Abstract
Zinc is necessary for successful gametogenesis in mammals; however the role of zinc in the gonad function of non-mammalian species has not been investigated. The genetic tractability, short generation time, and hermaphroditic reproduction of the nematode C. elegans offer distinct advantages for the study of impaired gametogenesis as a result of zinc deficiency. However the phenotypic reproductive effects arising from zinc restriction have not been established in this model. We therefore examined the effect of zinc deficiency on C. elegans reproduction by exposing worms to the zinc chelator N,N,N',N'-tetrakis (2-pyridylmethyl)ethane-1,2-diamine (TPEN). Treatment began at the early larval stage and continued until reproductive senescence. TPEN treatment reduced the total number of progeny produced by C. elegans hermaphrodites compared with control subjects, with the largest difference in output observed 48 hours after larval stage 4. At this time-point, zinc deficient worms displayed fewer embryos in the uterus and disorganized oocyte development when observed under DIC microscopy. DAPI staining revealed impaired oogenesis and chromosome dynamics with an expanded region of pachytene stage oocytes extending into the proximal arm of the gonad. This phenotype was not seen in control or zinc-rescue subjects. This study demonstrates that reproduction in C. elegans is sensitive to environmental perturbations in zinc, indicating that this is a good model for future studies in zinc-mediated subfertility. Aberrant oocyte development and disruption of the pachytene-diplotene transition indicate that oogenesis in particular is affected by zinc deficiency in this model.
Keywords: Zinc, C. elegans, oogenesis, fertility
1. Introduction
The essential micronutrient zinc is necessary for successful reproduction in vertebrate species. Adequate zinc bioavailability is required for sperm production (Hidiroglou and Knipfel, 1984), oocyte maturation (Kim et al., 2010; Bernhardt et al., 2011; Bernhardt et al., 2012; Kong et al., 2012; Tian and Diaz, 2012), fertilization (Kim et al., 2011; Que et al., 2014), and embryo development (Tian and Diaz, 2013; Tian et al., 2014). The periovulatory period surrounding oocyte maturation and ovulation is particularly sensitive to zinc insufficiency (Tian and Diaz, 2012; Tian and Diaz, 2013; Tian et al., 2014). A better understanding of the reproductive mechanisms that rely on zinc is a vital step in identifying and treating idiopathic infertility and optimizing assisted-reproduction techniques. New models are necessary to fully elucidate the mechanistic role of zinc in reproduction.
The invertebrate nematode C. elegans is a valuable research model where the role of zinc in reproduction has not yet been established. This genetically tractable model offers a unique life cycle that provides many advantages for the study of gonadal function. The majority of C. elegans are self-fertilizing hermaphrodites in which sperm and oocytes develop sequentially from a common gonad (Madl and Herman, 1979). The reproductive tract of the C. elegans hermaphrodite is U-shaped and composed of two gonadal arms joined by a common uterus. Each gonadal arm is independently capable of spermatogenesis, oogenesis, and fertilization (Hubbard and Greenstein, 2005). The ability to study sperm, oocytes, and embryos in the same subject, combined with the short generation time and well-annotated genome allow subtle reproductive phenotypes to be identified (Pazdernik and Schedl, 2013).
The lifecycle of the worm is well characterized (Corsi et al., 2015). Worms hatch in the first stage of larval development (L1) and then progress through 3 more larval stages (L2–L4) over the next 52 hours before reaching adulthood with a functional reproductive system. The distal tip of each gonadal arm in C. elegans is comprised of a mitotic zone in which germ cells are generated under the influence of NOTCH signaling (Austin and Kimble, 1987). As germ cells migrate away from the mitotic zone, they enter meiosis. Spermatogenesis begins during late L3 resulting in the production of approximately 300 sperm cells primarily generated during L4 (L'Hernault, 2006). These cells are stored in specialized structures known as spermatheca. Subsequently, the gonad begins oogenesis and the development of female germ cells exclusively. Early female germ cells develop in a syncytium, in which nuclei are not completely membrane-enclosed and open to a common cytoplasm known as the rachis. This syncytial arrangement persists through the pachytene stage of nuclear development, with oocyte differentiation and membrane enclosure occurring with the pachytene-diplotene transition at the beginning of the proximal gonad. The oocytes undergo further development including dynamic changes in chromosomal arrangement as they progress toward the spermatheca. Oocytes are ovulated sequentially into the spermatheca where they complete meiosis and are fertilized. The resulting embryos pass into the uterus and undergo mitotic divisions until being released from the adult worm around the 24-cell stage.
Germ cell and embryo development are well characterized in C. elegans (Hubbard and Greenstein, 2005; Lui and Colaiácovo, 2013), as are the effects of zinc deficiency on lifespan (Kumar et al., 2016). Storage and transport of zinc in intestinal cells have also been investigated under zinc deficient conditions (Roh et al., 2012; Roh et al., 2013), however the effects of zinc deficiency on C. elegans reproduction have not been investigated. We therefore undertook to induce and characterize the phenotypic effects of zinc deficiency on reproduction in C. elegans. The well-established zinc requirement for reproduction in mammalian species, as well as highly conserved mechanisms of oogenesis in both C. elegans and higher animals, led us to hypothesize that zinc restriction would decrease fertility in C. elegans hermaphrodites. Furthermore, given the emerging role of zinc in oocyte development prior to fertilization, we hypothesized that deficits in oogenesis would be evident in a zinc-deficient model.
2. Material and methods
2.1 C. elegans culture
Wild-type (strain N2) C. elegans were maintained under standard conditions on Nematode Growth Media (NGM)-agar plates seeded with Escherichia coli OP50 as a source of food (Stiernagle, 2006). Worms were maintained at 20°C. To create experimental conditions, NGM-agar plates were treated prior to addition of C. elegans. Zinc-deficient conditions were created with addition of N,N,N',N'-tetrakis(2-pyridylmethyl)ethane-1,2-diamine (TPEN) in 4% ethanol buffer; final concentration 50 µM/plate. Control plates were treated with ethanol buffer alone. A rescue group was created by treating plates with TPEN and ZnSO4 in ethanol buffer (final concentration 50 µM of both TPEN and Zn/plate). Animals were cultured on treatments for 24 hours prior to choosing L4 stage subjects for further treatment and analysis (Fig. 1). Worms treated with this protocol can be estimated to enter treatment during the late L2/early L3 stage of development. Animals subsequently remained on treatment for the duration of the experiment.
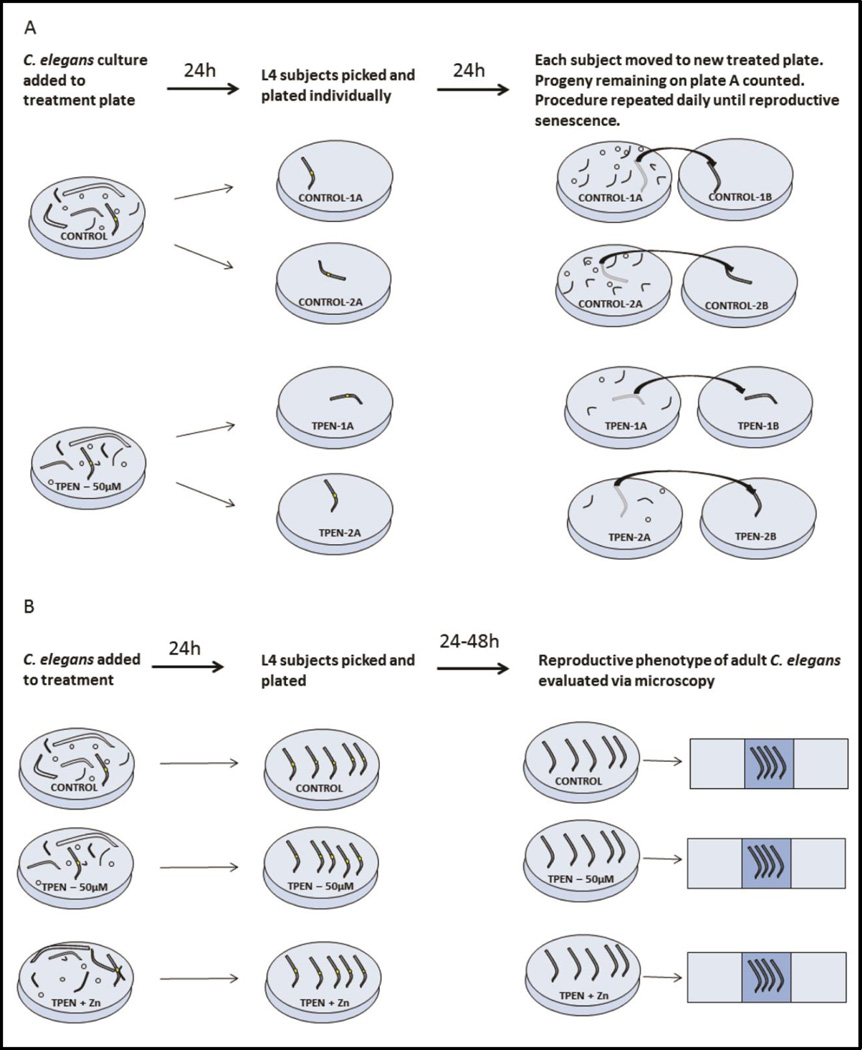
Figure 1. Experimental Protocol.
A. To test the effect of TPEN on reproduction in C. elegans, high numbers of N2 eggs, larvae, and adults were added to NGM plates treated with either TPEN (50µM) or buffer as a control. Twenty-four hours later, L4 animals were identified by size and vulval morphology and transferred individually to new treated plates. Animals were subsequently moved to a new plate every day and progeny released over the previous 24 hours was quantified. This procedure continued until reproductive senescence. B. Experiments conducted after the timecourse study were performed using a similar protocol. Large numbers of N2 animals were placed onto control, TPEN (50µM), or TPEN+Zn (50µM each) treated plates. After 24 hours L4 subjects were isolated. Subjects remained on treatment for an additional 24 or 48 hours before being processed for DIC or fluorescent microscopy. In all experiments, animals were placed onto treatments 24 hours prior to the L4 stage and remained on treatment until the experimental endpoint.
2.2 Effect of TPEN exposure on progeny production
L4 Worms were picked 24 hours after exposure to experimental conditions and placed singly on either TPEN-treated (n=4) or control plates (n=5). Worms were moved to a new plate (either TPEN-treated or control) daily and the number of progeny produced during the previous 24 hours was recorded. Progeny numbers were calculated as live worms remaining on each plate after transferring the adult worm (Fig. 1A). Plates were re-examined on multiple days to ensure that all progeny were counted. This procedure was repeated daily until reproductive output reached zero, which required 5 days for both control and TPEN treated groups.
2.3 Embryo Number and Oocyte Development
L4 worms were picked 24 hours after treatment with TPEN (n=38 worms), TPEN+ZN (n=38 worms), or buffer only (n=36 worms). Animals were subsequently maintained on treatment plates for the duration of the experiment (Fig 1B). Results of the prior progeny counting experiment showed the largest difference in reproductive performance was on day two of egg-laying. Therefore, at this time point worms were placed onto slides and observed via DIC on Zeiss Axioplan2 microscope at 40× magnification. The number of embryos developing in the uterus was recorded for each worm. Defects in oocyte development were also noted and imaged at this time. The stage of embryo development and rate of gonadal deformity was observed but did not differ between treatments.
2.4 DAPI Staining
Worms from each treatment group – TPEN, control, or TPEN +Zn – were created as before. 24 hours post L4, worms were fixed in Carnoy’s fixative (60% ethanol, 30% glacial acetic acid, 10% chloroform) and stained with DAPI (4',6-diamidino-2-phenylindole). Worms were mounted on 15 mm etched ring slides for fluorescent imaging using an Olympus FV10i confocal microscope at 60× magnification. We chose this timepoint in order to test if the reproductive defects in oogenesis noted 48 hours post L4 could be attributed to meiotic defects in the early adult worm.
2.5 Bright-Field Imaging for Representative Imaging
Worms were placed onto treatment 24 hours before reaching L4 as described above. Live worms were mounted on slides and imaged at 40× Zeiss axiocam2 DIC microscope. Images were taken for all groups once daily until egg-laying ceased.
2.6 Statistics
Daily progeny output data was analyzed using repeated measures ANOVA with an imposed autoregressive covariance structure. Treatment effects within day were compared using Student’s t-test. Treatment effects on total progeny released and embryo number were analyzed using one-way ANOVA and post-hoc Tukey pairwise comparisons. The effect of treatment on pachytene germ cells in the proximal gonad was tested using chi-square test for association. P value <0.05 was considered significant for all experiments.
3. Results
3.1 TPEN exposure reduces progeny output in C. elegans
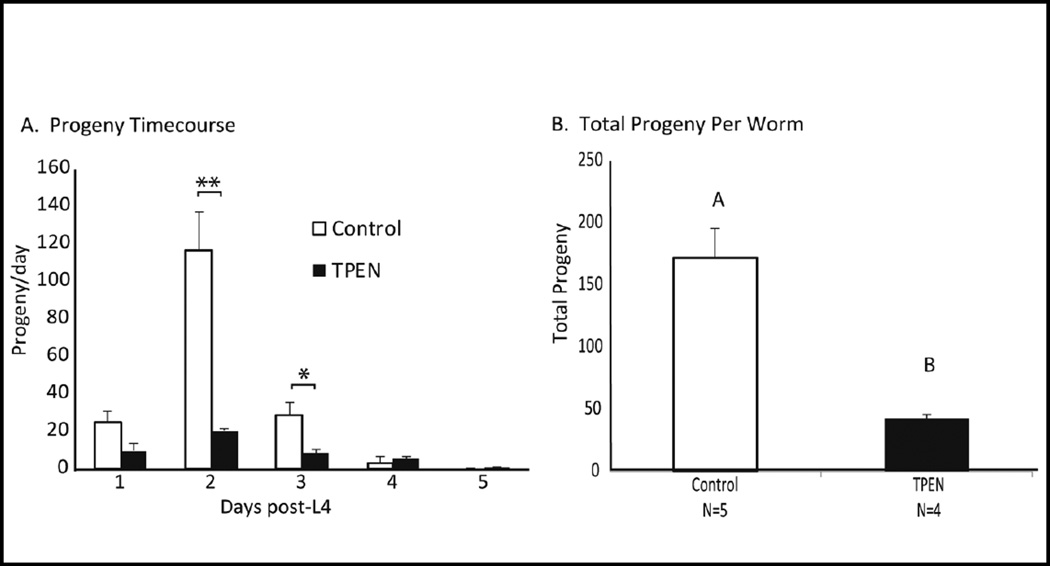
C. elegans hermaphrodites were raised individually on either control or TPEN treated plates. The number of progeny released from each subject per day was calculated from the onset of egg-laying until reproductive senescence. The total number of progeny was severely attenuated when worms were treated with TPEN (mean of 42.75±3.8 progeny compared with 171.8±23.8 under control conditions, Fig 2B). The largest deficit was seen on day 2 of egg-laying, 48h post-L4 (Fig. 2A). Repeated measures ANOVA showed a significant effect of treatment, day, and treatment by day interaction (p<0.0001). Due to the large treatment-induced effect in progeny output 48 hours post L4, this time point was selected to examine differences in embryo number and oocyte development in subsequent experiments.
Figure 2. TPEN treatment decreased fertility in C. elegans.
A. Number of progeny from worms placed on TPEN (50 µM) or control plates as early stage larva. Data was analyzed via repeated measures ANOVA (N=4–5) and showed significant effect of day (P<0.0001), treatment (P=0.0003) and treatment x day interaction (P<0.0001). *Indicated significant difference within day by student’s t-test, (*P<0.05; **P<.01). B. TPEN treatment significantly reduced the number of total progeny released. Subjects in the control group produced a mean of 171.8±23.8 progeny. TPEN treated subjects produced a mean of 42.75±3.8 progeny (P<0.01). Results analyzed by one-way ANOVA and Tukey’s post-hoc test.
3.2 Zinc restriction lowers the number of developing embryos in the uterus
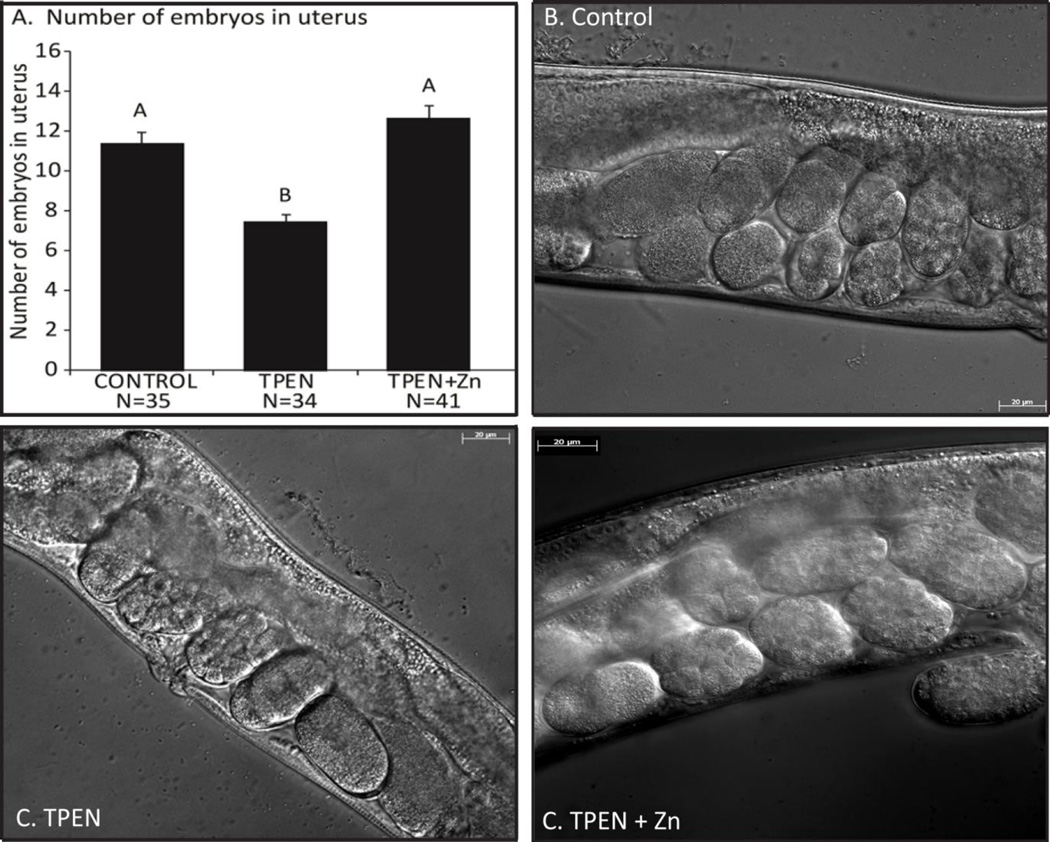
At 48 hours post-L4, zinc deficient worms had fewer developing embryos in-utero (7.5±0.4) compared with control (11.4±0.6) or rescue (12.7±0.6) groups (Fig. 3 p<0.001). Subjects were examined for disruption of embryo development or gonadal formation including: instances of intra-uterine larvae (bagging), gonadal atrophy, disrupted vulva formation, and malformation of either gonadal arm. These observations were combined and used to compare the rates of gonadal deformation between groups. There was no statistical difference between groups for this comparison. (Con – 22%, TPEN – 29%, Rescue – 27%; P=0.785 Chi square test for association).
Figure 3. Zinc restriction reduces the number of developing embryos.
A. Number of embryos in the uterus of control (11.4±0.6; n=41), TPEN (7.5±0.4; n=34), and TPEN plus zinc (12.7±0.6; n=41) worms 48 hours post-L4. abcIndicates significant difference by one-way ANOVA followed by Tukey’s post-hoc test, P<0.05. B–D. Representative DIC images (40×) from each group. abcIndicates significant difference by one-way ANOVA followed by Tukey’s post-hoc test. P<0.05.
3.3 Zinc restriction disrupts oocyte development
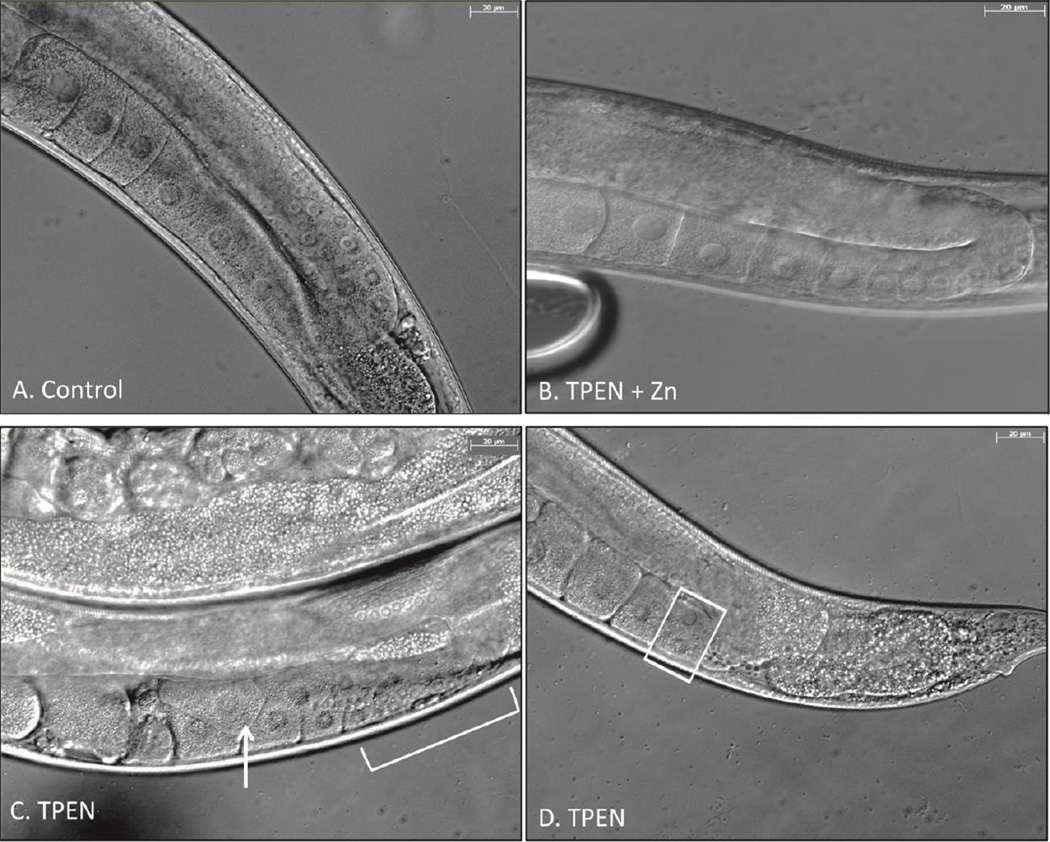
Under control conditions, female germ cells emerged from the rachis and began orderly, single-file oocyte development in the proximal gonadal arms (Fig 4A). Disrupted oogenesis was noted in 45% of TPEN treated subjects. Aberrant phenotypes included oocyte stacking, binucleate oocytes, and small (likely pachytene) oocytes extending past the gonadal turn (Fig 4C and 4D). Animals from the rescue group did not display disrupted oocyte development and resembled the phenotype of control worms (Fig 4B).
Figure 4. Zinc restriction disrupts oocyte development in the proximal gonad.
A. Representative DIC image (40×) of control gonad undergoing orderly meiotic progression. B. Representative image of rescue group. C. Image of TPEN-treated gonadal arm. Arrow – stacked and overlapping oocytes. Bracket – Apparent extended region of pachytene cells. D. TPEN treated gonadal arm. Boxed – binucleate oocyte.
3.4 Zinc restriction leads to an expanded region of pachytene germ cells in the proximal gonad
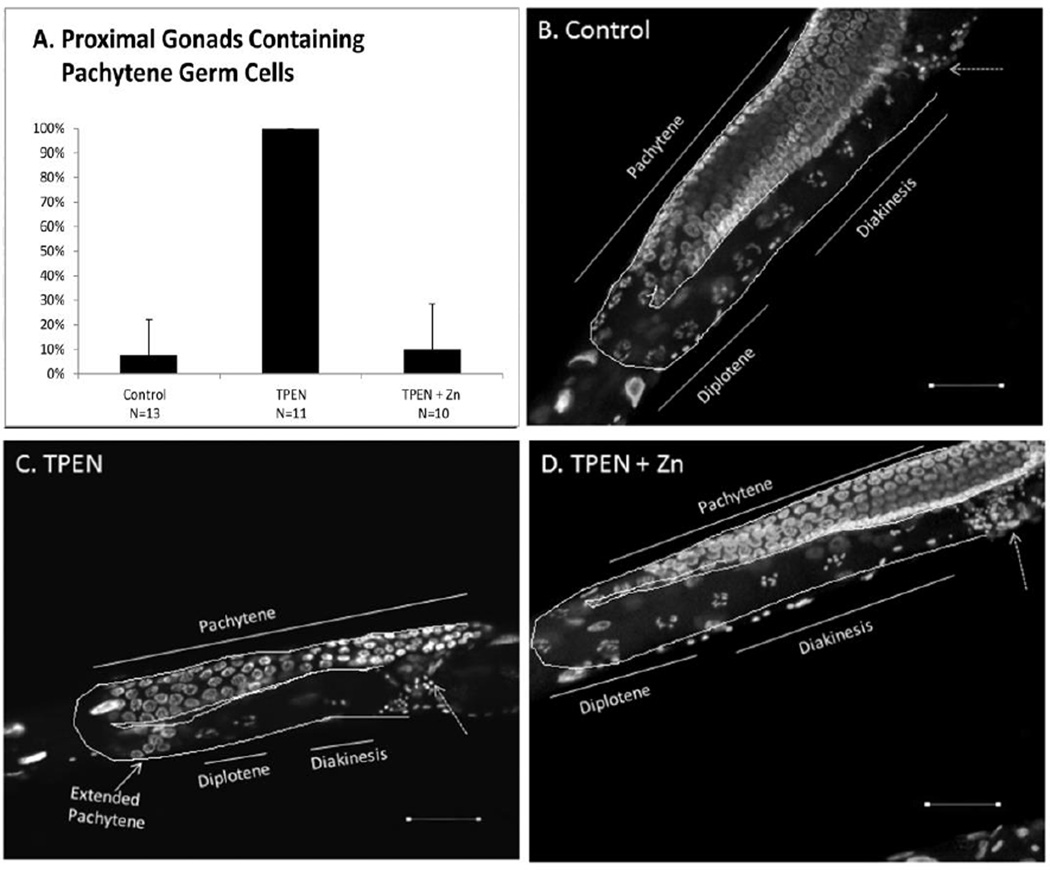
We used DAPI staining to examine meiotic progression and chromosomal dynamics directly and to investigate the suspected presence of pachytene oocytes in the proximal gonadal arm of zinc-deficient animals. When chromosomes were examined under fluorescence, all TPEN treated worms (100%) displayed an expanded region of pachytene-stage oocytes extending past the gonadal bend and into the proximal gonadal arm 24 hours post-L4. This morphology was rarely seen in control (7.6%) or TPEN+Zn (10%) subjects (Chi-square test for association: P<0.001, Fig. 5).
Figure 5. Zinc restriction extends the region of pachytene stage oocytes.
A. Percentage of proximal gonads containing pachytene oocytes. B–D. Representative DAPI images. Pachytene oocytes visible in proximal gonad in panel C. Dotted arrow points to the Spermatheca. Scale bar equals 20µm. Chi square test for association showed a significant effect of treatment on % extended pachytene phenotype (P<0.001). Error bars represent 95% confidence interval for control and rescue groups. Confidence interval cannot be calculated for proportions of 100% (TPEN).
4. Discussion
These findings clearly show an impaired reproductive phenotype resulting from zinc restriction in C. elegans. The ubiquity of zinc’s role in reproductive processes poses a challenge in elucidating the exact mechanism(s) responsible for the TPEN-induced reproductive defects. Studies of zinc restriction in other models indicate five critical points in reproduction that are vulnerable to zinc restriction: development of the reproductive tract (Martin et al., 1994), spermatogenesis (Abbasi et al., 1979), oogenesis (Tian and Diaz, 2012), fertilization (Kim et al., 2011), and embryo development (Tian and Diaz, 2013). The results of this study indicate oogenesis as the most obviously disrupted reproductive process in TPEN-treated C. elegans, however effects on other reproductive processes cannot be ruled out.
Animals began treatments as early-stage larvae, prior to reproductive tract development. However, TPEN treated subjects did not show gross reproductive tract deformity or disrupted development at a higher rate than control animals. Indeed, zinc-deficient animals began releasing progeny on the same day and persisted for the same duration as hermaphrodites in other treatment groups. This may indicate that C. elegans do not require labile zinc during reproductive tract development as in other species, or more likely that the zinc reserves identified by Roh et al (2012) in the digestive tract of C. elegans persisted through larval development and facilitated completion of a functional reproductive system. Regardless, the results of this study do not indicate any major changes in the anatomical development of the reproductive tract in TPEN treated C. elegans, and it is more likely that deficits in oogenesis, fertilization, and/or embryo development are responsible for the effects seen in this study.
Zinc plays a key role in fertilization and sperm dynamics. Liu et al (2013) demonstrated that zinc is sufficient to activate sperm in C. elegans and that the spermatheca is enriched in labile zinc. A unique aspect of reproduction in C. elegans is the hormonal action of the sperm cytoskeletal protein MSP-1. This protein acts outside of the spermatheca to initiate ovulation. MSP-1 simultaneously signals meiotic resumption in the diakinesis-arrested oocyte adjacent to the spermatheca and causes contraction of the gonadal sheath cells which push the oocyte into the spermatheca (Miller et al., 2001). The MSP-1 ovulation signal depends on the presence of two redundant zinc-finger proteins in the maturing oocyte – OMA-1 and OMA-2 (Detwiler et al., 2001). The phenotype of OMA-mutant C. elegans presents as an increased number of fully grown unfertilized diakinetic oocytes in the proximal gonadal arm. This phenotype is in contrast with the zinc-restriction phenotype described here which is characterized by an enlarged region of small pachytene-stage germ cells; making it unlikely that zinc-deficiency is acting primarily at the site of MSP signaling. Finally, although subjects in all groups were fertile, indicating the presence of sperm; sperm count and morphology were not evaluated. Therefore we cannot discount the possibility that differences in sperm quantity or quality may have affected reproductive output.
The most striking effect of zinc restriction in C. elegans hermaphrodites was seen in the female germ cells. The disorganization of oocyte progression in the gonad indicates impaired oogenesis in the zinc-restricted group, undoubtedly a contributing factor to lowered fertility in TPEN-treated subjects. DAPI staining also revealed an increase in the area of pachytene-stage germ cells, extending well past the gonadal turn. This phenotype may indicate meiotic disruption. Meiosis of female germ cells is driven by the MAPK cascade in C. elegans, a pathway previously shown to be affected by intracellular zinc concentration in C. elegans (Bruinsma et al., 2002; Yoder et al., 2004). MPK-1 (MAPK ortholog) signaling is required for progression past the pachytene arrest (Lee et al., 2007) and mutation of mpk-1 or mek-2 (mapkk ortholog) results in total pachytene arrest of female germ cells (Church et al., 1995). Interestingly, MPK-1 has been shown to interact with GLA-3, a protein containing two zinc-finger domains (Kritikou et al., 2006). gla-3 mutant worms are characterized by increased levels of oocyte apoptosis, however double mutants that lack both gla-3 and the apoptotic caspase gene ced-3 display a phenotype very similar to our zinc-restricted hermaphrodites. In both cases the region of pachytene nuclei was extended into the proximal gonadal arm, but diakinetic nuclei were still visible. The comparable germ cell phenotype, as well as the presence of zinc-finger domains make GLA-3 a likely target for disruption by zinc restriction. We will investigate the hypothesis that zinc sequestration interferes with germ cell progression by disrupting GLA-3 (and by extension MAPK) activity in future studies.
Reduced brood size and an extended pachytene region in the proximal gonadal arm are also an established phenotype after CPB-3 knock-down with siRNA (Boag et al., 2005). CPB-3 is an ortholog of cytoplasmic polyadenylation element binding protein (CPEB), a highly conserved RNA-binding protein involved in temporal control of mRNA translation in the developing oocyte (Tay et al., 2003). Oocytes in CPEB knockout mice do not progress beyond the pachytene stage (Racki and Richter, 2006) and synaptonemal complex formation is disrupted in both developing oocytes and spermatids (Tay and Richter, 2001). Hake et al (1998) demonstrated that CPEB requires a zinc cofactor in order to bind RNA, therefore it is possible that zinc deficiency impairs CPEB activity and interferes with the pachytene to diplotene transition. Future studies in our model will seek to confirm the relationship between zinc deficiency and CPEB activity.
Our experimental treatments were applied to NGM-agar plates seeded with E. coli OP50 as a food source. It is conceivable that TPEN treatment affected the density or quality of the bacterial lawn. However, this is unlikely since the bacterial lawn of TPEN – treated plates appeared normal under light microscopy, and a similar TPEN-treatment protocol has been used previously with no noted starvation effects (Roh et al.; Kumar et al., 2016). Moreover, the worms in our study do not display the reproductive phenotype associated with starvation described by Seidel and Kimble (2011); which includes intra-uterine larval hatching (bagging), germline shrinkage, constriction of the gonadal arms, and bulging germ cells. While starvation does disrupt the progression of oocytes in the proximal gonad, the starvation phenotype presents with only one oocyte per gonadal arm developing and ovulating before another oocyte begins to differentiate. This is distinct from our model and indicates that the reproductive effects of our study are a result of zinc-deficiency, rather than total nutrient deprivation.
In conclusion, our group has identified a novel C. elegans reproductive phenotype resulting from zinc restriction with the zinc chelator TPEN. Reproductive deficits were obvious and pronounced, indicating that C. elegans is a valuable model for zinc-restricted subfertility. The exact mechanism(s) of reduced fertility remains to be elucidated, but defective oogenesis is responsible for at least some of the reduced fertility. The TPEN-induced phenotype is similar to the phenotype seen in C. elegans with depleted GLA-3 or CPB-3 levels. Future studies will investigate whether zinc restriction impairs oogenesis though disruption the MAPK pathway via reduced GLA-3 activity, disrupted synaptonemal complex formation mediated by CPB-3, or a combination.
Acknowledgments
The authors would like to thank Avni Upadhyay for her expert guidance and instruction regarding C. elegans maintenance and technique. This work was funded in part by NIH Grant T32GM108563 and by the Huck Institutes of the Life Sciences through a J. Lloyd Huck Dissertation Research Grant.
Abbreviations
- NGM
Nematode Growth Media
- TPEN
N,N,N',N'-tetrakis(2-pyridylmethyl)ethane-1,2-diamine
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
The authors have no conflicts of interest to disclose.
References
- Abbasi AA, Prasad AS, Rabbani PR. Experimental zinc deficiency in man: effect on spermatogenesis. Trans Assoc Am Physicians. 1979;92:292–302. [PubMed] [Google Scholar]
- Austin J, Kimble J. glp-1 Is required in the germ line for regulation of the decision between mitosis and meiosis in C. elegans. Cell. 1987;51:589–599. doi: 10.1016/0092-8674(87)90128-0. [DOI] [PubMed] [Google Scholar]
- Bernhardt ML, Kim AM, O'Halloran TV, Woodruff TK. Zinc Requirement During Meiosis I–Meiosis II Transition in Mouse Oocytes Is Independent of the MOS-MAPK Pathway. Biology of Reproduction. 2011;84:526–536. doi: 10.1095/biolreprod.110.086488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernhardt ML, Kong BY, Kim AM, O'Halloran TV, Woodruff TK. A Zinc-Dependent Mechanism Regulates Meiotic Progression in Mammalian Oocytes. Biology of Reproduction. 2012 doi: 10.1095/biolreprod.111.097253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boag PR, Nakamura A, Blackwell TK. A conserved RNA-protein complex component involved in physiological germline apoptosis regulation in C. elegans. Development. 2005;132:4975–4986. doi: 10.1242/dev.02060. [DOI] [PubMed] [Google Scholar]
- Bruinsma JJ, Jirakulaporn T, Muslin AJ, Kornfeld K. Zinc ions and cation diffusion facilitator proteins regulate Ras-mediated signaling. Dev Cell. 2002;2:567–578. doi: 10.1016/s1534-5807(02)00151-x. [DOI] [PubMed] [Google Scholar]
- Church DL, Guan KL, Lambie EJ. Three genes of the MAP kinase cascade, mek-2, mpk-1/sur-1 and let-60 ras, are required for meiotic cell cycle progression in Caenorhabditis elegans. Development. 1995;121:2525–2535. doi: 10.1242/dev.121.8.2525. [DOI] [PubMed] [Google Scholar]
- Corsi AK, Wightman B, Chalfie M. A Transparent Window into Biology: A Primer on Caenorhabditis elegans. Genetics. 2015;200:387–407. doi: 10.1534/genetics.115.176099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Detwiler MR, Reuben M, Li X, Rogers E, Lin R. Two Zinc Finger Proteins, OMA-1 and OMA-2, Are Redundantly Required for Oocyte Maturation in C. elegans. Developmental Cell. 2001;1:187–199. doi: 10.1016/s1534-5807(01)00026-0. [DOI] [PubMed] [Google Scholar]
- Hake LE, Mendez R, Richter JD. Specificity of RNA binding by CPEB: requirement for RNA recognition motifs and a novel zinc finger. Mol Cell Biol. 1998;18:685–693. doi: 10.1128/mcb.18.2.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hidiroglou M, Knipfel JE. Zinc in Mammalian Sperm: A Review1. Journal of Dairy Science. 1984;67:1147–1156. doi: 10.3168/jds.S0022-0302(84)81416-2. [DOI] [PubMed] [Google Scholar]
- Hubbard EJA, Greenstein D. Introduction to the germ line. In: Community TCeR, editor. WormBook: WormBook. 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim AM, Bernhardt ML, Kong BY, Ahn RW, Vogt S, Woodruff TK, O’Halloran TV. Zinc Sparks Are Triggered by Fertilization and Facilitate Cell Cycle Resumption in Mammalian Eggs. ACS Chemical Biology. 2011;6:716–723. doi: 10.1021/cb200084y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim AM, Vogt S, O'Halloran TV, Woodruff TK. Zinc availability regulates exit from meiosis in maturing mammalian oocytes. Nat Chem Biol. 2010;6:674–681. doi: 10.1038/nchembio.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong BY, Bernhardt ML, Kim AM, O'Halloran TV, Woodruff TK. Zinc Maintains Prophase I Arrest in Mouse Oocytes Through Regulation of the MOS-MAPK Pathway. Biology of Reproduction. 2012 doi: 10.1095/biolreprod.112.099390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kritikou EA, Milstein S, Vidalain P-O, Lettre G, Bogan E, Doukoumetzidis K, Gray P, Chappell TG, Vidal M, Hengartner MO. C. elegans GLA-3 is a novel component of the MAP kinase MPK-1 signaling pathway required for germ cell survival. Genes & Development. 2006;20:2279–2292. doi: 10.1101/gad.384506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar J, Barhydt T, Awasthi A, Lithgow GJ, Killilea DW, Kapahi P. Zinc Levels Modulate Lifespan through Multiple Longevity Pathways in Caenorhabditis elegans. PLoS One. 2016;11:e0153513. doi: 10.1371/journal.pone.0153513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- L'Hernault SW. Spermatogenesis. WormBook. 2006:1–14. doi: 10.1895/wormbook.1.85.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M-H, Ohmachi M, Arur S, Nayak S, Francis R, Church D, Lambie E, Schedl T. Multiple Functions and Dynamic Activation of MPK-1 Extracellular Signal-Regulated Kinase Signaling in Caenorhabditis elegans Germline Development. Genetics. 2007;177:2039–2062. doi: 10.1534/genetics.107.081356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Chen L, Shang Y, Huang P, Miao L. The micronutrient element zinc modulates sperm activation through the SPE-8 pathway in Caenorhabditis elegans. Development. 2013;140:2103–2107. doi: 10.1242/dev.091025. [DOI] [PubMed] [Google Scholar]
- Lui DY, Colaiácovo MP. Meiotic Development in Caenorhabditis elegans. In: Schedl T, editor. Germ Cell Development in C. elegans. New York: Springer; 2013. pp. 133–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madl JE, Herman RK. Polyploids and sex determination in Caenorhabditis elegans. Genetics. 1979;93:393–402. doi: 10.1093/genetics/93.2.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin GB, White CL, Markey CM, Blackberry MA. Effects of dietary zinc deficiency on the reproductive system of young male sheep: testicular growth and the secretion of inhibin and testosterone. J Reprod Fertil. 1994;101:87–96. doi: 10.1530/jrf.0.1010087. [DOI] [PubMed] [Google Scholar]
- Miller MA, Nguyen VQ, Lee M-H, Kosinski M, Schedl T, Caprioli RM, Greenstein D. A Sperm Cytoskeletal Protein That Signals Oocyte Meiotic Maturation and Ovulation. Science. 2001;291:2144–2147. doi: 10.1126/science.1057586. [DOI] [PubMed] [Google Scholar]
- Pazdernik N, Schedl T. Introduction to germ cell development in Caenorhabditis elegans. Adv Exp Med Biol. 2013;757:1–16. doi: 10.1007/978-1-4614-4015-4_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Que EL, Bleher R, Duncan FE, Kong BY, Gleber SC, Vogt S, Chen S, Garwin SA, Bayer AR, Dravid VP, Woodruff TK, O'Halloran TV. Quantitative mapping of zinc fluxes in the mammalian egg reveals the origin of fertilization-induced zinc sparks. Nat Chem. 2014 doi: 10.1038/nchem.2133. advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racki WJ, Richter JD. CPEB controls oocyte growth and follicle development in the mouse. Development. 2006;133:4527–4537. doi: 10.1242/dev.02651. [DOI] [PubMed] [Google Scholar]
- Roh HC, Collier S, Deshmukh K, Guthrie J, Robertson JD, Kornfeld K. ttm-1 encodes CDF transporters that excrete zinc from intestinal cells of C. elegans and act in a parallel negative feedback circuit that promotes homeostasis. PLoS Genet. 2013;9:e1003522. doi: 10.1371/journal.pgen.1003522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roh HC, Collier S, Guthrie J, Robertson JD, Kornfeld K. Lysosome-Related Organelles in Intestinal Cells Are a Zinc Storage Site in C. elegans. Cell metabolism. 2012;15:88–99. doi: 10.1016/j.cmet.2011.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidel HS, Kimble J. The oogenic germline starvation response in C. elegans. PLoS One. 2011;6:e28074. doi: 10.1371/journal.pone.0028074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiernagle T. Maintenance of C. elegans. WormBook. 2006:1–11. doi: 10.1895/wormbook.1.101.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tay J, Hodgman R, Sarkissian M, Richter JD. Regulated CPEB phosphorylation during meiotic progression suggests a mechanism for temporal control of maternal mRNA translation. Genes & Development. 2003:1457–1462. doi: 10.1101/gad.1071403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tay J, Richter JD. Germ cell differentiation and synaptonemal complex formation are disrupted in CPEB knockout mice. Dev Cell. 2001;1:201–213. doi: 10.1016/s1534-5807(01)00025-9. [DOI] [PubMed] [Google Scholar]
- Tian X, Anthony K, Neuberger T, Diaz FJ. Preconception Zinc Deficiency Disrupts Postimplantation Fetal and Placental Development in Mice. Biology of Reproduction. 2014 doi: 10.1095/biolreprod.113.113910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian X, Diaz FJ. Zinc Depletion Causes Multiple Defects in Ovarian Function during the Periovulatory Period in Mice. Endocrinology. 2012;153:873–886. doi: 10.1210/en.2011-1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian X, Diaz FJ. Acute dietary zinc deficiency before conception compromises oocyte epigenetic programming and disrupts embryonic development. Developmental Biology. 2013;376:51–61. doi: 10.1016/j.ydbio.2013.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoder JH, Chong H, Guan KL, Han M. Modulation of KSR activity in Caenorhabditis elegans by Zn ions, PAR-1 kinase and PP2A phosphatase. EMBO J. 2004;23:111–119. doi: 10.1038/sj.emboj.7600025. [DOI] [PMC free article] [PubMed] [Google Scholar]