Abstract
Phosphoenolpyruvate carboxylase (PEPc) catalyzes the primary fixation of CO2 in Crassulacean acid metabolism plants. Flux through the enzyme is regulated by reversible phosphorylation. PEPc kinase is controlled by changes in the level of its translatable mRNA in response to a circadian rhythm. The physiological significance of changes in the levels of PEPc-kinase-translatable mRNA and the involvement of metabolites in control of the kinase was investigated by subjecting Kalanchoë daigremontiana leaves to anaerobic conditions at night to modulate the magnitude of malate accumulation, or to a rise in temperature at night to increase the efflux of malate from vacuole to cytosol. Changes in CO2 fixation and PEPc kinase activity reflected those in kinase mRNA. The highest rates of CO2 fixation and levels of kinase mRNA were observed in leaves subjected to anaerobic treatment for the first half of the night and then transferred to ambient air. In leaves subjected to anaerobic treatment overnight and transferred to ambient air at the start of the day, PEPc-kinase-translatable mRNA and activity, the phosphorylation state of PEPc, and fixation of atmospheric CO2 were significantly higher than those for control leaves for the first 3 h of the light period. A nighttime temperature increase from 19°C to 27°C led to a rapid reduction in kinase mRNA and activity; however, this was not observed in leaves in which malate accumulation had been prevented by anaerobic treatment. These data are consistent with the hypothesis that a high concentration of malate reduces both kinase mRNA and the accumulation of the kinase itself.
In plants with Crassulacean acid metabolism (CAM), phosphoenolpyruvate carboxylase (PEPc) (EC 4.1.1.31) catalyzes the nocturnal fixation of atmospheric CO2 (as HCO3−) into oxaloacetate, which is subsequently reduced to malate and stored in the vacuole. During the day, the decarboxylation of malate released from the vacuole generates a high intercellular partial pressure of CO2, which results in stomatal closure and the conservation of water. The fixation of this internally generated CO2 by Rubisco continues behind closed stomata until malate decarboxylation nears completion and the CO2 partial pressure drops. Stomata may subsequently re-open and atmospheric CO2 can then be fixed directly via the Calvin cycle.
The temporal separation of these C4 and C3 carboxylation processes, which distinguishes CAM from C4 photosynthesis, requires that the activity of PEPc be reduced during the day to curtail futile cycling of CO2 from concurrent malate synthesis and breakdown. The day/night regulation of flux through PEPc is achieved by reversible phosphorylation that reduces the sensitivity of the enzyme to inhibition by L-malate with the phosphorylated, malate-insensitive (active) form of PEPc present at night (Nimmo et al., 1984, 1986). The phosphorylation state of PEPc is determined by the presence or absence of a specific Ca2+-independent protein kinase termed PEPc kinase (Carter et al., 1991; Li and Chollet, 1994). Recently, Hartwell et al. (1996) used a novel approach in which the products of in vitro translation of leaf RNA were assayed directly for PEPc kinase activity to demonstrate that the activity of PEPc kinase reflects changes in the level of its translatable mRNA. Thus, levels of kinase mRNA were approximately 20-fold higher at night than during the daytime in leaves of the CAM plant Kalanchoë (Bryophyllum) fedtschenkoi (Hartwell et al., 1996).
While the levels of PEPc kinase mRNA in C3 and C4 plants appear to respond to photosynthesis and, thus, light-dark transitions, in CAM plants a circadian oscillator controls the levels of kinase activity and translatable mRNA under constant environmental conditions (Carter et al., 1991; Hartwell et al., 1996). This results in a circadian rhythm in the phosphorylation state of PEPc (Nimmo et al., 1987), which plays an important role in generating the endogenous rhythms of CO2 exchange in CAM plants first described by Wilkins (1959). To date, the exact nature of the circadian oscillator in CAM is unknown, but recent observations indicate that the timing of PEPc activation/deactivation varies between different CAM species grown under identical environmental conditions (Borland and Griffiths, 1997).
Physiological manipulations of dark CO2 uptake and malate accumulation have indicated that the storage capacity of the vacuole for malate plays a key role in determining the timing of the inactivation of PEPc (Winter and Tenhunen, 1982; Fischer and Kluge, 1984; Borland and Griffiths, 1997). Thus, in plants prevented from accumulating malate overnight in an atmosphere of N2, flux through PEPc increases substantially at the start of the day in ambient air, and the inactivation of PEPc is delayed by 2 to 3 h (Borland and Griffiths, 1997). Observations that the circadian rhythms of phosphorylation of PEPc and CO2 exchange can be disrupted and re-initiated by temperature changes have also pointed to a key role for the tonoplast in malate compartmentation, and for malate itself in the generation of the endogenous rhythm of PEPc activity (Wilkins, 1983; Carter et al., 1991; Grams et al., 1997). Malate inhibits PEPc kinase by binding to PEPc (Carter et al., 1991; Li and Chollet 1993, 1994), although it is not clear whether this effect is physiologically significant. Malate or other metabolites might also affect the phosphorylation of PEPc by acting at steps closer to the circadian oscillator. Such effects on the output from the oscillator could provide CAM plants with the flexibility to adjust C flux in response to changes in environmental conditions.
The aim of the present work was to study the relationship between leaf malate content, PEPc kinase activity, and levels of translatable kinase mRNA in intact plants of Kalanchoë daigremontiana Hamet et Perr. Physiological manipulations involving anaerobic treatments and temperature changes in the dark were used to modulate the magnitude of dark CO2 uptake and malate accumulation. The results highlight the physiological importance of changes in translatable PEPc kinase mRNA in the CAM cycle and suggest that metabolites, most likely malate, affect the phosphorylation of PEPc at several levels.
MATERIALS AND METHODS
Plant Material and Growth Conditions
Plants of Kalanchoë daigremontiana Hamet et Perr., which were approximately 1 year old and growing in 10-cm-diameter pots, were acclimated in the growth chamber for 4 weeks prior to experimentation. All measurements were conducted on the fourth leaf pair from the growing tip.
The plants were acclimated in a growth chamber (Fitotron, Sanyo Gallenkamp, Leicester, UK) programmed to provide gradual changes in temperature, humidity, and photsynthetic photon flux density (PPFD) at the start and end of the photoperiod in an attempt to mimic conditions found naturally at dawn and dusk. From 8:30 am until 12 pm, PPFD was increased to a maximum of 530 μmol m−2 s−1 at leaf height, the temperature was increased from 19°C to 27°C, and the relative humidity (RH) was decreased from 80% to 60% (the vapor pressure deficit was increased from 1.8–2.9 kPa). These conditions were maintained until 4 pm, when PPFD was decreased gradually until the lights were off at 7:30 pm, the temperature was decreased to 19°C, and the RH was increased to 80% (the vapor pressure deficit was 1.8 kPa). Over the 13-h dark period, the temperature (19°C) and RH (80%) remained constant.
Manipulation of CAM
Previous studies on K. daigremontiana have indicated that exposure of the plants to CO2-free air still permits the accumulation of malate (up to 25% of that observed in controls) through refixation of respiratory CO2 by PEPc (A. Borland, unpublished data). Thus, in order to completely inhibit PEP carboxylation at night, individual leaves of intact plants were enclosed in an atmosphere of N2 overnight, as described by Borland and Griffiths (1997), thereby preventing access to external CO2 and inhibiting the release of internal (respiratory) sources of CO2 (full N2). Some leaves were enclosed in an atmosphere of N2 for the first half of the dark period (until 2 pm) and then exposed to ambient air for the remainder (half N2). Control leaves were exposed to the ambient atmosphere in the growth chamber.
A set of plants, half of which were maintained in ambient air (control), and half with leaves enclosed in an atmosphere of N2 (half N2), was subjected to an increase in temperature from 19°C to 27°C in the middle of the dark period (2:30–3 am). The leaves enclosed in N2 were subsequently exposed to ambient air from 3 am onward, with the temperature maintained at 27°C and the RH at 70%.
Gas Exchange Measurements
Rates of net CO2 assimilation were measured continuously on the same leaf over 24 h. The leaf was enclosed in a porometer head that tracked the environmental conditions in the growth chamber with gas exchange parameters measured using an open infrared (IR) gas exchange system (H. Walz, GmbH Effeltrich, Germany) with a gas analyzer (Binos, H. Walz). Gas exchange parameters were calculated using DIAGAS software supplied by H. Walz. Each gas exchange curve presented is for a representative leaf from three replicate determinations.
Malate Content
Discs were punched from three replicate leaves, subjected to the various treatments at intervals over the dark and light periods, and immediately plunged into hot (80°C) methanol (80%, v/v). The methanolic extracts were heated for 1 h at 70°C before being evaporated to dryness, taken up in 100 mm N,N′-bis(2-hydroxyethylglycine) (Bicine), pH 7.8, and the malate content determined enzymatically using malate dehydrogenase, as described by Hohorst (1965).
PEPc and PEPc Kinase Assays
Leaf extracts were prepared and desalted as described by Hartwell et al. (1996). The activity of PEPc was assayed and its apparent Ki for l-malate estimated as described by Nimmo et al. (1984). PEPc kinase activity in desalted extracts was assayed according to the method of Carter et al. (1991) using purified dephosphorylated PEPc from Kalanchoë fedtschenkoi as the substrate. Incubations were for 30 min at 30°C.
Assay of PEPc-Kinase-Translatable mRNA
Following the method of Hartwell et al. (1996), RNA was isolated and translated in vitro using a rabbit reticulocyte lysate, and a sample of the translation products was assayed for PEPc kinase activity. The PEPc was isolated by immunoprecipitation, resolved by SDS gel electrophoresis, and the incorporation of 32P into PEPc was quantified by phosphor imaging. These values were corrected to take into account any differences in the efficiency of translation between the different samples, as estimated by the incorporation of [35S]Met into protein (Hartwell et al., 1996). The values are therefore equivalent to data from northern blot analysis corrected for RNA loading. Control experiments in which [γ-32P]ATP was omitted from the kinase assays showed that when K. daigremontiana RNA was used, small amounts of [35S]Met were incorporated into immunoprecipitated PEPc from de novo synthesis of PEPc during the translations. This incorporation was less than the amount of 32P incorporated into PEPc in controls using the products of translations with no added RNA. The background incorporation of 32P was as a result of trace contamination of the PEPc substrate with PEPc kinase. However, this was <4% of the maximum labeling obtained with samples containing RNA. All experiments were repeated at least twice, with similar results, and the data presented are from representative individual experiments.
RESULTS
Physiology of CAM and Manipulation by N2
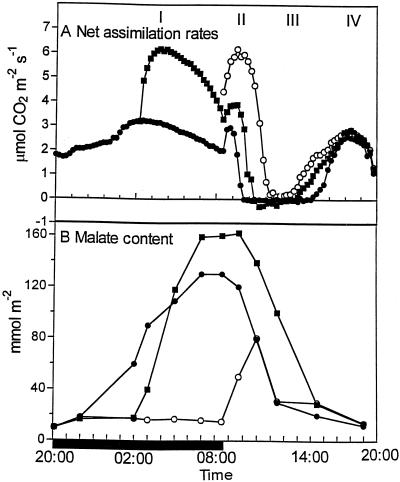
Figure 1A illustrates how the dark/light pattern of net CO2 uptake, which may be dissected into four phases (Osmond, 1978), was modulated in response to anaerobic conditions that were imposed for part or all of the dark period. Inhibiting CO2 uptake over the first half of the 13-h dark period by enclosing leaves in an atmosphere of N2 for 7.5 h resulted in a substantial increase in rates of net CO2 assimilation when darkened leaves were removed from N2 and transferred to ambient air (half N2) compared with control plants exposed to ambient air throughout the night. The malate content of the half-N2-treated leaves increased rapidly when darkened leaves were transferred to ambient air (Fig. 1B). After only 3 h in ambient air, the malate content of half N2 leaves was somewhat higher than that in control leaves that had accumulated malate over 9 h. At the end of the dark period, the malate content of the half-N2 leaves was about 25% higher than that measured in control leaves.
Figure 1.
Rates of net CO2 uptake and malate content in leaves exposed to anaerobic conditions for part or all of the dark period. A, Leaves were enclosed in an atmosphere of N2 for the first half (half N2) or entire duration (full N2) of the 13-h dark period before transfer to ambient air. Rates of net CO2 assimilation were measured. Control leaves were exposed to the ambient atmosphere in the growth chamber. Each gas exchange curve is representative of three replicate runs with se <10%. B, Malate content was measured in leaves subjected to the above treatments with each point being the mean of three replicates with se <10%. ●, Control leaves; ○, full-N2 leaves; ▪, half-N2 leaves. The solid bar on the x axis represents the period of darkness.
At the start of the 11-h photoperiod, leaves that had been exposed to N2 for the first half of the dark period (half N2) showed a small increase in the magnitude and duration of phase II net CO2 uptake compared with control plants (Fig. 1A). However, in leaves that had been enclosed in an atmosphere of N2 for the entire duration of the dark period (full N2), transfer to ambient air at the start of the photoperiod resulted in a substantial increase in the rates of net CO2 assimilation over both control and half-N2 leaves during phase II. Stomatal closure was delayed by about 2 h compared with controls, as judged by the time at which net CO2 assimilation fell to zero (Fig. 1A). Moreover, after transfer to ambient air at the start of the photoperiod, the full-N2 leaves accumulated about 60 mmol m−2 malate over the first 2.5 h of the photoperiod. Thus, in these leaves PEPc was still active at a period during which net breakdown of malate occurred in control and half-N2 leaves (Fig. 1B). Despite this accumulation of malate in full-N2 leaves during the photoperiod, the malate content attained only about 50% of that measured in control leaves, and the majority of decarboxylation was accomplished within 2 h. Consequently, during phase III, stomata remained closed for only 2 h in full-N2 leaves compared with 5 h in control leaves (Fig. 1A).
PEPc Kinase Activity, Translatable mRNA, and Manipulation by N2
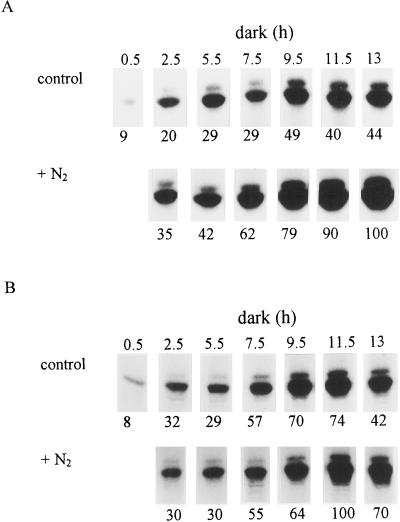
Figure 2 shows the changes in PEPc kinase activity and the level of translatable mRNA for the kinase in control and full-N2 leaves throughout the dark period. In control leaves, PEPc kinase activity increased over the first part of the dark period, reaching a plateau after 9.5 h in darkness (Fig. 2A). For leaves maintained in N2 during the entire dark period (in which malate content remained low; Fig. 1B), PEPc kinase activity increased steadily over the course of the dark period and was substantially higher than that measured in control leaves at comparable stages throughout the night. However, Figure 2B indicates that the levels of translatable PEPc kinase mRNA in control and full-N2 leaves were similar for the first 9.5 h of the dark period. Subsequently, levels of translatable mRNA in leaves enclosed in N2 were higher than those measured in control leaves.
Figure 2.
PEPc kinase activity and levels of translatable PEPc kinase mRNA under ambient and anaerobic conditions at night. Leaves were enclosed in an atmosphere of N2 overnight to prevent malate accumulation or maintained in ambient air. Samples for PEPc kinase assays (A) and RNA isolation and measurement of PEPc kinase translatable mRNA (B) were taken simultaneously from the same leaves at intervals over the 13-h dark period. Shown are autoradiographs of the 32P-labeled PEPc bands following SDS-PAGE. The doublet of PEPc bands is caused by the presence in a ratio of about 10:1 of two related subunits in K. fedtschenkoi PEPc, both of which are phosphorylated by PEPc kinase (Carter et al., 1991). The relative intensity of the PEPc bands, shown below each track, was determined by phosphor imaging. The total incorporation of [35S]Met into in vitro translation products using RNA isolated from control and N2-treated leaves was similar (data not shown). The results are from duplicate experiments
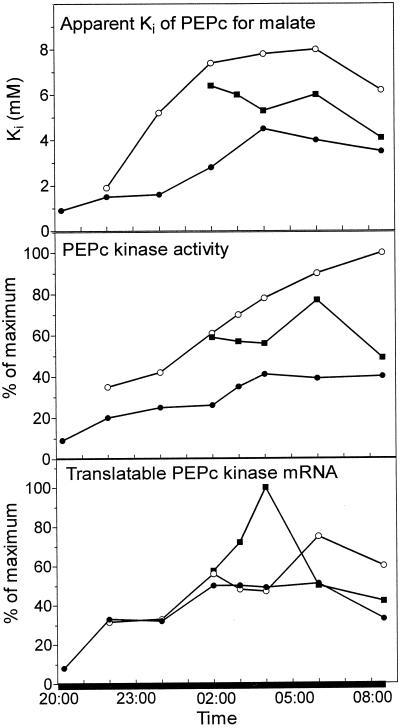
The time course of changes in PEPc kinase activity and translatable mRNA (Fig. 2), together with changes in the apparent Ki of PEPc for malate for control and full-N2 leaves, are illustrated in Figure 3. Changes in the apparent Ki for malate reflect the phosphorylation state of PEPc (Carter et al., 1991). In K. daigremontiana, the apparent Ki in control leaves increased from 0.5 to 5.0 mm during the night, compared with the range of 0.3 to 3.0 mm seen with K. fedtschenkoi in earlier work (Nimmo et al., 1984). However, the apparent Ki in full-N2 leaves reached 8 mm, implying that the enzyme was not fully phosphorylated in control leaves. The changes in apparent Ki closely followed changes in PEPc kinase activity. The increase in the apparent Ki measured in leaves enclosed in N2 compared with controls was reflected by an increased PEPc kinase activity in the full-N2 leaves. In control leaves, the levels of kinase-translatable mRNA reached a plateau at 2 am, whereas kinase activity and apparent Ki achieved maximum values 2 h later. In full-N2 leaves, a peak in kinase mRNA levels occurred at 6 am. The levels of mRNA were substantially higher than those measured in control leaves at this time. In both control and full N2 leaves, levels of translatable mRNA declined over the last part of the dark period.
Figure 3.
Apparent Ki of PEPc for l-malate, PEPc kinase activity, and translatable kinase mRNA under ambient and anaerobic conditions at night. Control leaves (●) were kept in ambient air throughout. Full-N2 leaves (○) were enclosed in an atmosphere of N2 overnight to prevent malate accumulation. Half-N2 leaves (▪) were enclosed in an atmosphere of N2 to prevent malate accumulation for the first half of the dark period before transfer to ambient air. Samples for PEPc and PEPc kinase assays and RNA isolation were taken simultaneously from the same leaves at intervals over the dark period. Kinase activity and translatable mRNA values are expressed as percentages of the maximum reached during the 13-h dark period. The results are from duplicate experiments.
Figure 3 also illustrates changes in apparent Ki for malate, PEPc kinase activity, and levels of translatable mRNA that occurred when leaves exposed to N2 for the first half of the dark period were subsequently transferred to ambient air for the remainder of the night. In these leaves, following transfer to ambient air, the apparent Ki for malate and PEPc kinase activity were appreciably higher than in the controls. In the 2 h following transfer of the half-N2 leaves to ambient air, rates of net CO2 uptake reached a maximum (Fig. 1A). Over this period, the level of kinase mRNA in the half-N2 leaves rose significantly. By 6 am, when malate content peaked (Fig. 1B), kinase mRNA had dropped to a level comparable to that measured in control leaves. The peak in kinase mRNA at 4 am preceded the time when maximum PEPc kinase activity was reached in half-N2 leaves at 6 am. There was little change in apparent Ki over this period.
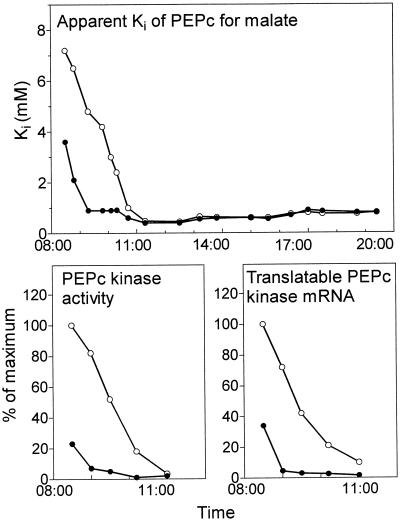
Figure 4 compares the changes that occurred at the start of the photoperiod in control leaves with those in leaves maintained in N2 throughout the dark period (full N2) but transferred to ambient air at the start of the photoperiod. In control leaves, the rapid down-regulation of PEPc activity was shown by a decrease in the apparent Ki of PEPc for l-malate and by the low level of PEPc kinase activity over the 1st h of the photoperiod as rates of net CO2 assimilation fell to zero and malate was broken down (Fig. 1). In the same leaves, the low levels of kinase mRNA detected at the start of the photoperiod declined to essentially zero after 100 min in the light. In contrast, the apparent Ki for malate, PEPc kinase activity, and kinase mRNA at the start of the photoperiod were substantially higher in leaves previously exposed to N2 overnight than in control leaves, and remained high well into the photoperiod as net CO2 uptake continued and malate was accumulated (Fig. 1).
Figure 4.
Changes in the apparent Ki for l-malate, PEPc kinase activity, and translatable kinase mRNA at the start of the photoperiod after a night in ambient or anaerobic conditions. Leaves that had been maintained in an atmosphere of N2 overnight to prevent malate accumulation were transferred to ambient air at the start of the photoperiod (○). Control leaves were maintained in ambient air (●). Samples for PEPc and PEPc kinase assays and RNA isolation were taken simultaneously from the same leaves at intervals over the light period. Kinase activity and translatable mRNA values are expressed as percentages of the maximum reached during the photoperiod. The results are from duplicate experiments.
Physiological Aspects of Temperature Manipulations
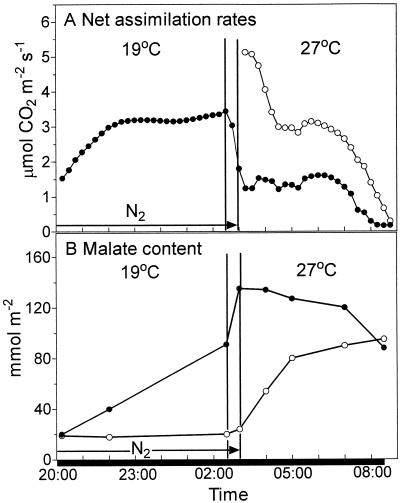
It has been suggested that the disruption of the circadian oscillations of CO2 exchange in CAM plants by high temperature may be a consequence of increased efflux of malate from the vacuole to the cytosol, the site of PEPc activity (Wilkins, 1983; Grams et al., 1997). Figure 5 illustrates the physiological consequences of exposing either control leaves or leaves prevented from accumulating malate over the first half of the dark period (half N2) to an 8°C increase in temperature in the middle of the night (from 2:30–3 am). In control leaves there was a rapid decline in the rate of net CO2 assimilation as the temperature was increased from 19°C to 27°C (Fig. 5A). The sharp increase in malate content over the 30-min rise in temperature (Fig. 5B) may be attributed to an increase in refixation of respiratory CO2 by PEPc. Overall, the maximum net assimilation rate at 27°C was <50% of that measured at 19°C in control leaves. Despite the continued net uptake of CO2, the malate content of the control leaves dropped slightly over the first few hours of exposure to the higher temperature, suggesting consumption of malate through increased rates of mitochondrial respiration. However, marked breakdown of malate was observed over the last hour of the dark period, when net CO2 assimilation had virtually ceased. Rates of net CO2 assimilation in leaves removed from N2 immediately after the temperature had been increased to 27°C were approximately 5-fold higher than those measured in control leaves. However, net assimilation rates dropped sharply during the first 1.5 h at the higher temperature in N2-treated leaves, reached a plateau for 3 h, and then decreased over the last hour of the dark period. Malate content in the N2-treated leaves showed a marked increase over the first 2 h at the higher temperature and a more gradual increase over the remaining 3.5 h. In contrast to control leaves, the net breakdown of malate in N2-treated leaves did not commence until the start of the photoperiod (data not shown).
Figure 5.
Modulation of net CO2 assimilation rates and malate accumulation by a temperature increase at night. Control leaves (●) were exposed to ambient air. Half-N2 leaves (○) were enclosed in an atmosphere of N2 for the first half of the dark period to prevent malate accumulation. Leaves were subjected to an 8°C rise in temperature from 2:30 to 3 am. The half-N2 leaves were subsequently exposed to ambient air at 27°C for the duration of the dark period. A, Rates of net CO2 uptake by leaves under the two treatments with each gas exchange curve representative of three replicate runs with se <10%. B, Malate content was measured in leaves subjected to the above treatments, with each point the mean of three replicates with se <10%. The solid bar on the x axis represents the period of darkness.
Modulation of PEPc Kinase Activity and Translatable mRNA by Temperature
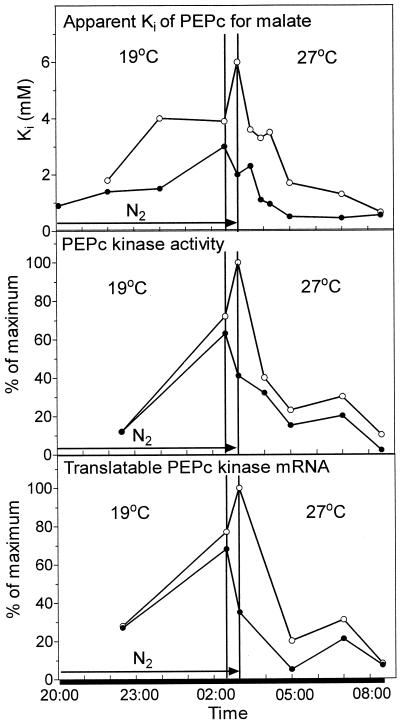
Figure 6 indicates that an 8°C rise in temperature over 30 min resulted in a decrease in PEPc kinase activity and kinase-translatable mRNA and a slight decrease in apparent Ki in control leaves. These parameters continued to fall over the following 70 min at 27°C. In contrast, in leaves prevented from accumulating malate over the first half of the dark period, an 8°C rise in temperature over 30 min resulted in an increase in apparent Ki, PEPc kinase activity, and kinase-translatable mRNA. However, transfer of the N2-treated leaves to ambient air after the temperature rise resulted in a marked decrease in translatable PEPc kinase mRNA, kinase activity, and the apparent Ki for l-malate over 70 min at the higher temperature as malate accumulated, presumably in the cytosol. Additional experiments in which the levels of PEPc kinase mRNA were measured at more frequent intervals after the temperature increase confirmed a steady decline in the levels of mRNA from 3 until 4:10 am (data not shown). From 4:10 until 7 am, the levels of kinase mRNA in half-N2 leaves were maintained at 20% of maximum. This was mirrored by a maintenance of PEPc kinase activity and by the plateau in net CO2 assimilation in half-N2 leaves (Fig. 5A). For the latter part of the dark period, the apparent Ki for l-malate and the levels of kinase activity and mRNA were somewhat higher in N2-treated leaves, in which the malate content was low but rising compared with controls, in which the malate content was high but declining (Fig. 5B).
Figure 6.
Modulation of the apparent Ki for l-malate, PEPc kinase activity, and translatable kinase mRNA by an increase in temperature at night. Control leaves (●) were exposed to ambient air. Half-N2 leaves (○) were enclosed in an atmosphere of N2 for the first half of the dark period to prevent malate accumulation. All leaves were subjected to an 8°C rise in temperature from 2:30 to 3 am. The half-N2 leaves were subsequently exposed to ambient air at 27°C for the duration of the dark period. Samples for PEPc and PEPc kinase assays and RNA isolation were taken simultaneously from the same leaves at intervals over the dark period. Kinase activity and translatable mRNA values are expressed as percentages of the maximum reached in leaves during a normal dark period at 19°C. The results are from duplicate experiments.
DISCUSSION
In this work we have manipulated intact plants to affect the magnitude of dark CO2 uptake and malate accumulation, and monitored the effects of these manipulations on the levels of PEPc kinase mRNA and activity. The results allow a number of conclusions about the control of PEPc kinase to be drawn. First, the data clearly demonstrate the physiological significance of PEPc phosphorylation, as shown by the close correlation between the activity in vitro of PEPc kinase, net CO2 uptake by PEPc, and malate accumulation in vivo under ambient air and after transfer from anaerobic conditions to ambient air. For example, leaves prevented from accumulating malate overnight in an atmosphere of N2 exhibited an extended period of CO2 uptake by PEPc for 2 to 3 h at the start of the photoperiod under ambient air (Fig. 1) (Borland and Griffiths, 1997). Under these conditions, kinase activity remained detectable and PEPc remained phosphorylated (as judged by its malate sensitivity) for several hours into the photoperiod (Fig. 4). In leaves moved from N2 to ambient air midway through the dark period, malate accumulated significantly faster, PEPc kinase activity was higher, and PEPc was more highly phosphorylated than in control leaves. (Figs. 1 and 3). The data presented here support and extend those of Hartwell et al. (1996) on B. (K.) fedtschenkoi in showing that these physiologically significant changes in PEPc kinase activity reflect changes in the translatable mRNA for this protein. Moreover, recent work using northern analysis with a PEPc kinase cDNA has shown that there are very similar changes in the level of PEPc kinase transcripts (J. Hartwell, A.M. Borland, G.I. Jenkins, and H.G. Nimmo, unpublished data).
Previous work has demonstrated clearly that PEPc kinase mRNA and activity and the phosphorylation state of PEPc are under circadian control (Nimmo et al., 1987; Carter et al., 1991; Hartwell et al., 1996). These effects contribute to the well-established circadian control of CO2 fixation in CAM plants (e.g. Wilkins, 1992). The influence of a circadian oscillator, rather than light/dark control, is illustrated by the fact that in K. fedtschenkoi in an 8-h photoperiod, both the increase and decrease in PEPc kinase mRNA and activity occur during the dark period (Hartwell et al., 1996). In the present work using K. daigremontiana in an 11-h photoperiod, the increase in PEPc kinase mRNA and activity also occured during the dark period (Figs. 2 and 3). The decline in PEPc kinase mRNA commences during the dark period, but the decline in kinase activity occurs only at the start of the light period.
The data in this paper allow a further conclusion to be drawn about the control of PEPc kinase. The circadian control of kinase mRNA and activity can be influenced by metabolic status, specifically by treatments that affect the content or compartmentation of malate. For example, in leaves that cannot accumulate malate, PEPc kinase activity is significantly higher than in control leaves, even though PEPc kinase mRNA levels are similar (Figs. 2 and 3). Although subjecting leaves to an anaerobic environment under N2 could in itself affect mRNA abundance, the data shown here present a number of testable hypotheses. Thus, in leaves with a high malate content, translation of PEPc kinase mRNA is reduced, the rate of inactivation (possibly by turnover) of PEPc kinase is increased, or both. The mechanisms(s) responsible could involve sensing of malate itself or of another metabolite the level of which correlates with the total leaf malate content.
Another effect of the prevention of malate accumulation was observed in experiments in which the temperature was increased from 19°C to 27°C in the middle of the dark period. In control leaves, this increase in temperature was accompanied by a reduction in the level of PEPc kinase mRNA. In contrast, in leaves in which malate accumulation had been prevented, there was a marked increase in kinase mRNA as temperature increased (Fig. 6). Experiments conducted with K. fedschenkoi have indicated that low temperature (i.e. 4°C) stabilizes the levels of kinase mRNA and postpones de-phosphorylation (Hartwell et al., 1996).
The effect of increased temperature on circadian rhythms of CO2 fixation has been ascribed to increased permeability of the tonoplast to malate and efflux of malate to the cytoplasm (Wilkins, 1983, 1992). There is direct experimental support for this hypothesis (Friemert et al., 1988). One possible explanation of our data is that PEPc-kinase-translatable mRNA is negatively regulated by cytosolic malate. However, it must be emphasized that no direct measurements of cytosolic malate have been made in CAM plants, and we have not been able to ascertain whether the temperature increase reduced PEPc-kinase-translatable mRNA through an increase in total malate (Fig. 5B), an increase in cytosolic malate, a lowering of cytosolic pH, or a change in another metabolite. Either transcription of the PEPc kinase gene or the stability of the kinase mRNA could be affected. Presumably, the relevant metabolite level in control leaves was insufficient to reduce the accumulation of PEPc kinase mRNA observed during the first 10 h of darkness (Fig. 3). PEPc kinase mRNA started to decline later in control leaves than in half-N2 leaves (6 and 4 am, respectively) (Fig. 3). Because the total leaf malate contents were actually similar at these times in the two treatments (Fig. 1B), there may be a threshold level of total malate in the dark (at about 120 mmol m−2) above which malate is sufficient to reduce PEPc kinase mRNA. However, PEPc kinase mRNA starts to decline after 6 am, even in leaves treated with full N2 and unable to accumulate malate (Fig. 3), so at least part of the decline at this time may reflect circadian control.
Overall, the control of flux through PEPc is multilayered. Fine control is achieved by changes in cytosolic levels of pH and opposing metabolic effectors such as malate (negative) and Glc 6-P (positive), whereas the phosphorylation of PEPc represents a means for coarse control of flux through this enzyme. The timing of phosphorylation is set by a circadian oscillator. The data in this paper show that circadian control can be overridden by metabolite control, probably in various ways. Our data are consistent with the view that metabolites can affect PEPc kinase gene expression or mRNA stability, and perhaps the stability of the kinase itself. Such metabolite effects may influence entrainment of the circadian rhythm to environmental conditions that support photosynthetic plasticity and survival through temporarily optimizing CO2 uptake. Identification of the factors responsible will require measurement of the amount and distribution of a number of key metabolites, including malate.
ACKNOWLEDGMENT
A.M.B. is grateful to Professor H. Griffiths (Department of Agricultural and Environmental Sciences, University of Newcastle) for his continued interest in this work.
Footnotes
Financial support was provided by the Natural Environment Research Council and the Biological and Biotechnological Science Research Council, United Kingdom.
LITERATURE CITED
- Borland AM, Griffiths H. A comparative study on the regulation of C3 and C4 carboxylation processes in the constitutive Crassulacean acid metabolism (CAM) plant Kalanchoë daigremontiana and the C3-CAM intermediate Clusia minor. Planta. 1997;201:368–378. doi: 10.1007/s004250050079. [DOI] [PubMed] [Google Scholar]
- Carter PJ, Nimmo HG, Fewson CA, Wilkins MB. Circadian rhythms in the activity of a plant protein kinase. EMBO J. 1991;10:2063–2068. doi: 10.1002/j.1460-2075.1991.tb07737.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer A, Kluge M. Studies on carbon flow in Crassulacean acid metabolism during the initial light period. Planta. 1984;160:121–128. doi: 10.1007/BF00392860. [DOI] [PubMed] [Google Scholar]
- Friemert V, Heininger D, Kluge M, Zeigler H. Temperature effects on malic-acid efflux from the vacuoles and on the carboxylation pathways in Crassulacean acid metabolism plants. Planta. 1988;174:453–461. doi: 10.1007/BF00634473. [DOI] [PubMed] [Google Scholar]
- Grams TEE, Borland AM, Roberts A, Griffiths H, Beck F, Lüttge U. On the mechanism of reinitiation of endogenous Crassulacean acid metabolism rhythm by temperature changes. Plant Physiol. 1997;113:1309–1317. doi: 10.1104/pp.113.4.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartwell J, Smith LH, Wilkins MB, Jenkins GI, Nimmo HG. Higher plant phosphoenolpyruvate carboxylase kinase is regulated at the level of translatable mRNA in response to light or a circadian rhythm. Plant J. 1996;10:1071–1078. [Google Scholar]
- Hohorst HJ. l-(−)Malate: determination with malic acid dehydrogenase and DPN. In: Bergmeyer HU, editor. Methods of Enzymatic Analysis. London: Academic Press; 1965. pp. 328–332. [Google Scholar]
- Li B, Chollet R. Resolution and identification of C4 phosphoenolpyruvate-carboxylase protein-kinase polypeptides and their reversible light activation in maize leaves. Arch Biochem Biophys. 1993;307:416–419. doi: 10.1006/abbi.1993.1609. [DOI] [PubMed] [Google Scholar]
- Li B, Chollet R. Salt induction and the partial purification/characterisation of phosphoenolpyruvate carboxylase protein-serine kinase from an inducible Crassulacean acid metabolism (CAM) plant, Mesembryanthemum crystallinum L. Arch Biochem Biophys. 1994;314:247–254. doi: 10.1006/abbi.1994.1437. [DOI] [PubMed] [Google Scholar]
- Nimmo GA, Nimmo HG, Fewson CA, Wilkins MB. Diurnal changes in the properties of phosphoenolpyruvate carboxylase in Bryophyllum leaves: a possible covalent modification. FEBS Lett. 1984;178:199–203. [Google Scholar]
- Nimmo GA, Nimmo HG, Hamilton ID, Fewson CA, Wilkins MB. Purification of the phosphorylated night form and dephosphorylated day form of phosphoenolpyruvate carboxylase from Bryophyllum fedtschenkoi. Biochem J. 1986;239:213–220. doi: 10.1042/bj2390213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimmo GA, Wilkins MB, Fewson CA, Nimmo HG. Persistent circadian rhythms in the phosphorylation state of phosphoenolpyruvate carboxylase from Bryophyllum fedtschenkoi leaves and in its sensitivity to inhibition by malate. Planta. 1987;170:408–415. doi: 10.1007/BF00395034. [DOI] [PubMed] [Google Scholar]
- Osmond CB. Crassulacean acid metabolism: a curiosity in context. Annu Rev Plant Physiol. 1978;29:379–414. [Google Scholar]
- Wilkins MB. An endogenous rhythm in the rate of dark fixation of carbon dioxide in leaves of Bryophyllum. II. The effects of light and darkness on the phase and period of the rhythm. J Exp Bot. 1959;10:377–390. [Google Scholar]
- Wilkins MB. The circadian rhythm of carbon-dioxide metabolism in Bryophyllum: the mechanism of phase-shift induction by thermal stimuli. Planta. 1983;157:471–480. doi: 10.1007/BF00397205. [DOI] [PubMed] [Google Scholar]
- Wilkins MB. Circadian rhythms: their origin and control. New Phytol. 1992;121:347–375. doi: 10.1111/j.1469-8137.1992.tb02936.x. [DOI] [PubMed] [Google Scholar]
- Winter K, Tenhunen JD. Light-stimulated burst of carbon dioxide uptake following nocturnal acidification in the Crassulacean acid metabolism plant Kalanchoë daigremontiana. Plant Physiol. 1982;70:1718–1722. doi: 10.1104/pp.70.6.1718. [DOI] [PMC free article] [PubMed] [Google Scholar]