Abstract
Obesity is associated with the inappropriate activation of the renin-angiotensin-system (RAS), which increases arterial pressure, impairs insulin secretion, and decreases peripheral tissue insulin sensitivity. RAS blockade reverses these detriments; however, it is not clear whether the disease state of the organism and treatment duration determine the beneficial effects of RAS inhibition on insulin secretion and insulin sensitivity. Therefore, the objective of this study was to compare the benefits of acute vs. chronic angiotensin receptor type 1 (AT1) blockade started after the onset of obesity, hyperglycemia, and hypertension on pancreatic function and peripheral insulin resistance. We assessed adipocyte morphology, glucose intolerance, pancreatic redox balance and insulin secretion after 2 and 11-weeks of AT1 blockade in the following groups of rats: (1) untreated Long-Evans Tokushima Otsuka (lean control; n = 10), (2) untreated Otsuka Long-Evans Tokushima Fatty (OLETF; n = 12) and (3) OLETF + ARB (ARB; 10 mg olmesartan/kg/d by oral gavage; n = 12). Regardless of treatment duration, AT1 blockade decreased systolic blood pressure, fasting plasma triglycerides and the insulin resistance index, whereas chronic AT1 blockade decreased fasting plasma glucose, glucose intolerance and the relative abundance of large adipocytes by 22%, 36%, and 70%, respectively. AT1 blockade, however, did not improve pancreatic oxidative stress or reverse impaired insulin secretion. Collectively, these data show that AT1 blockade after the onset of obesity, hyperglycemia, and hypertension improves peripheral tissue insulin sensitivity, but cannot completely reverse the metabolic derangement characterized by impaired insulin secretion once it has been compromised.
Keywords: Insulin resistance, hypertension, renin-angiotensin-system, adiposity, reactive oxygen species
Introduction
Obesity affects 35% of males and 40% of females in the United States (Flegal, et al. 2016) and predisposes individuals to the development of cardiovascular disease (Gaal, et al. 2006) (CVD) and type 2 diabetes mellitus (T2DM) (Kahn, et al. 2006). Another detriment of obesity is the inappropriate activation of the renin-angiotensin system (RAS) (Engeli, et al. 2005). Inappropriately activated RAS disrupts the actions of insulin in peripheral tissues. In L6 and primary myotubes, elevated Ang II levels decrease insulin-stimulated glucose uptake and glucose transporter 4 (GLUT4) translocation (Csibi, et al. 2010; Wei, et al. 2008; Wei, et al. 2006). In primary human preadipocytes, elevated Ang II levels decrease cell differentiation leading to the formation of large adipocytes (Janke, et al. 2002), while in male Wistar rats, elevated Ang II levels increase hepatic glucose output (Rao 1996), ultimately these events may contribute to the development of T2DM. On the contrary, angiotensin-converting enzyme inhibitors (ACEi) and angiotensin receptor blockers (ARB) reduced the onset of T2DM in individuals with cardiovascular risk factors or CVD (McMurray, et al. 2010; Tocci, et al. 2011). Moreover, in individuals without CVD, but with impaired fasting glucose or glucose intolerance, treatment with an ACEi increased the regression to normoglycemia (Bosch, et al. 2006) suggesting that the state of the disease at the onset of RAS inhibition may determine the effectiveness of the intervention on glucose regulation.
Healthy β-cells compensate for glucose intolerance and insulin resistance by insulin hypersecretion. However, β-cell dysfunction, the inability of β-cells to sustain this compensatory response, ultimately leads to the development of T2DM. Many factors such as elevated angiotensin II (Ang II) levels may result in β-cell dysfunction (Chhabra, et al. 2013; Chu, et al. 2006; Habibi, et al. 2008; Lastra, et al. 2007; Sauter, et al. 2015). Elevated Ang II levels also increase the expression and activity of the oxidant-generating enzyme NADPH oxidase 2 (Nox2) in L6 myotubes (Lastra et al. 2007; Wei et al. 2006). To manage tolerable levels of oxidants, antioxidant enzymes such as superoxide dismutase (SOD) neutralize elevated levels of superoxide. Pancreatic islets of male Wistar rats contain moderate but physiologically sufficient levels of SOD in the cytoplasm and mitochondria (Tiedge, et al. 1997). However, levels of the hydrogen peroxide removing enzymes, glutathione peroxidase (GPx) and catalase, are extremely low in pancreatic islets, composing less than 1% of the expression levels in the liver (Tiedge et al. 1997). Consequently, excessive generation of reactive oxygen species (ROS) impairs β-cell function leading to decreased glucose-stimulated insulin secretion (GSIS) (Li, et al. 2012). Nevertheless, this condition was reversed by AT1 blockade in young db/db mice (Chu, et al. 2007) suggesting that activation of AT1 contributes to the manifestation of insulin resistance via oxidative injury to the pancreas and associated impaired GSIS. However, the degree to which AT1 blockade can correct the pancreatic dysfunction present during the later progression of insulin resistance and early onset T2DM is not well established.
Although there has been substantial progress in delineating the mechanisms by which RAS activation impairs pancreatic β-cell function and peripheral insulin signaling; whether the disease state of the organism and treatment duration determine the beneficial effects of RAS inhibition is unknown. The objective of this study was to compare the benefits of acute vs. chronic AT1 blockade started after the onset of obesity, hyperglycemia, and hypertension on pancreatic function and peripheral insulin resistance. Using Otsuka Long-Evans Tokushima Fatty (OLETF) rats, a model characterized by hyperphagia, obesity, hyperglycemia, hypertension, dyslipidemia, and elevated RAS (Kawano, et al. 1992; Montez, et al. 2012; Nishiyama, et al. 2008), we tested the hypothesis that chronic AT1 blockade after the onset of obesity, hyperglycemia, and hypertension decreases fasting plasma glucose and glucose intolerance by improving adipocyte morphology, and that these effects are independent of improvements in pancreatic function.
Concise Methods
Detailed methods are available in the supplemantary material.
All experimental procedures were reviewed and approved by the institutional animal care and use committees of Kagawa Medical University (Kagawa, Japan), and the University of California, Merced (Merced, CA).
Animals
Eight-week-old male, Long-Evans Tokushima Otsuka (LETO) and OLETF rats were studied (Japan SLC Inc., Hamamatsu, Japan). Rats were randomly assigned to their study groups based on body mass (BM), so that mean BM in each group was within 5% of each other at the onset. The study groups were: (1) untreated LETO (lean control; n=5/time point) + vehicle (0.5% methylcellulose by oral gavage once daily; 1μL/g), (2) untreated OLETF (n=6/time point) + vehicle, and (3) OLETF + ARB (ARB; 10 mg olmesartan/kg/d by oral gavage at a volume of 1μL/g; for 2 or 11 wks; n=6/time point). For ARB administration, olmesartan was suspended in distilled water using 0.5% methylcellulose to achieve a concentration of 10 mg/mL and was kept at 4°C for less than 5 days. The two ARB dosing regiments represented acute (2 weeks) and chronic (11 weeks) treatments. All animals were housed in a specific pathogen-free facility under controlled temperature (23 ºC) and humidity (55%) with a 12-h light, 12-h dark cycle. All animals had free access to water and standard laboratory rat chow consisting of 5% fat, 24% protein, and 54% carbohydrates (MF; Oriental Yeast Corp., Tokyo, Japan).
Oral Glucose Tolerance Test (oGTT)
At −4, 2 and 11 weeks, following a 12 hour fast (21:00 – 09:00) oGTTs were performed 09:00 – 12:00 to assess glucose tolerance as previously described (Rodriguez, et al. 2012). The positive incremental area under the curve for glucose (AUCglucose) and insulin (AUCinsulin) were calculated by the trapezoidal method (Allison, et al. 1995) and used to calculate the insulin resistance index (IRI).
Pancreatic insulin and insulin secretion
For the measurement of total pancreatic insulin, 80 mg of frozen pancreatic tissue were homogenized in 250 uL of cold RIPA buffer, containing PIC (Thermo, Waltham, MA). The homogenized tissue was centrifuged (20,000g x 10 min at 4°C), and the aqueous layer was transferred to a separate tube and stored at −80 C for later analysis. Insulin secretion was calculated using the total area under the curve for insulin divided by the total area under the curve for glucose from the oGTT as previously described (Maki, et al. 2009; Retnakaran, et al. 2008).
Western Blot
Cytosolic and membrane proteins were extracted as previously described (Viscarra, et al. 2011) and assayed as described in the supplementary material. Membranes were scanned in an Odyssey infrared imager (LI-COR Biosciences, Lincoln, NE).
Statistics
Means (± standard error) were calculated using all samples unless otherwise noted. Baseline measurements were compared using an independent sample t-test. We used a one-way ANOVA at each time point for adiposity measurements with treatment group as a between subject factor. For NEFA measurements following an oGTT, we used a three factor ANOVA with group and time (wks) as between-subject factors and time after administration as a within-subject factor. For all other data, we used a two factor ANOVA with group and time as between-subject factors unless otherwise specified in the figure legend. When significant differences were observed, pair-wise comparisons were carried out using a Bonferroni correction. Glucose tolerance was assessed by comparing mean AUC values obtained from the glucose profiles during the oGTTs. Statistical significance was set at p < 0.05. Statistical analyses were performed with SPSS version 24 (IBM, Armonk, NY).
Results
Baseline characteristics of OLETF rats
Food intake, fasting plasma glucose (FPG), triglycerides (TG), non-esterified fatty acids (NEFA), glucose tolerance, insulin secretion, and the IRI were measured to assess the disease state at the onset of the study before intervention. At baseline, OLETF rats were characterized by higher food intake and FPG as compared to LETO (Table S1).
Effects of AT1 blockade on SBP and heart rate
SBP and heart rate were measured to assess the effects of AT1 blockade on cardiovascular function. SBP measured by tail cuff. SBP was greater at baseline in OLETF compared to LETO and remained elevated for the duration of the study. AT1 blockade decreased SBP at 2 weeks and remained lower throughout the study (Fig. 1A). Heart rate measure by tail cuff. There was a significant time, but not group effect on heart rate. Mean heart rate decreased with time (Fig. 1B). SBP measured by telemetry: SBP was greater at -3 weeks in OLETF compared to LETO, and AT1 blockade normalized it (Fig. S1). However, because the loss of battery life in most telemeters, comparisons could only be made until 5 weeks (Fig. S1).
Figure 1.
AT1 blockade decreases systolic blood pressure. Mean (±SE) (A) systolic blood pressure and (B) heart rate measured by tail cuff of Long-Evans Tokushima Otsuka (LETO; n=4), Otsuka Long-Evans Tokushima Fatty (OLETF; n=5), and OLETF + ARB (n=5). Comparisons were assessed using a two factor ANOVA with group as between-subjects factor and time as a within-subjects factor. a p < 0.05 vs. LETO; b p < 0.05 vs. OLETF; # p < 0.05 vs. baseline.
Chronic AT1 blockade decreases fasting plasma glucose
FPG, TG, NEFA, adiponectin, and IRI were measured to assess whether the timing of AT1 blockade influenced the biochemical parameters of metabolic syndrome and systemic insulin resistance. At 2-weeks, mean FPG was 51% greater in OLETF compared to LETO, and ARB had no significant effect. At 11-weeks, mean FPG was 63% higher in OLETF compared to LETO, and ARB reduced it 22% compared to OLETF (Table 1). There was a significant group, but not time effect on TG. Mean TG were greater in OLETF compared to LETO, while ARB reduced them (Table 1). At 2-weeks, mean plasma NEFA were 56% higher in OLETF compared to LETO, while ARB had no significant effect (Table 1). At 11-weeks, mean plasma NEFA were not different among the groups (Table 1). There was a significant time, but not group effect on plasma adiponectin. Mean plasma adiponectin decreased with time (Table 1). At 2-weeks, mean IRI was not different among the groups (Table 1). At 11-weeks, mean IRI was 3.7-fold greater in OLETF compared to LETO and ARB normalized it (Table 1). Collectively, these data demonstrate that the improvements of parameters related to metabolic syndrome are independent of the timing or duration of AT1 blockade, except FPG and IRI, which improved only after chronic blockade.
Table 1.
Mean (± SE) morphometrical, biochemical, and hormone measurements in LETO, OLETF, and OLETF + ARB male rats.
| 2 wks | 11 wks | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| LETO (n=5) | OLETF (n=6) | OLETF ARB (n=6) | LETO (n=5) | OLETF (n=6) | OLETF ARB (n=6) | |
| FI (g) | 17.7 ± 0.5 | 25.2 ± 0.7a | 24.3 ± 0.9a | 19.4 ± 0.5 | 29.3 ± 1.1a | 26.8 ± 0.3a |
| Retro fat mass (g)1 | 4.5 ± 0.4 | 11.4 ± 1.1c | 10.8 ± 1.7c | 7.7 ± 0.5 | 23.9 ± 2.0e | 16.8 ± 1.8e,f |
| Epi fat mass (g)1 | 4.6 ± 0.6 | 9.7 ± 0.9c | 7.1 ± 1.0 | 7.8 ± 0.7 | 13.9 ± 1.0e | 11.6 ± 1.1 |
| Glucose (mmol/L) | 4.1 ± 0.3 | 6.2 ± 0.1c | 6.4 ± 0.4c | 4.1 ± 0.3 | 6.7 ± 0.6e | 5.2 ± 0.2f,h |
| TG (mmol/L) | 0.6 ± 0.1 | 1.1 ± 0.1a | 0.8 ± 0.1a,b | 0.4 ± 01 | 1.3 ± 0.2a | 0.9 ± 0.1a,b |
| NEFA (mEq/L) | 0.57 ± 0.05 | 0.89 ± 0.07c | 0.79 ± 0.04c | 0.63 ± 0.05 | 0.71 ± 0.04g | 0.80 ± 0.10 |
| Adiponectin (ug/mL) | 2.43 ± 0.11 | 2.92 ± 0.32 | 3.08 ± 0.32 | 1.90 ± 0.10§ | 2.50 ± 0.18§ | 2.07 ± 0.13§ |
| IRI (r.u. x 106) | 4.2 ± 1.9 | 4.9 ± 1.4 | 9.1 ± 3.3 | 3.5 ± 8.6 | 16.5 ± 4.8e,g | 4.2 ± 1.6f |
Comparisons were assessed using a two factor ANOVA with group and time as between-subject factors or 1a one-way ANOVA at each time point.
FI, food intake; BM, body mass; TG, triglycerides; NEFA, non-esterified fatty acids; IRI, insulin resistance index.
Main effects:
p < 0.05 vs. LETO;
p < 0.05 vs. OLETF;
p < 0.05 vs. 2 wks.
Pairwise comparisons:
p < 0.05 vs. LETO 2 wks;
p < 0.05 vs. LETO 11 wks;
p < 0.05 vs. OLETF 11 wks;
p < 0.05, OLETF 2 wks vs. OLETF 11 wks;
p <0.05, OLETF ARB 2 wks vs. OLETF ARB 11 wks.
The metabolic syndrome-like phenotype is associated with oxidative stress and impaired antioxidant capacity in the pancreas
Markers of oxidative damage and antioxidant enzyme activities were measured to assess the effects of the metabolic syndrome-like phenotype and AT1 signaling on pancreatic redox balance. There was a significant group, but not time effect on pancreatic lipid peroxidation (4-HNE levels). Mean 4-HNE levels were higher in OLETF compared to LETO and ARB had no significant effect (Fig. 2A). Mean pancreatic nitrotyrosine levels did not change among the groups or different time points (data not shown). There was a significant group, but not time effect on pancreatic SOD, catalase and GPx activities. Mean SOD, catalase and GPx activities decreased in OLETF compared to LETO, and ARB had no significant effect (Fig. 2B, C & D). Collectively, these data demonstrate that the metabolic syndrome-like phenotype is associated with suppression of pancreatic antioxidant capacity and increased lipid peroxidation and is unaffected by AT1 blockade.
Figure 2.
Effects of AT1 blockade on redox balance in the pancreas. Mean (± SE) (A) 4-hydroxynonenal, and the representative dot blot, (B) activity of pancreatic superoxide dismutase (C) catalase and (D) glutathione peroxidase in pancreas of Long-Evans Tokushima Otsuka (LETO), Otsuka Long-Evans Tokushima Fatty (OLETF), and OLETF + ARB. Comparisons were assessed using a two factor ANOVA with group and time as between-subject factors. a p < 0.05 vs. LETO.
The metabolic syndrome-like phenotype is associated with blunted insulin secretion
Pancreatic insulin content, glucose transporter 2 (Glut 2) expression, and insulin secretion were measured to assess the effects of a pro-oxidant environment and AT1 signaling on pancreatic function. There were no group or time effects on pancreatic Glut 2 protein expression (data not shown). At 2-weeks, mean pancreatic insulin content was 2.9-fold greater in OLETF compared to LETO, and ARB normalized it (Fig. 3A). There was a significant group, but not time effect on insulin secretion. Mean insulin secretion was reduced in OLETF compared to LETO, and ARB had no significant effect (Fig. 3B). Collectively, these results suggest that AT1 blockade started after the onset of obesity, hyperglycemia, and hypertension is not able to recover impaired pancreatic insulin secretory capacity.
Figure 3.
Metabolic syndrome blunts insulin secretion. Mean (± SE) (A) pancreatic insulin, and (B) insulin secretion of Long-Evans Tokushima Otsuka (LETO), Otsuka Long-Evans Tokushima Fatty (OLETF), and OLETF ARB. Comparisons were assessed using a two factor ANOVA with group and time as between-subject factors. a p < 0.05 vs. LETO; c p < 0.05 vs. LETO 2 wks; d p < 0.05 vs. OLETF 2 wks; g p < 0.05, OLETF 2 wks vs. OLETF 11 wks.
Effects of AT1 blockade on adiposity and adipocyte morphology
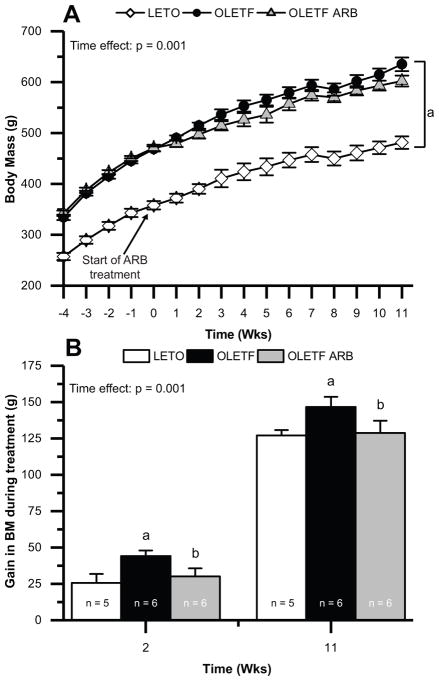
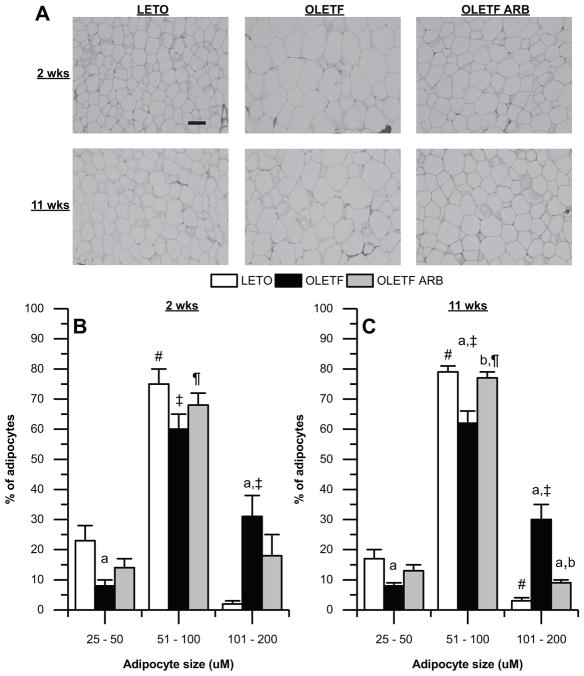
BM, Food intake, retroperitoneal and epidydimal fat masses, and adipocyte morphology were measured to assess whether the timing of AT1 blockade influenced the beneficial effects on BM and adipocyte morphology. Mean weekly BM was greater at −4 weeks in OLETF compared to LETO, remaining so for the duration of the study (Fig. 4A). AT1 blockade regardless of treatment duration had no significant effect compared to OLETF (Fig. 4A). We further evaluated the gain in BM during the treatment durations. There were significant group and time effects on gain in BM during treatment. At 2 and 11-weeks, the gain in BM during the treatment was greater in OLETF compared to LETO and ARB treatment normalized it (Fig. 4B). At 2-weeks, retroperitoneal, and epidydimal fat masses were greater in OLETF compared to LETO, and ARB had no significant effect compared to OLETF (Table 1). At 11-weeks, retroperitoneal, and epidydimal fat masses were greater in OLETF compared to LETO, while ARB reduced retroperitoneal fat mass 30% (Table 1). There was a significant group, but not time effect on food intake. Mean food intake was greater in OLETF compared to LETO (Table 1). At 2-weeks, the relative abundance of adipocytes between 25–50 and 101–200 μm were 65% lower and 14.5-fold greater, respectively, in OLETF compared to LETO, and ARB had no significant effect compared to OLETF (Fig. 5A & B). At 11-weeks, the relative abundance of adipocytes between 25–50 and 51–100 μm were 53% and 22%, respectively, lower in OLETF compared to LETO. The relative abundance of adipocytes between 51–100 μm was normalized in ARB (Fig. 5A & C). The relative abundance of adipocytes between 101–200 μm was 9-fold greater in OLETF compared to LETO, and ARB reduced it 70% compared to OLETF (Fig. 5A & C). Collectively, these data demonstrate that ARB treatment reduced gain in BM regardless of treatment duration, but only chronic ARB decreased retroperitoneal fat mass and improved adipocyte morphology.
Figure 4.
AT1 blockade decreases gain in BM. Mean (± SE) (A) weekly BM and (B) gain in BM during treatment of Long-Evans Tokushima Otsuka (LETO), Otsuka Long-Evans Tokushima Fatty (OLETF), and OLETF + ARB. Comparisons were assessed using a two factor ANOVA with group as between-subjects factor and time as a within-subjects factor for (A) and with group and time as between-subject factors for (B). a p < 0.05 vs. LETO; b p < 0.05 vs. OLETF
Figure 5.
Chronic AT1 blockade decreases the relative abundance of large adipocytes. (A) Representative images of adipocytes from retroperitoneal adipose. Mean (±SE) adipocyte size distribution after (B) 2-weeks of treatment in Long-Evans Tokushima Otsuka (LETO; n=4), Otsuka Long-Evans Tokushima Fatty (OLETF; n=6), and OLETF + ARB (n=4) and, (C) after 11-weeks of treatment in LETO (n=4), OLETF (n=6), and OLETF + ARB (n=5). Comparisons were assessed using a two factor ANOVA at the two-time points with group as between-subjects factor and adipocyte size as a within-subjects factor. a p < 0.05 vs. LETO; b p < 0.05 vs. OLETF; # p < 0.05 vs. small adipocytes (25–50 μm) for LETO; ‡ p < 0.05 vs. small adipocytes for OLETF; ¶ p < 0.05 vs. small adipocytes for OLETF + ARB. Scale bar: 100 μm.
TNF-α does not contribute to insulin resistance in OLETF rats
Plasma tumor necrosis factor-alpha (TNF-α) and epidydimal fat transmembrane TNF-α were measured to assess the effects of decreased adiposity associated with chronic AT1 blockade on systemic and local inflammation. There were no group or time effects on plasma TNF-α (Fig. 6A). There were significant group and time effects on transmembrane TNF-α. Mean transmembrane TNF-α was not different between LETO and OLETF; nonetheless, ARB increased it compared to LETO (Fig. 6B). These results suggest that systemic nor local TNF-α contribute to the development of insulin resistance in obese OLETF rats.
Figure 6.
Effects of AT1 blockade on systemic and local TNF-α. Mean (±SE) (A) plasma TNF-α and (B) epidydimal fat transmembrane TNF-α (% change from LETO at 2 weeks) and the representative western blot bands of Long-Evans Tokushima Otsuka (LETO), Otsuka Long-Evans Tokushima Fatty (OLETF), and OLETF + ARB. Comparisons were assessed using a two factor ANOVA with group and time as between-subject factors. a p < 0.05 vs. LETO.
Chronic AT1 blockade ameliorates the progression of glucose intolerance in OLETF rats
oGTTs were performed to determine whether the improvements in adiposity and adipocyte morphology associated with chronic ARB translated to an improvement in glucose intolerance. At 2-weeks, mean AUCglucose was not different between LETO and OLETF (Fig. 7A & E). At 11-weeks, mean AUCglucose was 2.2-fold greater in OLETF compared to LETO, and chronic ARB decreased it 36% (Fig. 7C & E). At 2-weeks, mean AUCinsulin was not different among the groups (Fig. 7B & F). At 11-weeks, mean AUCinsulin was not different between LETO and OLETF, nevertheless, ARB reduced it 72% compared to OLETF (Fig. 7B, D & F). These data suggest that chronic AT1 blockade protects against the progression of glucose intolerance in OLETF rats but is not sufficient to completely reverse the impairment.
Figure 7.
Chronic AT1 blockade attenuates the progression of glucose intolerance. The response of blood glucose and plasma insulin after (A & B, respectively) 2-weeks and (C & D, respectively) 11-weeks of treatment to an oral glucose tolerance test following an overnight-fast. The mean (±SE) (E) area under the curve for glucose and (F) insulin of Long-Evans Tokushima Otsuka (LETO), Otsuka Long-Evans Tokushima Fatty (OLETF), and OLETF + ARB. Comparisons were assessed using a two factor ANOVA with group and time as between-subject factors. c p < 0.05 vs. LETO 2 wks; e p < 0.05 vs. LETO 11 wks; f p < 0.05 vs. OLETF 11 wks; g p < 0.05, OLETF 2 wks vs. OLETF 11 wks.
Chronic AT1 blockade decreases the NEFA response to an oGTT
Plasma NEFA were measured during oGTT to assess the effects of improvements in adiposity and adipocyte morphology associated with chronic AT1 blockade on lipid metabolism. At 2-weeks, there was no difference in plasma NEFA in response to the glucose challenge between any of the groups (Fig. 8A). At 11-weeks, plasma NEFA was greater at 15, 30, and 60 minutes following the glucose challenge in OLETF compared to LETO, and this effect was reversed in ARB (Fig. 8B). These results demonstrate that the development of insulin resistance in OLETF rats is associated with the inability to suppress NEFA levels in response to glucose administration. Furthermore, chronic, but not acute AT1 blockade normalized NEFA levels in response to a glucose challenge suggesting that activation of AT1 contributes to impaired lipid metabolism.
Figure 8.
Effects of glucose administration on plasma NEFA. The response of NEFA to an oral glucose tolerance test following an overnight-fast (A) after 2-weeks of treatment in Long-Evans Tokushima Otsuka (LETO; n=5), Otsuka Long-Evans Tokushima Fatty (OLETF; n=6), and OLETF + ARB (n=6), and (B) after 11-weeks of treatment in LETO (n=5), OLETF (n=6), and OLETF + ARB (n=6). Comparisons were assessed using a three factor ANOVA with group and time (wks) as between-subject factors and time after administration as a within-subjects factor. e p < 0.05 vs. LETO 11 wks; f p < 0.05 vs. OLETF 11 wks; g p < 0.05, OLETF 2 wks vs. OLETF 11 wks.
Hepatic PEPCK and G6Pase protein expression are not modulated by AT1 signaling in OLETF rats
Fasting plasma insulin (FPI) along with hepatic proteins involved in insulin signaling and gluconeogenesis were measured to assess the impact of AT1 blockade on hepatic insulin signaling. There was a significant group, but not time effect on FPI and hepatic phosphorylated (p)-insulin receptor (IR):IR ratio. Mean FPI and p-IR:IR ratio were greater in OLETF compared to LETO; however, ARB had no significant effect (Fig. S2A & B). There was no group or time effects on the mean expressions of hepatic phosphoenolpyruvate carboxykinase (PEPCK-C) and glucose 6-phosphatase (G6Pase) (Fig. S2C & D). Collectively, these results suggest that altered expression of hepatic PEPCK-C and G6Pase may not contribute to the increase in FPG in OLETF rats.
Discussion
Inappropriately elevated RAS contributes to the dysregulation of glucose homeostasis in part by impairing β-cell function and peripheral insulin signaling (Favre, et al. 2015). Conversely, inhibition of RAS improves β-cell function and peripheral insulin signaling (Chu et al. 2006; Henriksen, et al. 2001; Nagai, et al. 2009; Shiuchi, et al. 2004; Wei et al. 2006), which can delay the onset of T2DM (Tocci et al. 2011). Nonetheless, it is not clear whether the disease state of the organism and treatment duration determine the beneficial effects of RAS inhibition on pancreatic function and insulin sensitivity. Therefore, the aim of this study was to determine whether acute and chronic AT1 blockade started after the onset of obesity, hyperglycemia, and hypertension would have beneficial effects on pancreatic function and peripheral insulin resistance. To this end, the present study demonstrates that regardless of treatment duration, AT1 blockade decreases SBP, BM, and fasting plasma TG. Moreover, chronic AT1 blockade was associated with the additional benefits of decreased FPG, AUCglucose, AUCinsulin, IRI, retroperitoneal fat mass, and a beneficial shift in adipocyte size. Despite these benefits, chronic AT1 blockade did not have an effect on pancreatic oxidative stress or insulin secretion in our rat model. These results suggest that regardless of the disease state, AT1 blockade can improve peripheral insulin resistance but cannot reverse impaired pancreatic function.
We previously demonstrated that 6-weeks of ARB treatment in 9-week old OLETF rats, when the initial detriments of the metabolic syndrome are just appearing in the phenotype, increased insulin secretion associated with a rise in pancreatic GLP-1r protein expression (Rodriguez et al. 2012). An improvement in insulin secretion is one of many avenues by which disruption of RAS can improve glucose intolerance (Chu et al. 2006; Cole, et al. 2010). Notwithstanding, in the present study (treatment started at 13 weeks of age), the improvement in glucose tolerance after chronic AT1 blockade was independent of increased insulin secretion. This disparity between the two studies is an important distinction because it may highlight the significance of the timing of treatment on pancreatic function. In support of this view, ACE2 overexpression in 8-week-old db/db mice improved glucose tolerance, pancreatic function, and prevented β-cell apoptosis; however, these beneficial effects were not replicated in 16-week-old db/db mice (Bindom, et al. 2010). Similarly, pioglitazone and/or liraglutide treatment in 7 to 9-week-old db/db mice increased β-cell function and mass, and increased the expression of various genes involved in the regulation of β-cell function; however, again, these effects were attenuated in 16 to 18-week-old db/db mice (Kimura, et al. 2015). Collectively, these data suggest that early events that harm the pancreas may be sufficiently detrimental to hinder the ability of targeted treatments to reverse impaired pancreatic function.
Inappropriately elevated angiotensin II (Ang II) levels result in β-cell dysfunction in C57BL/6N mice (Lastra et al. 2007; Sauter et al. 2015), and increase the expression and activity of Nox2 in L6 myotubes (Wei et al. 2006). In the pancreas, an increase in oxidant production shifts the oxidant/antioxidant balance to a pro-oxidant state leading to an increase in lipid peroxidation since islets have low levels of antioxidant enzymes (Tiedge et al. 1997). 4-HNE, a by-product of lipid peroxidation, decreases islet insulin and DNA content (Suarez-Pinzon, et al. 1996). In the present study, pancreatic 4-HNE levels were increased in OLETF rats, and pancreatic SOD, catalase, and GPx enzyme activities were decreased. Collectively, these results demonstrate a chronic pro-oxidant state of the pancreas in obese pre-diabetic OLETF rats. Furthermore, these results suggest that the decreased in pancreatic insulin content in OLETF may be a result of the pro-oxidant state of the pancreas. Moreover, OLETF rats also exhibited decreased insulin secretion despite increased insulin levels after 2-weeks of treatment, suggesting that defective glucose sensing may be responsible for this impairment. To this effect, β-cell glucose toxicity decreases the expression of GLP-1r and GLUT2 leading to impaired insulin secretion (Kawashima, et al. 2011; Thorens, et al. 1992; Xu, et al. 2007). Additionally, 10-week-old, ad libitum fed OLETF rats have increased levels of plasma dipeptidyl peptidase-4 activity compared to food restricted OLETF rats (Kirino, et al. 2011), which would decrease plasma GLP-1 (Rodriguez et al. 2012). Collectively, these data demonstrate that early in the development of metabolic syndrome OLETF rats are afflicted by an increased pro-oxidant state in the pancreas and decreased plasma GLP-1 leading to an impairment in glucose-stimulated insulin secretion. It is important to note that while acute or chronic AT1 blockade cannot reverse these detriments, RAS inhibition before the onset of dysregulated insulin secretion is protective (Nakayama, et al. 2005; Rodriguez et al. 2012; Zhang, et al. 2013), suggesting that these detriments are irreversible without sufficiently early intervention. If so, identifying appropriate targets for improving therapies to reverse dysregulated pancreatic function associated with the manifestation of metabolic syndrome will be especially necessary.
Obesity inappropriately activates RAS in animal models and humans (Boustany, et al. 2004; Engeli et al. 2005). RAS inhibition decreases BM and visceral fat mass in animal models of metabolic syndrome (Benson, et al. 2004; Miesel, et al. 2012; Müller-Fielitz, et al. 2012; Müller-Fielitz, et al. 2014; Müller-Fielitz, et al. 2015). These beneficial effects on BM and visceral fat mass are partially mediated by Mas receptor activation (Schuchard, et al. 2015) and are attributed to multiple factors including the prevention of leptin resistance (Müller-Fielitz et al. 2014; Müller-Fielitz et al. 2015) and increased circulating levels of adiponectin (Weisinger, et al. 2009; Zorad, et al. 2006). In the present study, we did not observe a reduction in BM regardless of treatment duration; nevertheless, ARB treatment blunted BM gain during the treatment period. The difference in our findings may be attributed to the diets used in the aforementioned studies, which were higher in carbohydrates and fat as compared to the standard chow used in our study. Likewise, 4 weeks of telmisartan treatment did not reduce BM in spontaneously hypertensive (SHR) rats fed a standard chow (Li, et al. 2006). Moreover, the BM reducing effects of ARB are dependent on the ARB dose and intact leptin signaling, with higher doses decreasing food intake and BM when leptin signaling is normal (Müller-Fielitz, et al. 2011). Although OLETF rats have intact leptin signaling at 5-weeks of age; by 8-weeks of age they develop peripheral but not central leptin resistance (Niimi, et al. 1999) which may be a result of an acquired impairment in the transport of leptin across the blood brain barrier (Banks, et al. 2003). While AT1 blockade regardless of treatment duration did not decrease BM, we found that chronic AT1 blockade decreased retro fat mass and the relative abundance of large adipocytes and normalized the relative abundance of medium adipocytes indicative of a beneficial shift in adipose morphology. Nevertheless, acute AT1 blockade had no detectable effect on adipocyte morphology, glucose intolerance, or FPG suggesting that: 1) at this stage in the development of metabolic syndrome, other factors beyond AT1 activation contribute to a greater extent or 2) at this stage of the condition, acute disruption of RAS is insufficient to overcome the progression of the metabolic syndrome. Although, we only observed an improvement in adipocyte morphology after chronic AT1 blockade previous studies have shown similar results regardless of treatment duration (Furuhashi, et al. 2004; Iwai, et al. 2010; Mori, et al. 2007; Müller-Fielitz et al. 2012; Muñoz, et al. 2009; Nagai et al. 2009; Tomono, et al. 2008). This discrepancy may be due to the use of different animal models and/or RAS inhibitor, or the time frame of treatment necessary to detect an effect.
Adipose expansion as seen in obesity is mediated by adipocyte hypertrophy, hyperplasia, or both. Similarly the detrimental effects of obesity on metabolic and cardiovascular health are associated with a higher abundance of hypertrophic adipocytes (Choe, et al. 2016). These large adipocytes have lower GLUT4 translocation in response to insulin (Franck, et al. 2007), higher lipolysis rates (Laurencikiene, et al. 2011), and higher pro-inflammatory adipokine expression and secretion (Skurk, et al. 2007). Therefore, large adipocytes are associated with the development of insulin resistance and type 2 diabetes (Acosta, et al. 2015; Kim, et al. 2015). In the present study, chronic AT1 blockade decreased the relative abundance of large adipocytes. This positive shift in adipocyte morphology may partially explain the improvement in glucose homeostasis. Recent evidence shows that these effects are independent of the blood pressure lowering effects of AT1 blockade (Müller-Fielitz et al. 2014). However, the improvement in glucose homeostasis may be independent of increased glucose uptake into adipocytes as adipose itself makes a minor contribution to glucose disposal in response to feeding (Baron, et al. 1988). In support of this view, a previous study found that glucose uptake in adipocytes was not altered by telmisartan treatment in Sprague Dawley (SD) rats or transgenic rats with low brain angiotensinogen fed a cafeteria diet (Winkler, et al. 2016). Collectively, these results suggest that the improvement in glucose homeostasis may be the result of other factors associated with a positive shift in adipocyte morphology such as increased expression and secretion of anti-inflammatory adipokines and decreased expression and secretion of pro-inflammatory adipokines (Skurk et al. 2007). In the present study, OLETF rats did not show increased systemic or local inflammation. Additionally, adiponectin levels decreased with time, which may be a result of increased BM as obesity is associated with decreased circulating levels of adiponectin (Arita, et al. 1999). This is significant because adiponectin is important for increasing peripheral insulin sensitivity (Yamauchi, et al. 2001) suggesting that the observed improvement in glucose homeostasis after chronic AT1 blockade is not mediated by adiponectin. In the same way, a previos study found that AT1 blockade improves glucose homeostasis in SHR rats and that these effects are not mediated by peroxisome proliferator-activated receptor (PPAR)-γ (Müller-Fielitz et al. 2012), a transcriptional regulator of adiponectin (Iwaki, et al. 2003). Furthermore, we found that at 11 weeks a glucose challenge did not suppress circulating NEFA levels and that chronic AT1 blockade reversed this impairment suggesting that the improvement in glucose homeostasis may result from increased adipogenesis, decreased lipolysis or both, in smaller insulin-sensitive adipocytes (Laforest, et al. 2015). Previous studies have demonstrated that Ang II inhibits lypolis (Goossens, et al. 2006), however, a different study demonstated that in individuals with impaired glycemia, valsartan treatment for 26 weeks suppressed posprandial free fatty acids (Moors, et al. 2013) suggesting a suppression of lypolysis. Furthermore, a study in primary cultured human preadipocytes demonstrated that AT1 blockade increased the lipid accumulaiton and differentiation of these cells (Janke et al. 2002). Collectively these results suggest that AT1 blockade may protect against ectopic lipid deposition in skeletal muscle by suppresing lypolysis and increasing preadipocyte differentiation leading to an improvement in insulin sensitivity an ultimately on glycemia (Sharma, et al. 2002). Nonetheless, we cannot rule out that other mechanisms such as blunting of the hypothalamic pituitary axis activity in response to stress may contribute to the AT1 associated improvements in glucose homeostasis (Armando, et al. 2001; Miesel et al. 2012).
Conclusion
In summary, the present study demonstrates that after the onset of obesity, hyperglycemia, and hypertension in OLETF rats, chronic AT1 blockade results in a beneficial shift in adipocyte morphology, and improved FPG and glucose intolerance regardless of the glycemic state at the onset of the treatment. Despite this, AT1 blockade cannot reverse impaired insulin secretion suggesting that once the pancreas has been compromised, recovery will be particularly challenging, and the severity of the condition may be masked by the apparent improvement in systemic glucose tolerance. Should these findings in a rat model recapitulate in humans, early RAS inhibition may be crucial to preserving pancreatic function, and identifying the other factors that contribute to the defined impairments in pancreatic function will be critical for proper management of metabolic syndrome especially as it relates to the timing of treatment.
Supplementary Material
AT1 blockade decreases systolic blood pressure. Mean (±SE) systolic blood pressure measured by telemetry of Long-Evans Tokushima Otsuka (LETO; n=3), Otsuka Long-Evans Tokushima Fatty (OLETF; n=4), and OLETF + ARB (n=4). Comparisons were assessed using a two factor ANOVA with group as between-subjects factor and time as a within-subjects factor. a p < 0.05 LETO, vs. OLETF; b p < 0.05 vs. OLETF.
Effects of AT1 on fasting plasma insulin along with hepatic proteins involved in insulin signaling and gluconeogenesis. Mean (± SE) (A) fasting plasma insulin concentrations and liver (B) P-IR to total IR-β ratio, (C) PEPCK-C, and (D) G6Pase (% change from LETO at 2 weeks) and the representative western blot bands of Long-Evans Tokushima Otsuka (LETO), Otsuka Long-Evans Tokushima Fatty (OLETF), and OLETF + ARB. Comparisons were assessed using a two factor ANOVA with group and time as between-subject factors. a p < 0.05 vs. LETO.
Acknowledgments
Funding
R. Rodriguez was supported by NIH NCMHD 9T37MD001480 and by a Faculty Mentor Program Fellowship from the University of California Merced Graduate Division. J.N. Minas was supported by NIH NCMHD 9T37MD001480. R.M. Ortiz was partially supported by NIH NHLBIK02HL103787. Research was partially funded by NIH NHLBIR01HL091767.
We would like to thank Dr. N. Pelisch, Dr. K. Kitada, Mr. A. Lee, Mr. M. Thorwald, Ms. P. Montez, M. Moreno and Ms. J. Aguil for their assistance during dissections and with laboratory analyses. We also thank Dr. J.A. Viscarra, Dr. H. Brooks, and Dr. S.H. Adams for their comments on a draft of the manuscript. Finally, we are grateful to Drs. J. Choi, M. Kitazawa, N. Oviedo, and Dr. S.H. Adams for their discussion of the results. Olmesartan was kindly donated by Daiichi-Sankyo (Tokyo, Japan) to A. Nishiyama.
Footnotes
Declaration of Interest
None of the authors have any conflicts of interest to disclose.
Author Contributions
RR, JPV-M, DN, AN, and RMO designed the research. RR and JNM performed the animal experiments. RR and JNM analyzed the samples and data. RR, JPV-M, DGP, SHA, and RMO interpreted the results. RR wrote the original draft of the manuscript. RR, JPV-M, DN, DGP, AN, and RMO edited and revised the manuscript. All authors approved the final version of the manuscript for submission.
References
- Acosta JR, Douagi I, Andersson DP, Bäckdahl J, Rydén M, Arner P, Laurencikiene J. Increased fat cell size: A major phenotype of subcutaneous white adipose tissue in non-obese individuals with type 2 diabetes. Diabetologia. 2015;59:560–570. doi: 10.1007/s00125-015-3810-6. [DOI] [PubMed] [Google Scholar]
- Allison DB, Paultre F, Maggio C, Mezzitis N, Pi-Sunyer FX. The use of areas under curves in diabetes research. Diabetes Care. 1995;18:245–250. doi: 10.2337/diacare.18.2.245. [DOI] [PubMed] [Google Scholar]
- Arita Y, Kihara S, Ouchi N, Takahashi M, Maeda K, Miyagawa J-i, Hotta K, Shimomura I, Nakamura T, Miyaoka K, et al. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochemical and Biophysical Research Communications. 1999;257:79–83. doi: 10.1006/bbrc.1999.0255. [DOI] [PubMed] [Google Scholar]
- Armando I, Carranza A, Nishimura Y, Hoe K-L, Barontini M, Terrón JA, Falcón-Neri A, Ito T, Juorio AV, Saavedra JM. Peripheral administration of an angiotensin ii at1 receptor antagonist decreases the hypothalamic-pituitary-adrenal response to isolation stress. Endocrinology. 2001;142:3880–3889. doi: 10.1210/endo.142.9.8366. [DOI] [PubMed] [Google Scholar]
- Banks WA, Farrell CL. Impaired transport of leptin across the blood-brain barrier in obesity is acquired and reversible. American Journal of Physiology-Endocrinology and Metabolism. 2003;285:E10–E15. doi: 10.1152/ajpendo.00468.2002. [DOI] [PubMed] [Google Scholar]
- Baron AD, Brechtel G, Wallace P, Edelman SV. Rates and tissue sites of non-insulin- and insulin-mediated glucose uptake in humans. American Journal of Physiology - Endocrinology And Metabolism. 1988;255:E769–E774. doi: 10.1152/ajpendo.1988.255.6.E769. [DOI] [PubMed] [Google Scholar]
- Benson SC, Pershadsingh HA, Ho CI, Chittiboyina A, Desai P, Pravenec M, Qi N, Wang J, Avery MA, Kurtz TW. Identification of telmisartan as a unique angiotensin ii receptor antagonist with selective pparγ–modulating activity. Hypertension. 2004;43:993–1002. doi: 10.1161/01.HYP.0000123072.34629.57. [DOI] [PubMed] [Google Scholar]
- Bindom SM, Hans CP, Xia H, Boulares HA, Lazartigues E. Angiotensin i–converting enzyme type 2 (ace2) gene therapy improves glycemic control in diabetic mice. Diabetes. 2010;59:2540–2548. doi: 10.2337/db09-0782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch J, Yusuf S, Gerstein HC, Pogue J, Sheridan P, Dagenais G, Diaz R, Avezum A, Lanas F, Probstfield J, et al. Effect of ramipril on the incidence of diabetes. The New England Journal of Medicine. 2006;355:1551–1562. doi: 10.1056/NEJMoa065061. [DOI] [PubMed] [Google Scholar]
- Boustany CM, Bharadwaj K, Daugherty A, Brown DR, Randall DC, Cassis LA. Activation of the systemic and adipose renin-angiotensin system in rats with diet-induced obesity and hypertension. American Journal of Physiology - Regulatory, Integrative and Comparative Physiology. 2004;287:R943–949. doi: 10.1152/ajpregu.00265.2004. [DOI] [PubMed] [Google Scholar]
- Chhabra K, Xia H, Pedersen K, Speth R, Lazartigues E. Pancreatic angiotensin-converting enzyme 2 improves glycemia in angiotensin ii-infused mice. American journal of physiology. Endocrinology and metabolism. 2013;304:E874–884. doi: 10.1152/ajpendo.00490.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choe SS, Huh JY, Hwang IJ, Kim JI, Kim JB. Adipose tissue remodeling: Its role in energy metabolism and metabolic disorders. Frontiers in Endocrinology. 2016;7:30. doi: 10.3389/fendo.2016.00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu KY, Lau T, Carlsson P-O, Leung PS. Angiotensin ii type 1 receptor blockade improves β-cell function and glucose tolerance in a mouse model of type 2 diabetes. Diabetes. 2006;55:367–374. doi: 10.2337/diabetes.55.02.06.db05-1022. [DOI] [PubMed] [Google Scholar]
- Chu KY, Leung PS. Angiotensin ii type 1 receptor antagonism mediates uncoupling protein 2-driven oxidative stress and ameliorates pancreatic islet β-cell function in young type 2 diabetic mice. Antioxidants & Redox Signaling. 2007;9:869–878. doi: 10.1089/ars.2007.1590. [DOI] [PubMed] [Google Scholar]
- Cole BK, Keller SR, Wu R, Carter JD, Nadler JL, Nunemaker CS. Valsartan protects pancreatic islets and adipose tissue from the inflammatory and metabolic consequences of a high-fat diet in mice. Hypertension. 2010;55:715–721. doi: 10.1161/HYPERTENSIONAHA.109.148049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csibi A, Communi D, Müller N, Bottari SP. Angiotensin ii inhibits insulin-stimulated glut4 translocation and akt activation through tyrosine nitration-dependent mechanisms. PloS one. 2010;5:e10070. doi: 10.1371/journal.pone.0010070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engeli S, Böhnke J, Gorzelniak K, Janke J, Schling P, Bader M, Luft FC, Sharma AM. Weight loss and the renin-angiotensin-aldosterone system. Hypertension. 2005;45:356–362. doi: 10.1161/01.HYP.0000154361.47683.d3. [DOI] [PubMed] [Google Scholar]
- Favre GA, Esnault VLM, Van Obberghen E. Modulation of glucose metabolism by the renin-angiotensin-aldosterone system. American Journal of Physiology - Endocrinology And Metabolism. 2015;308:E435–E449. doi: 10.1152/ajpendo.00391.2014. [DOI] [PubMed] [Google Scholar]
- Flegal KM, Kruszon-Moran D, Carroll MD, Fryar CD, Ogden CL. Trends in obesity among adults in the united states, 2005 to 2014. JAMA. 2016;315:2284–2291. doi: 10.1001/jama.2016.6458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franck N, Stenkula KG, Öst A, Lindström T, Strålfors P, Nystrom FH. Insulin-induced glut4 translocation to the plasma membrane is blunted in large compared with small primary fat cells isolated from the same individual. Diabetologia. 2007;50:1716–1722. doi: 10.1007/s00125-007-0713-1. [DOI] [PubMed] [Google Scholar]
- Furuhashi M, Ura N, Takizawa H, Yoshida D, Moniwa N, Murakami H, Higashiura K, Shimamoto K. Blockade of the renin–angiotensin system decreases adipocyte size with improvement in insulin sensitivity. Journal of Hypertension. 2004;22:1977–1982. doi: 10.1097/00004872-200410000-00021. [DOI] [PubMed] [Google Scholar]
- Gaal LF, Mertens IL, Block CE. Mechanisms linking obesity with cardiovascular disease. Nature. 2006;444:875–880. doi: 10.1038/nature05487. [DOI] [PubMed] [Google Scholar]
- Goossens GH, Blaak EE, Arner P, Saris WHM, Baak MAv. Angiotensin ii: A hormone that affects lipid metabolism in adipose tissue. International Journal of Obesity. 2006;31:0803388. doi: 10.1038/sj.ijo.0803388. [DOI] [PubMed] [Google Scholar]
- Habibi J, Whaley-Connell A, Hayden MR, DeMarco VG, Schneider R, Sowers SD, Karuparthi P, Ferrario CM, Sowers JR. Renin inhibition attenuates insulin resistance, oxidative stress, and pancreatic remodeling in the transgenic ren2 rat. Endocrinology. 2008;149:5643–5653. doi: 10.1210/en.2008-0070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henriksen EJ, Jacob S, Kinnick TR, Teachey MK, Krekler M. Selective angiotensin ii receptor antagonism reduces insulin resistance in obese zucker rats. Hypertension. 2001;38:884–890. doi: 10.1161/hy1101.092970. [DOI] [PubMed] [Google Scholar]
- Iwai M, Kanno H, Tomono Y, Inaba S, Senba I, Furuno M, Mogi M, Horiuchi M. Direct renin inhibition improved insulin resistance and adipose tissue dysfunction in type 2 diabetic kk-a(y) mice. Journal of Hypertension. 2010;28:1471–1481. doi: 10.1097/HJH.0b013e32833bc420. [DOI] [PubMed] [Google Scholar]
- Iwaki M, Matsuda M, Maeda N, Funahashi T, Matsuzawa Y, Makishima M, Shimomura I. Induction of adiponectin, a fat-derived antidiabetic and antiatherogenic factor, by nuclear receptors. Diabetes. 2003;52:1655–1663. doi: 10.2337/diabetes.52.7.1655. [DOI] [PubMed] [Google Scholar]
- Janke J, Engeli S, Gorzelniak K, Luft FC, Sharma AM. Mature adipocytes inhibit in vitro differentiation of human preadipocytes via angiotensin type 1 receptors. Diabetes. 2002;51:1699–1707. doi: 10.2337/diabetes.51.6.1699. [DOI] [PubMed] [Google Scholar]
- Kahn SE, Hull RL, Utzschneider KM. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature. 2006;444:840–846. doi: 10.1038/nature05482. [DOI] [PubMed] [Google Scholar]
- Kawano K, Hirashima T, Mori S, Saitoh Y, Kurosumi M, Natori T. Spontaneous long-term hyperglycemic rat with diabetic complications. Otsuka long-evans tokushima fatty (oletf) strain. Diabetes. 1992;41:1422–1428. doi: 10.2337/diab.41.11.1422. [DOI] [PubMed] [Google Scholar]
- Kawashima S, Matsuoka T-aA, Kaneto H, Tochino Y, Kato K, Yamamoto K, Yamamoto T, Matsuhisa M, Shimomura I. Effect of alogliptin, pioglitazone and glargine on pancreatic β-cells in diabetic db/db mice. Biochemical and Biophysical Research Communications. 2011;404:534–540. doi: 10.1016/j.bbrc.2010.12.021. [DOI] [PubMed] [Google Scholar]
- Kim J, Huh J, Sohn J, Choe S, Lee Y, Lim C, Jo A, Park S, Han W, Kim J. Lipid-overloaded enlarged adipocytes provoke insulin resistance independent of inflammation. Molecular and Cellular Biology. 2015;35:1686–1699. doi: 10.1128/MCB.01321-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura T, Kaneto H, Shimoda M, Hirukawa H, Okauchi S, Kohara K, Hamamoto S, Tawaramoto K, Hashiramoto M, Kaku K. Protective effects of pioglitazone and/or liraglutide on pancreatic β-cells in db/db mice: Comparison of their effects between in an early and advanced stage of diabetes. Molecular and cellular endocrinology. 2015;400:78–89. doi: 10.1016/j.mce.2014.11.018. [DOI] [PubMed] [Google Scholar]
- Kirino Y, Sato Y, Kamimoto T, Kawazoe K, Minakuchi K. Altered dipeptidyl peptidase-4 activity during the progression of hyperinsulinemic obesity and islet atrophy in spontaneously late-stage type 2 diabetic rats. American Journal of Physiology - Endocrinology And Metabolism. 2011;300:E372–379. doi: 10.1152/ajpendo.00319.2010. [DOI] [PubMed] [Google Scholar]
- Laforest S, Labrecque J, Michaud A, Cianflone K, Tchernof A. Adipocyte size as a determinant of metabolic disease and adipose tissue dysfunction. Critical Reviews in Clinical Laboratory Sciences. 2015;52:301–313. doi: 10.3109/10408363.2015.1041582. [DOI] [PubMed] [Google Scholar]
- Lastra G, Manrique C. The expanding role of oxidative stress, renin angiotensin system, and β-cell dysfunction in the cardiometabolic syndrome and type 2 diabetes mellitus. Antioxidants & Redox Signaling. 2007;9:943–954. doi: 10.1089/ars.2007.1615. [DOI] [PubMed] [Google Scholar]
- Laurencikiene J, Skurk T, Kulyté A, Hedén P, Åström G, Sjölin E, Rydén M, Hauner H, Arner P. Regulation of lipolysis in small and large fat cells of the same subject. The Journal of Clinical Endocrinology & Metabolism. 2011;96:E2045–E2049. doi: 10.1210/jc.2011-1702. [DOI] [PubMed] [Google Scholar]
- Li N, Li B, Brun T, Deffert-Delbouille C, Mahiout Z, Daali Y, Ma X-J, Krause K-H, Maechler P. Nadph oxidase nox2 defines a new antagonistic role for reactive oxygen species and camp/pka in the regulation of insulin secretion. Diabetes. 2012;61:2842–2850. doi: 10.2337/db12-0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y-Q, Ji H, Zhang Y-H, Ding D-Y, Ye X-L. Metabolic effects of telmisartan in spontaneously hypertensive rats. Naunyn-Schmiedeberg’s Archives of Pharmacology. 2006;373:264–270. doi: 10.1007/s00210-006-0069-y. [DOI] [PubMed] [Google Scholar]
- Maki KC, McKenney JM, Farmer MV, Reeves MS, Dicklin MR. Indices of insulin sensitivity and secretion from a standard liquid meal test in subjects with type 2 diabetes, impaired or normal fasting glucose. Nutr J. 2009;8:22. doi: 10.1186/1475-2891-8-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMurray JJ, Holman RR, Haffner SM, Bethel MA, Holzhauer B, Hua TA, Belenkov Y, Boolell M, Buse JB, Buckley BM, et al. Effect of valsartan on the incidence of diabetes and cardiovascular events. The New England Journal of Medicine. 2010;362:1477–1490. doi: 10.1056/NEJMoa1001121. [DOI] [PubMed] [Google Scholar]
- Miesel A, Müller-Fielitz H, Jöhren O, Vogt FM, Raasch W. Double blockade of angiotensin ii (at1)-receptors and ace does not improve weight gain and glucose homeostasis better than single-drug treatments in obese rats. British Journal of Pharmacology. 2012;165:2721–2735. doi: 10.1111/j.1476-5381.2011.01726.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montez P, Vázquez-Medina JP, Rodríguez R, Thorwald MA, Viscarra JA, Lam L, Peti-Peterdi J, Nakano D, Nishiyama A, Ortiz RM. Angiotensin receptor blockade recovers hepatic ucp2 expression and aconitase and sdh activities and ameliorates hepatic oxidative damage in insulin resistant rats. Endocrinology. 2012;153:5746–5759. doi: 10.1210/en.2012-1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moors CCM, Blaak EE, van der Zijl NJ, Diamant M, Goossens GH. The effects of long-term valsartan treatment on skeletal muscle fatty acid handling in humans with impaired glucose metabolism. The Journal of Clinical Endocrinology & Metabolism. 2013;98:E891–E896. doi: 10.1210/jc.2012-4067. [DOI] [PubMed] [Google Scholar]
- Mori Y, Itoh Y, Tajima N. Angiotensin ii receptor blockers downsize adipocytes in spontaneously type 2 diabetic rats with visceral fat obesity. American Journal of Hypertension. 2007;20:431–436. doi: 10.1016/j.amjhyper.2006.09.016. [DOI] [PubMed] [Google Scholar]
- Müller-Fielitz H, Landolt J, Heidbreder M, Werth S, Vogt FM, Jöhren O, Raasch W. Improved insulin sensitivity after long-term treatment with at1 blockers is not associated with pparγ target gene regulation. Endocrinology. 2012;153:1103–1115. doi: 10.1210/en.2011-0183. [DOI] [PubMed] [Google Scholar]
- Müller-Fielitz H, Markert A, Wittmershaus C, Pahlke F, Jöhren O, Raasch W. Weight loss and hypophagia after high-dose at1-blockade is only observed after high dosing and depends on regular leptin signalling but not blood pressure. Naunyn-Schmiedeberg’s Archives of Pharmacology. 2011;383:373–384. doi: 10.1007/s00210-011-0602-5. [DOI] [PubMed] [Google Scholar]
- Müller-Fielitz H, Hübel N, Mildner M, Vogt FM, Barkhausen J, Raasch W. Chronic blockade of angiotensin at1 receptors improves cardinal symptoms of metabolic syndrome in diet-induced obesity in rats. British Journal of Pharmacology. 2014;171:746–760. doi: 10.1111/bph.12510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller-Fielitz H, Lau M, Geißler C, Werner L, Winkler M, Raasch W. Preventing leptin resistance by blocking angiotensin ii at1 receptors in diet-induced obese rats. British Journal of Pharmacology. 2015;172:857–868. doi: 10.1111/bph.12949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muñoz MC, Giani JF, Dominici FP, Turyn D, Toblli JE. Long-term treatment with an angiotensin ii receptor blocker decreases adipocyte size and improves insulin signaling in obese zucker rats. Journal of Hypertension. 2009;27:2409–2420. doi: 10.1097/HJH.0b013e3283310e1b. [DOI] [PubMed] [Google Scholar]
- Nagai Y, Ichihara A, Nakano D, Kimura S, Pelisch N, Fujisawa Y, Hitomi H, Hosomi N, Kiyomoto H, Kohno M, et al. Possible contribution of the non-proteolytic activation of prorenin to the development of insulin resistance in fructose-fed rats. Experimental Physiology. 2009;94:1016–1023. doi: 10.1113/expphysiol.2009.048108. [DOI] [PubMed] [Google Scholar]
- Nakayama M, Inoguchi T, Sonta T, Maeda Y, Sasaki S, Sawada F, Tsubouchi H, Sonoda N, Kobayashi K, Sumimoto H, et al. Increased expression of nad(p)h oxidase in islets of animal models of type 2 diabetes and its improvement by an at1 receptor antagonist. Biochemical and Biophysical Research Communications. 2005;332:927–933. doi: 10.1016/j.bbrc.2005.05.065. [DOI] [PubMed] [Google Scholar]
- Niimi, Sato, Yokote, Tada, Takahara Effects of central and peripheral injection of leptin on food intake and on brain fos expression in the otsuka long-evans tokushima fatty rat with hyperleptinaemia. Journal of Neuroendocrinology. 1999;11:605–611. doi: 10.1046/j.1365-2826.1999.00368.x. [DOI] [PubMed] [Google Scholar]
- Nishiyama A, Nakagawa T, Kobori H, Nagai Y, Okada N, Konishi Y, Morikawa T, Okumura M, Meda I, Kiyomoto H, et al. Strict angiotensin blockade prevents the augmentation of intrarenal angiotensin ii and podocyte abnormalities in type 2 diabetic rats with microalbuminuria. Journal of Hypertension. 2008;26:1849–1859. doi: 10.1097/HJH.0b013e3283060efa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao RH. Pressor doses of angiotensin ii increase hepatic glucose output and decrease insulin sensitivity in rats. Journal of Endocrinology. 1996;148:311–318. doi: 10.1677/joe.0.1480311. [DOI] [PubMed] [Google Scholar]
- Retnakaran R, Shen S, Hanley AJ, Vuksan V, Hamilton JK, Zinman B. Hyperbolic relationship between insulin secretion and sensitivity on oral glucose tolerance test. Obesity. 2008;16:1901–1907. doi: 10.1038/oby.2008.307. [DOI] [PubMed] [Google Scholar]
- Rodriguez R, Viscarra JA, Minas JN, Nakano D, Nishiyama A, Ortiz RM. Angiotensin receptor blockade increases pancreatic insulin secretion and decreases glucose intolerance during glucose supplementation in a model of metabolic syndrome. Endocrinology. 2012;153:1684–1695. doi: 10.1210/en.2011-1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauter NS, Thienel C, Plutino Y, Kampe K, Dror E, Traub S, Timper K, Bédat B, Pattou F, Kerr-Conte J, et al. Angiotensin ii induces interleukin-1β–mediated islet inflammation and β-cell dysfunction independently of vasoconstrictive effects. Diabetes. 2015;64:1273–1283. doi: 10.2337/db14-1282. [DOI] [PubMed] [Google Scholar]
- Schuchard J, Winkler M, Stölting I, Schuster F, Vogt FM, Barkhausen J, Thorns C, Santos RA, Bader M, Raasch W. Lack of weight gain after angiotensin at1 receptor blockade in diet-induced obesity is partly mediated by an angiotensin-(1–7)/mas-dependent pathway. British Journal of Pharmacology. 2015;172:3764–3778. doi: 10.1111/bph.13172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma AM, Janke J, Gorzelniak K, Engeli S, Luft FC. Angiotensin blockade prevents type 2 diabetes by formation of fat cells. Hypertension. 2002;40:609–611. doi: 10.1161/01.hyp.0000036448.44066.53. [DOI] [PubMed] [Google Scholar]
- Shiuchi T, Iwai M, Li H-SS, Wu L, Min L-JJ, Li J-MM, Okumura M, Cui T-XX, Horiuchi M. Angiotensin ii type-1 receptor blocker valsartan enhances insulin sensitivity in skeletal muscles of diabetic mice. Hypertension. 2004;43:1003–1010. doi: 10.1161/01.HYP.0000125142.41703.64. [DOI] [PubMed] [Google Scholar]
- Skurk T, Alberti-Huber C, Herder C, Hauner H. Relationship between adipocyte size and adipokine expression and secretion. The Journal of Clinical Endocrinology & Metabolism. 2007;92:1023–1033. doi: 10.1210/jc.2006-1055. [DOI] [PubMed] [Google Scholar]
- Suarez-Pinzon WL, Strynadka K, Rabinovitch A. Destruction of rat pancreatic islet beta-cells by cytokines involves the production of cytotoxic aldehydes. Endocrinology. 1996;137:5290–5296. doi: 10.1210/endo.137.12.8940348. [DOI] [PubMed] [Google Scholar]
- Thorens B, Wu YJ, Leahy JL, Weir GC. The loss of glut2 expression by glucose-unresponsive beta cells of db/db mice is reversible and is induced by the diabetic environment. Journal of Clinical Investigation. 1992;90:77–85. doi: 10.1172/JCI115858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiedge M, Lortz S, Drinkgern J, Lenzen S. Relation between antioxidant enzyme gene expression and antioxidative defense status of insulin-producing cells. Diabetes. 1997;46:1733–1742. doi: 10.2337/diab.46.11.1733. [DOI] [PubMed] [Google Scholar]
- Tocci G, Paneni F, Palano F, Sciarretta S, Ferrucci A, Kurtz T, Mancia G, Volpe M. Angiotensin-converting enzyme inhibitors, angiotensin ii receptor blockers and diabetes: A meta-analysis of placebo-controlled clinical trials. American Journal of Hypertension. 2011;24:582–590. doi: 10.1038/ajh.2011.8. [DOI] [PubMed] [Google Scholar]
- Tomono Y, Iwai M, Inaba S, Mogi M, Horiuchi M. Blockade of at1 receptor improves adipocyte differentiation in atherosclerotic and diabetic models. American Journal of Hypertension. 2008;21:206–212. doi: 10.1038/ajh.2007.50. [DOI] [PubMed] [Google Scholar]
- Viscarra JA, Champagne CD, Crocker DE, Ortiz RM. 5′amp-activated protein kinase activity is increased in adipose tissue of northern elephant seal pups during prolonged fasting-induced insulin resistance. Journal of Endocrinology. 2011;209:317–325. doi: 10.1530/JOE-11-0017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Y, Sowers JR, Clark SE, Li W, Ferrario CM, Stump CS. Angiotensin ii-induced skeletal muscle insulin resistance mediated by nf-kappab activation via nadph oxidase. American journal of physiology. Endocrinology and metabolism. 2008;294:E345–E351. doi: 10.1152/ajpendo.00456.2007. [DOI] [PubMed] [Google Scholar]
- Wei Y, Sowers JR, Nistala R, Gong H, Uptergrove GME, Clark SE, Morris EM, Szary N, Manrique C, Stump CS. Angiotensin ii-induced nadph oxidase activation impairs insulin signaling in skeletal muscle cells. Journal of Biological Chemistry. 2006;281:35137–35146. doi: 10.1074/jbc.M601320200. [DOI] [PubMed] [Google Scholar]
- Weisinger RS, Stanley TK, Begg DP, Weisinger HS, Spark KJ, Jois M. Angiotensin converting enzyme inhibition lowers body weight and improves glucose tolerance in c57bl/6j mice maintained on a high fat diet. Physiology & Behavior. 2009;98:192–197. doi: 10.1016/j.physbeh.2009.05.009. [DOI] [PubMed] [Google Scholar]
- Winkler M, Schuchard J, Stölting I, Vogt FM, Barkhausen J, Thorns C, Bader M, Raasch W. The brain renin-angiotensin system plays a crucial role in regulating body weight in diet-induced obesity in rats. British Journal of Pharmacology. 2016;173:1602–1617. doi: 10.1111/bph.13461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu G, Kaneto H, Laybutt RD, Duvivier-Kali VF, Trivedi N, Suzuma K, King GL, Weir GC, Bonner-Weir S. Downregulation of glp-1 and gip receptor expression by hyperglycemia possible contribution to impaired incretin effects in diabetes. Diabetes. 2007;56:1551–1558. doi: 10.2337/db06-1033. [DOI] [PubMed] [Google Scholar]
- Yamauchi T, Kamon J, Waki H, Terauchi Y, Kubota N, Hara K, Mori Y, Ide T, Murakami K, Tsuboyama-Kasaoka N, et al. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nature Medicine. 2001;7:nm0801_0941. doi: 10.1038/90984. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Liu C, Gan Z, Wang X, Yi Q, Liu Y, Wang Y, Lu B, Du H, Shao J, et al. Improved glucose-stimulated insulin secretion by selective intraislet inhibition of angiotensin ii type 1 receptor expression in isolated islets of db/db mice. International Journal of Endocrinology. 2013;2013:10. doi: 10.1155/2013/319586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorad S, Dou J-t, Benicky J, Hutanu D, Tybitanclova K, Zhou J, Saavedra JM. Long-term angiotensin ii at1 receptor inhibition produces adipose tissue hypotrophy accompanied by increased expression of adiponectin and pparγ. European Journal of Pharmacology. 2006;552:112–122. doi: 10.1016/j.ejphar.2006.08.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
AT1 blockade decreases systolic blood pressure. Mean (±SE) systolic blood pressure measured by telemetry of Long-Evans Tokushima Otsuka (LETO; n=3), Otsuka Long-Evans Tokushima Fatty (OLETF; n=4), and OLETF + ARB (n=4). Comparisons were assessed using a two factor ANOVA with group as between-subjects factor and time as a within-subjects factor. a p < 0.05 LETO, vs. OLETF; b p < 0.05 vs. OLETF.
Effects of AT1 on fasting plasma insulin along with hepatic proteins involved in insulin signaling and gluconeogenesis. Mean (± SE) (A) fasting plasma insulin concentrations and liver (B) P-IR to total IR-β ratio, (C) PEPCK-C, and (D) G6Pase (% change from LETO at 2 weeks) and the representative western blot bands of Long-Evans Tokushima Otsuka (LETO), Otsuka Long-Evans Tokushima Fatty (OLETF), and OLETF + ARB. Comparisons were assessed using a two factor ANOVA with group and time as between-subject factors. a p < 0.05 vs. LETO.