Journal of Investigative Surgery, 26(2), 63–71. [DOI] [PubMed] [Google Scholar]
- Tanner K., Fujiki R., Dromey C., Merrill R., Robb W., Kendall K., … Sivasankar M. (2016). Laryngeal desiccation challenge and nebulized isotonic saline in healthy male singers and nonsingers: Effects on acoustic, aerodynamic, and self-perceived effort and dryness measures. Journal of Voice, 30(6), 670–676. [DOI] [PubMed] [Google Scholar]
- Tanner K., Roy N., & Merrill R. (2013). Comparing nebulized water versus saline after laryngeal desiccation challenge in Sjogren's Syndrome. The Laryngoscope, 123(11), 2787–2792. [DOI] [PubMed] [Google Scholar]
- Tanner K., Roy N., Merrill R., & Elstad M. (2007). The effects of three nebulized osmotic agents in the dry larynx. Journal of Speech, Language, and Hearing Research, 50(3), 635–646. [DOI] [PubMed] [Google Scholar]
- Tanner K., Roy N., Merrill R., Muntz R., Houtz D., Sauder C., … Wright-Costa J. (2010). Nebulized isotonic saline versus water following a laryngeal desiccation challenge in classically trained sopranos. Journal of Speech, Language, and Hearing Research, 53, 1555–1566. [DOI] [PubMed] [Google Scholar]
- Unoki T., Grap M., Sessler C., Best A., Wetzel P., Hamilton A., & Munro C. (2009). Autonomic nervous system function and depth of sedation in adults receiving mechanical ventilation. American Journal of Critical Care, 18(1), 42–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright R., Miller S., & Corsello B. (1990). Acid-induced esophagobronchial-cardiac reflexes in humans. Gastroenterology, 99(1), 71–73. [DOI] [PubMed] [Google Scholar]