Abstract
Fibroblast growth factor 21 (FGF21) is under investigation as a type 2 diabetes protein drug, but its efficacy is impeded by rapid in vivo clearance and by costly production methods. To improve the protein’s therapeutic utility, we recombinantly expressed FGF21 as a fusion with an elastin-like polypeptide (ELP), a peptide polymer that exhibits reversible thermal phase behavior. Below a critical temperature, ELPs exist as miscible unimers, while above, they associate into a coacervate. The thermal responsiveness of ELPs is retained upon fusion to proteins, which has notable consequences for the production and in vivo delivery of FGF21. First, the ELP acts as a solubility enhancer during E. coli expression, yielding active fusion protein from the soluble cell lysate fraction and eliminating the protein refolding steps that are required for purification of FGF21 from inclusion bodies. Second, the ELP’s phase transition behavior is exploited for facile chromatography-free purification of the ELP-FGF21 fusion. Third, the composition and molecular weight of the ELP are designed such that the ELP-FGF21 fusion undergoes a phase transition triggered solely by body heat, resulting in an immiscible viscous phase upon subcutaneous (s.c.) injection and thereby creating an injectable depot. Indeed, a single s.c. injection of ELP-FGF21 affords up to five days of sustained glycemic control in ob/ob mice. The ELP fusion partner massively streamlines production and purification of FGF21, while providing a controlled release method for delivery that reduces the frequency of injection, thereby enhancing the pharmacological properties of FGF21 as a protein drug to treat metabolic disease.
Keywords: Drug delivery, elastin-like polypeptide, recombinant fusion protein, subcutaneous depot, type 2 diabetes, fibroblast growth factor 21
Graphical abstract
Introduction
Over 30 million people in the United States are diabetic, and in 2015 alone, an additional 1.5 million new cases were diagnosed (1), underscoring both the significant health burden and the rapidly growing nature of the disease. Type 2 diabetes accounts for the majority of newly diagnosed cases, and is characterized by insufficient insulin production by the pancreas, leading to an inability to modulate blood glucose levels. It is estimated that 40–50% of type 2 diabetics fail to achieve their individualized glycemic goals (2), a statistic attributed both to ineffective treatment options and to poor patient compliance. There is hence an urgent need for more effective drugs that impose a minimal burden on the patient.
Fibroblast growth factor 21 (FGF21) was recently identified as an important metabolic regulator that improves insulin sensitivity (3–5) and lipid metabolism (6, 7). Pharmacological administration of FGF21 to diabetic mice and non-human primates reduces blood glucose levels, body weight, and plasma lipids (8, 9), and consequently FGF21 is being examined for its potential in treating type 2 diabetes. There are, however, two primary challenges to the use of FGF21 as a drug. First, the native protein exhibits poor pharmacokinetics, as it has only a one-hour plasma half-life that is attributed to rapid renal clearance and proteolytic processing (10, 11). Thus, efforts to increase FGF21’s therapeutic efficacy have included mutagenesis to promote stability (10, 12), as well as fusion to antibody fragments and PEGylation to prolong systemic circulation (10, 13, 14). The second drug development roadblock stems from inclusion body packaging of FGF21 during recombinant production in E. coli. Current methods for manufacturing necessitate denaturing and refolding of the insoluble protein product, resulting in decreased yields, increased costs, and further impeding the utility of FGF21 as a protein drug.
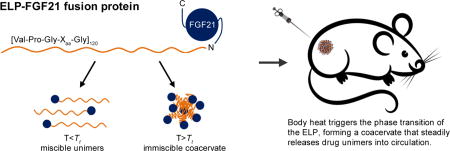
We demonstrate herein a solution to these production and delivery issues through the use of an elastin-like polypeptide (ELP) fusion partner. ELPs are unstructured peptide polymers consisting of a repeated [Val-Pro-Gly-Xaa-Gly] pentapeptide motif inspired by human tropoelastin. Our research group has demonstrated the application of ELPs in biotechnology and drug delivery, exploiting the biopolymer’s thermal responsiveness for chromatography-free purification of fused proteins (15, 16) and prolonged in vivo release of drugs -- notably the type 2 diabetes therapeutic glucagon-like peptide 1 (GLP-1) (17). Here, we show that an ELP tag enables: (1) soluble recombinant expression of active FGF21, (2) facile purification of the ELP-FGF21 fusion protein, and (3) an injectable slow-release depot platform -- all made possible through the intrinsic biophysical properties of the ELP. Subcutaneous (s.c.) administration of ELP-FGF21 to diabetic mice improves their metabolic parameters and confers blood glucose-lowering effects that are significant and sustained, thereby reducing the frequency of injection and increasing the feasibility of FGF21 as a protein drug to treat metabolic disease.
Materials and Methods
Expression vector synthesis
The nucleotide sequence encoding the 182 amino acid murine wild type (WT) FGF21 protein, minus the signal peptide, was codon optimized for E. coli expression and purchased from GeneArt (Thermo Fisher Scientific). Table S1 displays primer sequences used to introduce point mutations for amino acid substitutions L99R, P172G, and L173S, designed to enhance protein stability (10). The mutated Fgf21 gene was PCR amplified (Table S1), digested with BseRI, and ligated into a pET-24a+ vector modified for seamless fusion of genes (18).
Oligonucleotides encoding the peptide sequence ENLYFQG (Table S1) were purchased (Integrated DNA Technologies, Coralville, IA), annealed, and ligated into a modified pET-24a+ vector (18). Following the previously reported seamless cloning strategy (18), the gene encoding the ENLYFQG peptide (“tev”) was fused at the 3’-end to the mutated Fgf21, and at the 5’-end to a gene encoding an ELP consisting of 60 repeats of a [Val-Pro-Gly-Val-Gly] pentapeptide. The final vector encoded the polypeptide fusion “ELP-tev-FGF21” (see Table S2 for complete amino acid sequences). A similar process was followed for generating the vector encoding “ELPDepot-FGF21” (Table S2), however ELPDepot consisted of 120 repeats of a [Val-Pro-Gly-Xaa-Gly] pentapeptide -- where Xaa signifies a 4:1 ratio of Val:Ala -- and the gene encoding tev was omitted upstream of Fgf21.
Protein expression and purification
For production of ELPDepot without the fused FGF21, the ELP-encoding expression vector was transformed into Ultra BL21 (DE3) cells (Edge BioSystems, Gaithersburg, MD), and expression and inverse transition cycling (ITC) were carried out as described previously (16). ELPDepot-FGF21- and ELP-tev-FGF21-encoding expression vectors were transformed into SHuffle cells (New England Biolabs), and modified methods for expression and ITC were employed as follows.
A starter culture containing 50 mL of 55 g/L terrific broth (TB) plus kanamycin was inoculated and grown overnight at 37°C with orbital shaking at 250 rpm. The starter culture was centrifuged, resuspended in TB, and used to inoculate two 1-L volumes of TB plus kanamycin in 6 L Erlenmeyer flasks. The flasks were cultured at 25°C with orbital shaking at 200 rpm until they reached an OD600 of 0.1. Protein expression was then induced by addition of 250 µM IPTG. The culturing temperature was reduced to 16°C, and growth was allowed to proceed for an additional 18 h.
Bacterial cultures were centrifuged at 4°C for 10 min at 3365 rcf, and resuspended in cold PBS. Cell membranes were disrupted via sonication (Q500 sonicator, QSonica, Newtown, CT), and pulsed at 10 s on and 40 s off for a total sonication time of 90 s. DNA was precipitated by addition of 10% polyethylenimine, and cell lysate was separated into soluble and insoluble fractions by centrifugation at 4°C for 10 min at 23,645 rcf. The soluble fraction was brought to room temperature, and the ELP-FGF21 fusion protein was purified from solution via ITC. In this process, the phase transition of the ELP-FGF21 fusion protein was triggered by addition of 0.2 M (NH4)2SO4, producing a turbid suspension due to coacervation of the ELP fusion. The suspension was centrifuged at 25°C for 15 min at 23,426 rcf; this step is referred to as a “hot spin.” The supernatant was discarded and the pellet was resolubilized in PBS at 4°C with 25 rpm gentle rotation (R4045 Roto-Bot Programmable Rotator, Benchmark Scientific, Sayreville, NJ). The resulting solution was centrifuged at 4°C for 5 min at 18,407 rcf to pellet insoluble contaminants, and the supernatant was reserved; this step is referred to as a “cold spin.”
The ITC process was repeated by warming the solution to room temperature, adding (NH4)2SO4 to trigger the phase transition, centrifuging at 25°C for 8 min at 18,407 rcf to pellet the ELP-FGF21 fusion protein, resolubilizing the pellet in PBS at 4°C with gentle rotation, and centrifuging at 4°C for 5 min at 18,407 rcf. Three total rounds of ITC were necessary to yield a purified product.
For production of FGF21 without the fused ELP, ELP-tev-FGF21 was resuspended after its final round of ITC in TEV protease reaction buffer (19), substituting 3 mM glutathione for dithiothreitol. ELP-tev-FGF21 was incubated at 4°C overnight with an ELP-tagged TEV protease, denoted “ELP-protease” (see Supplementary Material for production methods, and Tables S3 and S4 for relevant primers and amino acid sequences) at an ELP-protease:ELP-tev-FGF21 OD280 ratio of 1:10. The phase transition of the ELPs was triggered by addition of 0.2 M (NH4)2SO4 and the resulting suspension containing immiscible ELP-tev and ELP-protease, as well as miscible FGF21 was centrifuged 15 min at 25°C. The supernatant containing FGF21 was dialyzed into PBS.
Endotoxin purification and testing
Recombinantly produced proteins were endotoxin-purified using Acrodisc Units (Pall Corporation, Port Washington, NY), and resulting endotoxin levels were tested using the Endosafe nexgen-PTS spectrophotometer (Charles River Laboratories, Wilmington, MA).
ERK phosphorylation assays
3T3-L1 murine fibroblasts (Zen-Bio Inc., Research Triangle Park, NC) were differentiated into adipocytes per manufacturer’s instructions. Cells were serum starved for 24 h and stimulated with 100 nM ELP-tev-FGF21 or FGF21 (ProSpec Protein Specialists, East Brunswick, NJ) as a function of time. Cells were lysed in RIPA buffer (Sigma-Aldrich) and soluble fractions were analyzed. Western blots were labeled with anti-phospho-ERK1/2 antibody, stripped by incubating 30 min in 50 mM beta mercaptoethanol plus 2% SDS, and reprobed with anti-ERK1/2 antibody (Cell Signaling Technology, Danvers, MA). For detection, an anti-rabbit HRP conjugate (Invitrogen) and the Pierce ECL system (Thermo Fisher Scientific) were employed.
For quantitative evaluation of ELP-FGF21 activity, a HEK293 cell line was generated that stably expressed murine KLB and FGFR1. For transfection details, see Supplementary Material. To verify FGFR1 and KLB expression in the resulting clonal cell lines, cells were harvested by a cell scraper, lysed in RIPA buffer, and soluble fractions were analyzed. Western blots were labeled with an anti-murine KLB antibody (R&D Systems, Minneapolis, MN) or an anti-murine FGFR1 antibody (Santa Cruz Biotechnology, Dallas, TX). Blots were stripped and reprobed with an anti-ERK1/2 antibody as a loading control. To ensure receptor functionality, stable clones expressing high levels of both KLB and FGFR1 were seeded at 5 × 104 cells/cm2 and adhered overnight. Cells were serum starved for 6 h and stimulated with 100 nM ELP-tev-FGF21 as a function of time. Cell lysates were prepared and probed via Western blot for ERK1/2 and phospho-ERK1/2 as described previously.
For the quantitative ERK phosphorylation assay, the KLB/FGFR1 HEK293 cells were seeded at 5 × 104 cells/cm2 and adhered overnight. After 6 h serum starvation, cells were treated with serial dilutions of FGF21 variants for 5 min. Cells were lysed and assessed for phospho-ERK1/2 and total ERK1/2 content using the AlphaLISA Surefire Ultra Assay Kits (PerkinElmer) and the EnSpire Alpha Plate Reader (PerkinElmer). Phospho-ERK1/2 levels were normalized by dividing the phospho-ERK1/2 signal by the total ERK1/2 signal. Data were fit to a three-parameter dose-response curve to determine EC50s using GraphPad Prism 6 software (La Jolla, CA).
Circular dichroism
Circular dichroism experiments were performed on ELPDepot, ELPDepot-FGF21, and FGF21 cleaved from ELP-tev-FGF21, using an Aviv Model 202 instrument and 1mm quartz cells (Hellma USA, Plainview, NY). Scans were carried out in PBS, pH 7.4, at 10°C with a protein concentration of 10 µM. Samples were scanned in triplicate from 260 nm to 190 nm in 1 nm steps with a 1 s averaging time. Data points with a dynode voltage above 500 V were omitted from analysis. Structural estimations were carried out using BeStSel (20), with an analysis range of 200–250 nm.
Phase behavior characterization
The lower critical solution temperature (LCST) phase transition behavior of ELPs and ELP-FGF21 fusion proteins was evaluated by monitoring the OD350 of solutions in PBS as a function of temperature on a Cary 300 UV-visible spectrophotometer equipped with a multicell thermoelectric temperature controller (Agilent Technologies, Santa Clara, CA). Heating and cooling were set to a rate of 1°C/min. Tt was defined as the temperature at which the optical density reached 50% of its maximal value (21).
Animals
In vivo studies were conducted in accordance with the AAALAC-accredited Duke Institutional Animal Care and Use Committee. Mice were purchased from Jackson Laboratory (Bar Harbor, ME) and maintained on a 12h/12h light/dark cycle with ad libitum access to food (LabDiet 5053) and water. For experiments involving 125I, water was supplemented with 0.4 wt% potassium iodide to block radionuclide accumulation in the thyroid.
Short-term efficacy studies in mice
Following 1 week acclimation, 7–9-week-old male ob/ob mice were randomized into control or treatment groups according to baseline blood glucose levels, and received a single injection of ELPDepot-FGF21, FGF21 cleaved from ELP-tev-FGF21, or vehicle into the s.c. space on the hind flank. Doses are reported in mg/kg, where “mg” represents the mass equivalent of FGF21 protein. Fed blood glucose levels were measured with an AlphaTRAK 2 Blood Glucose Meter (MEDIpoint, Mineola, NY) and body weights were recorded on days −1 and 6 of each experiment. Area under the curve (AUC) was calculated using GraphPad Prism 6 software using the trapezoidal rule and setting a Y=0 baseline.
Fluorescence tomography
ELPDepot-FGF21 or FGF21 cleaved from ELP-tev-FGF21 were fluorescently labeled on lysine residues with Alexa Fluor® 647 NHS Ester (Molecular Probes, Eugene, OR) per manufacturer’s instructions, and purified with Zeba Spin Desalting Columns (Thermo Fisher Scientific). Fluorophore conjugation stability was evaluated in vitro by incubating labeled protein in serum, and visualizing resulting products (see Supplementary Material for details).
Labeled protein was diluted with unlabeled protein to yield a fluorophore concentration of 1 µM, a concentration experimentally determined as optimal for depot imaging. 6-week-old male ob/ob mice were barbered below the midline and 20 mg/kg of the labeled protein solution was injected into the s.c. space on the hind flank. Mice were anesthetized with 2.5% isoflurane, and images of the injection sites were collected using a Fluorescence Molecular Tomography 4000 In Vivo Imaging System (Perkin Elmer). TrueQuant software (Perkin Elmer) was used to quantify fluorescence in regions of interest. Standards containing known amounts of fluorophore were imaged to create a calibration curve between fluorescence units and moles of Alexa 647, which could then be correlated to moles of drug at the injection site.
Pharmacokinetic studies
Tyrosine residues on ELPDepot-FGF21 or FGF21 cleaved from ELP-tev-FGF21 were reacted with Na125I radionuclide (Perkin Elmer) using Pierce Pre-Coated Iodination Tubes (Thermo Fisher Scientific) and the indirect method for iodination. Radiolabeled protein was purified with Zeba Spin Desalting Columns. Activities of radiolabeled constructs were measured with an Atomlab 400 Dose Calibrator (Biodex, Shirley, NY) and correlated to protein concentration.
7–9-week-old male ob/ob mice were injected i.v. with 1 mg/kg radiolabeled ELPDepot-FGF21 or FGF21 cleaved from ELP-tev-FGF21. The fusion was injected at a dilute concentration (10 µM) to avoid LCST phase transition of the ELP within the blood vessel. 10 uL blood samples were collected at frequent time points from the tail vein, and sample counts were measured at the end of the study on a Wallac Wizard 1480 Automatic Gamma Counter (Perkin Elmer). An activity vs. count standard curve was used to convert sample counts to activities, and subsequently to moles of drug. For tracking serum drug levels following a therapeutic dose, 9-week-old male ob/ob mice were injected via s.c. with 20 mg/kg radiolabeled ELPDepot-FGF21 or FGF21 cleaved from ELP-tev-FGF21. Blood was collected and counts were measured as previously described.
Pharmacokinetic analysis
I.v. data analysis: Co is the maximum serum concentration at time zero extrapolated from early time points. VD is the volume of distribution, calculated as VD = (drug dose injected i.v.)/(Co). AUC is the area under the concentration vs. time curve and was calculated by GraphPad Prism 6 software (La Jolla, CA) using the trapezoidal rule. AUC was estimated starting at Co and extrapolated to infinity based on a linear regression curve fit to the terminal portion of the log serum concentration vs. time curve. Terminal half-life (t1/2, elim) was estimated from the slope of the linear regression curve fit to the terminal portion of the log serum concentration vs. time curve. “CL” represents drug clearance, and is defined as CL = F*Dose/AUC, where F is bioavailability, equal to 1 for an i.v. drug bolus.
S.c. data analysis: The maximum serum concentration (Cmax) was recorded as observed, as well as time to reach Cmax (tmax). AUC was estimated utilizing a serum concentration of 0 nM at time zero and extrapolated to infinity based on a linear regression curve fit to the terminal portion of the log serum concentration vs. time curve. S.c. bioavailability is defined as F = (AUCs.c.*Dosei.v.)/(AUCi.v.*Doses.c.)*100%. CL is defined as above, using the s.c. bioavailability. Absorption half-life (t1/2, abs) was estimated from the slope of the linear regression curve fit to the terminal portion of the log serum concentration vs. time curve. When a drug administered at an extravascular site yields a terminal half-life greater than that calculated from an i.v. bolus, the terminal half-life reflects the absorption half-life (22).
All pharmacokinetic parameters are reported as means ± SEM.
Long-term efficacy studies in mice
7-week-old male ob/ob mice were randomized into control or treatment groups according to baseline blood glucose and percent glycosylated hemoglobin (%HbA1c) levels. Mice received an s.c. injection every 5 days of 20 mg/kg ELPDepot-FGF21, 20 mg/kg FGF21 cleaved from ELP-tev-FGF21, or vehicle control, for a duration of 8 weeks. Body weights and fed blood glucose levels were measured 1–2 times per week. Blood glucose AUC was calculated with GraphPad Prism 6 software using the trapezoidal rule and setting a Y=0 baseline. %HbA1c was measured on t = 0 and t = 55 d using a DCA Vantage Analyzer and DCA 2000 Reagent Kits (Siemens). Blood was collected at t = 30 d for analysis of serum insulin and triglyceride levels, which were measured using the Crystal Chem Ultra-Sensitive Mouse Insulin ELISA (Downers Grove, IL) and the Abcam Triglyceride Quantification Assay kit, respectively.
Statistical analyses
Data are presented as means ± SEM. In vitro activity EC50s were tested for statistical significance via unpaired t test. Blood glucose vs. time data were analyzed via two-way repeated measures ANOVA followed by uncorrected Fisher’s LSD at each discrete time point. Other metrics of in vivo efficacy were analyzed via one-way ANOVA followed by Dunnett’s multiple comparisons test.
Data availability
All raw data associated with this study have been deposited in the Mendeley Data repository, doi:10.17632/y3ybyx47sx.2 (23).
Results
FGF21 expression and purification is enhanced by ELP fusion
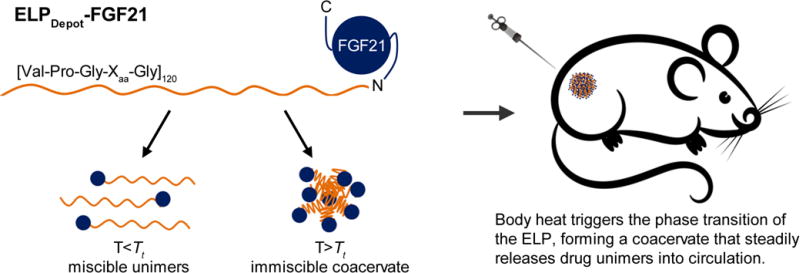
The WT murine FGF21 amino acid sequence was mutated at three sites, L99R, P172G, and L173S, to incorporate the amino acid substitutions previously identified in human FGF21 that inhibit proteolysis and aggregation (10). The N-terminus of FGF21 was selected for fusion to an ELP, as the C-terminus is believed to be essential for binding βklotho (11, 24), a single-pass transmembrane protein that mediates the interaction between FGF21 and its receptor (25). An ELP was chosen as a fusion partner due to its reversible LCST phase behavior in aqueous solution (21). ELPs have a distinct and tunable “transition temperature” (Tt), below which they exist as miscible unimers, and above which they associate into an immiscible coacervate (21). The thermal responsiveness of an ELP is retained when genetically fused to a peptide or protein (15, 26–29), with important consequences that are illustrated herein.
FGF21 was fused to one of two ELPs, each varying in composition and MW. The first consisted of 60 repeats of the Val-Pro-Gly-Val-Gly (VPGVG) pentapeptide and was designed for facile production of the fusion protein and subsequent removal of the ELP to yield free FGF21 with a minimal N-terminal scar. The tobacco etch virus (TEV) protease cleavage sequence, ENLYFQG (denoted as “tev” hereafter), was inserted between the ELP and FGF21, resulting in the fusion protein “ELP-tev-FGF21.”
The second ELP-FGF21 fusion was designed without the TEV protease cleavage motif, and the ELP sequence and MW were chosen such that the fusion protein exhibited a Tt below body temperature. This ELP consisted of 120 [Val-Pro-Gly-Xaa-Gly] pentapeptide repeats with a Val:Ala ratio of 4:1 at the Xaa position, and was chosen based on previous studies examining the effect of ELP composition on Tt (17, 29). We predicted that body heat would be sufficient to drive the ELP-mediated phase transition upon injection, forming a viscous coacervate in the s.c. space, thereby creating an injectable FGF21 depot (Fig. 1). This fusion was hence named “ELPDepot-FGF21.”
Fig. 1.
Schematic demonstrating the principles underlying the injectable FGF21 drug depot. An ELP consisting of 120 repeats of the [Val-Pro-Gly-Xaa-Gly] pentapeptide, with a Val:Ala ratio of 4:1 at the Xaa position, is genetically fused to the N-terminus of FGF21. At temperatures below the tunable transition temperature (Tt) of the ELP, the fusion exists as miscible unimers, while above the Tt it forms an immiscible coacervate. Tuning the Tt below 37°C allows body heat to trigger the phase transition in vivo, forming a depot from which unimers dissolve over time.
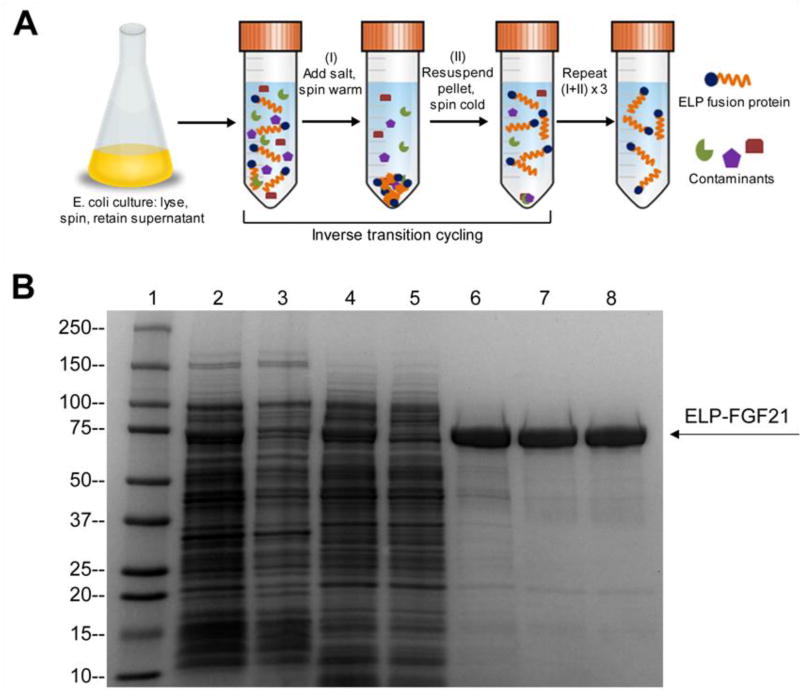
FGF21-encoding expression vectors were transformed into an E. coli expression system, and bacteria were cultured at reduced temperatures (25°C pre-induction, 16°C post-induction) to promote solubility of the fusion proteins. Cell lysates were separated through centrifugation, and the soluble fractions were subjected to ITC, a chromatography-free purification process developed by our research group that exploits the phase transition behavior of ELPs (15) (Fig. 2A). When samples retained throughout the purification process were visualized on a coomassie-stained SDS-PAGE gel, a 68.5 kDa band associated with ELPDepot-FGF21 was identified in the cell lysate and persisted in the soluble fraction (Fig. 2B). The ELP moiety therefore functioned as a solubility enhancer, preventing inclusion body packaging of FGF21 and obviating the need for protein refolding. ITC purification alone was sufficient to isolate ELP-FGF21 fusion proteins from contaminants (Fig. 2B), and this method of expression and purification yielded ~50 mg soluble fusion protein/L bacterial culture.
Fig. 2.
Purification of ELP-FGF21 by inverse transition cycling (ITC). (A) ITC purification schematic. Addition of salt (typically sodium chloride or ammonium sulfate) to the soluble fraction of the bacterial cell lysate triggers the LCST phase transition of the ELP fusion protein. Centrifugation at room temperature (“warm spin”) results in pelleting of the ELP fusion and separation from soluble contaminants. The ELP fusion pellet is then dissolved in cold buffer. Subsequent centrifugation at 4°C (“cold spin”) pellets the insoluble contaminants, while the ELP fusion remains in solution. The process is repeated by triggering the phase transition via salt addition at room temperature. Three to five rounds of ITC are typically sufficient to purify an ELP fusion protein from cell lysate. (B) SDS-PAGE analysis of ELP-FGF21 purification by ITC. ELPDepot-FGF21 was purified by ITC, and then evaluated for purity on a coomassie-stained SDS-PAGE gel. 1: MW ladder (kDa). 2: Cell lysate. 3: Insoluble fraction following DNA precipitation. 4: Soluble fraction following DNA precipitation. 5: Supernatant after first warm spin. 6: ITC round 1. 7: ITC round 2. 8: ITC round 3.
FGF21 was cleaved from ELP-tev-FGF21 by an ELP-tagged TEV protease (Fig. S1A), and ITC purification was further utilized to isolate the free FGF21 from the cleaved ELP and ELP-protease (Fig. S1B). For details regarding protease production, see Supplementary Material.
Recombinantly produced proteins were endotoxin purified and tested to ensure that levels were within the FDA guidelines (30). See Supplementary Material for a calculation of allowable endotoxin limits, and Fig. S2 for an endotoxin test result output.
ELP-fused FGF21 maintains structure and in vitro activity
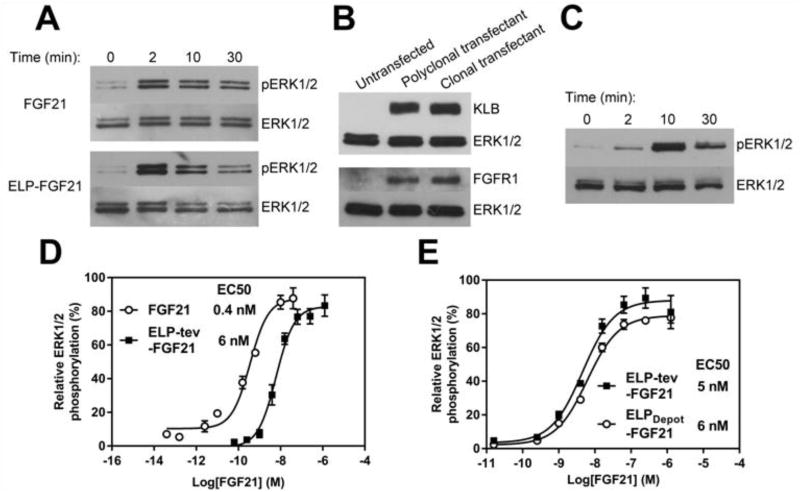
The in vitro activity of FGF21 is typically evaluated by quantifying ligand-induced ERK1/2 phosphorylation in a cell-based assay (10, 12, 14). Appropriate cell lines must express FGF21’s receptor complex, which includes FGF receptor 1c -- a widely expressed isoform of the receptor -- and βKlotho, present primarily in adipose tissue (31). Adipocytes were differentiated from murine fibroblasts and stimulated with ELP-tev-FGF21 or commercially purchased FGF21 for increasing times. Both FGF21 and the ELP fusion induced transient ERK1/2 phosphorylation (Fig. 3A), indicative of FGF21 activity. It should be noted that hereafter, FGF21 for use as a control was produced in-house due to inconsistent protein activities observed in commercially purchased FGF21.
Fig. 3.
ELP-FGF21 stimulates ERK1/2 phosphorylation. (A) Qualitative FGF21 activity assay in adipocytes. 3T3-L1 murine fibroblasts were differentiated into adipocytes, serum starved, then stimulated with 100 nM ELP-tev-FGF21 or 100 nM FGF21 for indicated times. Cell lysates were probed with antibodies against phosphorylated ERK1/2, as well as total ERK1/2 as a loading control. (B) HEK293 cell line stably expresses the FGF21 receptor complex. HEK293 cells were transfected with genes encoding murine FGFR1 and βKlotho (KLB), selected for stably expressing clones, and cell lysates were probed with antibodies against the respective receptors. (C) Qualitative FGF21 activity assay in transfected HEK293 cell line. A HEK293 cell line stably transfected with FGFR1 and KLB was serum starved, then stimulated with 100 nM ELP-tev-FGF21 for indicated times. Cell lysates were probed with antibodies against phosphorylated ERK1/2, as well as total ERK1/2 as a loading control. (D–E) Dose-response curves for ELP-FGF21 fusion proteins. A HEK293 cell line stably expressing the FGF21 receptor complex was serum starved then stimulated for 5 minutes with increasing concentrations of ELP-tev-FGF21, FGF21 cleaved from ELP-tev-FGF21, or ELPDepot-FGF21. Cells were lysed and assayed for phosphorylated and total ERK1/2 content. Data are presented as mean % phosphorylated ERK1/2 ± SEM, n=3. EC50s were determined by fitting a three-parameter dose-response curve.
For EC50 determination, a HEK293 cell line was generated that stably expressed murine FGF Receptor 1 (FGFR1) and murine βKlotho (KLB). For transfection details, see Supplementary Material. Receptor expression was confirmed in an isolated clone via Western blotting (Fig. 3B), and receptor function was verified through ligand-induced ERK1/2 phosphorylation (Fig. 3C). The resulting cell line was stimulated with serial dilutions of ELP-tev-FGF21, ELPDepot-FGF21, or FGF21 cleaved from ELP-tev-FGF21, and ERK1/2 phosphorylation was quantified. Fig. 3D shows a representative experiment (n=3 replicates) that was repeated in its entirety at least once with similar findings. ELP-tev-FGF21 and FGF21 cleaved from the ELP had EC50s of 6 nM and 0.4 nM, respectively, and the EC50 shift attributed to fusion of the ELP was statistically significant (p < 0.001). The EC50 calculated for ELPDepot-FGF21 was not significantly different (p > 0.05) from that of ELP-tev-FGF21 (Fig. 3E).
To identify potential alterations to the structure of FGF21 when fused to an ELP, circular dichroism (CD) experiments were carried out, comparing ELPDepot-FGF21 to an ELP control lacking FGF21 -- ELPDepot -- and to FGF21, cleaved from ELP-tev-FGF21. Mean residue ellipticity peaks for ELPDepot (Fig. S3A) were consistent with previous studies involving ELPs (32–34), showing a high degree of disorder and a ‘shelf’ at 220 nm, associated with the presence of beta turns. FGF21 was largely unstructured (Fig. S3A), which was anticipated based on previous findings (11, 35), and the ELPDepot-FGF21 fusion appeared a combination of FGF21 and ELPDepot (Fig. S3A). The additive relationship between FGF21, ELPDepot, and the ELPDepot-FGF21 fusion was more apparent in the unnormalized data (Fig. S3B). The individual spectra from FGF21 and ELPDepot were summed and the resulting “Addition” was plotted with the ELPDepot-FGF21 fusion with nearly perfect overlap (Fig. S3C). Secondary structure percentages were quantified (Fig. S3D), and further demonstrated negligible changes in FGF21 structure when fused to the ELP.
Phase transition behavior of ELP-FGF21 is suitable for depot formation
Previous work by our group with s.c. drug depots of a GLP1-ELP fusion has shown that a Tt near 30°C results in a depot -- triggered by body heat -- with optimal release kinetics of the GLP1-ELP fusion (17). In contrast, an ELP fusion with a Tt≪30°C forms an excessively stable coacervate that exhibits poor drug release, while a fusion with a Tt≥35°C (the temperature of the s.c. space (36)) exhibits a bolus-type release (17). The Tt is dependent on ELP composition (37), MW (38), and concentration (38), and we have developed analytical correlations for predicting an ELP’s LCST phase behavior based on these parameters. Fusion of an ELP to a protein can, however, alter its phase behavior, depending upon the balance of hydrophobic and charged solvent-accessible residues of the fusion partner (26). Thus, achieving a precise Tt for an ELP fusion requires iterative cycles of de novo design, characterization, and adjustment of the ELP to ultimately arrive at a fusion protein with the desired LCST phase transition profile.
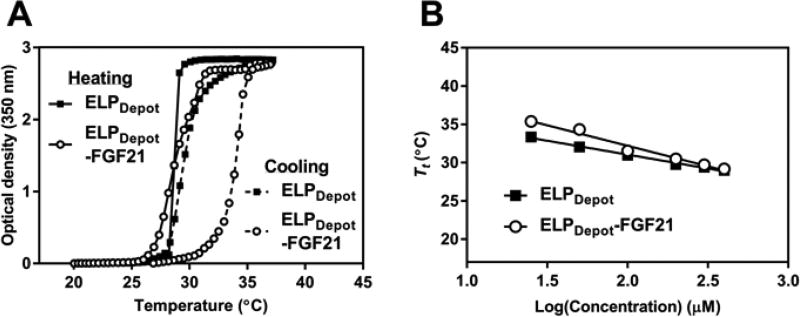
When designing an ELP-FGF21 fusion with optimal depot-forming characteristics, we selected a 120 pentapeptide ELP with a Val:Ala ratio of 4:1 in the guest residue position, termed “ELPDepot.” Fusion Tt profiles were generated by monitoring the optical turbidity as a function of temperature and concentration (Fig. 4A, Fig. S4). The LCST phase transition behavior of ELPDepot-FGF21 was found to be reversible (Fig. 4A) and concentration-dependent (Fig. 4B, Fig. S4), and the fusion exhibited a Tt close to 30°C at injection-relevant concentrations (Fig. 4B). Reversibility and the inverse dependence of Tt on fusion concentration are important characteristics for an ELP-based drug depot: as fusions at the depot margin are diluted by interstitial flow, their Tt raises above body temperature, thereby reversing the LCST phase transition and allowing release of fusion unimers from the coacervate.
Fig. 4.
LCST phase transition behavior of a depot-forming ELP-FGF21 fusion protein. (A) Turbidity profiles as a function of solution temperature for a depot-forming ELP, alone and fused to FGF21. The optical density at 350 nm of ELPDepot or ELPDepot-FGF21, each prepared at 400 µM in PBS, was measured as a function of temperature, with a temperature ramp up to 37°C then down to 20°C at a rate of 1°C/min. (B) Transition temperature (Tt) as a function of concentration for a depot-forming ELP, alone and fused to FGF21. The optical density at 350 nm of indicated dilutions of ELPDepot or ELPDepot-FGF21 was measured as a function of temperature, as described in (A). The Tt was defined as the temperature corresponding to the 50% maximum optical density, and was plotted against ELP concentration. Data are presented as mean ± SEM, n=3.
Depot-forming ELP-FGF21 exhibits prolonged in vivo efficacy
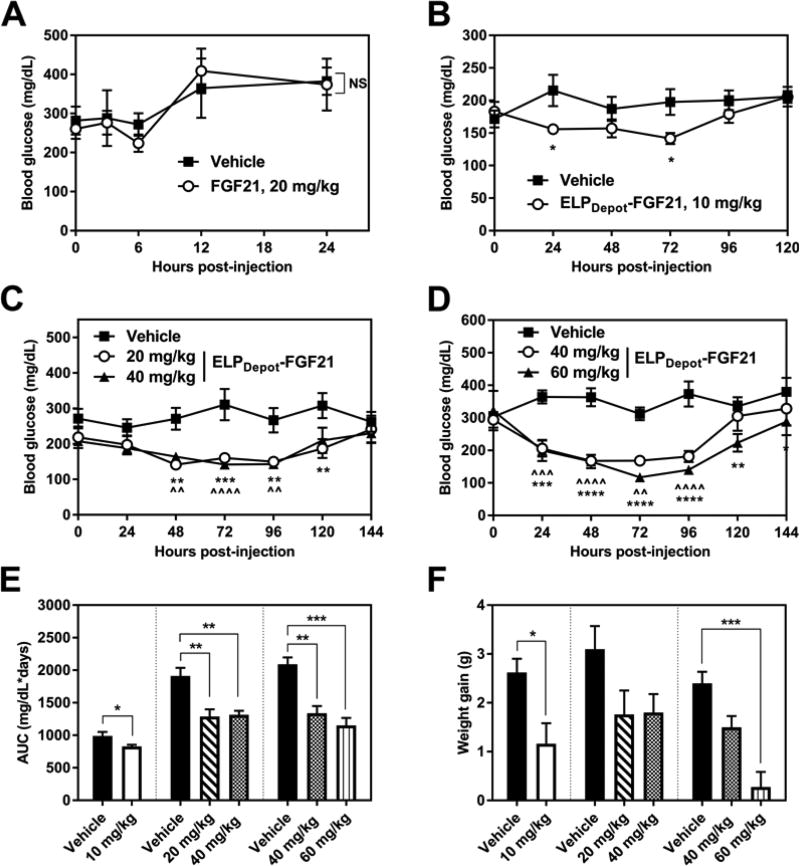
For studies evaluating the short-term in vivo efficacy of a depot-forming ELP-FGF21 fusion, ob/ob mice received a single s.c. injection of ELPDepot-FGF21 at 400 µM. This concentration correlates with a Tt of 29°C (Fig. 4B) and hence should result in the LCST phase transition of the fusion in the s.c. space. PBS vehicle was employed as a control, and treatment with FGF21 cleaved from ELP-tev-FGF21 was included for comparison. It has been established that a single administration of FGF21 has a transient, if any, effect on blood glucose levels in a diabetic mouse model (10, 14). We confirmed these findings, as a 20 mg/kg FGF21 s.c. injection failed to significantly depress blood glucose levels in ob/ob mice (Fig. 5A). Treatment with ELPDepot-FGF21, however, caused significant and dose-dependent blood glucose reductions (Fig. 5B–D). At 20 mg/kg doses and above, the effects were sustained as long as 5 d post-injection (Fig. 5C–D). Significant reductions in glucose AUC resulted from all ELPDepot-FGF21 doses tested (Fig. 5E), and modest decreases in weight gain were observed at all doses, with the highest dose nearly inhibiting any weight increase (Fig. 5F).
Fig. 5.
Short-term efficacy of ELPDepot-FGF21 in a diabetic mouse model. (A–D) Fed blood glucose levels following a single injection of FGF21 or ELPDepot-FGF21. 7–9-week-old ob/ob mice (n=4–5) were injected via s.c. with indicated dose of ELPDepot-FGF21, FGF21, or vehicle control and blood glucose levels were monitored. Data were analyzed via two-way repeated measures ANOVA followed by uncorrected Fisher’s LSD tests at each discrete time point. Data are presented as mean ± SEM (*p<0.05, **p<0.01, ***p<0.001, ****p<0.0001; ^ indicates significance for 40 mg/kg cohort; NS = treatment effects are not statistically significant). (E–F) Effects of ELPDepot-FGF21 on blood glucose area under the curve (AUC) and weight gain. AUC was calculated from the blood glucose vs. time data reported in B–D; weights were measured 1 day prior to injection and on day 6 post-injection. Data are presented as mean ± SEM, and were analyzed for statistical significance via one-way ANOVA followed by Dunnett’s multiple comparisons test (*p<0.05, **p<0.01, ***p<0.001).
maging confirms formation of an ELP-FGF21 drug depot upon s.c. injection
ELPDepot-FGF21 and FGF21 cleaved from ELP-tev-FGF21 were fluorescently labeled with a near-infrared dye for visualization of depot formation and dissolution. Stability of the fluorophore conjugate was first evaluated in vitro by incubation of labeled protein in serum at 37°C as a function of time. When reaction products were separated by SDS-PAGE and visualized by fluorescence imaging, the MW of the observed bands were consistent with the respective calculated MWs of FGF21 (Fig. S5) and ELPDepot-FGF21 (Fig. S6). A band associated with free Alexa 647 dye was visible only in positive control lanes (Fig. S5, Fig. S6), confirming the integrity of the amide bond conjugating the fluorophore to the primary amine groups on FGF21.
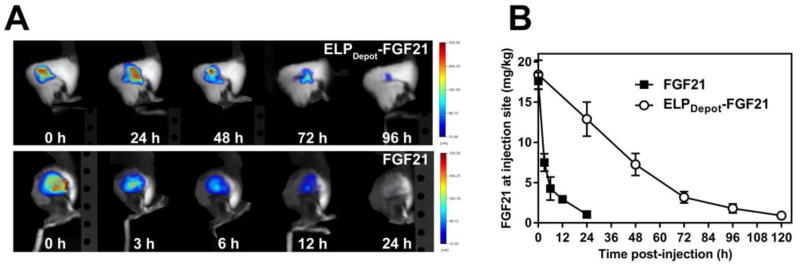
Molar equivalent doses of fluorescently labeled ELPDepot-FGF21 or FGF21 were administered to ob/ob mice via s.c. injection on the hind flank, and images were captured at injection sites at subsequent time points. Fluorescence was quantified within regions of interest as well as from standards, which revealed a linear correlation between moles of dye and fluorescence units (Fig. S7). This correlation was used to estimate the amount of FGF21 remaining in the s.c. space based on the dye:drug molar ratio upon injection. While the bulk of FGF21 was absorbed within 24 h (Fig. 6A: bottom panel, Fig. 6B, Fig. S8), the absorption rate of ELPDepot-FGF21 appeared greatly prolonged, with the majority of the fusion dissipating from the s.c depot by 4 d post-injection (Fig. 6A: top panel, Fig. 6B, Fig. S9).
Fig. 6.
Fluorescence tomography imaging and quantification of ELP-FGF21 depots. (A) Male ob/ob mice were injected via s.c. with 20 mg/kg fluorescently labeled ELPDepot-FGF21 or FGF21. Injection sites were imaged by fluorescence molecular tomography every 24 h for the ELPDepot-FGF21 cohort (top panel) or at indicated time points for FGF21 cohort (bottom panel). (B) Image analysis software was used to quantify fluorescence within assigned regions of interest, and these data were correlated to protein drug residing in the s.c. space for each respective cohort (n=5). Data are presented as means ± SEM.
ELP fusion improves FGF21 pharmacokinetics in mice
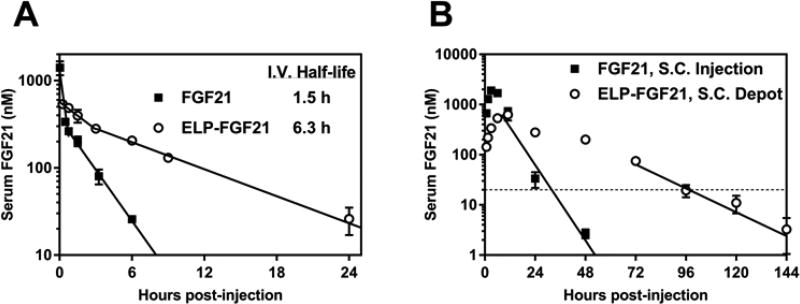
For tracking drug levels in circulation, ELPDepot-FGF21 and FGF21 cleaved from ELP-tev-FGF21 were radiolabeled and administered either i.v. or s.c. to ob/ob mice. I.v. drug was injected at a low concentration to prevent fusion coacervation within the vessel via the LCST phase transition, while s.c. drug was administered at a therapeutic dose and a concentration concomitant with ELP-based depot formation. Blood was collected at frequent time points, and gamma counts from samples were correlated to radiolabeled protein content. Serum FGF21 concentration was plotted against time, and regression curves were fit to the data (Table S5). Analysis of i.v. pharmacokinetic data (Fig. 7A, Table 1) showed that fusion of FGF21 to an ELP decreased the clearance rate from 2.0 to 0.46 mL/h, and increased the terminal half-life from 1.5 to 6.3 h. S.c. injection of a depot-forming ELP fusion further improved pharmacokinetic parameters of the protein (Fig. 7B, Table 1). ELPDepot-FGF21 had an absorption half-life of 16.6 h compared to 4.8 h for FGF21. Molar equivalent doses of s.c.-injected FGF21 and ELPDepot-FGF21 resulted in equivalent AUCs, however FGF21 reached a Cmax value three-fold higher than that observed for ELPDepot-FGF21. The depot-forming fusion remained in circulation 48–72 h longer than FGF21 before dropping below its minimum effective concentration of 20 nM, which was estimated based on efficacy data and reports in the literature for other FGF21 analogs (see Supplementary Material). ELPDepot-FGF21 had a calculated s.c. bioavailability of 26.8%.
Fig. 7.
Serum concentration vs. time profiles for ELP-fused FGF21. Male ob/ob mice (n=3–4) were injected with radiolabeled FGF21 or ELPDepot-FGF21 via (A) i.v. at 1 mg/kg or (B) s.c. at 20 mg/kg. Blood samples were collected at indicated times and radiolabeled protein was quantified via gamma counting. Data are presented as mean ± SEM. Solid lines represent a linear regression fit to the terminal portions of the i.v. and s.c. data, as well as early i.v. time points for extrapolation of initial serum concentration. The horizontal dotted line indicates the estimated minimum therapeutically effective concentration for ELPDepot-FGF21.
Table 1.
Pharmacokinetic parameters for ELP-fused FGF21 following i.v. or s.c. administration to mice.
| Construct | Co or Cmax (nM) | tmax (h) | VD (ml) | AUC (nM*h) | t1/2, elim or t1/2, abs (h) |
CL (ml/h) |
F (%) |
|---|---|---|---|---|---|---|---|
| ELP-FGF21 1 mg/kg, i.v. | 571 ± 35 | N/A | 3.3 ± 0.2 | 4029 ± 275 | 6.3 ± 1.0 | 0.46 ± 0.03 | 100 |
| FGF21 1 mg/kg, i.v. | 1387 ± 241 | N/A | 1.9 ± 0.5 | 1157 ± 100 | 1.5 ± 0.1 | 2.0 ± 0.2 | 100 |
| ELPDepot-FGF21 20 mg/kg, s.c. | 639 ± 143 | 9.3 ± 1.7 | N/A | 21617 ± 1376 | 16.6 ± 3.9 | 0.56 ± 0.01 | 26.8 ± 1.7 |
| FGF21 20 mg/kg, s.c. | 1904 ± 118 | 3 | N/A | 20076 ± 1552 | 4.8 ± 0.2 | 2.0 ± 0.1 | 86.8 ± 6.7 |
Data are reported as means ± SEM. Co, extrapolated serum concentration at time zero; Cmax, observed maximum serum concentration; tmax, time to Cmax; VD, blood volume of distribution; AUC, area under the curve; t1/2, elim, elimination half-life; t1/2, abs, absorption half-life; CL, clearance rate; F, bioavailability; N/A, not applicable.
Long-term treatment with ELPFGF21 improves metabolic parameters in mice
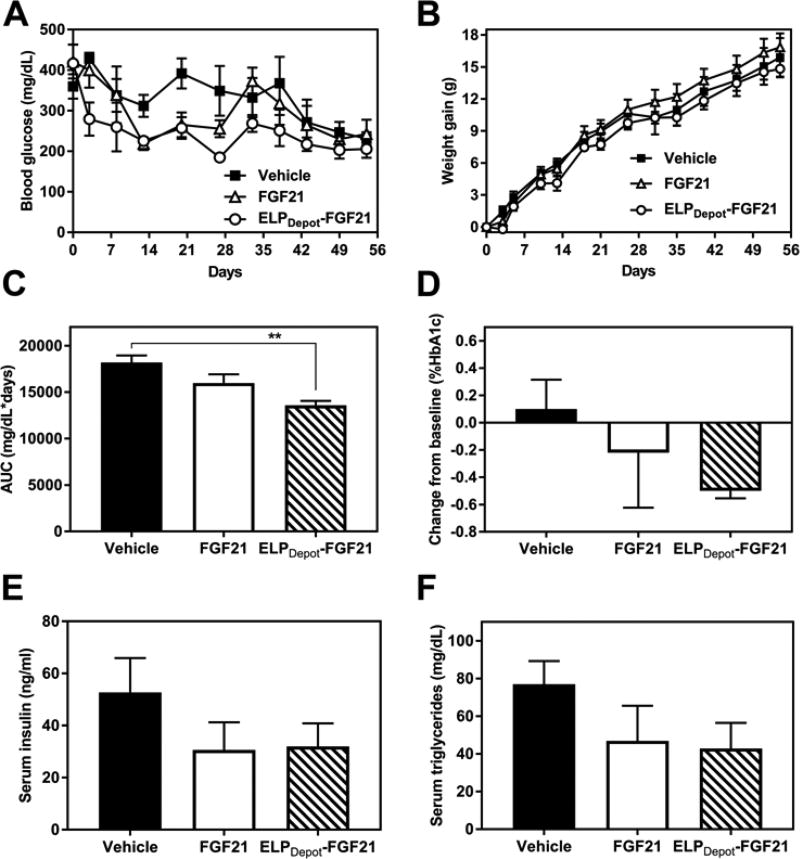
To evaluate long-term efficacy of the ELP fusion, molar equivalent doses of ELPDepot-FGF21 or FGF21 cleaved from ELP-tev-FGF21 were repeatedly administered to ob/ob mice every 5 days for 8 weeks. ELPDepot-FGF21 was injected at 400 µM to ensure its LCST phase transition in the s.c. space. Fed blood glucose levels and body weights (Fig. 8A–B) were monitored throughout the study, and blood draws were performed at t = 0, 30, and 55 d for evaluation of additional circulating metrics of efficacy.
Fig. 8.
Long-term efficacy of ELPDepot-FGF21 in a diabetic mouse model. 7-week-old ob/ob mice (n=5) received repeated s.c. injections of FGF21 or ELPDepot-FGF21 at 20 mg/kg every 5 days for 8 weeks. Fed blood glucose levels (A) and body weights (B) were monitored throughout the treatment period. (C) Blood glucose AUC was calculated from the blood glucose vs. time data reported in A. (D) %HbA1c was measured on day 55, 5 days following the final dose, and is reported as a magnitude change in %HbA1c compared to t=0. (E–F) Serum insulin and triglyceride levels were measured on day 30, prior to a scheduled treatment dose. Data are presented as mean ± SEM, and were analyzed for statistical significance via one-way ANOVA followed by Dunnett’s multiple comparisons tests (**p<0.01).
Mice treated with both the ELP fusion and FGF21 displayed reductions in blood glucose levels, however glucose control conferred by the fusion was more consistent and sustained than that from FGF21 (Fig. 8A). ELPDepot-FGF21 treatment significantly reduced blood glucose AUC compared to vehicle control, while the effects of FGF21 without the fused ELP were not significant (Fig. 8C). Furthermore, over the course of the 8 week study, a consistent reduction in HbA1c was observed in the depot cohort only (Fig. 8D), demonstrating superior glucose control afforded by ELPDepot-FGF21 compared to FGF21.
FGF21 treatment has been shown to reduce baseline insulin levels in diabetic mice (6, 12, 39, 40) -- an indication of increased insulin sensitivity -- while simultaneously decreasing circulating lipids (6, 8, 13, 14, 39, 40). Indeed, both the ELPDepot-FGF21 and FGF21 treatment cohorts displayed reduction trends for these metabolic markers. After 6 dosing cycles (t=30 d), mean insulin and triglyceride levels were ~40% lower in treatment groups compared to vehicle control (Fig. 8E–F), indicating that the metabolic effects of long-term ELPDepot-FGF21 treatment extended beyond glycemic control.
Discussion
Ever since the identification of FGF21 as a metabolic regulator, enthusiasm over its therapeutic potential has gained momentum, with applications expanding from diabetes to obesity, hyperlipidemia, and non-alcoholic fatty liver disease (41). However, the native protein is poorly suited for drug development due to its rapid clearance and degradation in vivo. The majority of efforts to boost FGF21’s utility as a drug have thus focused on improving delivery, specifically by prolonging its systemic circulation.
PEGylation has been shown to effectively extend the serum half-life of FGF21, and conjugation of PEG at strategically chosen internal residues maintains FGF21 in vitro potency (14, 42). A similar strategy involves covalent conjugation of FGF21 at an internal residue to the Fab fragment of an antibody. This formulation, termed “CovX-Body,” retains full potency, while extending FGF21’s serum half-life to 37 h (13). Recombinant fusion to the Fc fragment has the benefit of simpler manufacturing, and an Fc-fused FGF21 displays prolonged circulation with >48 h of sustained blood glucose reduction after a single injection to diabetic mice (10).
While these delivery approaches have proven successful in increasing the half-life of FGF21, they fail to address some significant production issues associated with the protein drug. Recombinant FGF21 is typically produced in an E. coli expression system, where the protein is packaged in inclusion bodies, structures that sequester unfolded, insoluble and biologically inactive protein within the bacterial cell (43). Extraction of FGF21 from inclusion bodies requires chaotropes and refolding via dialysis, followed by multiple steps of chromatography to purify the protein (10, 13, 14, 42). An additional round of chromatography is required following conjugation of purified FGF21 to PEG or CovX-Bodies (13, 14, 42), inevitably decreasing product homogeneity and yield. The vast -- and global -- prevalence of metabolic disease argues for cost-effective and scalable manufacturing methods for new therapeutics, that in our view are simply not met by current production methods for FGF21.
Here, we have shown that fusion to a thermally responsive ELP provides an alternative method to improve delivery of FGF21, while concurrently improving all aspects of the protein drug production and purification processes. During bacterial expression, the ELP prevents accumulation of FGF21 in inclusion bodies such that high yields of soluble and biologically active fusion protein are obtained without the need for denaturing and in vitro refolding of the protein. While solubility enhancers typically must be removed from their fusion partners following expression, our ELP tag provides an attractive design feature by remaining fused and streamlining downstream processes. Following expression, the thermally responsive properties of the ELP are exploited for a facile chromatography-free FGF21 purification scheme that requires only the application of salt and centrifugation (15, 16). Since the ELP is genetically fused to FGF21, the final purified product is monodisperse.
FGF21 maintains its structure and retains functional activity throughout bacterial expression and ELP-based purification, though fusion to an ELP reduces FGF21 potency by 15-fold. The observed increase in EC50 is not unexpected; FGF21 exhibits a 4–5-fold increase in EC50 when conjugated to PEG (14, 42) or fused to the Fc fragment (10), while ELP-fused GLP-1 is 50-fold less active compared to the native peptide (29). Moreover, we show that the reduced in vitro potency of ELP-FGF21 is more than compensated for in an in vivo setting.
The ELP fusion confers two important therapeutic properties to FGF21: (1) the circulation half-life of FGF21 is significantly increased upon fusion to the ELP, and (2) absorption from the injection site into circulation is temporally controlled. This second feature is achieved by carefully tuning the Tt such that the ELP fusion remains miscible at ambient temperature -- i.e. in the syringe -- but changes phase with body heat to an immiscible coacervate phase. We hypothesize that interstitial flow in the s.c space dilutes the ELP fusion unimers at the margins of the depot, raising their Tt above body temperature -- as the Tt correlates inversely with concentration. Consequently, the LCST phase transition reverses, allowing the ELP-based depot to dissolve from the “outside-in” and release ELPDepot-FGF21 unimers into circulation.
The formation of a thermally triggered s.c. FGF21 depot, and its slow dissolution over time, results in up to 5 d of blood glucose control in ob/ob mice following a single injection. This level of sustained action, consistent with a once-weekly dosing regimen, is unmatched by the majority of published methods for prolonging FGF21 circulation. To our knowledge, the duration of blood glucose-lowering efficacy allowed by the ELP fusion is surpassed only by an Fc-fused analog incorporating a recently identified mutation to further prolong circulation (40), and by an FGF21 dual PEGylation strategy (42). It should be noted, however, that PEGylated FGF21 has been associated with vacuole formation in the kidneys -- whereas ELPs are biocompatible and biodegradable (44). Furthermore, PEGylated biologics are coming under increasing scrutiny as it has been estimated that as much as 40% of the population in the developed world has pre-existing antibodies against PEG (45), resulting from prior exposure to the polymer in food and consumer products. The presence of anti-PEG antibodies can result in premature clearance of PEGylated drugs, or in extreme cases, life-threatening hypersensitivity reactions (46).
Imaging and pharmacokinetic analyses confirm that the sustained action of ELPDepot-FGF21 is consistent with the formation of an s.c. drug depot that controls release of fusion unimers into circulation. Less than 6% of drug remains at 24 h following a bolus injection of FGF21, while the ELP depot retains 70% of the injected fusion at this time. As ELP-fused FGF21 residing in the s.c. depot declines over 72 h post-injection, serum drug levels stay fairly steady, strongly indicating that ELPDepot-FGF21 unimers dissolve from the depot and enter circulation. Although the AUC values remain constant between the fusion and the free drug, the ELPDepot-FGF21 treatment results in a Cmax value that is three-fold lower than that observed for FGF21. These data indicate that the depot mediates absorption to inhibit burst release, an undesirable yet often inevitable consequence when delivering biologics via s.c. injection. Once in circulation, the ELP fusion slows clearance of FGF21 by four-fold, while maintaining circulating levels of ELP-FGF21 above the therapeutic threshold at least 48 h longer than those for FGF21.
The s.c. bioavailability of native FGF21 -- without modifications to promote stability -- is 78% in mice (9), while FGF21-antibody fragment conjugates/fusions have bioavailabilities of 52–69% (10, 13). Here we show that ELP-fused FGF21, delivered in the form of an s.c. depot, has a bioavailability of just under 30%. This value, while somewhat lower than that seen for other FGF21 formulations, is comparable to those reported for GLP-1 delivery from an ELP-based depot (17). Protein processing in the s.c. space is still poorly understood, however local catabolism has been observed following s.c. administration of insulin to pigs (47). The reduction in bioavailability associated with ELP-based drug depots may be a product of the extended residence in the s.c. space conferred by the ELP, thereby increasing the vulnerability of fused protein drugs to enzymatic processing and phagocytosis by resident cells. Nevertheless, we believe that the numerous advantages afforded by our ELP platform, to FGF21 production and delivery, more than compensate for the reduction in bioavailability observed for ELPDepot-FGF21.
To investigate the long-term effects of an ELPDepot-FGF21 treatment regimen, the depot-forming fusion was administered to ob/ob mice every 5 days for 8 weeks. The injection frequency was chosen based on efficacy and imaging data suggesting that a single s.c. injection of ELPDepot-FGF21 results in a depot that persists for ~5 days. Treatment with the fusion reduced blood glucose levels to a greater magnitude compared to a cohort receiving repeated molar equivalent doses of FGF21. Moreover, glucose levels in the FGF21 group appeared more erratic, displaying spikes and troughs characteristic of bolus drug injection therapy, while the ELP depot-based platform afforded tighter blood glucose control. Furthermore, a consistent 0.5% drop in %HbA1c -- a metric for evaluating long-term glycemic control -- was observed in the ELPDepot-FGF21 cohort only.
Both ELPDepot-FGF21 and FGF21 groups showed reductions in mean serum insulin and triglyceride levels, expanding the potential applications for ELPDepot-FGF21 to a broader range of metabolic diseases. Unfortunately the study was not sufficiently powered to achieve significance in the observed insulin and triglyceride reductions between the FGF21 and ELP-FGF21 groups, as the ob/ob mouse model typically displays a high degree of metabolic variance. However, clear trends emerged, as long-term treatment with ELP-FGF21 lowered serum insulin and triglycerides by ~40%. Thus we can conclude that the reduced injection frequency allowed by the ELP depot maintains a degree of in vivo efficacy comparable to that reported in the literature resulting from more frequent FGF21 dosing regimens (6, 8, 12, 14, 39).
Conclusions
As the incidence of metabolic disorders increases exponentially, novel drugs that target the underlying disease pathophysiology are greatly in need. However, the therapeutic feasibility of a new drug will depend not only on its efficacy but on the cost of production and ease of administration. Here we show that fusion of an ELP to the investigational protein drug FGF21 serves a triple function: the ELP enhances protein expression, massively streamlines purification, and seamlessly incorporates a controlled release drug delivery platform that is consistent with once-weekly dosing. In conclusion, an ELP-based approach to therapeutics provides a cost-effective method for drug production, as well as an innovative strategy for drug delivery, making this platform highly desirable from both a manufacturing perspective and from the perspective of the patient.
Supplementary Material
Acknowledgments
The authors thank Ian Cumming for assistance with cell sorting, performed at the Duke Human Vaccine Institute Flow Cytometry Facility. Depot imaging was performed on shared equipment in the Optical Molecular Imaging and Analysis core facility managed by the Duke Cancer Institute. We thank Dr. Michael R. Zalutsky, Duke University, for providing laboratory space and equipment for radiolabeling, as well as Xinghai Li for assistance with radiolabeling procedures. We also thank Dr. Terrence G. Oas, Duke University, for providing equipment for the circular dichroism studies. C.A.G. thanks Kelli M. Luginbuhl, Duke University, and Jeffrey L. Schaal, Duke University, for insightful discussions on in vitro and in vivo experimental design. This work was supported by the National Institute of Health [grant number R01-DK091789]. The funding source had no involvement in study design or preparation of the article.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest. A.C. is a scientific advisor and serves on the board of directors for PhaseBio Pharmaceuticals, Inc., which has licensed the ELP technology for drug delivery applications from Duke University.
Author Contributions. S.R. assisted with circular dichroism and analyzed associated data. C.A.G. performed all other research, data analysis, and wrote the manuscript. A.C. conceived the study, assisted with data analysis, and edited the manuscript.
References
- 1.Centers for Disease Control and Prevention. National Diabetes Statistics Report, 2017. U.S. Dept of Health and Human Services; Atlanta: 2017. [Google Scholar]
- 2.Bailey CJ. The Current Drug Treatment Landscape for Diabetes and Perspectives for the Future. Clin Pharmacol Ther. 2015;98(2):170–184. doi: 10.1002/cpt.144. [DOI] [PubMed] [Google Scholar]
- 3.Xu J, Stanislaus S, Chinookoswong N, Lau YY, Hager T, Patel J, Ge H, Weiszmann J, Lu S-C, Graham M, Busby J, Hecht R, Li Y-S, Li Y, Lindberg R, Véniant MM. Acute glucose-lowering and insulin-sensitizing action of FGF21 in insulin-resistant mouse models—association with liver and adipose tissue effects. Am J Physiol Endocrinol Metab. 2009;297(5):E1105–E1114. doi: 10.1152/ajpendo.00348.2009. [DOI] [PubMed] [Google Scholar]
- 4.Arner P, Pettersson A, Mitchell PJ, Dunbar JD, Kharitonenkov A, Rydén M. FGF21 attenuates lipolysis in human adipocytes – A possible link to improved insulin sensitivity. FEBS Lett. 2008;582(12):1725–1730. doi: 10.1016/j.febslet.2008.04.038. [DOI] [PubMed] [Google Scholar]
- 5.Lin Z, Tian H, Lam Karen SL, Lin S, Hoo Ruby CL, Konishi M, Itoh N, Wang Y, Bornstein Stefan R, Xu A, Li X. Adiponectin Mediates the Metabolic Effects of FGF21 on Glucose Homeostasis and Insulin Sensitivity in Mice. Cell Metab. 2013;17(5):779–789. doi: 10.1016/j.cmet.2013.04.005. [DOI] [PubMed] [Google Scholar]
- 6.Xu J, Lloyd DJ, Hale C, Stanislaus S, Chen M, Sivits G, Vonderfecht S, Hecht R, Li Y-S, Lindberg RA, Chen J-L, Young Jung D, Zhang Z, Ko H-J, Kim JK, Véniant MM. Fibroblast Growth Factor 21 Reverses Hepatic Steatosis, Increases Energy Expenditure, and Improves Insulin Sensitivity in Diet-Induced Obese Mice. Diabetes. 2009;58(1):250–259. doi: 10.2337/db08-0392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fisher FM, Chui PC, Nasser IA, Popov Y, Cunniff JC, Lundasen T, Kharitonenkov A, Schuppan D, Flier JS, Maratos-Flier E. Fibroblast Growth Factor 21 Limits Lipotoxicity by Promoting Hepatic Fatty Acid Activation in Mice on Methionine and Choline-Deficient Diets. Gastroenterology. 2014;147(5):1073–1083.e1076. doi: 10.1053/j.gastro.2014.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kharitonenkov A, Shiyanova TL, Koester A, Ford AM, Micanovic R, Galbreath EJ, Sandusky GE, Hammond LJ, Moyers JS, Owens RA, Gromada J, Brozinick JT, Hawkins ED, Wroblewski VJ, Li D-S, Mehrbod F, Jaskunas SR, Shanafelt AB. FGF-21 as a novel metabolic regulator. J Clin Invest. 2005;115(6):1627–1635. doi: 10.1172/JCI23606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kharitonenkov A, Wroblewski VJ, Koester A, Chen Y-F, Clutinger CK, Tigno XT, Hansen BC, Shanafelt AB, Etgen GJ. The Metabolic State of Diabetic Monkeys Is Regulated by Fibroblast Growth Factor-21. Endocrinology. 2007;148(2):774–781. doi: 10.1210/en.2006-1168. [DOI] [PubMed] [Google Scholar]
- 10.Hecht R, Li Y-S, Sun J, Belouski E, Hall M, Hager T, Yie J, Wang W, Winters D, Smith S, Spahr C, Tam L-T, Shen Z, Stanislaus S, Chinookoswong N, Lau Y, Sickmier A, Michaels ML, Boone T, Véniant MM, Xu J. Rationale-Based Engineering of a Potent Long-Acting FGF21 Analog for the Treatment of Type 2 Diabetes. PLoS One. 2012;7(11):e49345. doi: 10.1371/journal.pone.0049345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Micanovic R, Raches DW, Dunbar JD, Driver DA, Bina HA, Dickinson CD, Kharitonenkov A. Different roles of N- and C- termini in the functional activity of FGF21. J Cell Physiol. 2009;219(2):227–234. doi: 10.1002/jcp.21675. [DOI] [PubMed] [Google Scholar]
- 12.Kharitonenkov A, Beals JM, Micanovic R, Strifler BA, Rathnachalam R, Wroblewski VJ, Li S, Koester A, Ford AM, Coskun T, Dunbar JD, Cheng CC, Frye CC, Bumol TF, Moller DE. Rational Design of a Fibroblast Growth Factor 21-Based Clinical Candidate, LY2405319. PLoS One. 2013;8(3):e58575. doi: 10.1371/journal.pone.0058575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang J, Ishino T, Chen G, Rolzin P, Osothprarop TF, Retting K, Li L, Jin P, Matin MJ, Huyghe B, Talukdar S, Bradshaw CW, Palanki M, Violand BN, Woodnutt G, Lappe RW, Ogilvie K, Levin N. Development of a Novel Long-Acting Antidiabetic FGF21 Mimetic by Targeted Conjugation to a Scaffold Antibody. J Pharmacol Exp Ther. 2013;346(2):270–280. doi: 10.1124/jpet.113.204420. [DOI] [PubMed] [Google Scholar]
- 14.Mu J, Pinkstaff J, Li Z, Skidmore L, Li N, Myler H, Dallas-Yang Q, Putnam A-M, Yao J, Bussell S, Wu M, Norman TC, Rodriguez CG, Kimmel B, Metzger JM, Manibusan A, Lee D, Zaller DM, Zhang BB, DiMarchi RD, Berger JP, Axelrod DW. FGF21 Analogs of Sustained Action Enabled by Orthogonal Biosynthesis Demonstrate Enhanced Antidiabetic Pharmacology in Rodents. Diabetes. 2012;61(2):505–512. doi: 10.2337/db11-0838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meyer DE, Chilkoti A. Purification of recombinant proteins by fusion with thermally-responsive polypeptides. Nat Biotechnol. 1999;17(11):1112–1115. doi: 10.1038/15100. [DOI] [PubMed] [Google Scholar]
- 16.Hassouneh W, Christensen T, Chilkoti A. Elastin-Like Polypeptides as a Purification Tag for Recombinant Proteins. Curr Protoc Protein Sci. 2010;6.11:6.11.11–16. doi: 10.1002/0471140864.ps0611s61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Luginbuhl KM, Schaal JL, Umstead B, Mastria EM, Li X, Banskota S, Arnold S, Feinglos M, D’Alessio D, Chilkoti A. One-week glucose control via zero-order release kinetics from an injectable depot of glucagon-like peptide-1 fused to a thermosensitive biopolymer. Nat Biomed Eng. 2017;1:0078. doi: 10.1038/s41551-017-0078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McDaniel JR, MacKay JA, Quiroz FG, Chilkoti A. Recursive Directional Ligation by Plasmid Reconstruction Allows Rapid and Seamless Cloning of Oligomeric Genes. Biomacromolecules. 2010;11(4):944–952. doi: 10.1021/bm901387t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Waugh DS. [Accessed 15 March 2016];TEV Protease FAQ. 2010 Available at: http://mcl1.ncifcrf.gov/waugh_tech/faq/tev.pdf.
- 20.Micsonai A, Wien F, Kernya L, Lee Y-H, Goto Y, Réfrégiers M, Kardos J. Accurate secondary structure prediction and fold recognition for circular dichroism spectroscopy. Proceedings of the National Academy of Sciences. 2015;112(24):E3095. doi: 10.1073/pnas.1500851112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Urry DW. Physical Chemistry of Biological Free Energy Transduction As Demonstrated by Elastic Protein-Based Polymers. J Phys Chem B. 1997;101(51):11007–11028. [Google Scholar]
- 22.Jambhekar SS, Breen PJ. Basic Pharmacokinetics. 2 Pharmaceutical Press; London: 2012. [Google Scholar]
- 23.Gilroy CA. Dataset for: Fusion of fibroblast growth factor 21 to a thermally responsive biopolymer forms an injectable depot with sustained anti-diabetic action. Mendeley Data. 2018;v2 doi: 10.1016/j.jconrel.2018.03.015. [dataset] http://dx.doi.org/10.17632/y3ybyx47sx.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yie J, Hecht R, Patel J, Stevens J, Wang W, Hawkins N, Steavenson S, Smith S, Winters D, Fisher S, Cai L, Belouski E, Chen C, Michaels ML, Li Y-S, Lindberg R, Wang M, Véniant M, Xu J. FGF21 N- and C-termini play different roles in receptor interaction and activation. FEBS Lett. 2009;583(1):19–24. doi: 10.1016/j.febslet.2008.11.023. [DOI] [PubMed] [Google Scholar]
- 25.Lee S, Choi J, Mohanty J, Sousa LP, Tome F, Pardon E, Steyaert J, Lemmon MA, Lax I, Schlessinger J. Structures of β-klotho reveal a ‘zip code’-like mechanism for endocrine FGF signalling. Nature. 2018;553:501. doi: 10.1038/nature25010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Trabbic-Carlson K, Meyer DE, Liu L, Piervincenzi R, Nath N, LaBean T, Chilkoti A. Effect of protein fusion on the transition temperature of an environmentally responsive elastin-like polypeptide: a role for surface hydrophobicity? Protein Eng Des Sel. 2004;17(1):57–66. doi: 10.1093/protein/gzh006. [DOI] [PubMed] [Google Scholar]
- 27.Trabbic-Carlson K, Liu L, Kim B, Chilkoti A. Expression and purification of recombinant proteins from Escherichia coli: Comparison of an elastin-like polypeptide fusion with an oligohistidine fusion. Protein Sci. 2004;13(12):3274–3284. doi: 10.1110/ps.04931604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bellucci JJ, Amiram M, Bhattacharyya J, McCafferty D, Chilkoti A. Three-in-One Chromatography-Free Purification, Tag Removal, and Site-Specific Modification of Recombinant Fusion Proteins Using Sortase A and Elastin-like Polypeptides. Angew Chem Int Ed Engl. 2013;52(13):3703–3708. doi: 10.1002/anie.201208292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Amiram M, Luginbuhl KM, Li X, Feinglos MN, Chilkoti A. A depot-forming glucagon-like peptide-1 fusion protein reduces blood glucose for five days with a single injection. J Control Release. 2013;172(1):144–151. doi: 10.1016/j.jconrel.2013.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.U.S. Food & Drug Administration Department of Inspections Compliance Enforcement and Criminal Investigations. [Accessed 1 March 2018];Inspection Technical Guides: Bacterial Endotoxins/Pyrogens. 2015 Available at: https://www.fda.gov/ICECI/Inspections/InspectionGuides/InspectionTechnicalGuides/ucm072918.htm.
- 31.Kurosu H, Choi M, Ogawa Y, Dickson AS, Goetz R, Eliseenkova AV, Mohammadi M, Rosenblatt KP, Kliewer SA, Kuro-o M. Tissue-specific Expression of βKlotho and Fibroblast Growth Factor (FGF) Receptor Isoforms Determines Metabolic Activity of FGF19 and FGF21. J Biol Chem. 2007;282(37):26687–26695. doi: 10.1074/jbc.M704165200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reichheld SE, Muiznieks LD, Keeley FW, Sharpe S. Direct observation of structure and dynamics during phase separation of an elastomeric protein. Proceedings of the National Academy of Sciences. 2017;114(22):E4408. doi: 10.1073/pnas.1701877114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reichheld SE, Muiznieks LD, Stahl R, Simonetti K, Sharpe S, Keeley FW. Conformational Transitions of the Cross-linking Domains of Elastin during Self-assembly. J Biol Chem. 2014;289(14):10057–10068. doi: 10.1074/jbc.M113.533893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roberts S, Dzuricky M, Chilkoti A. Elastin-like Polypeptides as Models of Intrinsically Disordered Proteins. FEBS Lett. 2015;589(1900):2477–2486. doi: 10.1016/j.febslet.2015.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xu R, Ori A, Rudd TR, Uniewicz KA, Ahmed YA, Guimond SE, Skidmore MA, Siligardi G, Yates EA, Fernig DG. Diversification of the Structural Determinants of Fibroblast Growth Factor-Heparin Interactions: IMPLICATIONS FOR BINDING SPECIFICITY. J Biol Chem. 2012;287(47):40061–40073. doi: 10.1074/jbc.M112.398826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Trammell RA, Cox L, Toth LA. Markers for Heightened Monitoring, Imminent Death, and Euthanasia in Aged Inbred Mice. Comp Med. 2012;62(3):172–178. [PMC free article] [PubMed] [Google Scholar]
- 37.Urry DW. The change in Gibbs free energy for hydrophobic association: Derivation and evaluation by means of inverse temperature transitions. Chem Phys Lett. 2004;399:177–183. [Google Scholar]
- 38.Meyer DE, Chilkoti A. Quantification of the Effects of Chain Length and Concentration on the Thermal Behavior of Elastin-like Polypeptides. Biomacromolecules. 2004;5(3):846–851. doi: 10.1021/bm034215n. [DOI] [PubMed] [Google Scholar]
- 39.Hale C, Chen MM, Stanislaus S, Chinookoswong N, Hager T, Wang M, Véniant MM, Xu J. Lack of Overt FGF21 Resistance in Two Mouse Models of Obesity and Insulin Resistance. Endocrinology. 2012;153(1):69–80. doi: 10.1210/en.2010-1262. [DOI] [PubMed] [Google Scholar]
- 40.Stanislaus S, Hecht R, Yie J, Hager T, Hall M, Spahr C, Wang W, Weiszmann J, Li Y, Deng L, Winters D, Smith S, Zhou L, Li Y, Véniant MM, Xu J. A Novel Fc-FGF21 With Improved Resistance to Proteolysis, Increased Affinity Toward β-Klotho, and Enhanced Efficacy in Mice and Cynomolgus Monkeys. Endocrinology. 2017;158(5):1314–1327. doi: 10.1210/en.2016-1917. [DOI] [PubMed] [Google Scholar]
- 41.Kharitonenkov A, DiMarchi R. FGF21 Revolutions: Recent Advances Illuminating FGF21 Biology and Medicinal Properties. Trends Endocrinol Metab. 2015;26(11):608–617. doi: 10.1016/j.tem.2015.09.007. [DOI] [PubMed] [Google Scholar]
- 42.Xu J, Bussiere J, Yie J, Sickmier A, An P, Belouski E, Stanislaus S, Walker KW. Polyethylene Glycol Modified FGF21 Engineered to Maximize Potency and Minimize Vacuole Formation. Bioconjug Chem. 2013;24(6):915–925. doi: 10.1021/bc300603k. [DOI] [PubMed] [Google Scholar]
- 43.Baneyx F, Mujacic M. Recombinant protein folding and misfolding in Escherichia coli. Nat Biotech. 2004;22(11):1399–1408. doi: 10.1038/nbt1029. [DOI] [PubMed] [Google Scholar]
- 44.Chilkoti A, Christensen T, MacKay JA. Stimulus responsive elastin biopolymers: applications in medicine and biotechnology. Curr Opin Chem Biol. 2006;10(6):652–657. doi: 10.1016/j.cbpa.2006.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yang Q, Lai SK. Anti-PEG immunity: emergence, characteristics, and unaddressed questions. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2015;7(5):655–677. doi: 10.1002/wnan.1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ganson NJ, Povsic TJ, Sullenger BA, Alexander JH, Zelenkofske SL, Sailstad JM, Rusconi CP, Hershfield MS. Pre-existing anti–polyethylene glycol antibody linked to first-exposure allergic reactions to pegnivacogin, a PEGylated RNA aptamer. J Allergy Clin Immunol. 2016;137(5):1610–1613-e1617. doi: 10.1016/j.jaci.2015.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Richter WF, Jacobsen B. Subcutaneous Absorption of Biotherapeutics: Knowns and Unknowns. Drug Metabolism and Disposition. 2014;42(11):1881. doi: 10.1124/dmd.114.059238. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All raw data associated with this study have been deposited in the Mendeley Data repository, doi:10.17632/y3ybyx47sx.2 (23).