Abstract
For an examination of the progression of cavitation in large-diameter earlywood vessels of a deciduous ring-porous tree, potted saplings of Fraxinus mandshurica var japonica Maxim. were frozen and then thawed. The changes in the amount and distribution of water in the lumina of the current year's earlywood vessels during the course of the freezing and thawing were visualized by cryo-scanning electron microscopy. When samples were frozen, most of the current year's earlywood vessels were filled with water. After the subsequent thawing, the percentage of cavitated current-year earlywood vessels gradually increased with time. All of the current year's earlywood vessels were cavitated within 24 h, and only limited amounts of water remained in the lumina of earlywood vessels. Similar cavitation of earlywood vessels was observed after thawing of frozen, excised stem pieces. In contrast, many vessels of the current year's latewood retained water in the lumina during freezing and thawing. These observations indicate that the cavitation of the current year's earlywood vessels is not produced during freezing but progresses during rewarming after freezing in F. mandshurica var japonica.
Cavitation, which interferes with the transport of water in the xylem, occurs as a result of water stress and freezing of the xylem sap (Tyree and Sperry, 1989; Sperry, 1993). In dicotyledonous trees growing in northern temperate zones, water transport in the xylem is disrupted during the period from autumn to winter. Such dysfunction of water transport during the period from autumn to winter has been explained in terms of cavitation that is caused by freezing of the xylem sap (Sperry et al., 1994). It was assumed that this type of cavitation progresses as a consequence of two processes. When the xylem sap in conduits freezes, air bubbles appear as a result of the difference between the solubility of air in water and in ice. After the ice melts, the retained air bubbles expand in conduits if tension forces are generated in the vascular system (Tyree and Sperry, 1989; Sperry, 1993).
Changes in water transport in the xylem have been investigated mainly by measurements of hydraulic conductivity (Tyree and Dixon, 1986; Sperry et al., 1987, 1988a). Considerable loss of hydraulic conductivity during the winter has been reported in many species (Sperry et al., 1988b, 1994; Sperry, 1993; Tognetti and Borghetti, 1994; Magnani and Borghetti, 1995). In particular, ring-porous trees growing in northern temperature zones experience drastic losses of hydraulic conductivity after the first cold spell during the period from autumn to winter (Cochard and Tyree, 1990; Cochard et al., 1992; Sperry and Sullivan, 1992; Hacke and Sauter, 1996). By contrast, in diffuse-porous trees growing in northern temperate zones, hydraulic conductivity is lost gradually during the course of the winter season (Sperry et al., 1988b, 1994). The cited experiments provide important quantitative information about water transport in the xylem. However, to our knowledge, changes in the location of water in the xylem during the period from autumn to winter have not been monitored at the cellular level.
Cryo-scanning electron microscopy (cryo-SEM) is a powerful tool for monitoring the distribution of water in situ (Ohtani and Fujikawa, 1990; Sano et al., 1995; Canny, 1997a, 1997b; McCully et al., 1998; Buchard et al., 1999; McCully, 1999; Pate and Canny, 1999; Shane and McCully, 1999). Cavitation during the period from autumn to winter has been visualized by cryo-SEM in one species of ring-porous tree and in two species of diffuse-porous trees (Utsumi et al., 1996, 1998). The current year's large earlywood vessels of a ring-porous tree (Fraxinus mandshurica var japonica Maxim.), which had contained water during the growth season, were shown to lose water during the period from October to November (Utsumi et al., 1996). During this period, most of the leaves fell from the tree and the first cold spell was recorded. Thus, freezing appeared to cause the disappearance of water from the lumina of the current year's earlywood vessels. In contrast, the vessels of the outer annual rings of diffuse-porous trees, which are smaller in diameter than those of ring-porous trees, gradually lost water during the period from January to March (Utsumi et al., 1998). Many vessels in the outer annual rings of the diffuse-porous trees retained water in their lumina after the first cold spell in November. In diffuse-porous trees, repeated cycles of freezing and thawing probably cause cavitation in the vessels of outer annual rings, with the gradual resultant progression of cavitation.
The occurrence of cavitation during the period from autumn to winter has been revealed in some species, but the precise time course of cavitation caused by freezing under natural conditions remains to be evaluated, and details of the process of cavitation caused by freezing are not yet fully understood. Under experimental conditions, increased losses of hydraulic conductivity after freezing and subsequent thawing have been reported in some species (LoGullo and Salleo, 1993; Langan et al., 1997; Pockman and Sperry, 1997). However, changes in the amount and distribution of water in the vessel lumina as cavitation progresses remain to be determined.
Our goal in the present study was to determine whether cavitation is actually caused by freezing, and to monitor the progression of cavitation during freezing and subsequent thawing. Potted saplings of a deciduous ring-porous tree were frozen and thawed in the laboratory. Changes in the distribution of water in the lumina of the current year's earlywood vessels were visualized during the freeze-thaw cycle by cryo-SEM.
MATERIALS AND METHODS
Plant Materials
Thirty-six 4-year-old specimens of Fraxinus mandshurica var japonica, grown in the nursery of Hokkaido University, were used in this study. The height of each sample tree was about 2 m. The diameter of stems 80 cm above the ground, where the samples were taken, was about 2 cm. These sample trees were used during October to November, when most of the current year's earlywood vessels were filled with water and most of the leaves had fallen off.
Collection, Treatment, and Preparation of Samples
Small, cylindrical samples of stems (about 15 cm long) were collected from four sample trees grown in the nursery as controls. The small cylinders were collected after the xylem sap had been stabilized by freezing the stems on living trees by a previously described procedure (Utsumi et al., 1996). Watertight collars were made with plastic funnels to serve as containers for liquid nitrogen (LN2). The funnels were fitted to the sample stems and then the collars were filled with LN2 and the stems were allowed to freeze for approximately 5 min. Frozen stems were immediately removed from the sample trees and stored in a container with LN2.
Thirty-two trees that had been planted in pots were transferred to a low-temperature room (−20°C) and allowed to freeze for 30 min. This temperature is much lower than the freezing point of the water in the lumina of dead cells (Zimmermann, 1964, 1983). Small cylinders were removed, in the frozen state, from four of the frozen trees in the low-temperature room and stored in LN2. The remaining 28 trees were transferred to the laboratory (20°C). Four small cylinders were collected one from each of four trees at 1, 2, 4, 6, 12, 18, and 24 h after thawing by a procedure similar to that used for the collection of control samples.
To examine movements of water from vessels during cavitation in isolated stem pieces, 14 cylindrical stems 15 cm in length were excised from four frozen trees that had been stored in the low-temperature room for 30 min. Both cut ends of each excised stem were coated with petroleum jelly and covered with laboratory film (Parafilm, American National Can, Neenah, WI) to prevent dehydration, and the cylinders were transferred to the laboratory and kept at 20°C for thawing. Samples were taken 1, 2, 4, 6, 12, 18, and 24 h later in the same way as the planted samples.
Cryo-SEM
The sample stems that had been stored in LN2 were transferred to a low-temperature room kept at −20°C and divided into small segments (1 cm in length). These segments were cut into small blocks (5 × 5 × 5 mm) that included part of the outer two annual rings, cambial cells, and phloem. We selected one to four blocks from each stem. Transverse or tangential surfaces of each block were cleanly planed with steel blades on a sliding microtome (Yamato Koki, Tokyo) to expose cell lumina (Sano et al., 1993, 1995). Then the blocks were attached to specimen holders with a drop of glycerol and fastened with a screw. The specimen holder with the sample, immersed in LN2, was transferred to cryo-SEM system (model JSM840-a, JEOL, Tokyo) equipped with a freeze-etching unit. The holder and sample were fixed on the cold stage of the freeze-etching unit, which was maintained under a vacuum of approximately 1 × 10−4 Pa and equilibrated to −108°C. The specimen was freeze-etched under these conditions for about 10 min to eliminate contamination by frost, and then it was rotary-shadowed with a platinum-carbon pellet. The sample was transferred to the cold stage of the SEM, which was kept at about −160°C, and secondary electron images were observed and recorded at an accelerating voltage of 5 kV (Fujikawa et al., 1988; Utsumi et al., 1996, 1998).
For quantitative evaluation of the occurrence of cavitation during freezing and thawing, more than 50 current-year earlywood vessels were selected at random from the cryo-SEM photographs of transversely cut specimens at each sampling time. The vessels that had cavities larger than 20 μm in tangential diameter in their lumina were defined as cavitated vessels. The number of vessels filled with water and the number of cavitated vessels were determined, and the percentage of cavitated vessels was calculated.
RESULTS
Cavitation of Planted Saplings during Freezing and Thawing
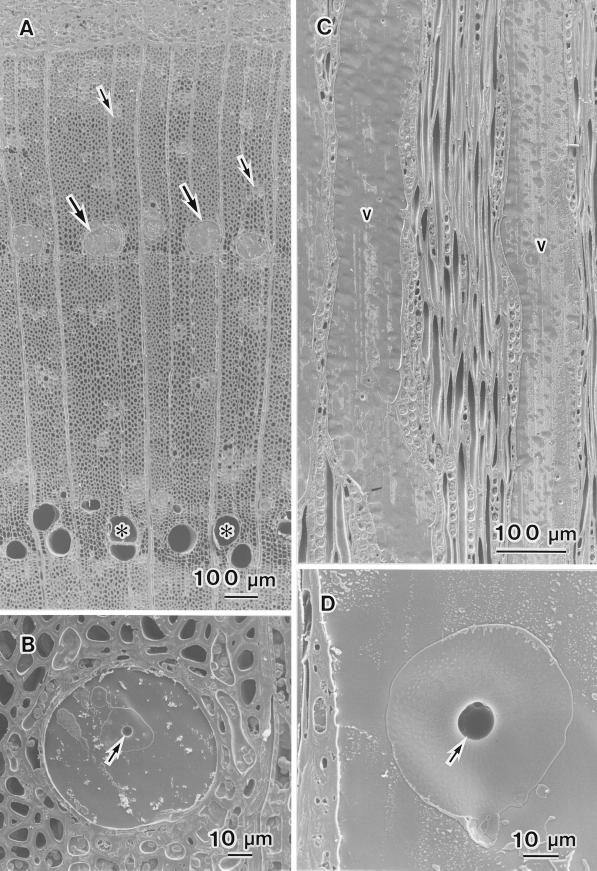
The distribution of water in the outer two annual rings was similar in control samples that had been taken from the nursery before freezing and in frozen samples that had been kept in the low-temperature room (−20°C) for 30 min. Most of the current year's earlywood vessels were filled with water (Fig. 1A, large arrows), while none of the earlywood vessels of the previous year's xylem contained water (Fig. 1A, asterisks). Many of the current year's latewood vessels were filled with water (Fig. 1A, small arrows). The water in some of the current year's earlywood vessels contained small air bubbles less than 15 μm in diameter. The sizes of these air bubbles were different between control samples and frozen samples. In control samples, many air bubbles were less than 1 μm in diameter; in the frozen samples, many were about 10 μm in diameter (Fig. 1B, arrow). On tangential surfaces, we also noted that lumina of most of the current year's earlywood vessels were filled with water (Fig. 1C, v). Some small air bubbles were visible in the water that filled the lumina of some of the current year's earlywood vessels (Fig. 1D, arrow), as shown in the transverse section in Figure 1B.
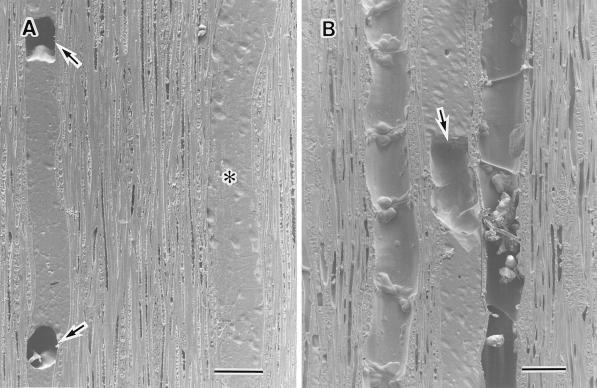
Figure 1.
Cryo-SEM photographs of planted intact samples of F. mandshurica var japonica after freezing. A, Transverse surface of two outer annual rings, the cambial zone, and phloem. The lumina of the current year's earlywood vessels (large arrows) are filled with water and the lumina of earlywood vessels of the previous year's xylem (asterisks) contain no water. The lumina of the current year's latewood vessels are filled with water (small arrows). B, Transverse surface of a current year's earlywood vessel that is filled with water. One small air bubble (arrow) is visible in the center of the vessel lumen. C, Tangential surface of the current year's earlywood vessels (v). The lumina of the current year's vessels are filled with water. D, Tangential surface of part of a current year's earlywood vessel. There is one air bubble (arrow) in the lumen.
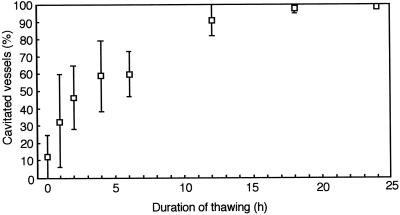
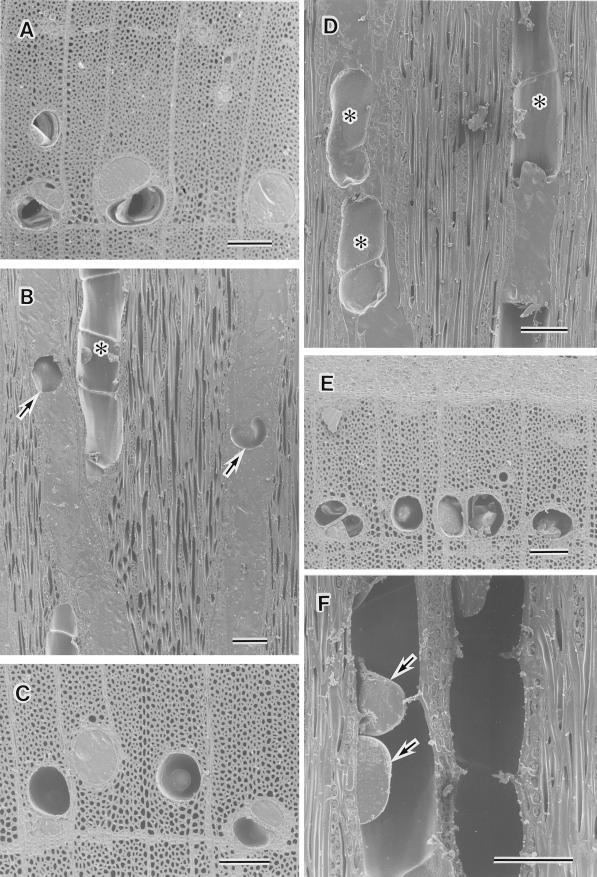
After thawing, we detected pronounced changes in the distribution of water in the current year's earlywood vessels, but no such changes were noted in the latewood vessels. Water in the lumina of the current year's earlywood vessels gradually disappeared with time. After thawing for 1 h, the percentage of cavitated vessels of the current year's earlywood was about 30% (Fig. 2). After thawing for 2 h, the percentage of cavitated vessels in the current year's earlywood increased to about 50% (Fig. 2). On transverse surfaces, we found that some of the current year's earlywood vessels had water in their lumina, while others had air in their lumina (Fig. 3A). Some current-year earlywood vessels contained large cavities of about 80 μm in tangential diameter (Fig. 3B, arrows), while others had only a limited amount of water in their lumina (Fig. 3B, asterisk). After thawing for 4 h, the percentage of cavitated vessels in the current year's earlywood was about 60% (Fig. 2). On transverse surfaces, we found that some current-year earlywood vessels were still filled with water, while others had air in their lumina (Fig. 3C). After thawing for 6 h, the percentage of cavitated vessels in the current year's earlywood was about 60% (Fig. 2). After 12 h, it was about 90% (Fig. 2) and large cavities were evident in almost all current-year earlywood vessels (Fig. 3D, asterisks). After thawing for 18 h, the percentage of cavitated vessels of the current year's earlywood was about 95% (Fig. 2). After thawing for 24 h, the vessels were almost entirely filled with air (Fig. 3E) and there was only a small amount of water in the lumina (Fig. 3F, arrows).
Figure 2.
Changes in the percentage of cavitated current-year earlywood vessels after thawing in planted intact samples. Mean values of four samples are shown, with 95% confidence intervals.
Figure 3.
Cryo-SEM photographs of planted intact samples of F. mandshurica var japonica after thawing. A, Transverse surface of the current year's xylem after thawing for 2 h. Some vessels have air in their lumina. B, Tangential surface of the current year's earlywood vessels after thawing for 2 h. Two vessels contain cavities (arrows) in their lumina. One vessel (asterisk) contains only a little water in its lumen. C, Transverse surface of the current year's xylem after thawing for 4 h. Some vessels have air in their lumina. D, Tangential surface of the current year's earlywood vessels after thawing for 12 h. Large cavities (asterisks) are visible in the vessel lumina. E, Transverse surface of the current year's xylem after thawing for 24 h. All earlywood vessels contain only a little water. F, Tangential surface of the current year's earlywood vessels after thawing for 24 h. Only a little water is visible in the vessel lumina (arrows). All bars represent 100 μm.
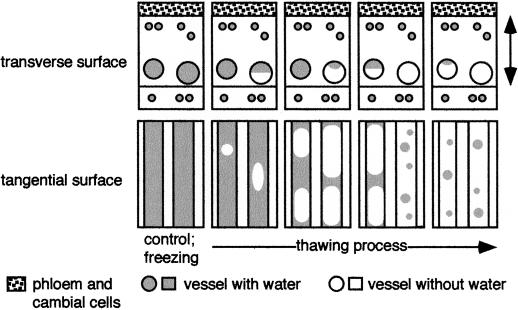
The progression of cavitation upon freezing and thawing in the current year's earlywood vessels is shown schematically in Figure 4.
Figure 4.
Schematic representation of the progression of cavitation during freezing and thawing of the current year's earlywood vessels in F. mandshurica var japonica. The double-headed arrow indicates the current year's xylem.
Cavitation of Excised Frozen Cylindrical Samples of Stems after Thawing
Changes in the distribution of water in the current year's earlywood vessels of excised cylindrical samples of stems after thawing were similar to those of planted intact stems. The relative amount of water in the lumina of the current year's earlywood vessels in excised samples decreased with time. Figure 5A shows the tangential surface of the current year's earlywood vessels after thawing for 1 h. One vessel lumen is filled with water (Fig. 5A, asterisk) while another has large cavities (Fig. 5A, arrows). Figure 5B shows the tangential surface of the current year's earlywood vessels after thawing for 4 h. One vessel has a large cavity in its lumen (arrow), while two other vessels have only a little water in their lumina. After thawing for 24 h, all of the current year's earlywood vessels were cavitated.
Figure 5.
Cryo-SEM photographs of excised samples of F. mandshurica var japonica after thawing. A, Tangential surface of the current year's earlywood vessels after 1 h of thawing. One vessel lumen is filled with water (asterisk) and another vessel lumen has cavities (arrows). B, Tangential surface of the current year's earlywood vessels after thawing for 6 h. One vessel has a large cavity in its lumen (arrow), and two vessels have a limited amount of water in their lumina. Both bars represent 100 μm.
DISCUSSION
We used cryo-SEM to examine the progression of cavitation in saplings of F. mandshurica var japonica that had been frozen and then thawed. The progression of cavitation in the current year's earlywood vessels corresponded for the most part to the model of cavitation proposed by many earlier researchers. According to this model, gases that are dissolved in the xylem sap appear as air bubbles as a result of their low solubility when the xylem sap freezes (Lybeck, 1959; Sucoff, 1969; Zimmermann, 1983). When the frozen sap thaws, if tension forces are generated in xylem conduits, the retained air bubbles expand and interfere with the transport of water in the xylem (Tyree and Sperry, 1989; Sperry, 1993). In agreement with this model, our observations showed clearly that most of the current year's earlywood vessels were filled with water in frozen samples, as they were in control samples, and that only small air bubbles were present in the water. This result indicated that cavitation did not progress during freezing. It was clear that the expansion of air bubbles occurred in the current year's earlywood vessels during and after the subsequent thawing.
The percentage of cavitated vessels in the current year's earlywood increased with the duration of time from rewarming and all vessels had only small amounts of water in their lumina after thawing for 24 h (Figs. 2 and 3). It has been suggested that in ring-porous trees growing in north temperate zones, water transport is disrupted soon after the first cold spell, as autumn turns to winter (Cochard and Tyree, 1990; Sperry and Sullivan, 1992; Sperry et al., 1994; Hacke and Sauter, 1996; Utsumi et al., 1996). However, details of the time course of cavitation have not been fully clarified. In particular, it was unclear how many hours or days are required for earlywood vessels to lose water during and after thawing once freezing has occurred. Our present results show clearly that the current year's earlywood vessels of F. mandshurica var japonica became almost empty within 1 d after the subsequent thawing.
In contrast to the earlywood vessels, many of the current year's latewood vessels retained water in their lumina during our study. This result indicates that latewood vessels are less vulnerable than earlywood vessels to cavitation due to freezing and subsequent thawing. There is a relationship between vulnerability to cavitation caused by freezing and conduit diameter (Ewers, 1985; Sperry and Sullivan, 1992; Tyree et al., 1994; Tyree and Cochard, 1996). Ewers (1985) found that air bubbles remained for a longer time in large-diameter than in small-diameter glass capillary tubes after thawing once freezing had occurred. Sperry and Sullivan (1992) suggested that cavitation caused by freezing would occur more easily at lower tensions in larger conduits than in small ones. When cavitation occurs in earlywood vessels of ring-porous trees, latewood vessels, which have relatively smaller diameters than earlywood vessels, should contribute to the water transport system. In a previous study, we found that, in F. mandshurica var japonica, most latewood vessels formed during the previous year retained water in their lumina throughout the next year (Utsumi et al., 1996). Latewood vessels formed during the previous year might play a major role in water transport at the onset of the growth season in early spring, when newly formed earlywood vessels are not yet functional.
We also examined the progression of cavitation after thawing in excised cylindrical samples of stems. In this analysis, both cut ends were heavily coated with petroleum jelly and covered with laboratory film to prevent the long-range vertical movement of water. However, cavitation progressed after thawing and the earlywood vessels became almost empty within a single day (Fig. 5), just as in planted intact samples. These results indicate that the long-range longitudinal movement of water does not take place in the lumina of earlywood vessels in intact stems when cavitation is in progress. When cavitation occurs in earlywood vessels, most fibers surrounding the earlywood vessels are empty and have the capacity to absorb the water from vessel lumina (Fig. 1A). The water from vessel lumina might move laterally during the progression of cavitation. In this study, some wood fibers of the current year's xylem were filled with water in some thawed samples (Fig. 3A). However, in some control and frozen samples, some wood fibers of the current year's xylem were filled with water (data not shown). Therefore, we were unable to conclude that water migrated from earlywood vessels to the fibers surrounding the earlywood vessels during freezing and thawing. However, refilling of the lumina of fibers that surround earlywood vessels with water has been observed in F. mandshurica var japonica under natural conditions (Utsumi et al., 1996). It is likely that water moves from earlywood vessels to the surrounding fibers when cavitation occurs during and after thawing.
Water might migrate from the current year's earlywood vessels to the fibers that surround earlywood vessels via two possible pathways. First, water in the lumina of earlywood vessels might migrate directly from the earlywood vessels to the fibers. Second, water might migrate from the earlywood vessels to surrounding fibers via parenchyma cells. F. mandshurica var japonica has both vasicentric parenchyma cells and scanty paratracheal parenchyma cells, which surround the vessels, so limited regions of earlywood vessels make contact with fibers (Sano and Fukazawa, 1994). Barnett et al. (1993) injected a solution of safranin into Quercus cellis L. and observed its rapid movement from vessels to ray parenchyma cells. They suggested that the protective layer might play an important role in the apoplastic pathway of water transport. When cavitation caused by freezing and thawing progresses in the current year's earlywood vessels, water in the lumina of earlywood vessels might migrate from earlywood vessels to fibers via the protective layer of parenchyma cells. Alternatively, if water in the lumina of earlywood vessel were to migrate directly from earlywood vessels to fibers, water might migrate through the vessel-fiber pit pairs. However, since F. mandshurica var japonica has few of these pit pairs (Sano and Fukazawa, 1994), it is unlikely that water in the lumina of earlywood vessels migrates directly to fibers.
Footnotes
This study was supported by Grants-in-Aid for Scientific Research from the Ministry of Education, Science and Culture, Japan (nos. 08456083 and 10306010), by the Japan Society for the Promotion of Science (grant no. JSPS–RFTF 96L00605), and by a Research Fellowship from the Japan Society for the Promotion of Science for Young Scientists (no. 10–2491).
LITERATURE CITED
- Barnett JR, Cooper P, Bonner LJ. The protective layer as an extension of the apoplast. IAWA J. 1993;14:163–171. [Google Scholar]
- Buchard C, McCully ME, Canny MJ. Daily embolism and refilling of root xylem vessels in three dicotyledonous crop plants. Agronomie. 1999;19:97–106. [Google Scholar]
- Canny MJ. Vessel contents of leaves after excision: a test of Scholander's assumption. Am J Bot. 1997a;84:1217–1222. [PubMed] [Google Scholar]
- Canny MJ. Vessel contents during transpiration: embolisms and refilling. Am J Bot. 1997b;84:1223–1230. [PubMed] [Google Scholar]
- Cochard H, Bréda N, Granier A, Aussenac G. Vulnerability to air embolism of three European oak species [Quercus petraea (Matt.) Liebl., Q. pubescens Willd., Q. robur L.] Ann Sci For. 1992;49:225–233. [Google Scholar]
- Cochard H, Tyree MT. Xylem dysfunction in Quercus: vessel sizes, tyloses, cavitation and seasonal changes in embolism. Tree Physiol. 1990;6:393–407. doi: 10.1093/treephys/6.4.393. [DOI] [PubMed] [Google Scholar]
- Ewers FW. Xylem structure and water conduction in conifer trees, dicot trees, and lianas. IAWA Bull New Ser. 1985;6:309–317. [Google Scholar]
- Fujikawa S, Suzuki T, Ishikawa T, Sakurai S, Hasegawa Y. Continuous observation of frozen biological materials with cryo-scanning electron microscope and freeze-replica by a new cryo-system. J Electron Microsc. 1988;37:315–322. [PubMed] [Google Scholar]
- Hacke U, Sauter JJ. Xylem dysfunction during winter and recovery of hydraulic conductivity in diffuse-porous and ring-porous trees. Oecologia. 1996;105:435–439. doi: 10.1007/BF00330005. [DOI] [PubMed] [Google Scholar]
- Langan SJ, Ewers FW, Davis SD. Xylem dysfunction caused by water stress and freezing in two species of co-occurring chaparral shrubs. Plant Cell Environ. 1997;20:425–437. [Google Scholar]
- LoGullo MA, Salleo S. Different vulnerabilities of Quercus ilex L. to freeze- and summer drought-induced xylem embolism: an ecological interpretation. Plant Cell Environ. 1993;16:511–519. [Google Scholar]
- Lybeck BR. Winter freezing in relation to the rise of sap in tall trees. Plant Physiol. 1959;34:482–486. doi: 10.1104/pp.34.4.482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnani F, Borghetti M. Interpretation of seasonal changes of xylem embolism and plant hydraulic resistance in Fagus sylvatica. Plant Cell Environ. 1995;18:689–696. [Google Scholar]
- McCully ME. Root xylem embolisms and refilling: relation to water potentials of soil, roots, and leaves, and osmotic potentials of root xylem sap. Plant Physiol. 1999;119:1001–1008. doi: 10.1104/pp.119.3.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCully ME, Huang CX, Ling LEC. Daily embolism and refilling of xylem vessels in the roots of field-grown maize. New Phytol. 1998;138:327–342. doi: 10.1046/j.1469-8137.1998.00101.x. [DOI] [PubMed] [Google Scholar]
- Ohtani J, Fujikawa S. Cryo-SEM observation on vessel lumina of a living tree: Ulmus davidiana var. Japonica. IAWA Bull New Ser. 1990;11:183–194. [Google Scholar]
- Pate JS, Canny MJ. Quantification of vessel embolisms by direct observation: a comparison of two methods. Plant Physiol. 1999;141:33–44. [Google Scholar]
- Pockman WT, Sperry JS. Freezing-induced xylem cavitation and the northern limit of Larrea tridentata. Oecologia. 1997;109:19–27. doi: 10.1007/s004420050053. [DOI] [PubMed] [Google Scholar]
- Sano Y, Fujikawa S, Fukazawa K. Studies on mechanisms of frost crack formation in tree trunks. Jpn J Freezing Drying. 1993;39:13–21. [Google Scholar]
- Sano Y, Fujikawa S, Fukazawa K. Detection and features of wetwood in Quercus mongolica var. grosseserrata. Trees. 1995;9:261–268. [Google Scholar]
- Sano Y, Fukazawa K. Structural variations and secondary changes in pit membranes in Fraxinus mandshurica var. japonica. IAWA J. 1994;15:283–291. [Google Scholar]
- Shane MW, McCully ME. Root xylem embolisms: implications for water flow to the shoot in single-rooted maize plants. Aust J Plant Physiol. 1999;26:107–114. [Google Scholar]
- Sperry JS. Winter xylem embolism and spring recovery in Betula cordifolia, Fagus grandifolia, Abies balsamera and Picea rubens. In: Borghetti M, Grace J, Raschi A, editors. Water Transport in Plants under Climatic Stress. Cambridge, UK: Cambridge University Press; 1993. pp. 86–98. [Google Scholar]
- Sperry JS, Donnelly JR, Tyree MT. A method for measuring hydraulic conductivity and embolism in xylem. Plant Cell Environ. 1988a;11:35–40. [Google Scholar]
- Sperry JS, Donnelly JR, Tyree MT. Seasonal occurrence of xylem embolism in sugar maple (Acer saccharum) Am J Bot. 1988b;75:1212–1218. [Google Scholar]
- Sperry JS, Holbrook NM, Zimmermann MH, Tyree MT. Spring filling of xylem vessels in wild grapevine. Plant Physiol. 1987;83:414–417. doi: 10.1104/pp.83.2.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperry JS, Nichols KL, Sullivan JEM, Eastlack SE. Xylem embolism in ring-porous, diffuse-porous and coniferous trees of northern Utah and interior Alaska. Ecology. 1994;75:1736–1752. [Google Scholar]
- Sperry JS, Sullivan JEM. Xylem embolism in response to freeze-thaw cycles and water stress in ring-porous, diffuse-porous, and conifer species. Plant Physiol. 1992;100:605–613. doi: 10.1104/pp.100.2.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sucoff E. Freezing of conifer xylem and the cohesion-tension theory. Physiol Plant. 1969;22:423–424. [Google Scholar]
- Tognetti R, Borghetti M. Formation and seasonal occurrence of xylem embolism in Alnus cordata. Tree Physiol. 1994;14:241–250. doi: 10.1093/treephys/14.3.241. [DOI] [PubMed] [Google Scholar]
- Tyree MT, Cochard H. Summer and winter embolism in oak: impact on water relations. Ann Sci For. 1996;53:173–180. [Google Scholar]
- Tyree MT, Davis SD, Cochard H. Biophysical perspectives of xylem evolution: is there a tradeoff of hydraulic efficiency for vulnerability to dysfunction? IAWA J. 1994;15:335–360. [Google Scholar]
- Tyree MT, Dixon MA. Water stress induced cavitation and embolism in some woody plants. Physiol Plant. 1986;66:397–405. [Google Scholar]
- Tyree MT, Sperry JS. Vulnerability of xylem to cavitation and embolism. Annu Rev Plant Physiol Plant Mol Biol. 1989;40:19–38. [Google Scholar]
- Utsumi Y, Sano Y, Fujikawa S, Funada R, Ohtani J. Visualization of cavitated vessels in winter and refilled vessels in spring in diffuse-porous trees by cryo-scanning electron microscopy. Plant Physiol. 1998;117:1463–1471. doi: 10.1104/pp.117.4.1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utsumi Y, Sano Y, Ohtani J, Fujikawa S. Seasonal changes in the distribution of water in the outer growth rings of Fraxinus mandshurica var. japonica: a study by cryo-scanning electron microscopy. IAWA J. 1996;17:113–124. [Google Scholar]
- Zimmermann MH. Effect of low temperature on ascent of sap in trees. Plant Physiol. 1964;39:568–572. doi: 10.1104/pp.39.4.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann MH. Xylem Structure and the Ascent of Sap. Berlin: Springer-Verlag; 1983. [Google Scholar]