Abstract
Biological models are necessary tools for gaining insight into underlying mechanisms governing complex pathologies such as cancer in the bone. Models range from in vitro tissue culture systems to in vivo models and can be used with corresponding epidemiological and clinical data to understand disease etiology, progression, driver mutations, and signaling pathways. In bone cancer, as with many other cancers, in vivo models are often too complex to study specific cell-cell interactions or protein roles, and 2D models are often too simple to accurately represent disease processes. Consequently, researchers have increasingly turned to 3D in vitro tissue engineered models as a useful compromise. In this review, tissue engineered 3D models of bone and cancer are described in depth and compared to 2D models. Biomaterials and cell types used are described, and future directions in the field of tissue engineered bone cancer models are proposed.
Keywords: Bone, Bone Marrow, Bone Marrow Adipose Tissue, Osteotropism, Breast Cancer Bone Metastasis, Biomaterials, Silk, Scaffolds
Graphical Abstract
Introduction
The goal of using disease models for bone cancer, or cancers that spread to bone, is to gain maximal information about how cancer and bone cells interact with each other. The accuracy of these models is crucial for researchers to determine how these interactions support cancer growth and lead to cancer-induced bone disease (e.g., osteolysis, as found in myeloma and metastatic breast cancer, or osteoblastic disease, as seen in prostate cancer metastasis). Tissue engineered disease models allow us to identify novel molecular targets, drug delivery mechanisms and disease biomarkers, which leads to better therapeutics or diagnostics for translation into the clinical setting. Cancer models in general are especially essential as cancer is the second leading cause of death in the United States and is responsible for nearly 1 of every 4 deaths.1 According to the American Cancer Society, about 1 million new cancer cases were diagnosed in 2016, and about 595,690 Americans died of cancer in 2016.2 However, cancer death rates have decreased by 23% between 1991 and 2012. This can be attributed to better screening and earlier diagnoses as well as the development of novel cancer therapies, supported by improved preclinical cancer research.2
Breast and prostate cancer, the most common cancers in women and men respectively,2 are fatal due to their highly metastatic potential during late/advanced disease stage. Commonly these cancers metastasize to the bone, wreaking havoc on the endocrine system, skeletal mechanical stability, hematopoiesis, and immune function. Metastasis is responsible for 90% of cancer mortality with solid tumors.2 Bone is one of the most common sites of metastasis in breast, prostate, and lung cancer. Bone is also frequently the site of metastasis in other cancers such as thyroid cancer, renal cancer, lymphoma, and Ewing’s sarcoma.2 Multiple myeloma not only initiates in the bone marrow (BM), but also disseminates from its original location and spreads throughout the BM; thus myeloma can also be thought of as a bone-metastatic cancer. Myeloma and other bone-metastatic cancer cells interact with BM cells that support their proliferation, chemoresistance, and evolution. Understanding the roles of these bone cells (osteoblasts, osteocytes, osteoclasts, hematopoietic cells, mesenchymal cells, and immune cells) and how they network and interact with tumor cells has proven useful in developing therapies for primary bone cancer and metastases. This phenomenon of microenvironmental support of tumors falls in line with Dr. Paget’s “seed and soil” hypothesis in which neoplastic cells (or “seeds”) only grow in hospitable bone marrow (a suitable “soil”).3 Making the soil less suitable for tumor cell homing or colonization inhibits tumor cell proliferation and disease progression. BM is rich in chemokines and growth factors, contains unique immune and stromal cell composition and has fenestrated blood vessels expressing specific molecules that uniquely facilitate tumor homing.4–11 In the bone and BM, tumor cells can influence mesenchymal and hematopoietic progenitors and mature cells, which can contribute to disease progression in many ways.1 Osteolytic tumors typically induce a vicious cycle by inhibiting osteoblastic activity while stimulating osteoclastic activity.12 Osteoblastic tumors typically do the opposite; these stimulate osteoblasts to rapidly produce excess bone matrix, leading to mechanically weak and dysfunctional bone matrix. Bone metastasis is especially insidious because tumor cells can lie dormant for a variable amount of time (from months to years) before becoming activated.3 Currently there is a need for a better understanding of the molecular pathways that govern bone metastasis to lead to better therapies and clinical outcomes for patients.
The standard method for cancer research is 2D cell culture, animal modeling, and subsequently clinical trials. More research into the efficiency of this pipeline has demonstrated that many findings in 2D culture do not translate to patients, in part due to differences between in vitro and in vivo culture as well as differences between 2D and 3D culture. Numerous researchers have found that gene expression and cell function in 2D cell culture do not closely mimic physiological gene expression found in vivo and many normal and malignant cells sense and respond to 3D environments differently than 2D environments.5
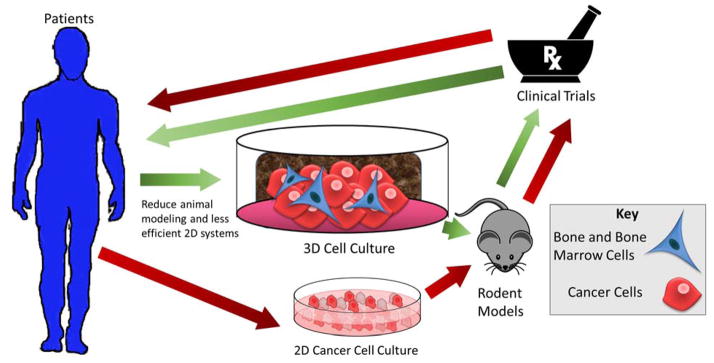
In the past few decades, a plethora of novel tissue engineered disease models of cancer have been developed through optimization of the biomaterials, bioreactors, and culture conditions for 3D culture.13 These models provided the field with new ways to accelerate the rate of cancer research and make clinically relevant discoveries about cancer biology by decreasing time wasted in unsuccessful in vivo models or failed clinical trials (Figure 1). This review discusses the numerous approaches in developing models to interrogate bone metastasis and growth in terms of cell-to-cell interactions, molecular pathway signaling, gene expression, and metabolism. Overall, this review provides a synopsis of the different types of biomaterials that are used and their relative advantages and disadvantages in the current undertaking to recapitulate the bone tumor microenvironment as accurately as possible. It concludes with some recommendations and potential future directions.
FIGURE 1.
Traditional methods of designing and testing therapeutics utilize 2D cell culture, then animal models, and finally clinical trials in patients (longer red pathway). Using 3D cell culture (shorter green pathway) allows scientists to bypass lower efficacy 2D models, minimize large-scale and expensive animal models, decrease false positives, and improve clinical research by identifying promising candidates or therapeutics more rapidly.
3D Models
Using 3D cell culture to recapitulate the tumor bone microenvironment provides an in-depth understanding of bone metastasis and cancer pathogenesis by more accurately modeling dynamic cell-cell and cell-ECM interactions. However, there are still many avenues to explore to optimize 3D cell culture by finding more suitable biomaterials for models in terms of cellular growth, reproducibility, scalability, and cost. When choosing a biomaterial for a 3D model, there are many properties to consider including longevity, biocompatibility, method of degradation, mechanics(strength, elasticity/plasticity, termed “viscoelasticity”), porosity, molecular gradients and mass transport (access to oxygen, nutrients and various soluble factors), optics, adhesion or signaling sites, surface roughness, shape, type (e.g., sponge or hydrogel), and source (natural vs. synthetic).12,14–16 Synthetic materials may allow for more control, but natural materials often promote more native cell-cell and cell-matrix signaling. The correct biomaterial must correctly regulate cellular behavior to more realistically recapitulate the biochemical signals, physiological environment, and topographical cues seen in vivo. The scaffold must provide the correct mechanical and functional environmental signals (e.g., surface chemistry and architecture)17 while encouraging cell adhesion, proliferation, and migration without evoking an inflammatory or apoptotic response. These properties are critical in recreating distinct tumor niches that are reproducible and relevant to in vivo physiology. Table 1 summarizes the biomaterials currently used for 3D bone cancer models.
Table 1.
Biomaterials used in Tissue Engineered 3D Bone Models
| Biomaterial | Scaffold Type | Cancer | References |
|---|---|---|---|
|
| |||
| Natural Materials | |||
| Silk fibroin | Scaffold, hydrogel, mat | MM, prostate, breast, osteosarcoma | 12,15,34,35 |
|
| |||
| Collagen | Hydrogel, scaffold | Breast, prostate, osteosarcoma | 38,40,91 |
|
| |||
| Chitosan-Alginate | Scaffold | Prostate, glioblastoma, hepatocellular carcinoma | 43,45 |
|
| |||
| Hyaluronic Acid | Scaffold, hydrogel | Renal cell carcinoma, MM | 22,48 |
|
| |||
| Bacterial nanocellulose (BNC) | Scaffold, hydrogel | Neuroblastoma, osteosarcoma, prostate, renal cancer, breast | 50,51 |
|
| |||
| Native ECM | Scaffold | MM, breast | 58,59 |
|
| |||
| ECM/cartilaginous matrix/Matrigel | Scaffold, hydrogel | Breast | 55 |
|
| |||
| Chitosan (with or without HA or collagen) | Scaffold | Breast | 92 |
|
| |||
| Cell sheets over medical-grade polycaprolcatone-tricalcium phosphate | Scaffold | Prostate | 93 |
|
| |||
| Synthetic Materials | Hydrogel | Breast, prostate | 64,68,94 |
| Poly(ethylene) glycol (PEG) | |||
|
| |||
| Poly(ε-caprolactone) PCL | Hydrogel | Breast, prostate, osteosarcoma, Ewing’s Sarcoma | 66,69,70 |
|
| |||
| Poly(amino acid)-based polymers | Hydrogel | Osteosarcoma | 62 |
|
| |||
| PLG non-mineralized PLG mineralized with HA |
Scaffold | Breast | 95 |
Abbreviations: BM, bone marrow; ECM, extracellular matrix; HA, hydroxyapatite; MM, multiple myeloma
Scaffold-Based Modeling Techniques
Scaffolds provide robust support for cell growth and they can simulate native extracellular matrix (ECM) in many of their properties. They can be made from natural sources such as collagen, Matrigel, silk, and agarose, but they can also come from synthetic materials such as poly(ethylene glycol) (PEG), poly(lactic-co-glycolic acid) (PLGA), or a combination of materials.18 These materials can be used alone or in combination (polyblended) to form different types of scaffolds such as sponges and hydrogels.
Hydrogels
Hydrogels are hydrophilic polymer networks that closely mimic properties of soft tissues in their viscoelastic properties and interstitial flow. They can be made of natural polymers (like hyaluronic acid (HA), collagen, silk, alginate, or chitosan), synthetic polymers, or a combination of polymers like chitosan and alginate, or PEG and silk.19,20 Hydrogels can also be reinforced with fibrous materials, like silk fibers, or microparticles to improve their strength. Hydrogels have been widely used to investigate tumor formation due to their easily tunable biophysical and mechanical properties, ease of synthesis, and reproducibility.19. For example, HA-based hydrogels have been used to test chemotherapeutics for prostate and endometrial cancers21 as well as myeloma.22 Commercially available hydrogels also exist and include Matrigel5, Tisseel, Qgel™23, ECMgel, Corgel™ BioHydrogel24, Bio-Oss® Collagen25, and Nano Dox™26. Challenges with pre-made hydrogels are their proprietary mixtures and potential lot-to-lot variations.
Sponges
Sponges, or sponge-like scaffolds, are stronger and more durable than hydrogel scaffolds and can also be made of one, or more than one, biomaterial. Sponges have a large surface area for cellular attachment and robust mechanical stability enabling long-term culture without disintegration.12,27 They also provide a comparatively high inter-fiber distance or pore size, compared to hydrogels, which facilitates nutrient and gas exchange as well as cell infiltration, which supports long-term culture.12,28 These properties make sponges useful for cancer cell culture. Silk sponges have been shown to induce significantly different angiogenic factor expression in osteosarcoma models versus 2D cell culture.29,30 Sponges with high mechanical stiffness also better recapitulate the ECM of tumors, and they can also be functionalized to stimulate tumor growth to reproduce in vivo conditions.19 Many studies agree that the properties of sponges enable better modeling of cancer-cell-ECM interactions and more accurate screening for cancer cell-drug outcomes.31
Natural Biomaterials
Silk
Silk scaffolds are made from fibroin fibers processed from raw silk that can be crafted into various morphologies such as sponges, hydrogels, films, mats, and other shapes, with various degradation profiles based on cross-linking degree and other chemistries.31 Silk scaffolds are a promising platform for 3D bone modeling due to their strength, toughness, biocompatibility, support for cell adhesion,32 thermal and chemical stability,15,31 and porosity.32 Fibroblasts, osteoblasts, hepatocytes, neurons, macrophages, keratinocytes, chondrocytes, endothelial cells, and mesenchymal stem cells have all been successfully cultured on silk scaffolds.15 Silk fibroin can be isolated from Bombyx mori or Antheraea mylitta silkworms and processed by one of two main methods, aqueous or solvent-based processing, to form silk sponges. The choice of method depends on pore size, degradation profile, mechanical strength, and surface roughness desired. Correia et al.33 compared different aqueous methods and a solvent (HFIP) method to each other and also compared these to decellurized bovine trabecular bone, in their research optimizing silk scaffolds for bone formation. Improved osteogenic induction and bone tissue formation were demonstrated by HFIP-based silk scaffold models as compared to the aqueous models. This improvement was confirmed via bone protein production (osteopontin (OPN), collagen type I, and bone sialoprotein), calcium deposition, and total bone volume assays. Architectural changes and mechanical compression from the beginning to the end of culture were also enhanced on HFIP scaffolds, determined by μCT and Young’s modulus calculations, due to the deposition of a more robust extracellular matrix.33 However, the aqueous-based structures were also similar to native bone scaffolds.33 Scaffold properties of architecture and mechanical stiffness play an important role in how bone formation occurs. The HFIP-derived models demonstrated a higher stiffness than aqueous-based scaffolds thereby providing a better platform for bone formation. Both models provided architecture that altered cellular activity and bone formation, outcomes of which were dependent on pore morphology of the scaffold.33 As with most biomaterials, methods of manufacturing silk scaffolds are highly dependent on application since different processes yield different mechanical strength and porosities.
Tan et al.34 used silk scaffolds to show differential gene expression related to chemosensitivity in 2D versus 3D osteosarcoma cultures. Doxorubicin and cisplatin are common therapeutics used in the treatment of osteosarcoma. Doxorubicin is a cell cycle specific agent while cisplatin is a cell cycle non-specific agent. The osteosarcoma cells grown on the 3D scaffold was less resistance to doxorubicin than the 2D model even though their MDR1 (multidrug resistance gene) levels stayed the same. The amount of doxorubicin needed to kill tumors in vivo was greater than the 2D cultures could be exposed to without massive cell death. However, when treated with cisplatin, a cell cycle stage non-specific drug, both 3D cell culture and 2D cell culture were similar in sensitivity to the drug. The 3D models exhibited decreased responsivity to doxorubicin similar to clinical in vivo responses, unlike 2D models, which implies that drug development using 3D culture would improve drug screening and drug development processes.34 Gene expression profiles of 3D cultured cells closely reflected mouse xenograft flank tumors and osteosarcoma tumor cells on the scaffold were more resistant to cell cycle specific chemotherapeutics, and the 3D models were much closer to in vivo studies in drug resistance than 2D models were.34 Hence, 3D silk scaffold models are a better comparison to in vivo tumor drug resistance than 2D models based on their ability to better approximate osteosarcoma physiology.34
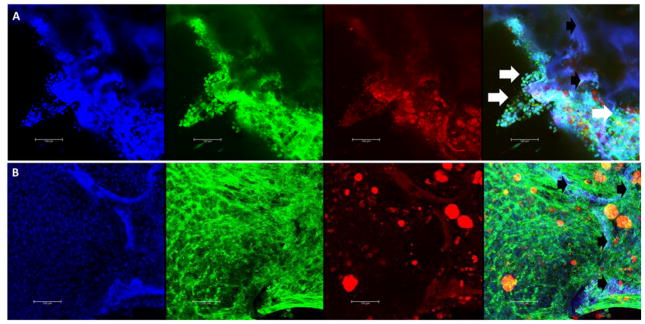
Likewise, silk scaffolds have also used to model breast cancer cell metastasis to create targeted therapeutics. Kundu et al.35 used a silk scaffold-based model for drug testing and screening using drug-loaded folate conjugated nanoparticles with MDA-MB-231 breast cancer cells and MG63 osteoblasts. They explain that, to mitigate the inconsistent and conflicting results of testing drugs in 2D cell culture and to minimize expensive animal testing, the 3D culture was utilized.35 The targeted, drug-loaded nanoparticles effectively targeted the cancer cells in the 3D model and showed a decrease in cancer cell populations after treatment, while the bone cells remained mostly normal functioning. 3D silk cell culture systems have been used in the investigation of prostate cancer metastasis by Kwon et al.36, as 2D models insufficiently demonstrated information for cellular function and tissue structure and function. Kwon et al.36 used PC3 prostate cancer cells and 3D silk sponges to create a sustainable 3D tissue system that enabled tumor cells to display an in vivo-like behavior, undergoing cell migration and invasion of the engineered bone (silk scaffold) similarly to bone metastasis.36 They hypothesized that prostate cancer cells have bone cell-like properties that allow them to survive, proliferate, migrate, and invade the bone microenvironment in a process called osteomimicry.36 This hypothesis was supported by findings from gene expression and cell migration studies on silk scaffolds.36 The authors’ system was a powerful tool for understanding how prostate cancer metastasizes, thereby supporting the development of novel diagnostic methods and therapeutics. Lastly, our lab developed a 3D bone marrow model using silk scaffolds with mechanical properties similar to bone in order to mimic how myeloma cells inhibit osteogenic differentiation.12 Silk scaffolds supported formation of dense calcified tissue and long-term culture of primary patient myeloma cells.12 Scaffolds were used to identify microRNAs aberrantly expressed by mesenchymal stem cells (MSCs) co-cultured with myeloma cells, which were found to contribute to the decreased osteoblastic activity seen in myeloma patient bones.12 We have recently developed a model for 3D bone marrow adipose tissue co-culture with tumor cells, as shown in Figure 2, which allows the researcher the ability to study both the effects of bone marrow adipose on myeloma, and myeloma on bone marrow adipose tissue.
FIGURE 2.
Confocal Imaging of Silk Scaffold Tissue Engineered Bone-Cancer Model. A heterogeneous population of mouse bone marrow stromal cells, containing mouse pre-bone marrow adipocytes, was seeded onto porous silk sponges, cultured for 9 days in growth media, and then switched to an adipogenic media for 10 days. Next, scaffolds were seeded with human green fluorescent-protein expression (GFP+) MM1S myeloma cells (A) or cultured alone (B) for 7 days. Scaffolds were formalin-fixed and stained with DAPI (for nuclei, blue), phalloidin (for actin, green), and Oil Red O (for lipids, red). Cellular phenotype was used to distinguish between green MM1S cells (round with large nuclei) and stromal cells (elongated). Confocal images show a maximum projection of blue, green, red and overlay channels (left to right). Silk is autofluorescent in every channel and thus appears purple in the overlay. White arrows indicate tumor cells. Black arrows indicate silk scaffold. The scale bar represents 100 μm in each image. This 3D model of BMAT and myeloma is one example of a 3D model that can be used to investigate the interactions and effects of myeloma on BMAT, and vice versa, in a realistic, long-term co-culture model. Such cultures provide unique insight into interactions between cancer cells and other cells of the bone and bone marrow that can then be applied to drug discovery.
Collagen
Another commonly used biomaterial for 3D modeling is collagen, which is biocompatible and biodegradable, and can be engineered and cross-linked to have stronger mechanical properties.37 Collagen I comprises approximately 95% of the organic matrix of bone and in vivo is reinforced with calcium and phosphate ions in the form of hydroxyapatite crystals to give bone its characteristic strength.37 Herroon et al. explored a novel 3D culture model using collagen to examine the interaction of prostate cancer cells with bone marrow-derived adipocytes, based on the fact that adipocytes in the bone marrow may contribute to tumor growth and metastatic progression of cancers.38 This team found that 2D cultures did not adequately recapitulate the limited diffusion-driven access to nutrients, growth factors, and signaling molecules in the bone tumor microenvironment.38 Furthermore, they found that tumor architecture in vivo was regulated by 3D spatial confirmation that 2D cell culture did not address.38 The researchers presented two new 3D models using collagen I gels to investigate bone tumor microenvironment through the interaction of co-cultured BM adipose tissue and metastatic tumor cells.38
Tumor cells in the 3D collagen I culture models had a more realistic metabolic response to adipocytes than the 2D culture models, illustrated by immunofluorescence analysis of metabolism-associated proteins, such as carbonic anhydrase 9 and hexokinase 2.38
In Fitzgerald et al.,37 three collagen-based 3D scaffolds were developed to evaluate a more physiologically relevant model of in vitro prostate cancer-bone metastasis. Two of the models incorporated different concentrations of nanohydroxyapatite (nHA) to recapitulate the the inorganic component of bone in order to improve their osteoconductive and osteoinductive capacity.37 The third collagen scaffold incorporated glycosaminoglycans, which are involved in cell adhesion, migration, proliferation, and differentiation.37 The 3D collagen models were compared to their 2D counterparts for their ability to support transfection of gene-based delivery vehicles for RNAi therapies in prostate cancer. The 3D models behaved more like in vivo tumors as shown in their gene expression, resistance to docetaxel treatment, and cell proliferation than 2D cell cultures.37 To further improve the efficacy of the collagen models for prostate cancer, in a separate study Fitzgerald et al.39 then co-cultured osteoblasts with prostate cancer cells in collagen-nHA scaffolds. The co-culture allowed direct cell-to-cell contact between osteoblasts and prostate cells as the interaction occurs in vivo, therefore producing a more clinically relevant model. Cell proliferation was reduced as compared to 2D cell culture, and MMP9, a marker of prostate cancer invasiveness, was enhanced. The investigators conclude that their ability to co-culture cancer and bone cells in a more native in vivo-like arrangement will accelerate development of novel next-generation cancer therapeutics.39
Many collagen/breast cancer models have also been developed. For example, Sameni et al. used a 3D co-culture called MAME (mammary architecture and microenvironment engineering) to investigate the progression of pre-invasive breast lesions into an invasive phenotype.40 The 3D model was constructed with a base of multiple layers of collagen I covered with a basement membrane layer. On top of this, cells were seeded and then covered with another layer of basement membrane. This model was unique in the addition of a temporal parameter using live cell imaging to monitor real-time cell-to-cell interactions. The MAME co-culture extended the time available for analysis of tumor microenvironmental changes and tumor growth, invasion, and proteolysis, compared to 2D culture.
Another collagen breast cancer model developed by Chen et al.41 examined the malignant phenotypes of MCF-7 cells in both 3D and 2D culture. The team investigated pro-angiogenic growth factor secretion, matrix metalloproteinase (MMP) transcription, epithelial to mesenchymal (EMT) transition phenotype, and cancer stem cell property acquisition. The investigators found that cells cultured in the 3D collagen scaffolds had significantly increased malignant phenotypes versus 2D culture. MCF-7 cells in 3D culture overexpressed pro-angiogenic factors and MMPs, which are associated with cancer ECM degradation, metastasis and angiogenesis. Another important factor the 3D culture provided was the hypoxic conditions as found in vivo, which aids in metastatic transformation and tumor invasion. Overall, collagen scaffolds enhanced tumorigenicity of the cells to better mimic in vivo conditions, and gave insight into metastatic processes.
In Hambach et al., collagen I scaffolds successfully facilitated growth of tumors with complex morphology akin to that found in vivo.42 Single human tumors of different cancer types (breast, kidney, and skin) were generated using human tumor cell lines on collagen I scaffolds.42 The models underwent multiparametric analysis after exposure to cellular immunotherapy. The resulting 3D tumor models resembled clinical tumors in their morphological aspects, growth patterns, and heterogeneity of target antigen expression and growth niche composition.42 The corresponding 2D cultures lacked the similarity to clinical models and differed in gene expression and intracellular signaling from the 3D tumor models.42 The ability to survey several parameters in treatment responses provided a valuable test of the efficacy of immunotherapeutics. Overall, 3D collagen models have thus proven very useful for cancer modeling.
Chitosan, alginate, and chitosan-alginate (CA) blends
Chitosan and alginate are natural polymers that have a structure similar to glycosaminoglycans, a principal constituent of ECM.43 Alginate polymers can be crosslinked to form hydrophilic hydrogels that support high cell loading densities and viable long-term cultures.44 Several cell types have been cultured in alginate scaffolds such as neuronal cells, osteoblasts, chondrocytes, and myoblasts.44 Chitosan is a natural polymer that is renewable, osteoconductive,45 biodegradable, biocompatible, non-antigenic, nontoxic, and biofunctional.46 However, chitosan, like alginate, lacks the proper mechanical strength for hard tissue engineering applications, such as bone modeling. Therefore, these materials are commonly reinforced with various ceramic phases like ollastonite, hydroxyapatite, and beta tricalcium phosphate. Chitosan can also be blended with natural polymers like silk, gelatin, and alginate.45,46 The combination of chitosan and alginate produces a mechanically strong chitosan-alginate (CA) scaffold with potentially osteoconductive properties.45 CA scaffolds have been demonstrated to replicate in vivo tumor microenvironments for human glioblastoma and hepatocellular carcinoma more effectively than 2D or Matrigel cultures.43 Blended CA scaffolds are easily dissolved in cell compatible solutions for cellular release and analysis and have long-term stability in cell culture media.43
In one study, prostate cancer cells were cultured on CA 3D scaffolds, 3D Matrigel matrix, or in 2D tissue culture dishes for 15 days and evaluated by proliferation assays and scanning electron microscopy (SEM) for morphology assessment.43 Prostate cancer cells, with or without primary lymphocytes, in Matrigel formed spheroids, similar to what is seen in vivo, while cells on the 2D surface remained flat, linear and elongated. The investigators also performed longitudinal studies on the 3D models to evaluate stability and cell growth over a long time (55 days) using live-cell fluorescence imaging. Matrigel degraded at 7 days and could not be further imaged, even with different seeding densities. However, robust CA scaffolds remained intact and supported large tumor spheroids after 55 days. The investigators summarize that the CA scaffolds generated a more clinically relevant response than the 2D cell cultures and that CA 3D scaffolds are an improved prostate cancer in vitro tumor model than 3D Matrigel matrix or 2D culture. Wang et al.47 also used 3D CA scaffolds with prostate cancer cells and confirmed the utility of these for long-term culture and 3D spheroid formation that mimicked in vivo morphologies. Gene expression of ECM and EMT gene markers was upregulated in the 3D CA scaffold. The CA scaffolds were also beneficial models for predicting in vivo targeting efficiency, which was not true in 2D.47 In sum, the CA 3D scaffolds are able to sustain long-term culture of prostate cancer cells and support immune cell interactions, suggesting that this type of model could be applied to screening immunotherapies in an array of cancers.
Hyaluronic acid
Hyaluronic Acid (HA) is a structural molecule that is ubiquitously distributed in the ECM and provides tissues with many of its physiomechanical properties.24 It does not invoke an immune response, is non-toxic, and is readily synthesized and metabolized via normal physiological pathways.24 HA can be cross-linked to form hydrogels with different mechanical properties, depending on the desired application.24 For cells to adhere and migrate, the substrate they grow on must have a certain stiffness and cell-matrix adhesion proteins. For HA hydrogels the compressive modulus can range from 9.3kPa-22.6kPa.28 Narayanan et al.22 tested matrix composition and stiffness of 3D, HA-based hydrogels for applications in BM MSCs and myeloma cell cultures. The percent survival of cells grown on medium stiffness hydrogels was higher than lower or higher stiffness samples, but cell viability was the same across all stiffness levels. The tunability of the HA 3D scaffolds is advantageous since they can be tailored to one’s desired tumor model. T. Pan et al48 investigated renal cell carcinoma (RCC) bone metastasis and found that cells remained viable over 24 days and formed spheroids in HA hydrogels, while in 2D culture the cells began to die after 2 days.48 Also, some genes strongly associated with RCC metastasis mechanisms and bone homeostasis were significantly higher in 3D versus 2D cell culture, again highlighting the utility of 3D culture.
Xu et al.49 created a novel HA hydrogel by designing a double network poly(Nε-acryloyl L-lysine)/hyaluronic acid (pLysAAm/HA) hydrogel to better replicate the breast tumor microenvironment found in vivo. The addition of the polymer improved the structural integrity and mechanical properties of the HA hydrogel allowing for long term. The elastic moduli of three pLysAAm/HA hydrogel formulations were 11.4 ± 0.8, 31.5 ± 1.5 and 46.3 ± 3.2 kPa, which are comparable to normal breast tissue, which can vary from 17.5 to 271.8 kPa. The pLysAAm/HA hydrogels also had good biocompatibility, and supported breast cancer cell growth and proliferation. When compared to their 2D counterparts, the hydrogels enhanced tumorigenic activity that mimicked in vivo behavior. The versatility of HA hydrogels with the ability to incorporate different materials and polymers makes them desirable for tumor engineering.
Bacterial nanocellulose
Bacterial nanocellulose (BNC) is a natural, nanofibrous polymer that is synthesized by the bacterium Acetobacter xylinum in virtually any microarchitecture and porosity during the bacteria fermentation process.50 BNC 3D cultures have unique and desirable properties including biocompatibility, high water affinity, unique in vivo integration due to non-degradability, and long shelf-life stability (over a year).51 BNC is inexpensive and has been used for 3D culture for cell types including neuroblastoma cells, MSCs, and osteosarcoma cells.51 It has also been used successfully in in vitro tumor engineered models with PC3 prostate cancer PC3 cells, renal cancer cells, and breast cancer cells. Xiong et al.50 used micropatterning to create macropores to overcome cell penetration issues in BNC. The patterned microporous BNC scaffolds promoted human MDA-MB-231 breast cancer cell proliferation into the scaffold, cell adhesion, and spreading. C. Gorgun et al.52 investigated the role of hypoxia on viability, morphology, and stemness of osteosarcoma stem cells using 3D bacterial cellulose scaffolds. The 3D bacterial cellulose scaffolds were able to support tissue microarchitecture, thus mimicking tumor structures and replicating in vivo conditions. The investigators discovered that hypoxia had no negative effect on cancer stem cell viability and that the subpopulation of osteosarcoma cells seen preserved their stemness and pluripotency. In sum, BNC 3D scaffolds are a new biomaterial currently being explored because of their potential in tumor and tissue engineering for long-term studies, high-throughput drug screening, and understanding cancer cell behavior.
Matrigel
Matrigel is a hydrogel that is derived from mouse tumor basement membrane, which is mostly constructed of collagen and laminin.53 Matrigel has been used in 2D culture as a thin layer, and in 3D culture as a hydrogel. It is typically mixed with other materials like collagen for 3D cell culture applications, since it has poor mechanical properties that may not be suitable to recapitulate the tumor microenvironment long-term by itself.54 For example, in studies by Kurup et al.,55 two methods of culturing breast cancer cells are explored: the overlay protocol (OP) and the embedded protocol (EP). For the OP, cells are cultured on a thin film of ECM made of Matrigel and collagen I. In the EP, the cells are completely submerged in this matrix. The authors found that even with identical ECM composition, the cells grown using the two different protocols were phenotypically different and differed in gene expression. Therefore, this work again demonstrated that a 3D physical environment and its mechanical properties are important factors in tumor formation and support. Eberle et al.56 used Matrigel for the investigation of cancer cell invasion in the formation of metastasis as it pertains to cancer treatments using X-irradiation therapy. The use of X-irradiation has been postulated to stimulate cancer cell motility/invasion and metastases by activating defined cellular and molecular mechanisms in the irradiated tissue microenvironment. The investigators used a commercially available 3D Matrigel model, an 8-μm pore size BD BioCoat™ Matrigel™ Invasion Chamber with human adenocarcinoma and prostate cancer cell lines. Each experiment in 3D also had a 2D counterpart done in parallel for comparison. Cell motility experiments using the Matrigel coated membranes assessed cell migration changes in response to irradiation. The investigators found that three of the four cancer cell lines grown in 3D, unlike the cancer cells grown in 2D, had a higher motility rate that better replicated in vivo conditions. However, the investigators found no significant effects of X-irradiation in either 2D or 3D cell cultures. Because previous studies using different cancer cell lines indicated that X-irradiation can affect migratory potentials57, it appears that cancer cell migration response to X-irradiation may be dependent on tumor type, and dose and type of X-irradiation. The ability to model different tumor types using 3D models is critical in discovering effective treatments for different types of cancers, and there is great benefit in using Matrigel in these models.
Bone-Cell Derived ECM and Native Bone
A recent area of interest in 3D cell culture is the use of native bone either with or without other biomaterials. To model the interactions between myeloma and the BM ECM, de la Puente et al.58 created 3DTEBM: 3D tissue engineered BM derived from BM supernatants from myeloma patient samples. There cultures used myeloma cells, stromal cells and endothelial cells. This model allowed for realistic oxygen and drug gradients, supported proliferation of tumor cells, and induced more drug resistance than 2D or commercial 3D tissue culture systems. Similarly, Templeton et al. used tissue fragments extracted from femoral bone via a rongeur without further processing, co-cultured with breast cancer cells to investigate cancer cell migration, colonization, and proliferation.59 The investigators discovered that the use of the bone fragments supported relevant cell types in the human bone microenvironment and were able to sustain cell viability. However, the 3D cultures had a short culture time (48–72 hours) which may reduce their applicability.
Another method of using native bone for 3D culture is decellularized bone matrix. Decellularization removes cells from the tissue of interest while preserving the structural and functional proteins of the ECM framework.60 The decellularized tissue can then be seeded with the cells of choice. Reichert et al.61 used decellularized mineralized matrix from human osteoblasts to create 3D prostate cancer models. The 3D model contained normal bone ECM proteins like osteocalcin, osteonectin, and osteopontin, all of which prostate cancer cells would encounter during bone metastasis. The use of native tissues and biomaterials is a novel avenue of research, but challenges to overcome include reproducibility, long-term culture and access to raw patient materials.
Synthetic Biomaterials
In addition to natural biomaterials, there are also synthetic biomaterials. Similar to natural biomaterials, synthetic biomaterials can be synthesized with specific mechanical and diffusion properties, and functionalized with ligands to promote cell-cell and cell-matrix communication and adhesion.62 Synthetic polymers have advantages over some natural materials because their properties are more easily controlled and they can be modulated to display specific responses to external stimuli like pH, temperature, salinity, or concentrations of small biomolecules.62 Here we describe some of the commonly used synthetic biomaterials.
Poly(ethylene) glycol: PEG
PEG is an inert, synthetic polymer popular for use in 3D cell culture because of its biocompatibility, high water content, tissue-like elasticity, and easily tunable biophysical and biomechanical properties.54 The mechanical stiffness of PEG hydrogels can be manipulated by polymer network cross-linking density for different applications and integration into different tissues. PEG can also integrate different cell-responsive motifs like integrin ligand RGD peptides, to promote cell attachment, or MMP- sensitive peptides, to allow cell-mediated matrix remodeling.54 Taubenberger et al.63 used MMP-degradable biohybrid PEG-heparin hydrogels to investigate the effect of biochemical cues on the microenvironment of early cancer events. Their team used this PEG-based hydrogel successfully for the design of tumor angiogenesis microenvironments of breast and prostate cancer cells with vascular endothelial cells and MSCs. PEG hydrogels were found to be useful in examining morphological changes, invasion, proliferation, and tumor angiogenesis in vitro. PEG can also be 3D printed for precise control of matrix/scaffold architecture for improved scalability and reproducibility. Zhu et al. designed 3D bone matrices by computer-aided design (CAD) software and printed them with a tabletop stereolithography-based 3D bioprinter.64 PEG and nHA hydrogels were fabricated at different weight percentages to change matrix stiffness and printed in three 400 μm layers. The 3D-printed PEG-multicellular based hydrogels supported spheroids that resemble natural tumor structure found in vivo while 2D cultures formed monolayers.
POLY(ε-CAPROLACTONE): PCL
PCL is a commonly used polymer for tissue engineering due to its biocompatibility, mechanical and structural properties, ease of fabrication, and long-term biodegradability.65 PCL can be fabricated to have the architecture and stiffness similar to a human tumor, and encourages cells to deposit their own matrix, thereby facilitating cell attachment and growth in 3D.66 Swaminathan et al.67 found that tumor stiffness correlates with invasiveness of cancer cells. The investigators observed that cancer cell lines displayed varying degrees of stiffness throughout a given population and this variation directly correlated to metastatic progression.67 Tumor cell stiffness values are highly dependent on the tissue they are growing in. Tissues in the body have an elastic modulus that ranges from approximately 50 Pa in neural tissue to 4 GPa in bone tissue. The ease of tunability of their physical properties, such as stiffness, and ease of integration with other biomaterials and polymers, makes PCL a desirable material for scaffolds. Sieh et al.68 designed a model using two constructs to study prostate cancer bone metastasis: a soft PEG hydrogel disc seeded with LNCaP prostate cancer cells adjacent to a hard tissue-engineered bone (TEB) construct made from mPCL-TCP (medical grade polycaprolactone –tricalcium phosphate) that was seeded with primary human osteoblasts which were grown in osteogenic media and produced mineralized bone matrix. The PEG hydrogel allowed for the prostate cancer cells to form multicellular masses that resembled avascular tumors, and the TEB produced osteoblast-derived signals to the cancer cells. Similar to the Kwon36 study on silk, this study also demonstrated osteomimicry by prostate cancer cells. The combined PEG and TEB co-culture model, unlike traditional 2D cell culture models, supported the regulation of androgen-responsive genes and increased the expression of genes associated with bone growth factors, matrix proteins, and bone remodeling enzymes, which are all also relevant to prostate cancer bone metastasis. This biphasic model has the potential to provide a bone microenvironment platform that can advance translational studies and high throughput testing of cancer therapies.
Balachander et al. also investigated the use of PCL 3D scaffolds as a model for breast cancer metastasis.66 MDA-MB-231 cells were grown on PCL scaffolds and compared to in vivo tumorigenicity and metastatic potential using gene expression microarrays, structural and mechanical characterization, cellular morphology using confocal microscopy, and cellular migration and invasion assays. PCL scaffolds resembled the topographical and mechanical properties of breast tumors due to their low elastic modulus and open pore architecture as compared with traditional 2D cell culture. Expression of gene genes related to metastatic initiation, progression, and colonization were similar for cells cultured on PCL scaffolds and cells in vivo, but this was not observed for 2D culture samples. The 3D cultured cells also had a strong pro-inflammatory gene expression signature, which could become a potential target for therapy since inflammation has been associated with cancer.66 PCL scaffolds increased the metastatic properties of breast cancer cells, therefore providing a better platform for the screening of therapeutics than traditional 2D cell culture.66 Thus, PCL scaffolds hold promise in serving as a platform for designing effective treatments and aiding in the understanding of cancer progression.
Similarly, in Holzapfel et al., PCL scaffolds coated with calcium phosphate promoted cell adhesion and osteogenic differentiation in vitro, and bone formation in vivo, to investigate advanced prostate cancer bone metastasis by creating a pre-metastatic bone marrow niche.69 In this study, PC3 prostate cancer cells were grown in static 3D culture on PCL scaffolds for 4 weeks and then transferred into a bi-axial bioreactor for another 4 weeks to increase fluid dynamics. After 8-weeks, the samples were grafted into mice. The researchers were able to increase metastatic capacity of the PC3 cells injected into the left cardiac ventricle of mice. This was accomplished by providing a humanized niche using PCL scaffolds seeded and cultured with human mesenchymal progenitor cells, for the PC3 cells to home to and develop macro-metastases in. In another study, Fong et al. grew human Ewing’s sarcoma TC-17 cells on 3D PCL scaffolds in vitro and found that this cultivated a well-differentiated Ewing’s sarcoma-like phenotype similar to that found in vivo.70 The model was compared to its 2D culture counterpart currently used as a pre-clinical gold standard for assessing drug efficacy. In the 3D PCL model, biochemical and morphological features, growth kinetics, and protein expression mirrored what is seen in vivo. On the other hand, 2D cell culture models poorly replicated Ewing’s sarcoma tumor biology.70 In two clinical trials the investigators conducted, the response to the antagonist used as a therapeutic had an unremarkable response in 2D cell culture but demonstrated tumor regression in a subset of EWS patients in the clinical trial. The 3D model appeared to be better at reproducing an in vivo-like response to therapeutics thus could potentially be better at identifying therapeutics for greater patient efficacy.
Poly(amino acid)-based polymers and poly(lactide-co-glycolide):(PLG)
Poly(amino acid)-based polymers are a new class of biomaterial used in tissue engineering because of their structural diversity, and because they are easy to synthesize and have chemical, biological, and mechanical properties that can be easily manipulated.62 Zrínyi et al. used poly(aspartic acid)-based (PASP) hydrogels as 3D scaffolds to investigate osteoblast adhesion and proliferation in osteosarcoma.62 PASP is a new scaffold material that has been shown to accelerate tissue regeneration and replace natural ECM by filling in damaged areas.62 Lynch et al.71 used PLG 3D models with and without hydroxyapatite to investigate osteogenic differentiation and OPN expression of BM-MSCs under controlled mechanical stimulation and breast cancer cell-derived paracrine factor influences. PLG scaffolds were cyclically mechanically compressed, which resulted in increased OPN expression in BM-MSCs undergoing osteogenic differentiation as compared to the static 2D cultures. Furthermore, when the samples were exposed to tumor-derived soluble factors, OPN expression was further enhanced. The enhancement indicates that OPN might not only be a factor in bone cell adhesion to the ECM, but also a primer for breast cancer cell adhesion or may in other ways support tumor progression.
Scaffold-Free, Bioreactors, and Dynamic Cultures
As described above, 3D culture systems are widely produced using natural and synthetic biomaterials. However, another emerging option is scaffold-free 3D culture, where cells grow typically in a sphere without an externally-applied biomaterial for support. The cells grown in spheroids are in different proliferative and metabolic states similar to in vivo conditions which improves testing and designing of drug therapies. These organoid cultures typically do not grow as large as cultures in scaffolds, and they risk becoming hypoxic in the center if they grow too large. Tumor spheroids, often formed using poly-HEMA (non-tissue culture-treated plastic) plates, often start from a single cell suspension and can be generated in a range of sizes depending on application and method.72 Larger spheroids starting from around 500 μm in diameter often display the cellular heterogeneity found in in vivo tumors.72 Organoid cellular clusters can be derived from primary tissue, embryonic stem cells, or induced pluripotent stem cells. Some are capable of self-renewal and self-organization and exhibit similar organ functionality to the tissue of origin.73 Organoids can be used for disease modeling, gene editing, personalized therapies, and analyzing stem cell behavior.73 For example, some 3D breast cancer organoid cultures have been found to be more innately resistant to treatments (neratinib and docetaxel) than 2D cultures.74 This may be due to differences in drug diffusion or signaling pathways that govern breast cancer response to drugs. Considerable research is now aimed at designing new assays for 3D tumor spheroid models that better account for the variability in the data and methods, but nutrient diffusion constraints will always limit the size that organoids will be able to reach.
Mechanical signals also play an important role in modulating tumor behavior in the bone microenvironment.71 Osteoblasts and osteocytes are mechanoreponsive and involved in the regulation of osteoclast differentiation and activity.71 Consequently, the mechanical loading of the skeleton plays an important role in bone metastasis. Bioreactors are devices developed to mimic in vivo physiology. They allow for precise and reproducible control over cellular environmental conditions, such as temperature, pH, flow rate, oxygen nutrient supply, and waste metabolite removal, and can provide mechanical loading to cells.75 In the vascular compartment the in vivo tissue conditions are not static thereby creating a need for a 3D models in bioreactors to better recapitulate tumor progression. Bioreactors for use in bone tissue models apply fluid sheer stress thorough porous scaffolds that activate cellular processes like differentiation and proliferation and aid in the perfusion of nutrients and oxygen transport.76 Another critical factor in bone formation and repair is oxygen concentration. For example, high dissolved oxygen concentrations of 5–10% are needed for mineralization.76 Bioreactors can supply the mechanical forces of sheer stress, pressure, gravity and oxygen concentrations needed to replicate physiological conditions for 3D models of bone. Bioreactors also overcome mass transfer limitations that are associated with static 3D culture.77 Moreover, perfusion and fluid sheer stress affect the developmental and differentiation of stem cells.78 Ferrarini et al. forced cells to grow in 3D using a dynamic culture bioreactor, called the Rotary Cell Culture System, where paclitaxel-releasing MSCs were found to inhibit the growth of multiple myeloma cells.79,80 Using novel bioreactors utilizing magnetic levitation and bioprinting method, another group forced cell spheroid formation using co-cultures of fibroblasts and tumor cells.81 The fibroblasts not only acted as a signaling stromal cell for the cancer cells, but they also produced ECM (collagen and fibronectin) such that the tumor cells were growing in an ECM ball. Although not yet used for cancer in bone, plates known as “3D Biomatrix hanging drop plates” have been used by other oncology researchers to investigate amidino-substituted benzimidazole and benzimidazo[1,2-a] quinoline derivatives’ effects on prostate and breast cancer cells in 2D and 3D culture systems.82 Changes in mechanical forces that can be controlled in a bioreactor potentially can signal pathways and cellular processes critical in understanding tumor formation and metastases. Further studies testing the effects of dynamic mechanical forces on bone metastatic pathogenesis using scaffolds may be useful in understanding how tumor cells respond to mechanical forces.
Microfluidic 3D cell culture systems can also be used to add a dynamic aspect to cultures. Microfluidic 3D cell cultures provide the user with precise control over experimental conditions and enable parallelization and automation, with low variability.83 These systems can mimic in vivo conditions by providing conditions to study cancer cell flow, attachment, transmigration, and colonization with fluid flow.84 Angiogenesis is critical for tumor growth and metastatic spread to bone and other organs; it is often modeled using microfluidic devices, such as described by Fu et al. to investigate leukemic cell-induced angiogenesis in vitro. The team engineered a device with microfluidic channels across a collagen matrix that would flow gradients of angiogenic factors secreted by leukemic cells that mimicked angiogenic induction.85 Leukemic cells were cultured with and without BM-MSCs in the 3D microfluidic angiogenesis chip in order to characterize angiogenic sprouting and morphogenesis differences induced by leukemic cells in the BM environment. This model could be useful for the evaluation of drug treatments of individual leukemia patients.
2D Versus 3D Culture Comparisons
It has become increasingly accepted that 3D cell cultures are more valuable than 2D cultures due to their capacity to mimic multidimensional tumor structures that are essential for correct cell-cell communication (e.g., integrin or notch signaling) and cell-matrix interaction (e.g., cytoskeletal rearrangements for pulling or pushing). The physical forms of cell cultures influence tumor growth rate, sustained tumor size, drug resistance, dormancy, and other characteristics. Moreover, cells grown in 2D cultures are forced to adopt a sheet-like morphology in which only a fraction of their cell membrane receives signals. By contrast, in a 3D environment, 100% of the membrane receives soluble signals. In 2D culture, cell polarization and phenotypes are abnormal and cell signaling via adhesion kinases, integrins, selectins, or selectin ligands is reduced compared to 3D cultures. This signaling and physical pressure on cells alters cell growth, migration, and apoptosis for both malignant and healthy cells. Still, 2D cultures are advantageous in that they are generally easier to maintain, simple, fast, and less expensive compared with 3D models and cost-intensive animal testing. This is due to the fact that all cells cultured in a 3D environment react differently and exhibit different phenotypic and genotypic properties compared to cells cultured in monolayers.86 For historical reasons, 2D culture is currently considered the “gold standard” of much biomedical research. Nonetheless, for bone metastatic cancers, as with many cancers, 3D culture methods are increasingly becoming the workhorse in data collection and are necessary to propel clinical and medical research forward.
Among tissue engineering researchers, there is growing consensus that 3D models better recapitulate in vivo conditions and cell responses than 2D models do.53 This review has provided an overview of the basis for this preference. Although academic laboratories have already begun to appreciate the suitability of 3D culture, and many industry and pharmaceutical laboratories are also benefiting from incorporating 3D culture into lab practice for drug development and screening research. This will likely improve the failure rate for oncology compounds entering clinical trials (currently about 90%), which is partially attributable to 2D and monoculture pre-clinical compound identification tests that produced misleading or non-predictive data for in vivo responses.87 Indeed, 3D models have recently shown significant growth in utilization in the pharmaceutical industry as 3D models have become better characterized.88
Conclusions
Animal models have some advantages over 3D systems since they can be used to study cancer spread and metastasis, shear flows/fluid forces, angiogenesis, cancer immunology, and metabolic or systemic influences on cancer all within the same animal. However, 3D models are becoming ever more sophisticated and bioreactors are being incorporated to better model in vivo fluid dynamics and mechanical stress. Moreover, the addition of endothelial cells, adipocytes, and immune cells to 3D cell cultures is now common, and media can be modified easily to study metabolites, hormones, or different energy sources. The understanding of immune responses has become increasingly important in our understanding of cancer and efficacy of drug therapies. The use of 3D cell culture models furthers this understanding by better replicating in vivo immune responses. Properties of 3D models used in investigating immune responses can be manipulated by the addition of immune cells and immune cell conditioned media. Modifications of biomaterial surface properties such as hydrophilicity, chemical modifications, roughness, and topography also can be used to optimize 3D models.89 Compared to in vivo models, 3D models are advantageous because inputs such as cell types, cell numbers, soluble factors, and matrices can be controlled more reliably.36 Moreover, animal models can have limited translatability to human physiology due to differences in mouse and human biology and protein/gene homology. Additionally, adequate animal models that can recapitulate the biology of disease in humans are often lacking. Conversely, 3D models can be completed with 100% human-derived components (scaffold material, media proteins and cells) increasing translational relevancy of the findings and side-stepping issues related to animal ethics (e.g., animal suffering). Overall, 3D tissue engineered models provide researchers with a compromise between 2D and in vivo models of bone cancer, creating a bridge for the gap between 2D and in vivo research findings.
In terms of cancer in the bone, it is clear that the choices of model system, co-culture cell types, and biomaterials are dependent on the scientific question at hand. For questions where osteolysis is being investigated, osteoclasts are a necessary cellular component. Where inhibition of bone formation is studied, osteoblasts or pre-osteoblasts must be included. Appropriate mechanical stiffness is crucial in osteogenic analyses, as biomaterial stiffness and strength directly relate to osteogenic differentiation.90 Weighing the advantages and disadvantages of each model system and biomaterial, it is also important to consider the experimental parameters for the system of interest, such as the source of tumor cells, metastatic pathways, and clinical applicability. Considering these parameters is crucial for designing the appropriate in vitro model. Typically, it is best to match as many properties as possible to in vivo conditions. However, models can be simplified pragmatically and less important cell types can be omitted to increase the usability, efficiency, and value of the model and decrease costs and complexity. There are many variables and options in designing a 3D model; for more information on previously used models and materials, please refer to Table 1.
In summary, 3D models are a promising approach for researching mechanisms and interactions of cancer within the BM microenvironment. 3D models better mimic in vivo conditions not only for cancer cells, but also for surrounding stromal cells. Co-cultured 3D models improve the bench-to-bedside translation of research. The use of 3D, co-culture assays, even as secondary screens after 2D assays, will accelerate discovery and decrease wasted time, effort, and materials in the drug discovery arena. New 3D models are currently being developed to reveal novel targets using co-culture/tri-culture systems with cells previously not explored deeply in relation to bone cancer, such as BM adipocytes, immune cells, and osteocytes. The 3D models described here, and in development in our lab and others, enable more in-depth and accurate investigations into bone cancer. The goal is that these 3D models will assist us in revealing novel mechanisms, detecting biomarkers, designing diagnostics, and developing therapeutics, all with the goal of improving patient prognosis and care, quality of life, and life expectancy.
Acknowledgments
The authors’ work is supported by the Maine Medical Center Research Institute (MMCRI) Start-up funds, the NIH/NIDDK (R24DK092759-01), and pilot project grants from the NIH/NIGMS (P30GM106391) and the American Cancer Society (Research Grant #IRG-16-191-33). The authors thank Dr. Michael Erard, Scientific Editor and Writing Consultant at MMCRI, and Heather Fairfield and Anne Ryan of the Reagan Lab, for editorial and figure design assistance.
Footnotes
Conflict of Interest: The authors declare that they have no conflict of interest.
References
- 1.American Cancer Society. [accessed Mar 8, 2015];Cancer Facts and Statistics. http://www.cancer.org/research/cancerfactsstatistics/cancerfactsfigures2015/index.
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66(1):7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 3.Paget S. The distribution of secondary growths in cancer of the breast. 1889. Cancer Metastasis Rev. 1989;8(2):98–101. [PubMed] [Google Scholar]
- 4.Reagan MR, Rosen CJ. Navigating the bone marrow niche: translational insights and cancer-driven dysfunction. Nat Rev Rheumatol. 2015;12(3):154–168. doi: 10.1038/nrrheum.2015.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Härmä V, Virtanen J, Mäkelä R, Happonen A, Mpindi J-P, Knuuttila M, Kohonen P, Lötjönen J, Kallioniemi O, Nees M. A Comprehensive Panel of Three-Dimensional Models for Studies of Prostate Cancer Growth, Invasion and Drug Responses. PLoS One. 2010;5(5):e10431. doi: 10.1371/journal.pone.0010431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kawano Y, Moschetta M, Manier S, Glavey S, Görgün GT, Roccaro AM, Anderson KC, Ghobrial IM. Targeting the bone marrow microenvironment in multiple myeloma. Immunol Rev. 2015;263(1):160–172. doi: 10.1111/imr.12233. [DOI] [PubMed] [Google Scholar]
- 7.Glavey S, Reagan M, Manier S, Moschetta M, Kawano Y, Sacco A, Roccaro AM, Robbins M, Ghobrial IM. Dissecting the Mechanisms of Activity of SLAMF7 and the Targeting Antibody Elotuzumab in Multiple Myeloma. Blood. 2014;124(21):3431. [Google Scholar]
- 8.Glavey SV, Manier S, Natoni A, Sacco A, Moschetta M, Reagan MR, Murillo LS, Sahin I, Wu P, Mishima Y, et al. The sialyltransferase ST3GAL6 influences homing and survival in multiple myeloma. Blood. 2014 doi: 10.1182/blood-2014-03-560862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roccaro AM, Hideshima T, Raje N, Kumar S, Ishitsuka K, Yasui H, Shiraishi N, Ribatti D, Nico B, Vacca A, et al. Bortezomib mediates antiangiogenesis in multiple myeloma via direct and indirect effects on endothelial cells. Cancer Res. 2006;66(1):184–191. doi: 10.1158/0008-5472.CAN-05-1195. [DOI] [PubMed] [Google Scholar]
- 10.Roccaro AM, Mishima Y, Sacco A, Moschetta M, Tai Y-T, Shi J, Zhang Y, Reagan MR, Huynh D, Kawano Y, et al. CXCR4 Regulates Extra-Medullary Myeloma through Epithelial-Mesenchymal-Transition-like Transcriptional Activation. Cell Rep. 2015 doi: 10.1016/j.celrep.2015.06.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reagan MR, Liaw L, Rosen CJ, Ghobrial IM. Dynamic Interplay between Bone and Multiple Myeloma: Emerging Roles of the Osteoblast. Bone. 2015;75:161–169. doi: 10.1016/j.bone.2015.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reagan MR, Mishima Y, Glavey SV, Zhang YY, Manier S, Lu ZN, Memarzadeh M, Zhang YY, Sacco A, Aljawai Y, et al. Investigating osteogenic differentiation in multiple myeloma using a novel 3D bone marrow niche model. Blood. 2014;124(22):3250–3259. doi: 10.1182/blood-2014-02-558007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fitzgerald KA, Malhotra M, Curtin CM, O’Brien FJ, O’Driscoll CM. Life in 3D is never flat: 3D models to optimise drug delivery. J Control Release. 2015;215:39–54. doi: 10.1016/j.jconrel.2015.07.020. [DOI] [PubMed] [Google Scholar]
- 14.Baker EL, Bonnecaze RT, Zaman MH. Extracellular Matrix Stiffness and Architecture Govern Intracellular Rheology in Cancer. Biophys J. 2009;97(4):1013–1021. doi: 10.1016/j.bpj.2009.05.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Talukdar S, Mandal M, Hutmacher DW, Russell PJ, Soekmadji C, Kundu SC. Engineered silk fibroin protein 3D matrices for in vitro tumor model. Biomaterials. 2011;32(8):2149–2159. doi: 10.1016/j.biomaterials.2010.11.052. [DOI] [PubMed] [Google Scholar]
- 16.Mastro AM, Vogler EA. A three-dimensional osteogenic tissue model for the study of metastatic tumor cell interactions with bone. Cancer Res. 2009;69(10):4097–4100. doi: 10.1158/0008-5472.CAN-08-4437. [DOI] [PubMed] [Google Scholar]
- 17.Arabnejad S, Burnett Johnston R, Pura JA, Singh B, Tanzer M, Pasini D. High-strength porous biomaterials for bone replacement: A strategy to assess the interplay between cell morphology, mechanical properties, bone ingrowth and manufacturing constraints. Acta Biomater. 2016;30:345–356. doi: 10.1016/j.actbio.2015.10.048. [DOI] [PubMed] [Google Scholar]
- 18.Pradhan S, Hassani I, Seeto WJ, Lipke EA. PEG-fibrinogen hydrogels for three-dimensional breast cancer cell culture. J Biomed Mater Res - Part A. 2017;105(1):236–252. doi: 10.1002/jbm.a.35899. [DOI] [PubMed] [Google Scholar]
- 19.Shologu N, Szegezdi E, Lowery A, Kerin M, Pandit A, Zeugolis DI. Recreating complex pathophysiologies in vitro with extracellular matrix surrogates for anticancer therapeutics screening. Drug Discov Today. 2016;21(9):1521–1531. doi: 10.1016/j.drudis.2016.06.001. [DOI] [PubMed] [Google Scholar]
- 20.Kapoor S, Kundu SC. Silk protein-based hydrogels: Promising advanced materials for biomedical applications. Acta Biomater. 2016;31:17–32. doi: 10.1016/j.actbio.2015.11.034. [DOI] [PubMed] [Google Scholar]
- 21.Saito T, Sadoshima J. Multi-Layered, Hyaluronic Acid-Based Hydrogel Formulations Suitable for Automated 3D High Throughput Drug Screening of Cancer-Stromal Cell Co-Cultures. Med Image Anal. 2016;116(8):1477–1490. doi: 10.1161/CIRCRESAHA.116.303790.The. [DOI] [Google Scholar]
- 22.Narayanan NK, Duan B, Butcher JT, Mazumder A, Narayanan BA. Characterization of multiple myeloma clonal cell expansion and stromal Wnt/β-catenin signaling in hyaluronic acid-based 3D hydrogel. In Vivo (Brooklyn) 2014;28(1):67–73. [PubMed] [Google Scholar]
- 23.Catelas I, Sese N, Wu BM, Dunn JCY, Helgerson Sa M, Tawil B, Ph D. Human Mesenchymal Stem Cell Proliferation and Osteogenic. Tissue Eng. 2006;12(8):2385–2396. doi: 10.1089/ten.2006.12.2385. [DOI] [PubMed] [Google Scholar]
- 24.Darr A, Calabro A. Synthesis and characterization of tyramine-based hyaluronan hydrogels. J Mater Sci Mater Med. 2009;20(1):33–44. doi: 10.1007/s10856-008-3540-0. [DOI] [PubMed] [Google Scholar]
- 25.Lohmann P, Willuweit A, Neffe AT, Geisler S, Gebauer TP, Beer S, Coenen HH, Fischer H, Hermanns-Sachweh B, Lendlein A, et al. Bone regeneration induced by a 3D architectured hydrogel in a rat critical-size calvarial defect. Biomaterials. 2017;113:158–169. doi: 10.1016/j.biomaterials.2016.10.039. [DOI] [PubMed] [Google Scholar]
- 26.Van Lieshout EMM, Van Kralingen GH, El-Massoudi Y, Weinans H, Patka P. Microstructure and biomechanical characteristics of bone substitutes for trauma and orthopaedic surgery. BMC Musculoskelet Disord. 2011;12(1):34. doi: 10.1186/1471-2474-12-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yao L, O’Brien N, Windebank A, Pandit A. Orienting neurite growth in electrospun fibrous neural conduits. J Biomed Mater Res - Part B Appl Biomater. 2009;90 B(2):483–491. doi: 10.1002/jbm.b.31308. [DOI] [PubMed] [Google Scholar]
- 28.Dadwal U, Falank C, Fairfield H, Linehan S, Rosen CJ, Kaplan DL, Sterling J, Reagan MR. Tissue-engineered 3D cancer-in-bone modeling: silk and PUR protocols. Bonekey Rep. 2016;5:842. doi: 10.1038/bonekey.2016.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Holle AW, Young JL, Spatz JP. In vitro cancer cell-ECM interactions inform in vivo cancer treatment. Adv Drug Deliv Rev. 2016;97:270–279. doi: 10.1016/j.addr.2015.10.007. [DOI] [PubMed] [Google Scholar]
- 30.Tan PHS, Aung KZ, Toh SL, Goh JCH, Nathan SS. Three-dimensional porous silk tumor constructs in the approximation of in vivo osteosarcoma physiology. Biomaterials. 2011;32(26):6131–6137. doi: 10.1016/j.biomaterials.2011.04.084. [DOI] [PubMed] [Google Scholar]
- 31.Koh LD, Cheng Y, Teng CP, Khin YW, Loh XJ, Tee SY, Low M, Ye E, Yu HD, Zhang YW, et al. Structures, mechanical properties and applications of silk fibroin materials. Prog Polym Sci. 2015;46:86–110. doi: 10.1016/j.progpolymsci.2015.02.001. [DOI] [Google Scholar]
- 32.HST, Shin C, Lok T, CHG, Suresh N. The Dominant Role of IL-8 as an Angiogenic Driver in a Three-Dimensional Physiological Tumor Construct for Drug Testing. Tissue Eng Part A. 2014;20(11–12):1758–1766. doi: 10.1089/ten.tea.2013.0245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Correia C, Bhumiratana S, Yan L-P, Oliveira AL, Gimble JM, Rockwood D, Kaplan DL, Sousa RA, Reis RL, Vunjak-Novakovic G. Development of silk-based scaffolds for tissue engineering of bone from human adipose-derived stem cells. Acta Biomater. 2012;8(7):2483–2492. doi: 10.1016/j.actbio.2012.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tan PHS, Chia SS, Toh SL, Goh JCH, Nathan SS. Three-dimensional spatial configuration of tumour cells confers resistance to chemotherapy independent of drug delivery. J Tissue Eng Regen Med. 2016;10(8):637–646. doi: 10.1002/term.1800. [DOI] [PubMed] [Google Scholar]
- 35.Subia B, Dey T, Sharma S, Kundu SC. Target specific delivery of anticancer drug in silk fibroin based 3D distribution model of bone-breast cancer cells. ACS Appl Mater Interfaces. 2015;7(4):2269–2279. doi: 10.1021/am506094c. [DOI] [PubMed] [Google Scholar]
- 36.Kwon H, Kim HJ, Rice WL, Subramanian B, Park S-H, Georgakoudi I, Kaplan DL. Development of an in vitro model to study the impact of BMP-2 on metastasis to bone. J Tissue Eng Regen Med. 2010;4(8):590–599. doi: 10.1002/term.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fitzgerald KA, Guo J, Tierney EG, Curtin CM, Malhotra M, Darcy R, O’Brien FJ, O’Driscoll CM. The use of collagen-based scaffolds to simulate prostate cancer bone metastases with potential for evaluating delivery of nanoparticulate gene therapeutics. Biomaterials. 2015;66:53–66. doi: 10.1016/j.biomaterials.2015.07.019. [DOI] [PubMed] [Google Scholar]
- 38.Herroon MK, Diedrich JD, Podgorski I. New 3D-culture approaches to study interactions of bone marrow adipocytes with metastatic prostate cancer cells. Front Endocrinol (Lausanne) 2016;7(JUL) doi: 10.3389/fendo.2016.00084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fitzgerald KA, Guo J, Raftery RM, Castaño IM, Curtin CM, Gooding M, Darcy R, O’Brien FJ, O’Driscoll CM. Nanoparticle-mediated siRNA delivery assessed in a 3D co-culture model simulating prostate cancer bone metastasis. Int J Pharm. 2016;511(2):1058–1069. doi: 10.1016/j.ijpharm.2016.07.079. [DOI] [PubMed] [Google Scholar]
- 40.Sameni M, Anbalagan A, Olive MB, Moin K, Mattingly RR, Sloane BF. MAME models for 4D live-cell imaging of tumor: microenvironment interactions that impact malignant progression. J Vis Exp. 2012;(60) doi: 10.3791/3661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen L, Xiao Z, Meng Y, Zhao Y, Han J, Su G, Chen B, Dai J. The enhancement of cancer stem cell properties of MCF-7 cells in 3D collagen scaffolds for modeling of cancer and anti-cancer drugs. Biomaterials. 2012;33(5):1437–1444. doi: 10.1016/j.biomaterials.2011.10.056. [DOI] [PubMed] [Google Scholar]
- 42.Hambach L, Buser A, Vermeij M, Pouw N, van der Kwast T, Goulmy E. Human Microtumors Generated in 3D: Novel Tools for Integrated In Situ Studies of Cancer Immunotherapies. Methods Mol Biol. 2016;1393:147–161. doi: 10.1007/978-1-4939-3338-9_15. [DOI] [PubMed] [Google Scholar]
- 43.Florczyk SJ, Liu G, Kievit FM, Lewis AM, Wu JD, Zhang M. 3D Porous Chitosan-Alginate Scaffolds: A New Matrix for Studying Prostate Cancer Cell-Lymphocyte Interactions In Vitro. Adv Healthc Mater. 2012;1(5):590–599. doi: 10.1002/adhm.201100054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fang X, Sittadjody S, Gyabaah K, Opara EC, Balaji KC, Shen Q, Goderie S, Jin L, Karanth N, Sun Y, et al. Novel 3D Co-Culture Model for Epithelial-Stromal Cells Interaction in Prostate Cancer. PLoS One. 2013;8(9):e75187. doi: 10.1371/journal.pone.0075187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sharma C, Dinda AK, Potdar PD, Chou CF, Mishra NC. Fabrication and characterization of novel nano-biocomposite scaffold of chitosan-gelatin-alginate-hydroxyapatite for bone tissue engineering. Mater Sci Eng C. 2016;64:416–427. doi: 10.1016/j.msec.2016.03.060. [DOI] [PubMed] [Google Scholar]
- 46.Adhikari U, Rijal NP, Khanal S, Pai D, Sankar J, Bhattarai N. Magnesium incorporated chitosan based scaffolds for tissue engineering applications. 2016 doi: 10.1016/j.bioactmat.2016.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang K, Kievit FM, Florczyk SJ, Stephen ZR, Zhang M. 3D Porous Chitosan–Alginate Scaffolds as an In Vitro Model for Evaluating Nanoparticle-Mediated Tumor Targeting and Gene Delivery to Prostate Cancer. Biomacromolecules. 2015;16(10):3362–3372. doi: 10.1021/acs.biomac.5b01032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pan T, Fong ELSS, Martinez M, Harrington DA, Lin S-H, Farach-Carson MC, Satcher RL. Three-dimensional (3D) culture of bone-derived human 786-O renal cell carcinoma retains relevant clinical characteristics of bone metastases. Cancer Lett. 2015;365(1):89–95. doi: 10.1016/j.canlet.2015.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xu W, Qian J, Zhang Y, Suo A, Cui N, Wang J, Yao Y, Wang H. A double-network poly(Nε-acryloyl l-lysine)/hyaluronic acid hydrogel as a mimic of the breast tumor microenvironment. Acta Biomater. 2016;33:131–141. doi: 10.1016/j.actbio.2016.01.027. [DOI] [PubMed] [Google Scholar]
- 50.Xiong G, Luo H, Gu F. A Novel in Vitro Three-Dimensional Macroporous Scaffolds from Bacterial Cellulose for Culture of Breast Cancer Cells. J Biomater Nanobiotechnol. 2013;4(October):316–326. doi: 10.4236/jbnb.2013.44040. [DOI] [Google Scholar]
- 51.Krontiras P, Gatenholm P, Daniel AH. Adipogenic differentiation of stem cells in three-dimensional porous bacterial nanocellulose scaffolds. 2014:195–203. doi: 10.1002/jbm.b.33198. [DOI] [PubMed] [Google Scholar]
- 52.Gorgun C, Ozturk S, Gokalp S, Vatansever S, Gurhan SID, Urkmez AS. Synergistic role of three dimensional niche and hypoxia on conservation of cancer stem cell phenotype. Int J Biol Macromol. 2015 doi: 10.1016/j.ijbiomac.2015.12.053. [DOI] [PubMed] [Google Scholar]
- 53.Liu Z, Vunjak-Novakovic G. Modeling tumor microenvironments using custom-designed biomaterial scaffolds. Curr Opin Chem Eng. 2016;11:94–105. doi: 10.1016/j.coche.2016.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ki CS, Shih H, Lin CC. Effect of 3D matrix compositions on the efficacy of EGFR inhibition in pancreatic ductal adenocarcinoma cells. Biomacromolecules. 2013;14(9):3017–3026. doi: 10.1021/bm4004496. [DOI] [PubMed] [Google Scholar]
- 55.Kurup A, Ravindranath S, Tran T, Keating M, Gascard P, Valdevit L, Tlsty TD, Botvinick EL, Provenzano PP, Inman DR, et al. Novel insights from 3D models: the pivotal role of physical symmetry in epithelial organization. Sci Rep. 2015;5:15153. doi: 10.1038/srep15153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Eberle F, Saulich MF, Leinberger FH, Seeger W, Engenhart-Cabillic R, Dikomey E, Hänze J, Hattar K, Subtil FSB. Cancer cell motility is affected through 3D cell culturing and SCF/c-Kit pathway but not by X-irradiation. Radiother Oncol. 2016;119(3):537–543. doi: 10.1016/j.radonc.2016.04.036. [DOI] [PubMed] [Google Scholar]
- 57.Suetens A, Moreels M, Quintens R, Soors E, Buset J, Chiriotti S, Tabury K, Gregoire V, Baatout S. Dose- and time-dependent gene expression alterations in prostate and colon cancer cells after in vitro exposure to carbon ion and X-irradiation. J Radiat Res. 2015;56(1):11–21. doi: 10.1093/jrr/rru070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.de la Puente P, Muz B, Gilson RC, Azab F, Luderer M, King J, Achilefu S, Vij R, Azab AK. 3D tissue-engineered bone marrow as a novel model to study pathophysiology and drug resistance in multiple myeloma. Biomaterials. 2015;73:70–84. doi: 10.1016/j.biomaterials.2015.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Templeton ZS, Bachmann MH, Alluri RV, Maloney WJ, Contag CH, King BL. Methods for Culturing Human Femur Tissue Explants to Study Breast Cancer Cell Colonization of the Metastatic Niche. J Vis Exp. 2015;(97):1–10. doi: 10.3791/52656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lee DJ, Diachina S, Lee YT, Zhao L, Zou R, Tang N, Han H, Chen X, Ko CC. Decellularized bone matrix grafts for calvaria regeneration. J Tissue Eng. 2016;7:204173141668030. doi: 10.1177/2041731416680306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Reichert JC, Quent VMC, Burke LJ, Stansfield SH, Clements JA, Hutmacher DW. Mineralized human primary osteoblast matrices as a model system to analyse interactions of prostate cancer cells with the bone microenvironment. Biomaterials. 2010;31(31):7928–7936. doi: 10.1016/j.biomaterials.2010.06.055. [DOI] [PubMed] [Google Scholar]
- 62.Juriga D, Nagy K, Jedlovszky-Hajdú A, Perczel-Kovách K, Chen YM, Varga G, Zrínyi M. Biodegradation and Osteosarcoma Cell Cultivation on Poly(aspartic acid) Based Hydrogels. ACS Appl Mater Interfaces. 2016;8(36):23463–23476. doi: 10.1021/acsami.6b06489. [DOI] [PubMed] [Google Scholar]
- 63.Taubenberger AV, Bray LJ, Haller B, Shaposhnykov A, Binner M, Freudenberg U, Guck J, Werner C. 3D extracellular matrix interactions modulate tumour cell growth, invasion and angiogenesis in engineered tumour microenvironments. Acta Biomater. 2016;36:73–85. doi: 10.1016/j.actbio.2016.03.017. [DOI] [PubMed] [Google Scholar]
- 64.Holmes B, Bulusu K, Plesniak M, Nowicki MA, Castro NJ, Plesniak MW, Zhu W, Castro NJ, Cui H, Zhou X, et al. A 3D printed nano bone matrix for characterization of breast cancer cell and osteoblast interactions. Nanotechnology. 27(31):1–9. doi: 10.1088/0957-4484/27/31/315103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Palomeras S, Rabionet M, Ferrer I, Sarrats A, Garcia-Romeu ML, Puig T, Ciurana J. Breast Cancer Stem Cell Culture and Enrichment Using Poly(ε-Caprolactone) Scaffolds. Molecules. 2016;21(4) doi: 10.3390/molecules21040537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Balachander GM, Balaji SA, Rangarajan A, Chatterjee K. Enhanced Metastatic Potential in a 3D Tissue Scaffold toward a Comprehensive in Vitro Model for Breast Cancer Metastasis. 2015 doi: 10.1021/acsami.5b09064. [DOI] [PubMed] [Google Scholar]
- 67.Swaminathan V, Mythreye K, Tim O’Brien E, Berchuck A, Blobe GC, Superfine R. Mechanical Stiffness grades metastatic potential in patient tumor cells and in cancer cell lines. Cancer Res. 2011;71(15):5075–5080. doi: 10.1158/0008-5472.CAN-11-0247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sieh S, Taubenberger AV, Lehman ML, Clements JA, Nelson CC, Hutmacher DW. Paracrine interactions between LNCaP prostate cancer cells and bioengineered bone in 3D in vitro culture reflect molecular changes during bone metastasis. Bone. 2014;63:121–131. doi: 10.1016/j.bone.2014.02.001. [DOI] [PubMed] [Google Scholar]
- 69.Holzapfel BM, Wagner F, Loessner D, Holzapfel NP, Thibaudeau L, Crawford R, Ling M-T, Clements JA, Russell PJ, Hutmacher DW. Species-specific homing mechanisms of human prostate cancer metastasis in tissue engineered bone. Biomaterials. 2014;35(13):4108–4115. doi: 10.1016/j.biomaterials.2014.01.062. [DOI] [PubMed] [Google Scholar]
- 70.Fong ELS, Lamhamedi-Cherradi S-E, Burdett E, Ramamoorthy V, Lazar AJ, Kasper FK, Farach-Carson MC, Vishwamitra D, Demicco EG, Menegaz BA, et al. Modeling Ewing sarcoma tumors in vitro with 3D scaffolds. Proc Natl Acad Sci U S A. 2013;110(16):6500–6505. doi: 10.1073/pnas.1221403110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lynch ME, Chiou AE, Lee MJ, Marcott SC, Polamraju PV, Lee Y, Fischbach C. Three-Dimensional Mechanical Loading Modulates the Osteogenic Response of Mesenchymal Stem Cells to Tumor-Derived Soluble Signals. Tissue Eng Part A. 2016;22(15–16):1006–1015. doi: 10.1089/ten.tea.2016.0153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zanoni M, Piccinini F, Arienti C, Zamagni A, Santi S, Polico R, Bevilacqua A, Tesei A. 3D tumor spheroid models for in vitro therapeutic screening : a systematic approach to enhance the biological relevance of data obtained. Nat Publ Gr. 2016;(November 2015):1–11. doi: 10.1038/srep19103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fatehullah A, Tan SH, Barker N. Organoids as an in vitro model of human development and disease. Nat Cell Biol. 2016;18(3):246–254. doi: 10.1038/ncb3312. [DOI] [PubMed] [Google Scholar]
- 74.Breslin S, O’Driscoll L, Breslin S, O’Driscoll L. The relevance of using 3D cell cultures, in addition to 2D monolayer cultures, when evaluating breast cancer drug sensitivity and resistance. Oncotarget. 2016;7(29) doi: 10.18632/oncotarget.9935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Antoni D, Burckel H, Josset E, Noel G. Three-dimensional cell culture: a breakthrough in vivo. Int J Mol Sci. 2015;16(3):5517–5527. doi: 10.3390/ijms16035517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Weyand B, Israelowitz M, Kramer J, Bodmer C, Noehre M, Strauss S, Schmälzlin E, Gille C, Von Schroeder HP, Reimers K, et al. Three-Dimensional Modelling inside a Differential Pressure Laminar Flow Bioreactor Filled with Porous Media. 2015;2015 doi: 10.1155/2015/320280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Xu X, Farach-Carson MC, Jia X. Three-dimensional in vitro tumor models for cancer research and drug evaluation. Biotechnol Adv. 2014;32(7):1256–1268. doi: 10.1016/j.biotechadv.2014.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Goodman SL, Lyden T, Li W-J, Yen T, Haycock JW, Goodman SL, Wendt KD, Kostrna MS, Radi C, Handorf AM, et al. 3D Cell Culture and Microscopy in a Capsule with Scaffolds, Tumors & Stem Cells. Microsc Microanal. 2016;22(S3):998–999. doi: 10.1017/S1431927616005833. [DOI] [Google Scholar]
- 79.Bonomi A, Steimberg N, Benetti A, Berenzi A, Alessandri G, Pascucci L, Boniotti J, Coccè V, Sordi V, Pessina A, et al. Paclitaxel-releasing mesenchymal stromal cells inhibit the growth of multiple myeloma cells in a dynamic 3D culture system. Hematol Oncol. 2016 doi: 10.1002/hon.2306. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 80.Ferrarini M, Steimberg N, Ponzoni M, Belloni D, Berenzi A, Girlanda S, Caligaris-Cappio F, Mazzoleni G, Ferrero E. Ex-Vivo Dynamic 3-D Culture of Human Tissues in the RCCS??? Bioreactor Allows the Study of Multiple Myeloma Biology and Response to Therapy. PLoS One. 2013;8(8):e71613. doi: 10.1371/journal.pone.0071613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Leonard F, Godin B. 3D In Vitro Model for Breast Cancer Research Using Magnetic Levitation and Bioprinting Method. Methods Mol Biol. 2016;1406:239–251. doi: 10.1007/978-1-4939-3444-7_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Brajša K, Vujasinović I, Jelić D, Trzun M, Zlatar I, Karminski-Zamola G, Hranjec M. Antitumor activity of amidino-substituted benzimidazole and benzimidazo[1,2-a]quinoline derivatives tested in 2D and 3D cell culture systems. J Enzym Inhib Med Chem. 2016;6:1139–1145. doi: 10.3109/14756366.2015.1101093. [DOI] [PubMed] [Google Scholar]
- 83.Willaert RG, Goossens K. Microfluidic Bioreactors for Cellular Microarrays. 2015 [Google Scholar]
- 84.Arrigoni C, Bersini S, Gilardi M, Moretti M. In Vitro Co-Culture Models of Breast Cancer Metastatic Progression towards Bone. Int J Mol Sci. 2016;17(9):1405. doi: 10.3390/ijms17091405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zheng Y, Sun Y, Yu X, Shao Y, Zhang P, Dai G, Fu J. Angiogenesis in Liquid Tumors: An In Vitro Assay for Leukemic-Cell-Induced Bone Marrow Angiogenesis. Adv Healthc Mater. 2016;5(9):1014–1024. doi: 10.1002/adhm.201501007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Abbott RD, Wang RY, Reagan MR, Chen Y, Borowsky FE, Zieba A, Marra KG, Rubin P, Ghobrial IM, Kaplan DL, et al. The Use of Silk as a Scaffold for Mature, Sustainable Unilocular Adipose 3D Tissue Engineered Systems. Adv Healthc Mater. 2016;5(13):1667–1677. doi: 10.1002/adhm.201600211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Edmondson R, Broglie JJ, Adcock AF, Yang L. Three-dimensional cell culture systems and their applications in drug discovery and cell-based biosensors. Assay Drug Dev Technol. 2014;12(4):207–218. doi: 10.1089/adt.2014.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Stock K, Estrada MF, Vidic S, Gjerde K, Rudisch A, Santo VE, Barbier M, Blom S, Arundkar SC, Selvam I, et al. Capturing tumor complexity in vitro: Comparative analysis of 2D and 3D tumor models for drug discovery. Sci Rep. 2016;6(February):28951. doi: 10.1038/srep28951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Damanik FFR, Rothuizen TC, van Blitterswijk C, Rotmans JI, Moroni L. Towards an in vitro model mimicking the foreign body response: tailoring the surface properties of biomaterials to modulate extracellular matrix. Sci Rep. 2014;4:6325. doi: 10.1038/srep06325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Linsley CS, Wu BM, Tawil B. Mesenchymal stem cell growth on and mechanical properties of fibrin-based biomimetic bone scaffolds. J Biomed Mater Res Part A. 2016;104(12):2945–2953. doi: 10.1002/jbm.a.35840. [DOI] [PubMed] [Google Scholar]
- 91.Titorencu I, Albu MG, Anton F, Georgescu A, Jinga VV. Scaffolds for Bone Tissue Engineering. 2012;555:208–217. doi: 10.1080/15421406.2012.635540. [DOI] [Google Scholar]
- 92.Zhu W, Wang M, Fu Y, Castro NJ, Fu SW, Zhang LG. Engineering a biomimetic three-dimensional nanostructured bone model for breast cancer bone metastasis study. Acta Biomater. 2015;14:164–174. doi: 10.1016/j.actbio.2014.12.008. [DOI] [PubMed] [Google Scholar]
- 93.Sieh S, Lubik AA, Clements JA, Nelson CC, Hutmacher DW. Interactions between human osteoblasts and prostate cancer cells in a novel 3D in vitro model. Organogenesis. 6(3):181–188. doi: 10.4161/org.6.3.12041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhu W, Holmes B, Glazer RI, Zhang LG. 3D printed nanocomposite matrix for the study of breast cancer bone metastasis. Nanomedicine Nanotechnology, Biol Med. 2016;12(1):69–79. doi: 10.1016/j.nano.2015.09.010. [DOI] [PubMed] [Google Scholar]
- 95.Pathi SP, Kowalczewski C, Tadipatri R, Fischbach C. A novel 3-D mineralized tumor model to study breast cancer bone metastasis. PLoS One. 2010;5(1):e8849. doi: 10.1371/journal.pone.0008849. [DOI] [PMC free article] [PubMed] [Google Scholar]