Abstract
Background
Several reports indicate that eosinophils are induced in chronic pancreatitis including patients with pancreatic malignancy. However, significance of eosinophilic pancreatitis (EP) is poorly understood and unexplored.
Aim
Accumulation and degranulation of eosinophils promote pancreatic fibrosis and malignancy.
Method
Human pancreatic tissue biopsy samples including chronic pancreatitis (n=3), malignant (n=4), non-malignant (n=3), and normal (n=3) were used for H&E, anti-MBP staining, anti-tryptase staining, anti-IgE staining and Mason’s trichrome staining.
Results
We show induced eosinophils and degranulated eosinophils indicated by the presence of anti-MBP stained extracellular granules in the malignant pancreatic (pancreatic cancer) and non-malignant human pancreatic tissues. A comparable number of eosinophils were observed in non-malignant and malignant pancreatic tissue sections, but the sections differed in degranulated eosinophils and the presence of extracellular granules. Additionally, induced mast cells and tissue-specific IgE positive cells were also detected in the tissue sections of malignant pancreatitis patients compared to non-malignant human pancreatic patients. Tissue-specific IgE induction is critical for the degranulation of eosinophils and mast cells that may lead to increased accumulation of collagen in malignant compared to non-malignant human pancreatic tissue samples. We show a large number of anti-tryptase stained extracellular granules in the tissue sections of malignant pancreatic cancer patients. Both IgE and eosinophil major basic proteins (MBP) are reported for the activation and degranulation of mast cells in tissues.
Conclusion
Taken together, our investigation concludes that eosinophils and mast cells accumulation and degranulation are critical in promoting pancreatitis pathogenesis that may lead to the development of pancreatic fibrosis and malignancy.
Keywords: Eosinophils, Mast cells, Cytokines, Chemokines, Pancreatitis, Fibrosis
Introduction
Several clinical reports have indicated induced eosinophils in the biopsies of pancreatitis patients that are termed as “eosinophilic pancreatitis (EP)” [1–7]. More recently, a microscopic examination of a pancreatic biopsy revealed that eosinophil infiltration occurs into the pancreatic duct, acini, and interstitium associated with fibrous connective tissue hyperplasia and accumulation of collagen [2]. Some clinical reports suggest that eosinophilic pancreatitis mimics pancreatic neoplasia, as a number of eosinophils are detected in pancreatic tumors [4, 8, 9]. However, yet no attempt has been made to understand the role of eosinophils in promoting pancreatitis, pancreatic fibrosis and pancreatic malignancy. Several investigators have reported eosinophilic pancreatitis inpatients, but still refer to it as a “rare disease”. The possibility of eosinophil accumulation in the pancreas was not noticed, as most of the pancreatic biopsies were performed using endoscopic ultrasound with fine needle aspirate, with the purpose to detect whether patients had developed pancreatic malignancy. These procedures did not provide sufficient tissue for pathological examination; therefore, the EP in patients may not be detected in most hospitals and clinics, hence it is ignored and termed a rare disease. Therefore, we tested the hypothesis that accumulation of eosinophils and eosinophil degranulation in the pancreas may be critical for promoting pancreatic fibrosis and pancreatic malignancy. Accordingly, we examined pancreatic tissue samples of pancreatitis, non-malignant, malignant, and normal tissue samples for eosinophil accumulation and degranulation. Herein, we report that tissue eosinophilia indeed occurs in pancreatitis and eosinophil degranulation in the pancreas is associated with pancreatic fibrosis and pancreatic malignancy. Taken together, we show that eosinophilic pancreatitis may not be considered a rare disease and needs appropriate attention to understand the role of eosinophils in pathogenesis of pancreatitis, fibrosis and malignancy. Of note, pancreatic fibrosis is the major concern for failed therapies in chronic pancreatitis and pancreatic malignancy.
Materials and Methods
Collection of patient tissue samples
A total of 13 human pancreatic tissue samples including chronic pancreatitis (n=3), malignant (cancer) (n=4), non-malignant (benign) (n=3), and normal (n=3) were obtained from the Biospecimen Core facility, Louisiana Cancer Research Consortium. The 3 specimens from normal that do not show any histological abnormalities in H&E stained tissue sections considers as control. Pancreatic tumor sections histopathologically evaluation and passed on pathological report differentiated for non-malignant and malignant pancreatic patients. Patients’ pancreatic tissues were collected during surgical resection of pancreatic tumors and surgical procedures performed in chronic pancreatitis. Approximately 100 mg segments of tumor and adjacent tissue were taken and immediately frozen in liquid nitrogen or 4% formaldehyde. Samples from those patients who had chemotherapy and radiotherapy were excluded from the studies. All 4% formaldehyde fixed tissues were used for paraffin embedding, sectioning and processing for H & E staining, immunostaining, and routine light microscopy. Further, the diagnosis of tumor grade was confirmed histologically. A written informed consent was obtained from each patient as per the Institutional Review Board approval for the study. The detailed clinical characteristics of each patient have been provided in Table 1.
Table 1.
Details of human pancreatic biopsies
| S.No. | Age | Gender | Lymphovascular invasion | Perineural invasion | % Tumor | Pathological Status |
|---|---|---|---|---|---|---|
| 1 | 45 | M | − | Normal tissue | ||
| 2 | 63 | F | − | Normal tissue | ||
| 3 | 57 | F | − | Normal tissue | ||
| 4 | 50 | F | − | Chronic pancreatitis | ||
| 5 | 67 | F | − | Chronic pancreatitis | ||
| 6 | 63 | M | − | ND | Chronic pancreatitis | |
| 7 | 72 | M | − | ND | 10 | Non-malignant |
| 8 | 55 | M | − | − | 15 | Non-malignant |
| 9 | 71 | M | − | ND | 10 | Non-malignant |
| 10 | 57 | F | ++ | ++ | 100 | Malignant |
| 11 | 66 | M | − | ++ | 80 | Malignant |
| 12 | 52 | F | ++ | ++ | 70 | Malignant |
| 13 | 65 | M | ++ | + | 100 | Malignant |
(−) absent, (+) less severe, (++) Severe, ND- Not defined, M- male, F –female
Analysis of tissue IgE, Eosinophils, and Eosinophil Degranulation
Tissue sections were immunostained with anti-human IgE (Vector Laboratories, CA, USA) and antiserum against mouse eosinophil major basic protein (MBP, i.e. eosinophil specific granule), a kind gift of Drs. James and Nancy Lee (Mayo Clinic, Scottsdale, AZ) as described [10–12]. In brief, endogenous peroxidase in the tissue was quenched with 0.3% hydrogen peroxide in methanol followed by nonspecific protein binding blocking with normal goat serum. Tissue sections were then incubated with goat anti-IgE (1:100) and rat anti-MBP (1:6000) overnight at 4°C, followed by 1:250 dilution of biotinylated horse anti-goat IgG and goat anti-rat IgG secondary antibody (Vector Laboratories, CA, USA) respectively and avidin-peroxidase complex (Vector Laboratories, CA, USA) for 30 minutes each. These slides were further developed with nickel diaminobenzidine-cobalt chloride solution (Vector Laboratories, CA, USA) to form a black precipitate, and counterstained with nuclear fast red (Poly Scientific R & D Corp, NY, USA). Negative controls include replacing the primary antibody with normal rabbit serum to check endogenous biotin and peroxidase activity. Quantification of the immunoreactive cells was performed using a video-assistant integrated computer software program Image Pro software analyzer (Media Cybenetics, PA, USA). The number of eosinophils and IgE positive cells were expressed as eosinophils/mm2 and IgE positive cells/mm2. A total of 4–5 high power fields in each pancreatic section were evaluated for eosinophils and IgE positive cells counting and analysis. Photomicrographs are presented as original magnification ×400 and ×1000.
Mast Cells Analysis
Mast cells were detected in the human pancreatic tissue sections by performing anti - tryptase immunofluorescence staining as described earlier [13]. In brief, anti-tryptase immunofluorescence was performed using monoclonal anti-tryptase antibody (Biorad, CA, USA) followed by PE-labeled secondary antibody (BD Biosciences, CA, USA) and mounted with nuclear staining DAPI mounting material. The images were captured using an Olympus BX51 microscope with appropriate filters and photomicrographs are presented as original magnification ×400 and ×1000.
Tissue collagen analysis
Collagen staining was performed on tissue sections by Masson’s trichrome staining (Poly Scientific R&D Corp, NY, USA) method for the detection of collagen fibers according to the manufacturer’s recommendations [14] and collagen tissue thickness was measured using a video-assistant integrated computer software program Image Pro software analyzer (Media Cybenetics, PA, USA) and collagen thickness is expressed in μm2.
Statistical analysis
The nonparametric Mann-Whitney U-test was employed for comparison of data between two groups, and Krustal-Wallis for comparison of more than two groups. Parametric data were compared using t -tests or analysis of variance. Values are reported as mean ± S.D. P -values < 0.05 were considered statistically significant.
Results
Eosinophils in human pancreatitis
The eosinophil accumulation in the pancreas of normal and pancreatitis patients’ tissue sections were examined in H& E stained tissue sections. We report that all pancreatitis patient tissue sections show varying magnitudes of eosinophilic accumulation compared to no eosinophils in normal tissue sections of pancreas (Figure 1 A, B). Several eosinophils were detected in hematoxylin and eosin (H&E) stained pancreatic tissue sections and marked with black arrows in the presented representative photomicrograph of pancreatitis patients (Figure 1B). Morphometric analysis was performed to quantitate tissue eosinophils in pancreatitis patients, and the data indicated ~200 eosinophils/mm2 were found in pancreatitis tissue sections (p=0.0001) compare to normal tissue sections (Figure 1 C). Data is expressed as mean ± S.D., Patients analyzed for each group, normal individual = 3, pancreatitis patient = 5.
Figure 1. Analysis of eosinophils in human pancreatitis tissue.
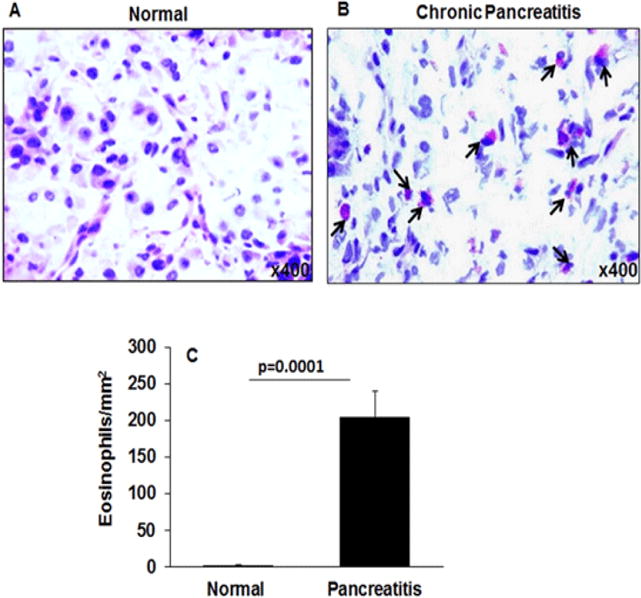
Representative H & E stained photomicrograph of a tissue section of normal pancreas (A) and pancreatitis patients’ tissue (B) showing accumulated eosinophils in pancreatitis tissue sections marked with black arrows. Eosinophils in the pancreatic tissue sections were quantitated and expressed as eosinophils/mm2 (C). Data presented as mean ± SD, n = 3 for normal individuals, and n = 3 for pancreatitis patients. Representative photomicrograph presented as ×400 of original magnification.
Detection of Extracellular Eosinophil Granules in Malignant (Cancer) and Non-Malignant (Benign) Pancreatic Tissue Sections
Eosinophil degranulation promotes disease pathogenesis including tissue fibrosis; therefore, we further examined the presence of degranulating eosinophils and eosinophil extracellular granules in the tissue sections by performing anti-MBP immunostaining. We report that a number of anti-MBP positive eosinophils, with micro abscesses were detected in both malignant and non-malignant pancreatic tissue sections compared to normal tissue sections (Figure 2 A–C). Induced anti-MBP stained eosinophils along with degranulated eosinophils were detected in malignant pancreatic tissue sections (Figure 2C, F) compared to non-malignant pancreatic tissue sections (Figure 2B, E); whereas no eosinophils were detected in the normal tissue sections (Figure 2A, D); all photomicrograph shown with ×400 and ×1000 magnification. Further, our analysis showed a large number of extracellular anti-MBP stained granules in the tissue sections of malignant pancreatic patients. We marked eosinophils with black arrows, degranulated eosinophils with green arrows, and several extracellular anti-MBP stained granules with yellow arrows in malignant pancreatic tissue sections. The higher magnification (×1000) photomicrograph of anti-MBP stained tissue sections are included to show more visible degranulated eosinophils and anti-MBP stained extracellular granules (Figure 2F). The extracellular MBP granules are eosinophil degranulation products, the characteristic feature of any eosinophil associated disease [15, 16]. The morphometric quantitation of eosinophils was comparable in non-malignant and malignant pancreatic tissue (p=0.001), however there was high eosinophil degranulation in malignant tissue sections compare to the non-malignant tissue sections (Figure 2G).
Figure 2. Eosinophil analysis in normal, malignant, and non-malignant pancreatic patients.
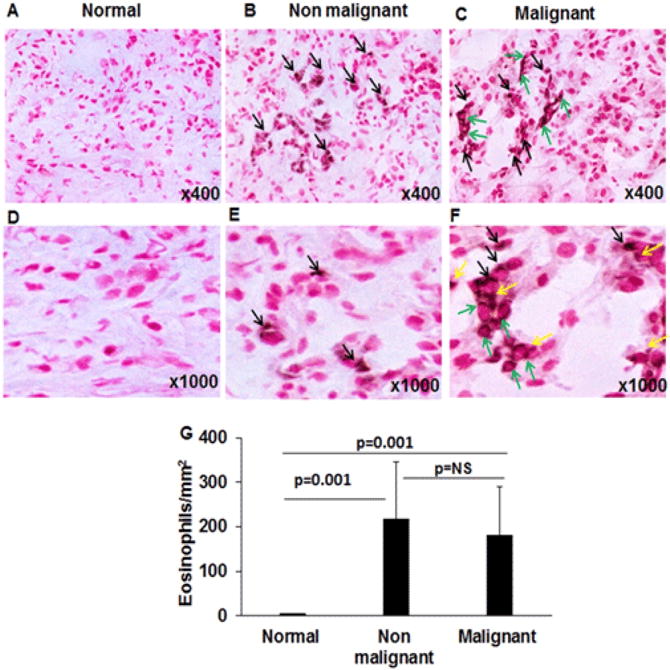
Anti-MBP immunostaining was performed to detect eosinophils in tissue sections, no MBP-positive eosinophils were observed in representative photomicrographs of normal individuals (A, D). Several anti-MBP stained eosinophils were detected in nonmalignant pancreatic tissue sections (B, E); whereas malignant pancreatic tissue showed a number of intact and degranulated eosinophils (C, F). Degranulation products are identified as anti-MBP stained extracellular granules. Black arrows mark intact eosinophils, green arrow indicates degranulated eosinophils, and yellow arrows indicate extracellular granules of MBP in the malignant tissue sections (C, F). The quantitation of eosinophil numbers was comparable in non-malignant and malignant pancreatic tissue sections compared to no eosinophils detected in normal pancreatic tissue sections (G). Representative photomicrographs presented as ×400 and ×1000 of original magnification. The quantitative data are expressed as mean± S.D, n=3 for normal and n = 4 & 3 for malignant and non-malignant patient respectively. NS=non significant.
Detection of mast cells in malignant and non-malignant pancreatic tissue sections
Mast cells were previously detected in pancreatic cancer [17, 18]; however, mast cell activation and degranulation processes are not studied in the microenvironment during disease progression and tumor development. Therefore, we examined the significance of mast cell degranulation and presence of its extracellular granules in the normal, non-malignant and malignant pancreatic tissue sections. We detected mast cells in the tissue by performing anti-tryptase immunofluorescence in the normal, non-malignant, and malignant pancreatic tissue sections. Our analysis has shown accumulation of a large number of tryptase-positive mast cells in both malignant and non-malignant pancreatic patient tissue sections (p=0.001) compare to very few numbers of tryptase-positive mast cells detected in normal pancreatic tissue sections (Figure 3A–G). Further, analysis revealed a significantly increased tryptase positive mast cell numbers in malignant pancreatic tissue sections (p=0.004) compare to non-malignant patients tissue sections (Figure 3G). In addition to mast cell accumulation, we also observed highly induced extracellular anti-tryptase stained granules in malignant pancreatic tissue sections (Figure 3C, F) compared to non-malignant pancreatic tissue sections (Figure 3B, E), all photomicrographs shown with ×400 and ×1000 magnification. In the photomicrograph, we differentiated intact mast cells with white arrows, degranulated mast cells with yellow arrows, and anti-tryptase stained extracellular granules of mast cells with green arrows. The higher magnification (×1000) photomicrograph of anti-tryptase stained tissue sections is included to show more visibly the degranulated mast cells and their extracellular anti-tryptase stained granules (Figure 2C, F). Very few mast cell granules were detected in non-malignant tissue sections (Figure 3B, E) and none were detected in normal pancreatic tissue sections (Figure 3 A, D). The morphometric analysis was performed to quantitate mast cell numbers and data was expressed as tryptase-positive mast cells/mm2 (Figure 3G).
Figure 3. Mast cells analysis in normal, malignant and non-malignant pancreatic patients.
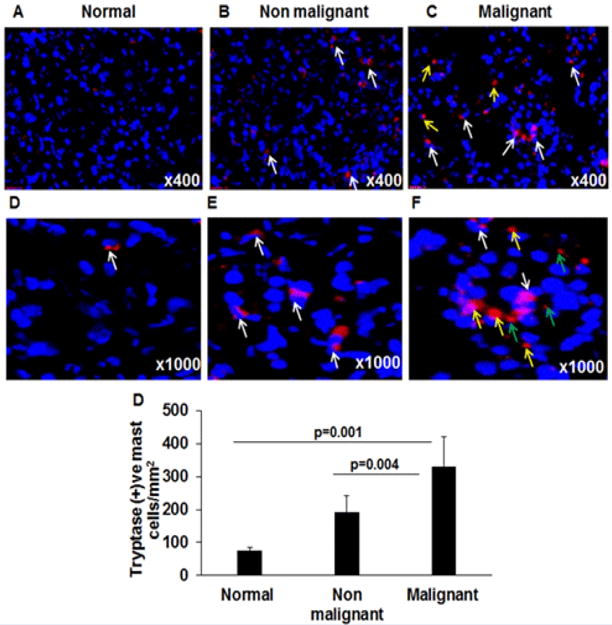
Anti-tryptase immunostaining was performed to detect mast cells in tissue sections and anti-tryptase positive mast cells were shown in a representative photomicrograph of normal (A, D), non-malignant (B, E), and malignant pancreatic tissue sections (C, F). White arrows indicate tryptase positive intact mast cells (A–D); degranulated mast cells (marked by yellow arrows) and extracellular tryptase granules (marked by green arrows) are detected only in malignant pancreatic tissue sections (C, F). The quantitation of mast cell numbers in normal, non-malignant and malignant pancreatic tissue sections were detected and found significantly induced compared to the normal pancreas tissue sections (G). Representative photomicrographs presented as ×400 and ×1000 of original magnification. The quantitative data are expressed as mean ± S.D, n=3 for normal and n = 4 for malignant and n=3 non-malignant patient.
Increase of tissue specific IgE in malignant and non-malignant pancreatic tissue sections
Induced blood IgE has been reported in pancreatitis as well as in pancreatic cancer; however, the tissue eosinophils and mast cells require tissue specific IgE induction. Therefore, we tested the hypothesis that the tissue specific IgE-producing cells are increased in malignant pancreatic tissues. Accordingly, we performed anti-IgE immunostaining in human pancreatic non-malignant and malignant tissue sections. Our immunostaining analysis for tissue IgE indicates that a large number of IgE positive cells were detected and marked by blue arrows in both non-malignant (p=0.0018) and malignant human pancreatic tissue sections (p=0.0005) compared to normal human pancreatic tissue section (Figure 4A–D). Further, analysis indicated a significant increase in anti-IgE positive cells in malignant pancreatic tissue sections (p=0.013) compare to non-malignant patients tissue sections (Figure 4B–D). The results indicate that induced IgE positive cells produce tissue specific IgE that binds with IgE receptors present on both eosinophils as well as mast cells, leading to activation and degranulation of these cells. Therefore, IgE-induced degranulation of both eosinophils and mast cells may be playing a critical role in the progression of pancreatic malignancy.
Figure 4. Tissue specific IgE analysis in normal, malignant and non-malignant pancreatic patients.
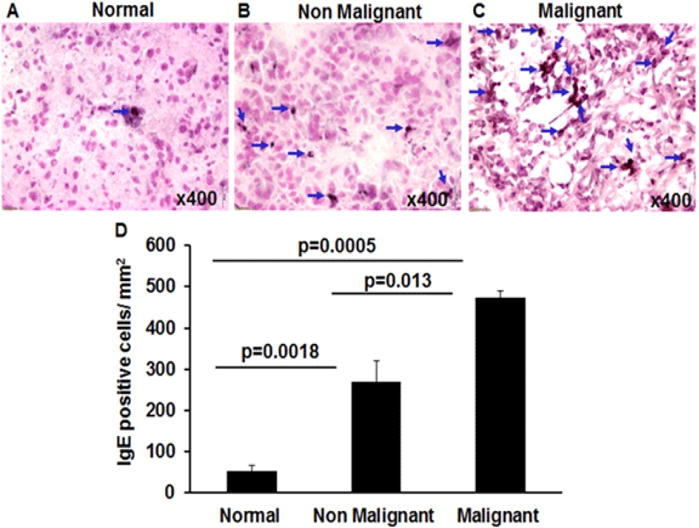
Human anti-IgE immunostaining was performed to detect IgE producing cells in tissue sections of normal, malignant, and non-malignant tissue sections. The large number of anti-IgE positive cells was detected in non-malignant and malignant pancreatic tissue sections compared to very few numbers in normal pancreas tissue sections (A–D). A significant increase in IgE positive cell was also observed in malignant pancreatic tissue sections compared to non-malignant tissue sections (B, C). Blue arrows mark IgE positive cells. The morphometric quantitation of IgE positive cells is shown as IgE positive cells/mm2 (D). Representative photomicrographs presented as ×400 of original magnification. The quantitative data are expressed as mean± S.D, n=3 for normal and n = 4 for malignant and n=3 non-malignant patient.
Analysis of fibrosis and collagen accumulation in malignant and non-malignant pancreatic tissue sections
Since highly induced eosinophil and mast cell degranulation has been observed in malignant pancreatic patients, therefore, we were next interested to determine whether IgE-induced degranulation of eosinophils and mast cells is associated with tissue collagen accumulation. Accordingly, we performed Massons’ trichrome staining in the tissue sections of normal, non-malignant, and malignant pancreatic patient tissue sections. Our analysis indicated induced collagen deposition in both malignant (p=0.001) and non-malignant pancreatic tissue sections (p=0.003) compared to the normal pancreatic tissue sections (Figure5A–D). Further, analysis indicated a significant increase in collagen deposition in malignant pancreatic tissue sections (p=0.012) compare to non-malignant patients tissue sections (Figure 5 B–D). The semi-quantitative collagen accumulation in the normal, non-malignant, and malignant pancreatic tissue was performed by using morphometric analysis to determine the thickness of perivascular collagen and represented collagen thickness in μm2 (Figure 5 D). Additionally, we provide a possible mechanistic pathway by which eosinophils and mast cells degranulate and contribute in promoting pancreatic malignancy in Figure 6.
Figure 5. Analysis of collagen accumulation in normal, malignant, and non-malignant pancreatic patients.
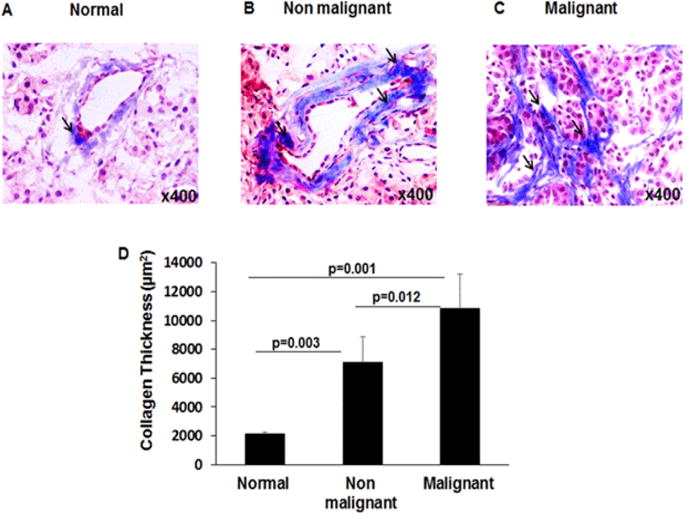
Masson’s trichrome staining was performed to analyze collagen accumulation in tissue sections and perivascular collagen is detected in a representative photomicrograph of tissue section in normal (A), non-malignant (B), and malignant pancreatic tissues (C). The quantitation of perivascular collagen thickness in non-malignant and malignant pancreatic tissue sections indicated significantly induced collagen accumulation compared to normal pancreas tissue sections (D). Representative photomicrographs presented as ×400 of original magnification. The quantitative data are expressed as mean ± S.D, n=3 for normal and n = 4 for malignant and n=3 non-malignant patient.
Figure 6.
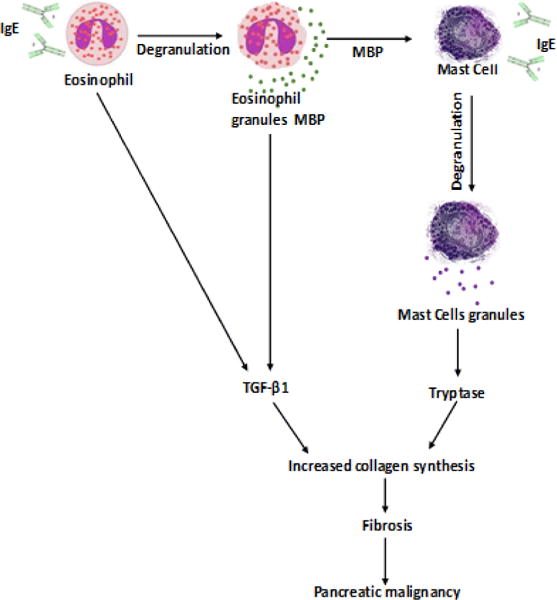
Schematic diagram showing the mechanistic pathways of role of eosinophils and mast cells in promoting pancreatic malignancy. IgE is causing the degranulation of eosinophils that secretes extracellular granules major basic protein (MBP). MBP and IgE further causing the degranulation of mast cells that secretes Tryptase. Furthermore, eosinophils secreted TGF β1 and mast cell secreted Tryptase induces collagen fiber synthesis and accumulation that leads the development of fibrosis and pancreatic malignancy.
Discussion
The etiology of eosinophilic pancreatitis (EP) is poorly understood and the role of eosinophils in the initiation and progression of pancreatitis has not been explored. The clinical symptoms and characteristics for EP patients are reported as levels of peripheral eosinophils >1.5×109 for >6 months, with no history of rhinitis, bronchial asthma, or other allergic diseases, and no eosinophilic infiltration in the heart, skin, and gastrointestinal tract, as well as the exclusion of leukemia, parasitic infection, and a diagnosis of hypereosinophilic syndrome as per the international standards [2, 19]. Some reports refer eosinophilic pancreatitis as a rare disease[20]. However, our presented data indicates that EP may not be rare, because it is not well studied and systematically diagnosed. Pancreatic biopsy procedures are normally based on endoscopic ultrasound with fine needle aspirate that lack tissue pathological analysis; therefore EP is an ignored disease entity. In the current study, we provided evidence that eosinophils indeed accumulated in the pancreatic tissue sections of non-malignant and malignant pancreatic tumors, and that induction of eosinophil degranulation was observed mainly in malignant tumors. In addition, mast cells were also observed in pancreatic tissue sections of non-malignant and malignant patients. Mast cells are well-known effector cells [21]. Interestingly, eosinophilic derived granules i.e. major basic protein (MBP) and IgE are capable of activating as well as degranulating the mast cells [15, 16]. We show tissue specific IgE producing cells are also increased in nonmalignant and malignant tissue sections. The induced IgE and degranulation of both inflammatory cells are more prominent in fibrotic malignant pancreatic tissue sections as compared to non-malignant pancreatic tissue sections of patients. Tryptase, a serine protease stored in mast cell granules that induces the synthesis of type I collagen in human fibroblasts [22] and stimulates fibroblast proliferation [23–25]and chemotaxis [22]. Further, our result indicates that highly induced collagen deposition and increased fibrosis observed during the progression of disease pathogenesis from non-malignant stage to malignant stage. The induced degranulation products of eosinophils and mast cells in malignant pancreatic tissue sections suggest that IgE-induced degranulation of eosinophils and mast cells have an important role in pancreatic tissue remodeling and fibrosis that may be critical in the further progression to pancreatic malignancy. Of note, the induction of systemic IgE was previously reported in pancreatic cancer [26, 27]; however, we show that induction of tissue specific IgE is critical for pathogenesis compared to systemic IgE. Systemic IgE induction occurs in response to any antigen. These findings suggest eosinophil and mast cell crosstalk including IgE association may have a critical role in promoting chronic pancreatitis including fibrosis and malignancy.
Taken together, we provided the evidence that accumulation of eosinophils and mast cells in the pancreas might not be a rare occurrence in human pancreatitis. Additionally, we show induction of tissue specific IgE and degranulation of eosinophils and mast cells are associated with pancreatic fibrosis and malignancy in human. Taken together, the current study highlights the need for detailed investigation on the mechanistic roles of tissue-specific IgE and eosinophils in promoting pancreatitis pathogenesis including fibrosis, and indicates EP may be an independent diseases entity in a group of particular pancreatitis patients that needs proper attention of health care providers.
Acknowledgments
This work was partially supported by the grants NIH R01 AI080581 (AM). Dr. Mishra is Endowed Schlieder Chair; therefore, we thank Edward G. Schlieder Educational Foundation for their support. We also thank Biospecimen Core facility, Louisiana Cancer Research Consortium for providing pancreatitis, nonmalignant, and malignant patients’ tissues.
References
- 1.Kakodkar S, Omar Hina, Cabrera Julio, Chi Kenneth. Eosinophilic Pancreatitis Diagnosed With Endoscopic Ultrasound. ACG Case Rep J. 2015;2(4):239–241. doi: 10.14309/crj.2015.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tian L, Fu Peng, Dong Xianghui, Jiping, Zhu Hong. Eosinophilic pancreatitis: Three case reports and literature review. Mol Clin Oncol. 2016;4(4):559–562. doi: 10.3892/mco.2016.760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abraham SC, Steven Leach, Yeo Charles J, Cameron John L, Murakata, Linda A, et al. Eosinophilic pancreatitis and increased eosinophils in the pancreas. Am J Surg Pathol. 2003;27(3):334–342. doi: 10.1097/00000478-200303000-00006. [DOI] [PubMed] [Google Scholar]
- 4.Cay A, Imamoglu M, Cobanoglu U. Eosinophilic pancreatitis mimicking pancreatic neoplasia. Can J Gastroenterol. 2006;20(5):361–364. doi: 10.1155/2006/386918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lyngbaek S, Adamsen S, Aru A, Bergenfeldt M. Recurrent acute pancreatitis due to eosinophilic gastroenteritis. Case report and literature review. JOP Pancreas. 2006;7(2):211–217. [PubMed] [Google Scholar]
- 6.Wereszczynska-Siemiatkowska U, Mroczko B, Siemiatkowski A. Serum profiles of interleukin-18 in different severity forms of human acute pancreatitis. Scand J Gastroenterol. 2002;37(9):1097–1102. doi: 10.1080/003655202320378310. [DOI] [PubMed] [Google Scholar]
- 7.Manohar M, Verma AK, Venkateshaiah SU, Sanders NL, Mishra A. Pathogenic mechanisms of pancreatitis. World J Gastrointest Pharmacol Ther. 2017;8(1):10–25. doi: 10.4292/wjgpt.v8.i1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hirata J, Koga T, Nishimura J, Ibayashi H. Pancreatic carcinoma associated with marked eosinophilia: a case report. Eur J Haematol. 1987;39(5):462–466. doi: 10.1111/j.1600-0609.1987.tb01457.x. [DOI] [PubMed] [Google Scholar]
- 9.Sah RP, Pannala R, Zhang L, Graham RP, Sugumar A, Chari TS. Eosinophilia and allergic disorders in autoimmune pancreatitis. Am J Gastroenterol. 2010;105(11):2485–2491. doi: 10.1038/ajg.2010.236. [DOI] [PubMed] [Google Scholar]
- 10.Matthews AN, Friend DS, Zimmermann N, Sarafi MN, Luster AD, Pearlman E, et al. Eotaxin is required for the baseline level of tissue eosinophils. Proc Natl Acad Sci U S A. 1998;95(11):6273–6278. doi: 10.1073/pnas.95.11.6273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mishra A, Hogan SP, Lee JJ, Foster PS, Rothenberg ME. Fundamental signals that regulate eosinophil homing to the gastrointestinal tract. J Clin Invest. 1999;103(12):1719–1727. doi: 10.1172/JCI6560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Venkateshaiah SU, Zhu X, Rajavelu P, Niranjan R, Manohar M, Verma AK, et al. Regulatory effects of IL-15 on allergen-induced airway obstruction. J Allergy Clin Immunol. 2017 doi: 10.1016/j.jaci.2017.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barbara G, Stanghellini V, De Giorgio R, Cremon C, Cottrell GS, Santini D, et al. Activated mast cells in proximity to colonic nerves correlate with abdominal pain in irritable bowel syndrome. Gastroenterology. 2004;126(3):693–702. doi: 10.1053/j.gastro.2003.11.055. [DOI] [PubMed] [Google Scholar]
- 14.Niranjan R, Rajavelu P, Ventateshaiah SU, Shukla JS, Zaidi A, Mariswamy SJ, et al. Involvement of interleukin-18 in the pathogenesis of human eosinophilic esophagitis. Clin Immunol. 2015;157(2):103–113. doi: 10.1016/j.clim.2015.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Butterfield JH, Weiler D, Peterson EA, Gleich GJ, Leiferman KM. Sequestration of eosinophil major basic protein in human mast cells. Lab Invest. 1990;62(1):77–86. [PubMed] [Google Scholar]
- 16.Niranjan R, Mavi P, Rayapudi M, Dynda S, Mishra A. Pathogenic role of mast cells in experimental eosinophilic esophagitis. Am J Physiol Gastrointest Liver Physiol. 2013;304(12):G1087–94. doi: 10.1152/ajpgi.00070.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ammendola M, Sacco R, Sammarco G, Donato G, Zuccalà V, Luposella M, et al. Mast cells density positive to tryptase correlates with angiogenesis in pancreatic ductal adenocarcinoma patients having undergone surgery. Gastroenterol Res Pract. 2014;2014:951–957. doi: 10.1155/2014/951957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guo X, Zhai L, Xue R, Shi J, Zeng Q, Gao C. Mast Cell Tryptase Contributes to Pancreatic Cancer Growth through Promoting Angiogenesis via Activation of Angiopoietin-1. Int J Mol Sci. 2016;17(6):E834. doi: 10.3390/ijms17060834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Valent P, Klion AD, Horny HP, Roufosse F, Gotlib J, Weller PF, et al. Contemporary consensus proposal on criteria and classification of eosinophilic disorders and related syndromes. J Allergy Clin Immunol. 2012;130(3):607–612. doi: 10.1016/j.jaci.2012.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Euscher E, Vaswani K, Frankel W. Eosinophilic pancreatitis: a rare entity that can mimic a pancreatic neoplasm. Ann Diagn Pathol. 2000;4(6):379–385. doi: 10.1053/adpa.2000.19371. [DOI] [PubMed] [Google Scholar]
- 21.Manohar M, Verma AK, Venkateshaiah SU, Mishra A. Immunological Responses Involved In Promoting Acute and Chronic Pancreatitis. J Clin Immunol Res. 2017;1(1):1–8. [Google Scholar]
- 22.Gruber BL, Kew RR, Jelaska A, Marchese MJ, Garlick J, Ren S, et al. Human mast cells activate fibroblasts: tryptase is a fibrogenic factor stimulating collagen messenger ribonucleic acid synthesis and fibroblast chemotaxis. J Immunol. 1997;158(5):2310–2317. [PubMed] [Google Scholar]
- 23.Akers IA, Parsons M, Hill MR, Hollenberg MD, Sanjar S, Laurent GJ, et al. Mast cell tryptase stimulates human lung fibroblast proliferation via protease-activated receptor-2. Am J Physiol Lung Cell Mol Physiol. 2000;278(1):L193–201. doi: 10.1152/ajplung.2000.278.1.L193. [DOI] [PubMed] [Google Scholar]
- 24.Schneider A, Haas SL, Hildenbrand R, Siegmund S, Reinhard I, Nakovics H, et al. Enhanced expression of interleukin-18 in serum and pancreas of patients with chronic pancreatitis. World J Gastroenterol. 2006;12(40):6507–6514. doi: 10.3748/wjg.v12.i40.6507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Deng X, Wang L, Elm MS, Gabazadeh D, Diorio GJ, Eagon PK, et al. Chronic alcohol consumption accelerates fibrosis in response to cerulein-induced pancreatitis in rats. Am J Pathol. 2005;166(1):93–106. doi: 10.1016/S0002-9440(10)62235-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fu SL, Pierre J, Smith-Norowitz TA, Hagler M, Bowne W, Pincus MR, et al. Emmunoglobulin E antibodies from pancreatic cancer patients mediate antibody-dependent cell-mediated cytotoxicity against pancreatic cancer cells. Clin Exp Immunol. 2008;153(3):401–409. doi: 10.1111/j.1365-2249.2008.03726.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Olson SH, Hsu M, Wiemels JL, Bracci PM, Zhou M, Patoka J, et al. Serum immunoglobulin e and risk of pancreatic cancer in the prostate, lung, colorectal, and ovarian cancer screening trial. Cancer Epidemiol Biomarkers Prev. 2014;23(7):1414–1420. doi: 10.1158/1055-9965.EPI-13-1359. [DOI] [PMC free article] [PubMed] [Google Scholar]


