Abstract
Purpose
Both theoretical models of infant language acquisition and empirical studies posit important roles for attention to speech in early language development. However, deaf infants with cochlear implants (CIs) show reduced attention to speech as compared with their peers with normal hearing (NH; Horn, Davis, Pisoni, & Miyamoto, 2005; Houston, Pisoni, Kirk, Ying, & Miyamoto, 2003), which may affect their acquisition of spoken language. The main purpose of this study was to determine (a) whether infant-directed speech (IDS) enhances attention to speech in infants with CIs, as compared with adult-directed speech (ADS), and (b) whether the degree to which infants with CIs pay attention to IDS is associated with later language outcomes.
Method
We tested 46 infants—12 prelingually deaf infants who received CIs before 24 months of age and had 12 months of hearing experience (CI group), 22 hearing experience–matched infants with NH (NH-HEM group), and 12 chronological age–matched infants with NH (NH-CAM group)—on their listening preference in 3 randomized blocks: IDS versus silence, ADS versus silence, and IDS versus ADS. We administered the Preschool Language Scale–Fourth Edition (PLS-4; Zimmerman, Steiner, & Pond, 2002) approximately 18 months after implantation to assess receptive and expressive language skills of infants with CIs.
Results
In the IDS versus silence block, all 3 groups looked significantly longer to IDS than to silence. In the ADS versus silence block, both the NH-HEM and NH-CAM groups looked significantly longer to ADS relative to silence; however, the CI group did not show any preference. In the IDS versus ADS block, whereas both the CI and NH-HEM groups preferred IDS over ADS, the NH-CAM group looked equally long to IDS and ADS. IDS preference quotient among infants with CIs in the IDS versus ADS block was associated with PLS-4 Auditory Comprehension and PLS-4 Expressive Communication measures.
Conclusions
Two major findings emerge: (a) IDS enhances attention to speech in deaf infants with CIs; (b) the degree of IDS preference over ADS relates to language development in infants with CIs. These results support a focus on input in developing intervention strategies to mitigate the effects of hearing loss on language development in infants with hearing loss.
Cochlear implantation provides children with severe-to-profound hearing loss access to speech and unprecedented opportunities for the acquisition of spoken language (Geers, Tobey, Moog, & Brenner, 2008; Houston & Bergeson, 2014; Houston et al., 2012). However, deaf children who received cochlear implants (CIs), even those implanted at very early ages, often lag behind their peers with normal hearing (NH) in a range of speech and language standardized tests (Conway et al., 2011; Geers, Strube, Tobey, Pisoni, & Moog, 2011; Holt, Beer, Kronenberger, Pisoni, & Lalonde, 2012; Houston & Bergeson, 2014). In addition, there is a considerable amount of variability in spoken language outcomes after cochlear implantation (Niparko et al., 2010). This may not be surprising because the acoustic signal transmitted to the auditory nerve by CIs is greatly impoverished and underspecified relative to the speech signal received by normally functioning cochlea (Zeng, 2004).
However, the degraded quality of speech may not be the only factor that leads to poor language outcomes in CI users. Recently, a growing body of evidence suggests that early auditory deprivation followed by degraded auditory input affects the development of the auditory neural pathway as well as other higher level cortical areas, which may have cascading effects for infants with CIs, who are processing already impoverished auditory input (Geers et al., 2011; Holt et al., 2012; Pisoni & Geers, 2000). Indeed, recent studies suggest that deaf infants with CIs show differences in a range of cognitive skills as compared with their peers with NH, such as visual memory, procedural learning, and executive function (Conway et al., 2011; Conway, Karpicke, & Pisoni, 2007; Houston & Bergeson, 2014; Pisoni, 2000).
Attention to Speech
One fundamental cognitive skill that is critical to the acquisition of spoken language is the ability to pay attention to speech (Glenn, Cunningham, & Joyce, 1981; Houston & Bergeson, 2014; Houston, Pisoni, Kirk, Ying, & Miyamoto, 2003; Vouloumanos & Werker, 2004, 2007). This ability may be especially important for infants with CIs to acquire spoken language because it has been shown that the processing of degraded speech depends critically on attention (Wild et al., 2012). However, previous studies suggest that infants with CIs show reduced attention to speech as compared with children with NH (Horn, Houston, & Miyamoto, 2007; Houston & Bergeson, 2014; Houston et al., 2003). For example, using the visual habituation paradigm, Houston et al. (2003) tested infants' attention to repetitions of a sound, such as hop hop hop or ahhh versus silence. They found that deaf infants with 6 months of CI experience showed reduced looking time to sound versus silence than did 6-month-old infants with NH. Using a similar paradigm, Horn et al. (2007) found that deaf infants both pre- and post-CI showed significantly reduced overall looking times to the speech stimuli than their chronological age–matched peers with NH in a speech discrimination test.
Reduced attention to speech in infants with CIs makes access to linguistically relevant units difficult; however, to become a successful language learner, the infant must be able to distinguish and attend to meaningful signals, speech in particular, among a range of sounds in the environment. To date, research has shown that attention to speech is innate or at least developed very early from infants' experience with the auditory input. For example, typically developing infants with NH prefer speech over filtered speech (Spence & DeCasper, 1987), noise (Butterfield & Siperstein, 1970), synthetic sinusoidal waves (Vouloumanos & Werker, 2004, 2007), silence (Houston et al., 2003), and even other naturally occurring sounds (Shultz & Vouloumanos, 2010). Recent theoretical models of infant language acquisition posit important roles for attention to speech in early language development. For example, according to the Word Recognition and Phonetic Structure Acquisition model, infants innately attend more to some aspects of the speech signal than others. What they attend to is important for encoding acoustic details into memory (Jusczyk, 1993). Although there is no direct evidence supporting the relationship between attention and speech processing in infants, data from adults provide some insights into our understanding of this relationship. For example, under conditions when attentional resources are depleted, adults' ability to segment words from a stream of speech based on statistical regularities is seriously compromised (Toro, Sinnett, & Soto-Faraco, 2005). In addition, using functional magnetic resonance imaging, Wild et al. (2012) assessed the degree to which spoken sentences were processed under distraction. They found that frontal regions were only engaged when listeners were attending to speech and that these regions exhibited elevated responses to degraded speech. These findings suggest that attention enhances the processing of degraded speech by engaging higher order mechanisms that modulate perceptual auditory processing.
Nevertheless, empirical studies indeed have revealed a relationship between attention to speech and language outcomes in children. For example, attention to speech over sinusoidal waves in infancy predicts later expressive vocabulary in both typically developing children and children with autism spectrum disorder (Kuhl, Coffey‐Corina, Padden, & Dawson, 2005; Molfese, 2000; Vouloumanos & Curtin, 2014). Given these findings, it is likely that reduced attention to speech in infants with CIs may affect the encoding of already impoverished acoustic–phonetic information into memory. This, in turn, may lead to deleterious effects on other aspects of language acquisition, such as sound discrimination, speech segmentation, and word learning, which are strong predictors of language outcomes. Nevertheless, it should be noted that the auditory stimuli in both Houston et al. (2003) and Horn et al. (2007) were nonwords, such as ahhh, hop hop hop, or seepug; therefore, it could be that reduced attention to speech exhibited by infants with CIs is tied to the specific qualities of the stimuli. Indeed, when presented with natural speech and nonspeech sounds, infants with CIs, similar to their peers with NH, preferred listening to child-directed speech over both white noise and time-reversed speech (Segal & Kishon-Rabin, 2011). Therefore, the first aim of this study was to expand previous studies and examine whether attention to speech exhibited by infants with CIs is modulated by different aspects of speech: infant-directed speech (IDS) and adult-directed speech (ADS), the speech styles that infants frequently encounter in their everyday listening environment.
Preference for IDS
IDS differs from ADS in a range of acoustic–phonetic properties, such as expanded vowel space, slower speaking rate, higher pitch, wider pitch range, and longer pauses (Albin & Echols, 1996; Bergeson, Miller, & McCune, 2006; Burnham, Kitamura, & Vollmer-Conna, 2002; Cristia, 2010; Dilley, Millett, McAuley, & Bergeson, 2014; Fernald, 1989; Fernald & Simon, 1984; Grieser & Kuhl, 1988; Papoušek & Hwang, 1991; Werker, Pegg, & McLeod, 1994). Several studies have shown that IDS is very effective in engaging and sustaining attention in young infants with NH (Fernald, 1985, 1989; Fernald & Simon, 1984; Kitamura, Thanavishuth, Burnham, & Luksaneeyanawin, 2001; Kuhl et al., 1997; Werker et al., 1994). For example, using a conditioned head-turn procedure, Fernald (1985) found that 4-month-old infants turned their heads more often in the direction necessary to activate a recording of IDS than ADS. The preference for IDS over ADS is very robust as it is present even when speech samples are presented in a foreign language (Werker et al., 1994), or in synthesized speech that preserves prosodic information (Fernald & Kuhl, 1987). This may not be surprising because engaging attention is found to be mostly implemented by the exaggerated prosodic properties, fundamental frequency (F0) in particular, of IDS (Fernald & Mazzie, 1991; Kuhl & Meltzoff, 1984; Mehler, Bertoncini, Barriere, & Jassik-Gerschenfeld, 1978). For example, when presented with low-pass-filtered and then resynthesized speech with different types of modulation, 4-month-old infants preferred speech with the F0 pattern of IDS, but not with the amplitude or duration patterns of IDS (Fernald & Kuhl, 1987).
The consequence of IDS modification and infant's preference for IDS may be profound because pieces of both direct and indirect evidence suggest that IDS has important effects on language development in infants with NH (Cristia & Seidl, 2014; Drotar & Sturm, 1988; Liu, Kuhl, & Tsao, 2003; Song, Demuth, & Morgan, 2010; Trainor, Austin, & Desjardins, 2000). For example, infants who experienced a larger amount of IDS at home became more efficient in word processing and had a larger expressive vocabulary by 24 months of age (Weisleder & Fernald, 2013). In addition, Ma, Golinkoff, Houston, and Hirsh-Pasek (2011) showed that 21-month-olds learned novel words only from IDS, but not from ADS. From a theoretical perspective, it has been suggested that the underlying mechanism of IDS to promote language development is that it boosts attention in general (Schachner & Hannon, 2011).
While much information about the role of IDS in directing and sustaining attention is available to infants with NH, there is a paucity of research on the perceptual preference for IDS exhibited by infants with CIs. Although it is tempting to assume that IDS may also enhance attention in infants with CIs, this is true only if the CI devices would allow deaf infants access to the unique acoustic properties of IDS. In a recent study, Robertson, von Hapsburg, and Hay (2013) compared whether infants with hearing impairment prefer IDS over ADS, the same way as children with NH do. Specifically, using a central fixation procedure, they examined the listening preference for IDS over ADS on nine 19.1-month-old infants with hearing impairment who had approximately 7.7 months' hearing experience using either CIs or hearing aids (HAs), as well as two control groups with NH: a younger NH control group with similar hearing experience, as well as an older control group with similar chronological age. The motivation to include the two control groups was twofold. First, research has demonstrated that mothers adjust their speech style to the children with CIs according to the perceived language level, rather than the chronological age, of the child (Bergeson et al., 2006). Therefore, there is reason to believe that children with CIs may display preferences that are consistent with their hearing experience, mirroring patterns of maternal input. Second, there is a developmental change of IDS preference such that older infants with NH who are over 13 months of age, in general, do not prefer IDS over ADS (Cooper & Aslin, 1990; Fernald, 1985). The results showed that infants with hearing impairment, similar to the younger control group, preferred listening to IDS over ADS. However, the older control group did not show any preference.
These findings are promising because they suggest that infants with hearing impairment may have sufficient access to the acoustic signal to differentiate types of speech in the ambient environment and develop a hearing age–equivalent listening preference for IDS over ADS. However, note that, in this study, the participants were not homogenous because five out of the nine infants with hearing impairment were fitted with HAs, making it difficult to determine whether the IDS preference is present in both CI and HA populations. There are reasons to suspect that the type of assistive device worn (i.e., CIs or HAs) may affect access to IDS significantly for the following reasons. First, infants with HAs, in general, have a larger amount of residual hearing before implantation and are fitted with the devices earlier than infants with CIs. This leads to more auditory experience with the ambient environment and more opportunities for them to encode and process speech sounds before they are fitted with devices. It is likely that experience with speech and the allocation of cognitive resources, specifically attention to speech, are linked. Second, due to the differences in how CI and HA devices process the auditory signal, the spectral information provided by CIs is less rich compared with the signal provided by the hair cells in the cochlea in infants with NH and HAs (Loizou, 2006). Therefore, the frequency resolution for infants with CIs is poorer, which may cause the cues of pitch properties to be less salient. These factors may lead to reduced IDS sensitivity in infants with CIs if they rely on pitch cues to distinguish between IDS and ADS. However, it is also possible that deaf children with CIs may develop other speech-processing strategies. For example, they may rely on temporal or contextual cues that can be transmitted via CI devices (Dorman, Dankowski, McCandless, Parkin, & Smith, 1991; Zeng, Rebscher, Harrison, Sun, & Feng, 2008) to cope with the impoverished auditory signal and thus show similar IDS preference.
Current Study
Taken together, research in the past has established that (a) infants with CIs show reduced attention to speech, which may lead to delayed spoken language development, and (b) IDS enhances attention to speech and facilitates language development in young infants with NH, as compared with ADS, across a variety of methods and cultures. Against this background, the first aim of this study was to examine whether attention to speech exhibited by infants with CIs is modulated by speech style, IDS and ADS in particular. Specifically, we would like to examine whether IDS enhances attention to speech in infants with CIs, relative to ADS. To answer this question, we tested infants with CIs and infants with NH on a preference test. We predict that if the CI devices would allow deaf infants access to the special acoustic properties of IDS, then they would show enhanced attention to IDS rather than ADS. The second aim of this study was to examine to which extent IDS preference in infants with CIs may be related to later language outcomes. This question is of profound clinical significance because it would allow us to identify those children with CIs who may be at additional risk of language delay. Therefore, we collected a measure of language outcome for the CI group. We reasoned that if IDS enhances attention to speech in deaf infants with CIs, then it is possible to observe an empirical association between IDS preference and a measure of language development.
Method
Participants
Three groups of infants participated in this study: a CI group, a hearing experience–matched control group with NH (NH-HEM), and a chronological age–matched control group with NH (NH-CAM). The CI group consisted of 12 prelingually deaf infants with approximately 12 months' hearing experience (9 boys, 3 girls; mean chronological age = 27.24 months, range = 21.97 33.17 months; mean hearing age = 11.88 months, range = 10.26–13.38 months; the CI group). 1 The NH-HEM group consisted of 22 infants with NH (10 boys, 12 girls; mean age = 11.68 months, range = 10.10 13.39 months); these infants had the same hearing age as the infants in the CI group and were matched by group. The NH-CAM group consisted of 12 infants with NH (7 boys, 5 girls; mean age = 27.55 months, range = 20.91–36.68 months), who were the same chronological age as the infants in the CI group. The infants in the NH-CAM group were matched to the CI group by pair, such that each infant in the NH group was matched to an infant with CI based on the chronological age.
The deaf infants received a CI prior to 24 months of age (mean implantation age = 15.35 months, range = 9.80–21.88 months). They were recruited from a CI program in a university's medical center in a Midwestern town. Additional demographic and audiological information for infants with CIs is shown in Table 1. The infants with NH were all healthy full-term with no known history of developmental delay or hearing impairment. Informed consent was given to the caregivers prior to testing.
Table 1.
Demographic information for infants with cochlear implants (CIs).
| Subject ID | Gender | Age at CI | Age at test | Hearing age | Mean PTA unaided (dB) | Com mode | Degree of HL |
|---|---|---|---|---|---|---|---|
| 3307 | M | 9.87 | 21.97 | 12.07 | 92.50 | OC | Sev/Prof |
| CI35 | F | 16.74 | 28.96 | 12.23 | 114.00 | OC | Sev/Prof |
| CI53 | M | 11.94 | 23.93 | 11.97 | 120.00 | OC | Sev/Prof |
| 3407 | F | 21.88 | 33.09 | 11.18 | 107.50 | TC | Sev/Prof |
| CI39 | M | 17.89 | 29.68 | 11.77 | 95.00 | OC | Sev/Prof |
| 3098 | M | 10.26 | 23.68 | 13.38 | 119.00 | OC | Sev/Prof |
| 4083 | M | 17.30 | 28.75 | 11.41 | 120.00 | OC | Sev/Prof |
| CI36 | M | 21.51 | 33.17 | 12.20 | 98.60 | TC | Sev/Prof |
| 3259 | F | 15.99 | 29.11 | 13.08 | 120.00 | TC | Sev/Prof |
| 4577 | M | 16.88 | 28.42 | 11.51 | 88.50 | OC | Sev/Prof |
| CI34 | M | 10.36 | 22.26 | 11.50 | 107.00 | OC | Sev/Prof |
| 3374 | M | 13.62 | 23.91 | 10.26 | 109.40 | Unknown | Sev/Prof |
| M (SD) | 15.61 (3.99) | 27.24 (3.96) | 11.88 (0.83) | 107.63 (11.55) |
Note. All ages are reported in months. PTA = pure-tone average before implantation (across the frequencies of 250, 500, 1000, 2000, and 4000 Hz); Com Mode = the type of communication program that the infant was following in speech-language therapy; HL = hearing loss; OC = oral communication (exclusively spoken); TC = total communication (a combination of spoken language and Signed Exact English); Sev/Prof = severe-to-profound.
Stimuli
The study used both auditory and visual stimuli. The auditory stimuli were natural speech samples of four sentences: Good morning! How are you today? What are you doing? Let's go for a walk. Four female adult native speakers of American English produced the speech stimuli in a sound-attenuated room. None of these speakers was the mother of an infant who participated in this study. They were instructed to produce the stimuli as if they were talking to an infant (IDS) and as if they were talking to an adult (ADS), for a total of eight passages (four IDS and four ADS passages, which were used in the IDS and ADS trials, respectively). Each speaker produced the passage several times, and one IDS and one ADS passage from each speaker were selected as experimental stimuli based on their acoustic clarity and appropriateness as speech directed to infants or to adults. They were then digitized at a sampling rate of 44.1 kHz. The average F0, F0 range, and sentence durations for the IDS and ADS speech stimuli for the four speakers are shown in Table 2.
Table 2.
Acoustic characteristics: Mean fundamental frequency (F0) in Hz, F0 range (Hz), and sentence durations (s) of infant-directed speech (IDS) and adult-directed speech (ADS) stimuli for each speaker.
| Speaker | Measures | Type | Sentences |
|||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |||
| 1 | F0 | IDS | 288 | 322 | 334 | 346 |
| ADS | 176 | 197 | 192 | 193 | ||
| F0 range | IDS | 172–521 | 168–498 | 222–523 | 203–506 | |
| ADS | 161–225 | 171–231 | 155–218 | 171–248 | ||
| Duration | IDS | 1.07 | 1.52 | 1.34 | 1.67 | |
| ADS | 0.77 | 1.03 | 0.82 | 0.94 | ||
| 2 | F0 | IDS | 284 | 229 | 291 | 307 |
| ADS | 176 | 203 | 208 | 182 | ||
| F0 range | IDS | 143–523 | 131–508 | 158–439 | 214–501 | |
| ADS | 155–242 | 75–274 | 141–262 | 115–291 | ||
| Duration | IDS | 0.84 | 1.40 | 1.25 | 1.63 | |
| ADS | 0.57 | 0.86 | 0.68 | 1.00 | ||
| 3 | F0 | IDS | 311 | 229 | 199 | 261 |
| ADS | 210 | 228 | 209 | 188 | ||
| F0 range | IDS | 139–442 | 151–512 | 147–511 | 174–496 | |
| ADS | 191–246 | 143–320 | 140–312 | 122–268 | ||
| Duration | IDS | 0.97 | 1.15 | 1.41 | 1.57 | |
| ADS | 0.61 | 0.77 | 0.70 | 0.95 | ||
| 4 | F0 | IDS | 274 | 221 | 246 | 275 |
| ADS | 224 | 211 | 225 | 222 | ||
| F0 range | IDS | 163–525 | 142–422 | 165–313 | 184–426 | |
| ADS | 191–311 | 76–329 | 83–268 | 186–286 | ||
| Duration | IDS | 1.50 | 2.31 | 2.05 | 1.87 | |
| ADS | 0.82 | 0.95 | 0.78 | 1.08 | ||
The visual stimuli consisted of an attention getter (a small dynamic video display of a blue-and-white expanding and shrinking wheel) and a visual display (a white-and-red static checkerboard pattern).
Experimental Design
There were three test blocks: IDS versus silence, in which infants received four IDS and four silent trials; ADS vs. silence, in which infants received four ADS and four silent trials; and IDS versus ADS, in which they received four IDS and four ADS trials. During each of the four IDS/ADS trials, one of the four speakers presented the IDS/ADS passage who was not the speaker in the rest of the three IDS/ADS trials. During the silent trials, no sound was played. The presentation order of the eight trials within each block was randomized across the participants, as well as the order of the three blocks (six orders in total).
Apparatus and Procedure
Infants were tested using the central fixation procedure (e.g., Best & McRoberts 2003; Houston et al., 2003). Each infant was seated on the caregiver's lap in front of a TV monitor in the middle of a quiet and comfortable double-walled sound booth by Industrial Acoustics Corp. The caregivers wore headphones, which played continuous music and speech babbles, and were therefore blind to the speech stimuli. Speech stimuli were presented to the infants via loudspeakers on the TV monitor at a comfortable level of 65 ± 5 dB SPL. A camera was located below the monitor to record the infant's behavior and feed the live video stream on a display in the control room. The experimenter was seated in the control room and watched the live video stream; she or he observed the infant's gaze direction and coded online whether the infants were looking at the monitor or looking away for each trial on the keyboard of a Macintosh computer. All the orientation data were stored in a computer data file.
All trials began by presenting the attention getter at the center of the monitor to draw infants' attention. Once the infant looked at the attention getter, the test trials were initiated. During the test trials, the infant was presented with the static checkerboard at the center of the monitor and the speech stimuli. Each trial continued (i.e., the passage would repeat) until the infant looked away for 1 s or more. If the infant turned away from the monitor for less than 1 s, that time was not included in the looking time, although the monitor continued to display the checkerboard and the loudspeaker to play auditory stimuli. The trial duration was determined by the duration of the infant's look to the checkerboard. The dependent measures were the average looking times across trials to each type of auditory stimuli within each block.
Language Skills
Approximately 20 months after implantation, the Preschool Language Scale–Fourth Edition (PLS-4; Zimmerman, Steiner, & Pond, 2002) was administered on infants with CIs by certified speech-language pathologists who had extensive experience testing children with hearing loss. The PLS-4 is a standardized individually administered test of receptive and expressive language skills that is suitable for use from birth to 6 years 11 months, and it is commonly used with children with hearing loss (Fitzpatrick, Durieux-Smith, Eriks-Brophy, Olds, & Gaines, 2007; Geers, Moog, Biedenstein, Brenner, & Hayes, 2009). Auditory Comprehension (PLS-AC) and Expressive Communication (PLS-EC) standard scores were obtained for nine out of the 12 children with CIs at the mean age of 34.13 (SD = 5.42) months and the mean device experience of 19.89 (SD = 3.07) months.
Results
Listening Preference
The means, standard deviations, and medians of the looking times to each stimulus type in all the three groups, namely, the CI, NH-HEM, and NH-CAM groups, are reported in Table 3, separated by Hearing Status and Block. We removed the data points whose difference values between the two types of the stimuli within each block were more than 2.5 SD. A repeated-measures analysis of variance (RM ANOVA) on looking time with Hearing Status (CI, NH-HEM, and NH-CAM), Gender (female, male), and Order (six test orders) as between-subject factors and Block (three blocks) and Type (IDS vs. silence, ADS vs. silence, IDS vs. ADS) as within-subjects factors revealed no main effects of or interactions with either Gender, F < 2.11, p > .168, or Order, F < 2.11, p > .168. Therefore, we removed these two factors from the model and reran the RM ANOVA. Results demonstrated a marginally significant three-way Block × Hearing Status × Type interaction, F(3.55, 58.57, Huynh–Feldt corrected values) = 2.64, p = .049, ηp 2 = .138. To explore the source of interaction, we reran three new RM ANOVAs on looking time with Hearing Status (CI, NH-HEM, and NH-CAM) as the between-subjects factor and Type as the within-subject factor for each block. In addition, we also conducted a nonparametric Wilcoxon signed-rank test to compare looking times to different types of stimuli in order to corroborate our parametric analyses.
Table 3.
Average, standard deviation, and median looking times (s) to different types of stimuli for the children with cochlear implants (CIs), their hearing experience–matched peers with normal hearing (NH-HEM), and their chronological age–matched peers with normal hearing (NH-CAM), separated by hearing status and block (the IDS versus silence, ADS versus silence, and IDS versus ADS blocks).
| Block | Type | Hearing status |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CI |
NH-HEM |
NH-CAM |
||||||||
| Average | SD | Mdn | Average | SD | Mdn | Average | SD | Mdn | ||
| IDS versus silence | IDS | 8.85 | 4.79 | 11.10 | 7.69 | 4.67 | 6.11 | 11.61 | 5.52 | 10.11 |
| Silence | 5.29 | 2.85 | 4.15 | 4.14 | 1.60 | 3.75 | 5.52 | 3.51 | 3.63 | |
| ADS versus silence | ADS | 6.05 | 3.61 | 4.23 | 6.12 | 2.42 | 6.40 | 8.46 | 4.57 | 7.54 |
| Silence | 5.99 | 3.74 | 4.18 | 3.91 | 1.43 | 3.99 | 4.60 | 2.81 | 4.24 | |
| IDS versus ADS | IDS | 6.83 | 4.09 | 4.90 | 7.27 | 4.76 | 6.10 | 7.15 | 3.98 | 6.00 |
| ADS | 3.78 | 2.77 | 3.38 | 5.28 | 3.98 | 4.20 | 7.26 | 5.37 | 7.20 | |
Note. IDS = infant-directed speech; ADS = adult-directed speech.
IDS Versus Silence
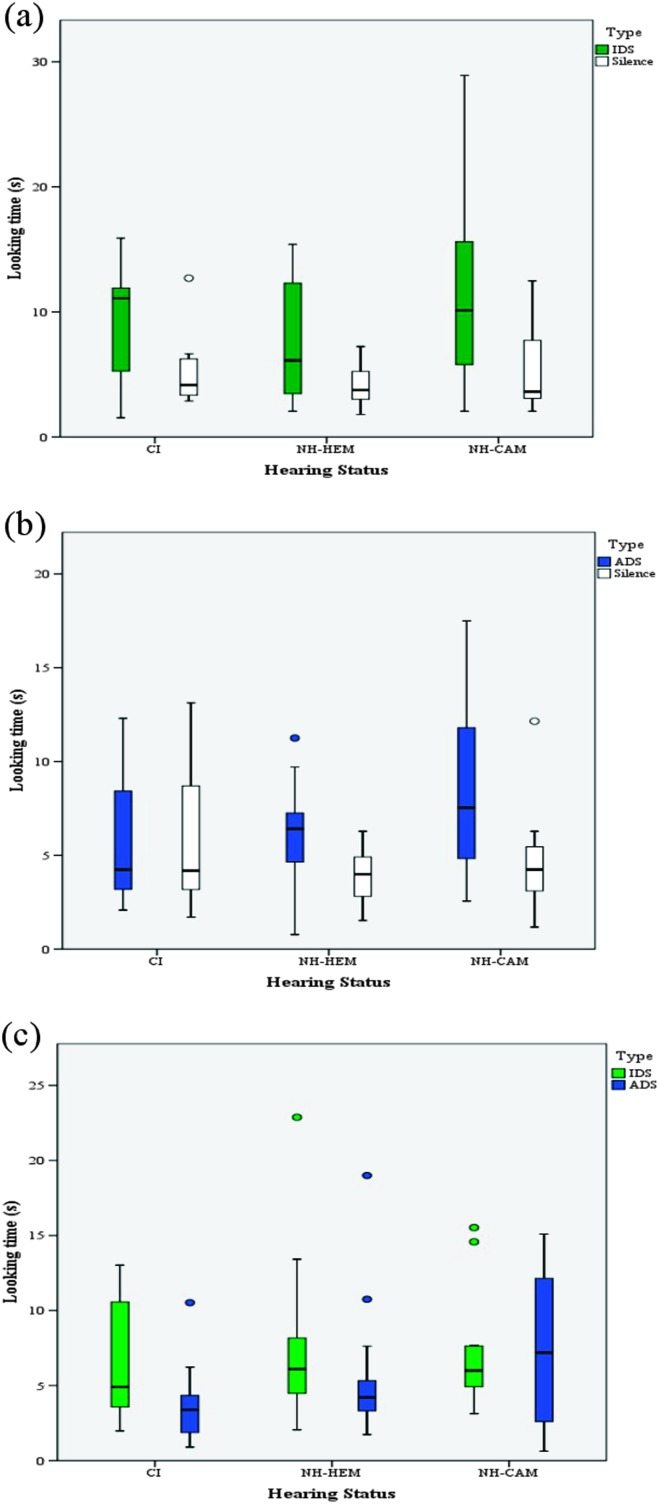
For the IDS versus silence block, the Hearing Status × Type interaction was not significant, F(2, 40) = 1.01, p = .373, nor was the main effect of Hearing Status, F (1, 40) = 2.02, p = .146. However, the main effect of Type was significant, F(1, 40) = 28.14, p < .001, ηp 2 = .413, because the three groups, in general, looked significantly longer to IDS (M = 9.08, SD = 5.89, Mdn = 8.40) than to silence (M = 4.82, SD = 2.60, Mdn = 3.83). A Wilcoxon signed-rank test also showed that looking time was significantly longer for IDS compared with silence, Z = 4.25, p < .001. Infants' looking times for the IDS and silence trials are presented in Figure 1(a). These results suggest that the three groups, in general, prefer IDS over silence.
Figure 1.
Box plots for the looking times (s) in (a) the infant-directed speech (IDS) versus silence block, (b) the adult-directed speech (ADS) versus silence block, and (c) the IDS versus ADS block, for three groups of children: children with cochlear implant (CIs), their hearing experience–matched peers with normal hearing (NH-HEM), and chronological age–matched peers with normal hearing (NH-CAM).
ADS Versus Silence
For the ADS versus silence block, there was a significant Hearing Status × Type interaction, F(1, 40) = 3.27, p = .048, ηp 2 = .140. In addition, the main effect of Type was significant, F(1, 40) = 13.23, p = .001, ηp 2 = .248, because children, in general, looked longer to ADS than to silence. However, the main effect of Hearing Status was not significant, F(1, 40) = 1.53, p = .228. To explore the source of Hearing Status × Type interaction, we conducted post hoc paired-samples t tests and the Wilcoxon signed-rank test for each group to compare their preference for ADS and silence. The comparisons were Bonferroni-adjusted at an alpha level of 0.017 per test (.05/3). The results showed that the CI group looked equally long to ADS and silence, t(10) = 0.07, p = .950, Z = 0.00, p = 1.00; however, both the NH-HEM and NH-CAM groups looked significantly longer to ADS than to silence, t(19) = 3.41, p = .001, Z = 3.06, p = .002, and t(11) = 2.75, p = .019, Z = 2.43, p = .015, respectively. Infants' looking times for the ADS and silence trials are presented in Figure 1(b). These results suggest that whereas the two control groups with NH prefer ADS over silence, the CI group does not show any preference.
IDS Versus ADS
For the IDS versus ADS block, there was a significant Hearing Status × Type interaction, F(2, 39) = 3.48, p = .041, ηp 2 = .151. In addition, the main effect of Type was significant, F(1, 39) = 12.18, p = .001, ηp 2 = .238, because children, in general, showed longer looking times to IDS (M = 7.12, SD = 4.28, Mdn = 5.99) than to ADS (M = 5.45, SD = 0.66, Mdn = 4.14). However, the main effect of Hearing Status was not significant, F(1, 39) = .644, p = .531. To explore the source of interaction, we conducted post hoc paired-samples t tests and Wilcoxon signed-rank tests to compare children's preference for IDS over ADS for each group, using the Bonferroni-adjusted alpha level of .017 per test (.05/3). The results showed that both CI and NH-HEM groups looked significantly longer for IDS, t(10) = 3.19, p = .010, Z = 2.40, p = .016, compared with ADS trials, t(18) = 3.44, p = .003, Z = 2.86, p = .004; however, the NH-CAM group looked equally long during IDS and ADS trials, t(11) = 1.12, p = .909, Z = −0.078, p = .937. Infants' looking times for the IDS and ADS trials are presented in Figure 1(c). These results suggest that the CI and NH-HEM groups prefer IDS over ADS; however, the NH-CAM group does not.
To determine which demographic factors contribute to explaining the listening preference of infants with CIs in this study, we computed correlational analyses between listening preference, age at implantation, and residual hearing (measured by pre-CI pure-tone average [PTA]). For this set of analyses, we used listening preference quotients, specifically IDS preference quotient in the IDS versus ADS block, calculated by (IDS − ADS)/(IDS + ADS), IDS preference quotient in the IDS versus silence block, calculated by (IDS − Silence)/(IDS + Silence), and ADS preference in the ADS versus silence block, calculated by (ADS − Silence)/(ADS + Silence), to assess listening preference. For example, the IDS preference quotient in the IDS versus ADS block was calculated by dividing the average looking time differences to IDS and ADS (IDS − ADS) by the total amount of looking time to both IDS and ADS (IDS + ADS) for each CI child. Positive numbers indicate a preference for the expected speech type.
The Pearson bivariate correlations between preference quotients and demographic variables that were continuous, namely, age at implantation and residual hearing, revealed a significantly negative correlation between preference quotient in the IDS versus ADS block and residual hearing, r(11) = −.744, p = .009; a marginally negative correlation between preference quotient in the IDS versus silence block and residual hearing, r(11) = −.603, p = .050. In addition, the correlation between preference quotients in the IDS versus silence block and the ADS versus silence block was significant, r(10) = .748, p = .013. However, the bivariate relationship between any of the other two measures was not significant, r < .306, p > .360. To examine the effect of communication mode—the communication program that the infant was following in speech-language therapy after implantation—on the listening preference for each block, three separate independent-samples t tests were conducted. No group difference was found, r < .29, p > .505. 2 Taken together, these results suggest that infants with a larger amount of residual hearing showed stronger IDS preference in both the IDS versus ADS and IDS versus silence blocks.
Attention to IDS and Language Outcomes in Deaf Infants With CIs
The question we next turn to is whether individual differences in speech preference are associated with language outcomes in the CI group. To explore the relationship between the preferences of children with CIs for different types of stimuli and their later language outcomes, we first ran bivariate correlations among listening preference quotients, PLS-AC, and PLS-EC. Correlation values are shown in Table 4. The IDS preference quotient in the IDS versus ADS block correlated significantly with the measures of PLS-AC, r(8) = .846, p = .008, and marginally significant with the measure of PLS-EC, r(8) = .702, p = .052. However, the correlations between the IDS preference quotient in the IDS versus silence block and the ADS preference quotient in the ADS versus silence block and the language outcome measures, though both in the positive direction, did not reach statistical significance.
Table 4.
Correlations among preference quotients (IDS preference quotient in the IDS vs. silence block, ADS preference quotient in the ADS vs. silence block, and IDS preference quotient in the IDS vs. ADS block) for children with cochlear implants in each block and PLS-AC and PLS-EC standard scores at 18 months after cochlear implantation.
| Measures | Preference quotient |
Language |
|||
|---|---|---|---|---|---|
| IDS vs. silence | ADS vs. silence | IDS vs. ADS | PLS-AC | PLS-EC | |
| Preference quotient | |||||
| IDS vs. silence | — | .748* | .745* | .648 † | .586 |
| ADS vs. silence | — | .315 | .346 | .371 | |
| IDS vs. ADS | — | .846** | .702 † | ||
| Language | |||||
| PLS-AC | — | .954** | |||
| PLS-EC | — | ||||
Note. IDS = infant-directed speech; ADS = adult-directed speech; PLS-AC = Preschool Language Scale–Auditory Comprehension; PLS-EC = Preschool Language Scale–Expressive Communication.
.5 < p < .10, two-tailed.
p < .05, two-tailed.
p < .01, two-tailed.
Discussion
The auditory environment of an infant includes a broad range of auditory signals, among which speech signal may be the most important source of information for infants to acquire spoken language. Typically developing infants are born with well-developed auditory systems capable of detecting the differences between these signals and showing a preference for speech sounds over nonspeech sounds. Although deaf infants with CIs are also able to distinguish between speech versus nonspeech sounds, they show reduced attention to speech, in general, as compared with their peers with NH (Horn et al., 2007; Houston & Bergeson, 2014; Houston et al., 2003). The ability to pay attention to speech may be especially important for their spoken language acquisition because infants with CIs have suboptimal perceptual access to the speech signal, whereas the processing of degraded speech depends critically on attention (Wild et al., 2012).
Therefore, the primary goal of this study was to examine whether IDS enhances attention to speech exhibited by infants with CIs as compared with ADS. Although infants frequently encounter both IDS and ADS in their everyday listening environment, young infants with NH pay more attention to IDS (Fernald & Mazzie, 1991; Kuhl & Meltzoff, 1984; Mehler et al., 1978). We found that deaf infants with CIs, similar to their hearing experience–matched peers with NH, showed increased attention to IDS relative to ADS or silence; however, they did not prefer ADS over silence; whereas both the younger and older control groups showed a preference for ADS over silence. These findings suggest not only that IDS and ADS are discriminable to infants with CIs but also that IDS enhances their attention to speech.
These results support and extend previous research in several ways. First, the findings that infants with CIs did not prefer ADS over silence is, in general, consistent with the findings that infants with CIs showed reduced attention to speech relative to their peers with NH (Horn et al., 2007; Houston & Bergeson, 2014; Houston et al., 2003). Second, our finding that infants with CIs showed preference for IDS over silence is consistent with and extends previous findings that infants with CIs preferred listening to child-directed speech over both white noise and time-reversed speech (Segal & Kishon-Rabin, 2011). Third, our finding that the chronological age–matched control group with NH showed a preference for IDS and ADS over silence, but did not show preference for IDS over ADS, is in line with the developmental change in infants' IDS preference, such that infants older than 13 months of age typically do not show IDS preference over ADS (Fernald, 1985; McRoberts, McDonough, & Lakusta, 2009). Fourth, infants with CIs displayed IDS preference similar to their hearing experience–matched peers, rather than their chronological age–matched peers. These may be largely related to the nature of the input because caregivers seemed to adjust their speech styles according to the hearing and developmental status of their infants with hearing impairment, rather than the chronological age (Bergeson et al., 2006; Wieland, Burnham, Kondaurova, Bergeson, & Dilley, 2015). For example, the increase in average and minimum pitch from ADS to IDS in mothers' speech to infants with CIs was more similar to that in speech to the control infants with matched hearing experience and distinct from control infants with matched chronological age (Bergeson et al., 2006).
Why does IDS enhance attention to speech exhibited by infants with CIs? One possibility is that infants with CIs, similar to infants with NH, have increased motivation to attend to IDS, either because IDS is innately reinforcing to them or because it contains more arousing properties than ADS. This possibility could only be true if the relevant acoustic properties of the speech are readily transmitted by the CI device, thus allowing infants with CIs to detect the difference between IDS and ADS. Previous studies seem to suggest that the CI device allows infants with CIs access to sufficient acoustic information to succeed in a range of speech perception tasks. For example, Hebrew 12- to 33-month-old deaf infants, implanted under 2.5 years of age, with 1–6 months of CI use, developed a bias for the more common weak/strong stress pattern in Hebrew (Segal, Houston, & Kishon-Rabin, 2016). In spite of the findings that the IDS preference in young infants with NH appears to be driven by changes in pitch (Fernald & Mazzie, 1991; Kuhl & Meltzoff, 1984; Mehler et al., 1978), the acoustic properties that drive the preference for IDS exhibited by infants with CIs may be different from their peers with NH. It is possible that deaf infants with CIs may develop different cue-weighting and speech-processing strategies due to early auditory deprivation and subsequent degraded speech input. For example, in a recent study, Peng et al. (2017) examined acoustic cue processing in CI recipients in a lexical tone recognition task. These CI recipients were prelingually deaf native Mandarin speakers who were between 6.6 and 21.4 years old. They found that CI recipients rely less on F0 contours, but more on durational patterns, than the NH control group, to recognize lexical tones. Given that our IDS stimuli were much slower than ADS stimuli in general (refer to Table 1), it is possible that the infants with CIs in our study relied on durational cues to distinguish between IDS and ADS. Therefore, one direction for future work would be to assess their weighting of each of the acoustic cues in a broad range of speech perception tasks, in order to illuminate our understanding of which cues are available to infants with CIs and what are the most crucial cues for them to process speech signals. Another possibility is that IDS is easier for infants with CIs to detect because it undergoes less degree of degradation or results in greater signal-to-noise ratio when transmitted to the CIs, as compared with ADS. Although no direct evidence is available to support this speculation, findings from infants' perception of IDS under noise conditions seem to support this argument (Barker & Newman, 2000; Colombo, Frick, Ryther, Coldren, & Mitchell, 1995; Fernald, 1984; Newman, 2003). For example, Colombo and colleagues found that infants performed better at detecting sweeping tones in noise when the tones resembled IDS intonational patterns. In addition, parents increased both pitch and pitch range when speaking to toddlers in a noise condition (Newman, 2003). If this were the case, then the IDS preference may be due to ease of perception. Although we are not able to tease apart these two explanations, the findings that IDS enhances attention to speech are encouraging because they suggest that infants with CIs possess basic auditory capacities for the discrimination of IDS and ADS and show enhanced attention to IDS relative to ADS.
One caveat of the current study is that, although looking time has long been used as the standard measure of infant attention, longer looking time may not necessarily imply higher level of attention. Therefore, another important future direction is to use more sophisticated behavioral, heart rate, or neurophysiological means to measure infant attention in speech perception studies.
In addition, we also found that the unaided PTA was associated with IDS preference; specifically, infants with a larger amount of residual hearing showed enhanced attention to IDS in both the IDS versus ADS and IDS versus silence blocks than infants with less amount of residual hearing before cochlear implantation. However, age at implantation was not correlated with listening preferences of infants with CIs. First of all, the finding that PTA was correlated with IDS preference is not surprising because it is possible that infants with a larger amount of residual hearing may have had more access to auditory information before receiving CIs, and this experience may be very helpful for them to develop IDS preference that is more similar to their peers with NH. Although these results should be interpreted with caution given the small sample size, other studies have similarly demonstrated that deaf infants with CIs, who had more residual hearing, showed enhanced attention to speech (Houston & Bergeson, 2014) as well as better vowel discrimination abilities (Phan, Houston, Ruffin, Ting, & Holt, 2016). Therefore, from a clinical perspective, it may be very helpful for deaf infants to receive HAs, prior to cochlear implantation, in order to amplify the limited amount of residual hearing as soon as they are diagnosed with hearing loss. Second, the finding that age at implantation was not correlated with IDS preference may seem to be somewhat surprising at first, because previous studies showed that infants implanted earlier consistently show better performance in speech perception tasks than infants who received CIs later (Fryauf-Bertschy, Tyler, Kelsay, & Gantz, 1997; Kirk, Miyamoto, Ying, Perdew, & Zuganelis, 2002; Miyamoto, Svirsky, & Robbins, 1997; Svirsky, Teoh, & Neuburger, 2004). For example, Fryauf-Bertschy et al. (1997) found that deaf children who received cochlear implantation prior to 5 years of age had significantly better open-set word recognition skills than those implanted after 5 years of age. However, it should be noted that the infants in our study received CIs much earlier; it seems that very early implantation may not play a very important role in the speech perception ability of deaf infants. This is consistent with other studies indicating that age at implantation does not have an effect on speech perception skills among children implanted before 2 years of age (Horn et al., 2007; Phan et al., 2016). However, this does not necessarily suggest that very early implantation may not have an effect on other processes related to language development. For example, Houston, Stewart, Moberly, Hollich, and Miyamoto (2012) found that toddlers who had their CIs activated between 7 and 14 months of age performed significantly better in a word-learning task than those who had their CIs activated between 16 and 22 months of age. Their findings suggest that early access to sound via a CI may facilitate the ability of deaf infants to learn novel words. Future studies are encouraged to explore the effects of very early implantation on the other processes related to language development and how these processes may be interrelated in explaining variabilities in language outcomes.
As the secondary goal, we examined the relationship between IDS preference exhibited by infants with CIs and later language outcomes. The findings showed that the extent to which infants with CIs pay attention to IDS in the IDS versus ADS block was correlated with later receptive and expressive language outcomes, suggesting that the attention to IDS in deaf infants may be helpful to multiple processes that are related to language development. Although a large body of literature demonstrates infants' preference for IDS, to our knowledge, this is the first study that explored how this preference might be related to later language development.
Why is IDS preference over ADS related to receptive and expressive language in the second year after cochlear implantation? There are at least two possibilities. The first possibility is that infants who showed enhanced attention to IDS rather than ADS have had more experience with IDS in their everyday listening environment, allowing them more access to the acoustic cues that are relevant to numerous dimensions of early language acquisition. Consequently, the bias toward IDS may lead to better encoding, storage, and retrieval of acoustic–phonetic and phonological information into memory. This process, in turn, accelerates the segmentation of words from continuous speech and eventually bootstraps language learning at higher levels. The second possibility is that the ability of infants with CIs to discriminate the two registers of speech varies with the function of their cognitive abilities; specifically, those infants with CIs with better encoding abilities may have been better able at distinguishing between IDS and ADS and orienting to IDS more. Indeed, listening preference for one stimulus over another is considered to reveal higher levels of processing, which requires not only discrimination (Kemler Nelson et al., 1995). There is an emergent line of research showing that higher level processing and encoding abilities gathered during infancy have a measurable effect on concurrent and subsequent language skills in children with NH and hearing impairment (Conway, Pisoni, Anaya, Karpicke, & Henning, 2011; Fernald, Perfors, & Marchman, 2006; Rose, Feldman, & Jankowski, 2009). However, these two possibilities are not mutually exclusive, and there might be a bidirectional relationship. The current study cannot tease apart these two alternative explanations. Future studies taking a multivariate approach to investigate the relationship between infants' speech perception abilities, IDS preference, and outcome measures would disentangle the factors that contribute to language development.
It is worth mentioning that, in the real world, infants hear speech in rich multimodal contexts and their experiences with language are above and beyond receiving acoustic signals alone. That is, IDS is not detached from other forms of infant-directed communication, but rather a part of a multimodal communication system that is replete with many interrelated social, emotional, tactile, and linguistic cues. There is an emerging line of research that shows that caregivers spontaneously perform multimodal behavior as much as 75%–99% of the time during the interaction with the infants (Gogate, Bahrick, & Watson, 2000; Nomikou & Rohlfing, 2011). The use of multimodal communication may not be incidental because a considerable body of research suggests that the ways in which caregivers interact with their infants play a key role in language development in both infants with NH (Masur, Flynn, & Eichorst, 2005; Rowe & Goldin-Meadow, 2009; Seidl, Tincoff, Baker, & Cristia, 2015) and deaf children (Niparko et al., 2010; Pressman, Pipp-Siegel, Yoshinaga-Itano, & Deas, 1999). Therefore, it could be that the infants who showed enhanced attention to IDS may also be involved in high-quality caregiver–infant interaction mediated by IDS at home. Future studies examining the relationship between multimodal parent–infant interaction and language development in deaf children will illuminate our understanding of the mechanisms that could explain why enhanced IDS preference is associated with receptive and expressive language in deaf infants with CIs.
Conclusions
In summary, the findings from this study suggest that a period of auditory deprivation followed by degraded auditory input may negatively affect the attention to speech in deaf infants with CIs; in spite of this, infants with CIs are able to discriminate IDS and ADS and show enhanced attention to IDS. In addition, this study revealed a direct link between IDS preference and language outcome. These findings have important clinical implications because they support a focus on linguistic input in developing intervention strategies to mitigate the effects of hearing loss on language development in infants with hearing impairment.
Acknowledgments
This research was supported in part by the National Institute on Deafness and Other Communication Disorders Grant (R01 DC008581) to Derek M. Houston and Laura C. Dilley.
Funding Statement
This research was supported in part by the National Institute on Deafness and Other Communication Disorders Grant (R01 DC008581) to Derek M. Houston and Laura C. Dilley.
Footnotes
Many of the infants were also tested at less than 3 months and 4–6 months after cochlear implantation. We decided to focus on the data from the 12-month post-CI testing because pilot testing of infants with NH revealed that 12-month-olds showed the expected preference for IDS, whereas younger infants with NH did not. This may seem surprising because previous studies showed that younger infants normally prefer IDS over ADS; however, the differences may depend on the characteristics of the particular IDS stimuli or the experimental paradigm (see Newman & Hussain, 2006, for more discussion).
Note that, given the small sample size (three out of the 12 infants with CIs followed total communication mode), the results should be interpreted with caution.
References
- Albin D. D., & Echols C. H. (1996). Stressed and word-final syllables in infant-directed speech. Infant Behavior and Development, 19(4), 401–418. https://doi.org/10.1016/S0163-6383(96)90002-8 [Google Scholar]
- Barker B. A., & Newman R. S. (2000). The cocktail party effect in infants: Following one's mother's voice. In Howell S. C., Fish S. A., & Keith-Lucas T. (Eds.), 24th Annual Boston University Conference on Language Development (pp. 92–103). Boston, MA: Cascadilla. [Google Scholar]
- Bergeson T. R., Miller R. J., & McCune K. (2006). Mothers' speech to hearing-impaired infants and children with cochlear implants. Infancy, 10(3), 221–240. [Google Scholar]
- Best C. C., & McRoberts G. W. (2003). Infant perception of non-native consonant contrasts that adults assimilate in different ways. Language and Speech, 46(Pt 2–3), 183–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnham D., Kitamura C., & Vollmer-Conna U. (2002). What's new, pussycat? On talking to babies and animals. Science, 296(5572), 1435. [DOI] [PubMed] [Google Scholar]
- Butterfield E. C., & Siperstein G. N. (1970). Influence of contingent auditory stimulation upon non-nutritional suckle. In Third symposium on oral sensation and perception: The mouth of the infant (pp. 313–334). Springfield, IL: Charles C. Thomas. [Google Scholar]
- Colombo J., Frick J. E., Ryther J. S., Coldren J. T., & Mitchell D. W. (1995). Infants' detection of analogs of “motherese” in noise. Merrill-Palmer Quarterly, 41(1), 104–113. [Google Scholar]
- Conway C. M., Karpicke J., Anaya E. M., Henning S. C., Kronenberger W. G., & Pisoni D. B. (2011). Nonverbal cognition in deaf children following cochlear implantation: Motor sequencing disturbances mediate language delays. Developmental Neuropsychology, 36(2), 237–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway C. M., Karpicke J., & Pisoni D. B. (2007). Contribution of implicit sequence learning to spoken language processing: Some preliminary findings with hearing adults. Journal of Deaf Studies and Deaf Education, 12(3), 317–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway C. M., Pisoni D. B., Anaya E. M., Karpicke J., & Henning S. C. (2011). Implicit sequence learning in deaf children with cochlear implants. Developmental Science, 14(1), 69–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper R. P., & Aslin R. N. (1990). Preference for infant-directed speech in the first month after birth. Child Development, 61(5), 1584–1595. [PubMed] [Google Scholar]
- Cristia A. (2010). Phonetic enhancement of sibilants in infant-directed speech. The Journal of the Acoustical Society of America, 128(1), 424–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cristia A., & Seidl A. (2014). The hyperarticulation hypothesis of infant-directed speech. Journal of Child Language, 41(04), 913–934. [DOI] [PubMed] [Google Scholar]
- Dilley L. C., Millett A. L., McAuley J. D., & Bergeson T. R. (2014). Phonetic variation in consonants in infant-directed and adult-directed speech: The case of regressive place assimilation in word-final alveolar stops. Journal of Child Language, 41(01), 155–175. [DOI] [PubMed] [Google Scholar]
- Dorman M. F., Dankowski K., McCandless G., Parkin J. L., & Smith L. (1991). Vowel and consonant recognition with the aid of a multichannel cochlear implant. The Quarterly Journal of Experimental Psychology, 43(3), 585–601. [DOI] [PubMed] [Google Scholar]
- Drotar D., & Sturm L. (1988). Prediction of intellectual development in young children with early histories of nonorganic failure-to-thrive. Journal of Pediatric Psychology, 13(2), 281–296. [DOI] [PubMed] [Google Scholar]
- Fernald A. (1984). The perceptual and affective salience of mothers' speech to infants. In Feagans L., Garvey C., & Golinkoff R. (Eds.), The origins and growth of communication (pp. 5–29). Norwood, NJ: Ablex. [Google Scholar]
- Fernald A. (1985). Four-month-old infants prefer to listen to motherese. Infant Behavior and Development, 8(2), 181–195. https://doi.org/10.1016/S0163-6383(85)80005-9 [Google Scholar]
- Fernald A. (1989). Intonation and communicative intent in mothers' speech to infants: Is the melody the message? Child Development, 60(6), 1497–1510. [PubMed] [Google Scholar]
- Fernald A., & Kuhl P. K. (1987). Acoustic determinants of infant preference for motherese speech. Infant Behavior and Development, 10(3), 279–293. https://doi.org/10.1016/0163-6383(87)90017-8 [Google Scholar]
- Fernald A., & Mazzie C. (1991). Prosody and focus in speech to infants and adults. Developmental Psychology, 27(2), 209–221. https://doi.org/10.1037/0012–1649.27.2.209 [Google Scholar]
- Fernald A., Perfors A., & Marchman V. A. (2006). Picking up speed in understanding: Speech processing efficiency and vocabulary growth across the 2nd year. Developmental Psychology, 42(1), 98–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernald A., & Simon T. (1984). Expanded intonation contours in mothers' speech to newborns. Developmental Psychology, 20(1), 104–113. https://doi.org/10.1037/0012-1649.20.1.104 [Google Scholar]
- Fitzpatrick E., Durieux-Smith A., Eriks-Brophy A., Olds J., & Gaines R. (2007). The impact of newborn hearing screening on communication development. Journal of Medical Screening, 14(3), 123–131. https://doi.org/10.1258/096914107782066248 [DOI] [PubMed] [Google Scholar]
- Fryauf-Bertschy H., Tyler R. S., Kelsay D. M. R., & Gantz B. J. (1997). Cochlear implant use by prelingually deafened children: The influences of age at implant use and length of device use. Journal of Speech and Hearing Research, 40, 183–199. [DOI] [PubMed] [Google Scholar]
- Geers A. E., Moog J. S., Biedenstein J., Brenner C., & Hayes H. (2009). Spoken language scores of children using cochlear implants compared to hearing age-mates at school entry. Journal of Deaf Studies and Deaf Education, 14(3), 371–385. https://doi.org/10.1093/deafed/enn046 [DOI] [PubMed] [Google Scholar]
- Geers A. E., Strube M. J., Tobey E. A., Pisoni D. B., & Moog J. S. (2011). Epilogue: Factors contributing to long-term outcomes of cochlear implantation in early childhood. Ear and Hearing, 32(1 Suppl), 82S–92S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geers A. E., Tobey E., Moog J., & Brenner C. (2008). Long-term outcomes of cochlear implantation in the preschool years: From elementary grades to high school. International Journal of Audiology, 47(S2), S21–30. https://doi.org/10.1080/14992020802339167 [DOI] [PubMed] [Google Scholar]
- Glenn S. M., Cunningham C. C., & Joyce P. F. (1981). A study of auditory preferences in non handicapped infants and infants with Down's Syndrome. Child Development, 52, 1303–1307. [PubMed] [Google Scholar]
- Gogate L. J., Bahrick L. E., & Watson J. D. (2000). A study of multimodal motherese: The role of temporal synchrony between verbal labels and gestures. Child Development, 71(4), 878–894. [DOI] [PubMed] [Google Scholar]
- Grieser D. L., & Kuhl P. K. (1988). Maternal speech to infants in a tonal language: Support for universal prosodic features in motherese. Developmental Psychology, 24(1), 14–20. https://doi.org/10.1037/0012-1649.24.1.14 [Google Scholar]
- Holt R. F., Beer J., Kronenberger W. G., Pisoni D. B., & Lalonde K. (2012). Contribution of family environment to pediatric cochlear implant users' speech and language outcomes: Some preliminary findings. Journal of Speech, Language, and Hearing Research, 55(3), 848–864. https://doi.org/10.1044/1092-4388(2011/11-0143) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn D. L., Davis R., Pisoni D., & Miyamoto R. (2005). Development of visual attention skills in prelingually deaf children who use cochlear implants. Ear and Hearing, 26, 389–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn D. L., Houston D. M., & Miyamoto R. T. (2007). Speech discrimination skills in deaf infants before and after cochlear implantation. Audiological Medicine, 5, 232–241. [Google Scholar]
- Houston D. M., Beer J., Bergeson T. R., Chin S. B., Pisoni D. B., & Miyamoto R. T. (2012). The ear is connected to the brain: Some new directions in the study of children with cochlear implants at Indiana University. Journal of the American Academy of Audiology, 23(6), 446–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houston D. M., & Bergeson T. R. (2014). Hearing versus listening: Attention to speech and its role in language acquisition in deaf infants with cochlear implants. Lingua, 139, 10–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houston D. M., Pisoni D. B., Kirk K. I., Ying E., & Miyamoto R. T. (2003). Speech perception skills of deaf infants following cochlear implantation: A first report. International Journal of Pediatric Otorhinolaryngology, 67, 479–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houston D. M., Stewart J., Moberly A., Hollich G., & Miyamoto R. T. (2012). Word learning in deaf children with cochlear implants: Effects of early auditory experience. Developmental Science, 15(3), 448–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jusczyk P. W. (1993). From general to language-specific capacities: The WRAPSA model of how speech perception develops. Journal of Phonetics, 21, 3–28. [Google Scholar]
- Kemler Nelson D. G., Jusczyk P. W., Mandel D. R., Myers J., Turk A., & Gerken L. (1995). The head-turn preference procedure for testing auditory perception. Infant Behavior and Development, 18(1), 111–116. [Google Scholar]
- Kirk K. I., Miyamoto R. T., Ying E. A., Perdew A. E., & Zuganelis H. (2002). Cochlear implantation in young children: Effects of age at implantation and communication mode. The Volta Review, 102, 127–144. [Google Scholar]
- Kitamura C., Thanavishuth C., Burnham D., & Luksaneeyanawin S. (2001). Universality and specificity in infant-directed speech: Pitch modifications as a function of infant age and sex in a tonal and non-tonal language. Infant Behavior and Development, 24(4), 372–392. https://doi.org/10.1016/S0163-6383(02)00086-3 [Google Scholar]
- Kuhl P. K., Andruski J. E., Chistovich I. A., Chistovich L. A., Kozhevnikova E. V., Ryskina V. L., … Lacerda F. (1997). Cross-language analysis of phonetic units in language addressed to infants. Science, 277(5326), 684–686. https://doi.org/10.1126/science.277.5326.684 [DOI] [PubMed] [Google Scholar]
- Kuhl P. K., Coffey‐Corina S., Padden D., & Dawson G. (2005). Links between social and linguistic processing of speech in preschool children with autism: Behavioral and electrophysiological measures. Developmental Science, 8(1), F1–F12. [DOI] [PubMed] [Google Scholar]
- Kuhl P. K., & Meltzoff A. N. (1984). The intermodal representation of speech in infants. Infant Behavior and Development, 7, 361–381. [Google Scholar]
- Liu H.-M., Kuhl P. K., & Tsao F.-M. (2003). An association between mothers' speech clarity and infants' speech discrimination skills. Developmental Science, 6(3), F1–F10. https://doi.org/10.1111/1467-7687.00275 [Google Scholar]
- Loizou P. (2006). Speech processing in vocoder-centric cochlear implants. Advances in Otorhinolaryngology, 64, 109–143. [DOI] [PubMed] [Google Scholar]
- Ma W., Golinkoff R. M., Houston D. M., & Hirsh-Pasek K. (2011). Word learning in infant- and adult-directed speech. Language Learning and Development, 7(3), 185–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masur E. F., Flynn V., & Eichorst D. L. (2005). Maternal responsive and directive behaviours and utterances as predictors of children's lexical development. Journal of Child Language, 32(01), 63–91. [DOI] [PubMed] [Google Scholar]
- McRoberts G. W., McDonough C., & Lakusta L. (2009). The role of verbal repetition in the development of infant speech preferences from 4 to 14 months of age. Infancy, 14(2), 162–194. [DOI] [PubMed] [Google Scholar]
- Mehler J., Bertoncini J., Barriere M., & Jassik-Gerschenfeld D. (1978). Infant recognition of mother's voice. Perception, 7, 491–497. [DOI] [PubMed] [Google Scholar]
- Miyamoto R. T., Svirsky M. A., & Robbins A. M. (1997). Enhancement of expressive language in prelingually deaf children with cochlear implants. Acta Oto-Laryngologica, 117(2), 154–157. https://doi.org/10.3109/00016489709117758 [DOI] [PubMed] [Google Scholar]
- Molfese D. L. (2000). Predicting dyslexia at 8 years of age using neonatal brain responses. Brain and Language, 72(3), 238–245. [DOI] [PubMed] [Google Scholar]
- Newman R. S. (2003). Prosodic differences in mothers' speech to toddlers in quiet and noisy environments. Applied Psycholinguistics, 24(04), 539–560. [Google Scholar]
- Newman R. S., & Hussain I. (2006). Changes in preference for infant-directed speech in low and moderate noise by 4.5- to 13-month-olds. Infancy, 10(1), 61–76. [DOI] [PubMed] [Google Scholar]
- Niparko J. K., Tobey E. A., Thal D. J., Eisenberg L. S., Wang N.-Y., Quittner A. L., … CDaCl Investigative Team. (2010). Spoken language development in children following cochlear implantation. The Journal of the American Medical Association, 303(15), 1498–1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomikou I., & Rohlfing K. J. (2011). Language does something: Body action and language in maternal input to three-month-olds. IEEE Transactions on Autonomous Mental Development, 3(2), 113–128. [Google Scholar]
- Papoušek M., & Hwang S.-F. C. (1991). Tone and intonation in Mandarin babytalk to presyllabic infants: Comparison with registers of adult conversation and foreign language instruction. Applied Psycholinguistics, 12(4), 481–504. https://doi.org/10.1017/S0142716400005889 [Google Scholar]
- Peng S. C., Lu H. P., Lu N., Lin Y. S., Deroche M. L., & Chatterjee M. (2017). Processing of acoustic cues in lexical-tone identification by pediatric cochlear-implant recipients. Journal of Speech, Language, and Hearing Research, 60(5), 1223–1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan J., Houston D. M., Ruffin C., Ting J., & Holt R. F. (2016). Factors affecting speech discrimination in children with cochlear implants: Evidence from early-implanted infants. Journal of the American Academy of Audiology, 27(6), 480–488. https://doi.org/10.3766/jaaa.15088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pisoni D. B. (2000). Cognitive factors and cochlear implants: Some thoughts on perception, learning, and memory in speech perception. Ear and Hearing, 21(1), 70–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pisoni D. B., & Geers A. E. (2000). Working memory in deaf children with cochlear implants: Correlations between digit span and measures of spoken language processing. The Annals of Otology, Rhinology & Laryngology, 185(Suppl.), 92–93. https://doi.org/11141023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pressman L., Pipp-Siegel S., Yoshinaga-Itano C., & Deas A. (1999). Maternal sensitivity predicts language gain in preschool children who are deaf and hard of hearing. Journal of Deaf Studies and Deaf Education, 4(4), 294–304. https://doi.org/10.1093/deafed/4.4.294 [DOI] [PubMed] [Google Scholar]
- Robertson S., von Hapsburg D., & Hay J. S. (2013). The effect of hearing loss on the perception of infant- and adult-directed speech. Journal of Speech, Language, and Hearing Research, 56(4), 1108–1119. https://doi.org/10.1044/1092-4388(2012/12-0110) [DOI] [PubMed] [Google Scholar]
- Rose S. A., Feldman J. F., & Jankowski J. J. (2009). A cognitive approach to the development of early language. Child Development, 80(1), 134–150. https://doi.org/10.1111/j.1467-8624.2008.01250.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe M. L., & Goldin-Meadow S. (2009). Differences in early gesture explain SES disparities in child vocabulary size at school entry. Science, 323(5916), 951–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schachner A., & Hannon E. E. (2011). Infant-directed speech drives social preferences in 5-month-old infants. Developmental Psychology, 47(1), 19–25. [DOI] [PubMed] [Google Scholar]
- Segal O., Houston D. M., & Kishon-Rabin L. (2016). Auditory discrimination of lexical stress patterns in hearing-impaired infants with cochlear implants compared with normal hearing: Influence of acoustic cues and listening experience to the ambient language. Ear and Hearing, 37(2), 225–234. https://doi.org/10.1097/AUD.0000000000000243 [DOI] [PubMed] [Google Scholar]
- Segal O., & Kishon-Rabin L. (2011). Listening preference for child-directed speech versus nonspeech stimuli in normal-hearing and hearing-impaired infants after cochlear implantation. Ear and Hearing, 32(3), 358–372. https://doi.org/10.1097/AUD.0b013e3182008afc [DOI] [PubMed] [Google Scholar]
- Seidl A., Tincoff R., Baker C., & Cristia A. (2015). Why the body comes first: Effects of experimenter touch on infants' word finding. Developmental Science, 18(1), 155–164. https://doi.org/10.1111/desc.12182 [DOI] [PubMed] [Google Scholar]
- Shultz S., & Vouloumanos A. (2010). Three-month-olds prefer speech to other naturally occurring signals. Language Learning and Development, 6(4), 241–257. [Google Scholar]
- Song J. Y., Demuth K., & Morgan J. (2010). Effects of the acoustic properties of infant-directed speech on infant word recognition. The Journal of the Acoustical Society of America, 128(1), 389–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spence M. J., & DeCasper A. D. (1987). Prenatal experience with low-frequency maternal-voice sounds influence neonatal perception of maternal voice samples. Infant Behavior and Development, 10, 133–142. [Google Scholar]
- Svirsky M. A., Teoh S. W., & Neuburger H. (2004). Development of language and speech perception in congenitally, profoundly deaf children as a function of age at cochlear implantation. Audiology and Neurotology, 9, 224–233. [DOI] [PubMed] [Google Scholar]
- Toro J. M., Sinnett S., & Soto-Faraco S. (2005). Speech segmentation by statistical learning depends on attention. Cognition, 97(2), B25–B34. [DOI] [PubMed] [Google Scholar]
- Trainor L. J., Austin C. M., & Desjardins R. N. (2000). Is infant-directed speech prosody a result of the vocal expression of emotion? Psychological Science, 11(3), 188–195. [DOI] [PubMed] [Google Scholar]
- Vouloumanos A., & Curtin S. (2014). Foundational tuning: How infants' attention to speech predicts language development. Cognitive Science, 38(8), 1675–1686. [DOI] [PubMed] [Google Scholar]
- Vouloumanos A., & Werker J. F. (2004). Tuned to the signal: The privileged status of speech for young infants. Developmental Science, 7(3), 270–276. [DOI] [PubMed] [Google Scholar]
- Vouloumanos A., & Werker J. F. (2007). Listening to language at birth: Evidence for a bias for speech in neonates. Developmental Science, 10(2), 159–164. https://doi.org/10.1111/j.1467-7687.2007.00549.x [DOI] [PubMed] [Google Scholar]
- Weisleder A., & Fernald A. (2013). Talking to children matters: Early language experience strengthens processing and builds vocabulary. Psychological Science, 24(11), 2143–2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werker J. F., Pegg J. E., & McLeod P. J. (1994). A cross-language investigation of infant preference for infant-directed communication. Infant Behavior and Development, 17(3), 323–333. [Google Scholar]
- Wieland E. A., Burnham E. B., Kondaurova M., Bergeson T. R., & Dilley L. C. (2015). Vowel space characteristics of speech directed to children with and without hearing loss. Journal of Speech, Language, and Hearing Research, 58(2), 254–267. https://doi.org/10.1044/2015_JSLHR-S-13-0250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wild C. J., Yusuf A., Wilson D. E., Peelle J. E., Davis M. H., & Johnsrude I. S. (2012). Effortful listening: The processing of degraded speech depends critically on attention. Journal of Neuroscience, 32(40), 14010–14021. https://doi.org/10.1523/JNEUROSCI.1528-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng F.-G. (2004). Trends in cochlear implants. Trends in Amplification, 8(1), 1–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng F. G., Rebscher S., Harrison W., Sun X., & Feng H. (2008). Cochlear implants: System design, integration, and evaluation. IEEE Reviews in Biomedical Engineering, 1, 115–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman I. L., Steiner V. G., & Pond R. E. (2002). Preschool Language Scale–Fourth Edition. San Antonio: The Psychological Corporation. [Google Scholar]