Abstract
By 2050, almost 16 million Americans may live with dementia and will rely predominantly on informal caregivers for support. In providing this support, caregivers frequently suffer a wide range of adverse consequences. Although established psychoeducation programs benefit caregivers, attending in-person programs is challenging for caregivers who must find time, transportation, and substitute care, all of which come with expenses. To address this challenge, the Savvy Caregiver Program, an evidence-based psychoeducation program with demonstrated effectiveness for caregiving and disease-related outcomes, was transformed into an on-line program, Tele-Savvy. This article describes the rationale for and design of a prospective longitudinal randomized controlled trial (n=270), currently underway. The trial aims to establish Tele-Savvy’s efficacy in (i) reducing the negative effects of caregiving on caregivers; (ii) promoting care recipients’ quality of life; (iii) improving caregiver mastery; and (iv) to explore Tele-Savvy’s efficacy among caregivers of different races/ethnicities. The mediating role of mastery on Aims 1 & 2 outcomes will be assessed. Participants are randomized to the active condition (immediate Tele-Savvy participation), attention control, or usual care. Participants in the two latter conditions complete Tele-Savvy six months post-baseline. Multilevel mixed effects models will be used to examine changes in outcomes and model group by time interactions (months since baseline). The exploratory aim will be addressed using analysis of covariance and qualitatively. This trial’s results may be used by healthcare and community organizations to implement Tele-Savvy into dementia care, increasing caregivers’ access to this evidence-based intervention.
Introduction
In 2017, approximately 5.5 million Americans lived with Alzheimer’s disease, accounting for 60–80% of all dementias, with other subtypes (frontotemporal, Lewy body dementia, vascular, and mixed) increasing total prevalence. The prevalence of these progressive neurodegenerative diseases will continue to grow (Alzheimer’s Association, 2017). Most persons living with dementia (PLWD) are supported by informal unpaid caregivers, usually relatives (Zhu et al., 2016).
Caregivers often experience poor physical and psychological health (Coffman, Resnick, & Lathan, 2017; Fonareva & Oken, 2014; Pinquart & Sörensen, 2007); compromised immune response (Bennett, Fagundes, & Kiecolt-Glaser, 2012); and strained employment and financial hardships (Alzheimer’s Association, 2017). Multiple programs have demonstrated improved caregiver- and patient-centered outcomes (Belle et al., 2006; Gallagher-Thompson, Gray, Dupart, Jimenez, & Thompson, 2008; Mittelman, Roth, Coon, & Haley, 2004). Many caregivers, however, do not benefit from these programs (Gitlin, Marx, Stanley, & Hodgson, 2015) for logistic or transportation reasons or because their life circumstances (income, employment, high caregiving demands) make them hard to reach (Navaie, 2011). Caregivers frequently link their day-to-day responsibilities with an inability to leave their person and their house (Brown & Alligood, 2004).
The Savvy Caregiver Program (SCP), an evidence-based in-person psychoeducation program, has demonstrated efficacy in reducing dementia caregivers’ burden, improving caregiver competence and management of the caregiving situation, and empowering caregivers in the midst of their person’s inevitable cognitive decline (Hepburn, Lewis, Sherman, & Tornatore, 2003; Hepburn, Lewis, Tornatore, Sherman, & Bremer, 2007). It has demonstrated efficacy for caregivers of diverse ethnic backgrounds (Kally et al., 2014) and internationally (Sepe-Monti et al., 2016). Along with the Resources for Enhancing Alzheimer’s Caregiver Health (REACH) (Belle et al., 2006) and New York University Caregiver Intervention (Mittelman et al., 2004), the SCP has been implemented as an evidence-based program in multiple states by the U.S. Administration for Community Living (Long, Gould, Hughes, O’Keefe, & Wiener, 2014; Karon et al., 2015).
To make the SCP more accessible, especially to caregivers with limited or no ability to attend in-person programs, we developed and pilot tested Tele-Savvy, an on-line version of the SCP. Based on pilot results of decreased caregiver burden, anxiety, depressive symptoms, and distress due to their persons’ behavioral and psychological symptoms of dementia (BPSD), and participants’ endorsement of the feasibility and acceptability of Tele-Savvy (Griffiths, Whitney, Kovaleva, & Hepburn, 2016; Kovaleva, Blevins, Griffiths, & Hepburn, 2017), we received support to conduct a large-scale randomized controlled trial (RCT) to test the efficacy of Tele-Savvy (R01AG054079; the clinical trials registration number is NCT03033875). This paper describes the design of the trial currently underway. The trial’s three specific aims seek to (i) reduce the negative effects of caregiving on caregivers; (ii) promote care recipients’ quality of life; and (iii) improve caregiver mastery. An exploratory aim examines Tele-Savvy’s efficacy among caregivers of different races/ethnicities.
Background
In 2017, over 15 million Americans served as dementia caregivers, providing over 18.2 billion hours of unpaid care valued at over $230.1 Billion. While caregiving may provide rewards (Lloyd, Patterson, & Muers, 2014; Roth, Fredman, & Haley, 2015), caregivers’ responsibilities increase over time (Alzheimer’s Association, 2017; Gillespie, Mullan, & Harrison, 2014; Spillman, Wolff, Freedman, & Kasper, 2011), adversely affecting caregivers’ health (Fonareva & Oken, 2014), psychological and social well-being (Schulz & Martire, 2004); and family relationships (Gaugler, Zarit, & Pearlin, 1999; Tremont, Davis, & Bishop, 2006). Although few are trained for this work, caregivers essentially play the role of a clinician and even a healthcare team. Importantly, caregivers express the need for care for their own physical, emotional, financial, and social well-being, for respite care, and for information about the disease (Ball et al., 2015; Brown & Alligood, 2004; McCabe, You, & Tatangelo, 2016).
Dementia caregiving differs from non-dementia caregiving in the prevalence and severity of BPSD – a wide range of symptoms and behaviors, some of which nearly all PLWD manifest at some point during illness. BPSD include mood disorders, sleep disruption, psychotic symptoms, and agitation (Desai, Schwartz, & Grossberg, 2012). Wandering, psychosis, and agitation are leading risk factors for institutionalization (Miller, Schneider, & Rosenheck, 2011). While the key cause of BPSD is the underlying neuropathology, frequently co-existing and reversible factors exacerbate them and interactions with caregivers can provoke or worsen them (Desai et al., 2012)
Numerous programs have been developed for dementia caregivers (Boots, de Vugt, van Knippenberg, Kempen, & Verhey, 2014; Wisniewski et al., 2003). The strategies include counseling (Mittelman, Ferris, Shulman, Steinberg, & Levin, 1996; Mittelman et al., 2004); cognitive-behavioral therapy (Gallagher-Thompson, Gray, Dupart, Jimenez, & Thompson, 2008; Gallagher-Thompson & Steffen, 1994); acceptance and commitment therapy (Losada et al., 2015); environmental modification (Gitlin, Corcoran, Winter, Boyce, & Hauck, 2001; Gitlin, Hauck, Dennis, & Winter, 2005); psychoeducation (Hepburn et al., 2007; Ostwald, Hepburn, Caron, Burns, & Mantell, 1999; Kajiyama et al., 2013); and multicomponent programs encompassing several of the aforementioned strategies, such as REACH (Belle et al., 2006; Eisdorfer et al., 2003; Gitlin et al., 2005).
While several programs are considered evidence-based by the U.S. Administration for Community Living (Link, 2015–2016) which supported their community implementation, they remain widely inaccessible (Hughes, Shuman, Wiener, & Gould, 2017). Multiple on-line programs for dementia caregivers exist (Parra-Vidales, Soto-Pérez, Perea-Bartolomé, Franco-Martin, & Munoz-Sanchez, 2017). They leverage the exponentially increasing internet usage among older adults, up from 14% in 2000 to 64% in 2016 (Pew Research Center, 2017). Evidence from RCTs about these on-line programs is scarce (Boots et al., 2014).
The Savvy Caregiver Program (SCP)
The SCP, based on the developers’ prior psychoeducation programs’ success in RCTs (Hepburn, Lewis, Narayan, Center, Tornatore, Bremer, & Kirk, 2005; Ostwald et al., 1999), is a theory-driven, evidence-based intervention that has demonstrated its efficacy in decreasing caregivers’ reaction to BPSD, caregiver burden, and distress, and increasing caregivers’ skills, confidence, and knowledge (Hepburn et al., 2003, 2007). In a six-week, 12-hour program, the SCP aids caregivers in fostering an environment for the PLWD that is as calm, safe, and as pleasant as possible. Instruction about dementia and its effect on cognition, behavioral and emotional control, performance of daily tasks, self-care, and decision-making is combined with at-home practice and in-class coaching to help caregivers develop a repertoire of caregiving strategies. SCP is offered in-person in multiple states (Alzheimer’s Association, n. d.) through the U.S. Administration for Community Living and Administration on Aging support (Association of State and Territorial Health Officials, n. d.).
Several reasons prompted the development of Tele-Savvy. Most immediately, our own local SCP efforts and those of SCP implementation sites around the country experienced repeated and frequent instances of caregivers’ being unable to attend the in-person program. These experiences are echoed by reports in the literature of numerous barriers to in-person program attendance, including rural residence, lacking substitute care, substantial caregiving responsibilities, working full- or part-time, transportation barriers, caregivers’ limited mobility (i.e., induced by poor physical health), and PLWD’s severe functional dependence (Brown & Alligood, 2004; Navaie, 2011). Tele-Savvy development was also reinforced by the overall trend of increasing telehealth use (Munro Cullum, Hynan, Grosch, Parikh, & Weiner, 2014; van den Berg, Schumann, Kraft, & Hoffman, 2012).
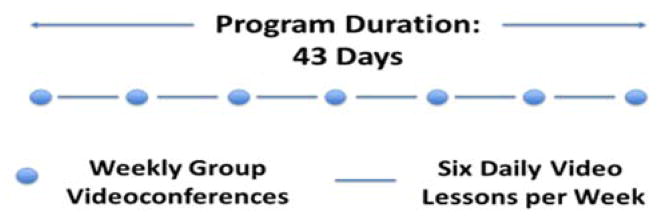
Tele-Savvy was designed to make SCP more accessible without sacrificing the building blocks that made it successful. To that end, the SCP structure was converted into a 43-day synchronous and asynchronous on-line program. Tele-Savvy combines seven weekly 75-minute group videoconferences interspersed with daily brief (8–20 minute) email-delivered “video lessons” (Griffiths et al., 2016; Kovaleva et al., 2017). Tele-Savvy provides virtual access to SCP’s curriculum and learning objectives by engaging facilitators and caregivers through digital means.
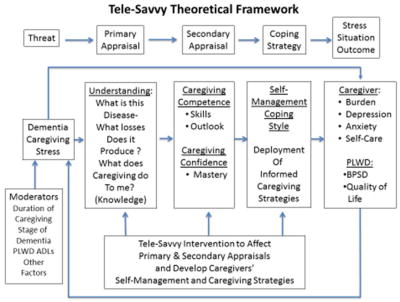
SCP is based on the stress process theoretical framework (Bandura, 1977, 1989; Folkman & Lazarus, 1988; Folkman, Lazarus, Gruen, & DeLongis, 1986) (Figure 1). The stress process framework and the chronic condition management framework (Anderson et al., 2015; Ryan & Sawin, 2009) underlie Tele-Savvy’s core premise: caregiving stress associated with the PLWD’s progressive dementia and the work of caregiving threaten the dyad’s well-being. Tele-Savvy seeks to promote positive outcomes for caregivers and PLWD by strengthening a range of caregivers’ coping capacities through a curriculum designed to enhance caregiving skills and knowledge and foster caregiving self-efficacy (mastery). Tele-Savvy posits that caregiving is a clinical role, for which most caregivers are unprepared. Incorporating the expertise of several disciplines, including nursing (Algase, Beattie, Antonakos, Beel-Bates, & Yao, 2010; Beck et al., 2002; Kovach, Noonan, Schildt, & Wells, 2005); psychology (Burgio, Stevens, Guy, Roth, & Haley, 2003; Coon, Thompson, Steffen, Sorocco, & Gallagher-Thompson, 2003; Teri, Logsdon, Uomoto, & McCurry, 1997); and occupational therapy (Gitlin, Kales, & Lyketsos, 2012; Gitlin, Winter, Burke, Chernett, Dennis, & Hauck, 2008; Gitlin, Winter, Corcoran, Dennis, Schinfeld, & Hauck, 2003), Tele-Savvy seeks to promote caregivers’ understanding that their person’s behavior is affected by dementia’s progressive impact on their cognition, function, behavior (e.g., BPSD), and personality. Information about dementia progression and etiology of associated changes in the person’s cognition and behavior is intended to alter caregivers’ primary appraisal of the threat. Social cognitive theory (Bandura, 1977) suggests that performance of an intended behavior, observation of peers’ performance of such behavior, and a respected authority’s support of the requisite behavior promote “mastery,” a person’s positive evaluation of his/her capacity for that behavior. All components of Tele-Savvy – synchronous and asynchronous instruction, caregivers’ off-line practice of recommended strategies, and facilitators’ coaching during videoconferences – are intended to strengthen caregiving mastery (secondary appraisal). They are also meant to facilitate development and implementation of self-derived successful caregiving and self-care/self-management coping strategies. Discussing these strategies’ implementation during the videoconferences allows caregivers to report on successful enactment, while providing peers with an example and receiving acknowledgment from the respected authority (facilitator). Improved coping (management of the threat – caregiving stress) is expected to improve caregiver- and PLWD-centered outcomes.
Figure 1.
Tele-Savvy theoretical framework. PLWD = person(s) living with dementia. BPSD = behavioral and psychological symptoms of dementia. ADL = activity of daily living
The SCP to Tele-Savvy transformation – described in detail elsewhere (Griffiths et al., 2016) – began with a story-boarding exercise by the co-principal investigators (KH & PG). In the exercise, the constituent parts of SCP were divided into those that required interactivity – give and take among the caregivers and between caregivers and a facilitator – and those that could be packaged into discrete units that could be taught without facilitation. This step identified the material to be covered in seven consecutive weekly group videoconferences and that to be covered through 36 daily video lessons to be viewed individually by caregivers. The basic flow of SCP provided the order in which materials were to be provided either synchronously or asynchronously. The co-PIs then outlined the content for the 36 video lessons and identified lessons in which enactments of the lesson principles would be useful. Volunteer actors were engaged to portray a variety video vignettes of family caregiving experiences and activities that exemplified or illustrated a principle or concept taught in the lesson. Concurrently, the co-PIs recruited a number of Emory faculty members (including themselves) and clinical experts who would serve as the “teacher” in the videos. They then developed scripts for every video lesson, rehearsed the teachers in their parts, and, with the technical help of a video crew, shot and edited the videos – adding the video vignettes, as appropriate. The 6–20 minute long daily video lessons can be watched and re-watched at any time during the program and up to two weeks post-program. Topic examples include the role of a caregiver; information on dementias (types, etiology, management, prognosis); dementia-produced losses in cognition and behavioral and emotional control; contented involvement as a key strategy to engage PLWDs in activities; dementia stages and fitting tasks to persons’ disease stage; decision-making in dementia caregiving; and building a support network for the caregiver. The sixth video in each week focuses on caregivers’ self-care (breathing, meditation).
In the main, the synchronous group videoconferences followed the structure of the selected elements of SCP; the debriefing, talks, and exercises that relied on interaction in SCP were brought over, whole cloth, into Tele-Savvy. A brief review of the week’s video lessons was added to the videoconferences to allow for caregivers’ questions and responses. The original SCP is taught in six sessions. Tele-Savvy entails seven group sessions; an orientation videoconference was added (“session 0”) to introduce participants to the on-line format and discuss program logistics.
Assignments – viewing daily videos, reading the manual, and doing workbook exercises – were not mandatory. During orientation videoconference, facilitators explained that participants were strongly encouraged to complete all assignments. But, importantly, it was emphasized that participants were free to complete assignments as their caregiving situations permitted, not to create additional pressure for caregivers.
Once Tele-Savvy was developed, an expert panel of six SCP authors and experienced instructors evaluated the fidelity of the Tele-Savvy curriculum to SCP. The evaluation employed several criteria, including Tele-Savvy’s fidelity to SCP’s guiding premise and theory, overall curriculum, class time, emphasis on Savvy vocabulary, caregiver sharing and group interactions, and caregiver manual and workbook. They also viewed several of the daily videos. The panel was asked to rate Tele-Savvy along these dimensions, using a Likert-type scale (highly faithful to not at all faithful) and to provide commentary on Tele-Savvy’s strengths and weaknesses on these dimensions. Although we did not establish an a priori cutoff for acceptable fidelity ratings, our expectation was that any dimension receiving an unacceptable rating by two or more reviewers would require corrective action. No such actions were required. Several qualitative comments – for example, paying special attention to promoting “groupness” in an on-line environment – did result in slight program adjustments.
In a subsequent no control pre-post pilot test, 58 caregivers demonstrated decreased caregiver burden (p=0.003), depressive symptoms (p=0.007), and distress due to BPSD (p=0.012), and increased caregiver competence (p=0.001). Caregivers’ competence mediated caregiver burden reduction (p=0.006). Qualitative findings from the pilot supported Tele-Savvy’s feasibility and attested to strong connectedness in the on-line setting. All Tele-Savvy videoconference lectures, video lessons, caregiver manual, and other accompanying materials were updated in response to pilot participants’ feedback (Kovaleva et al., 2017).
Research Design and Methods
Specific Aims
The current trial uses a longitudinal, randomized, three-arm design and pursues these aims:
Aim 1. Establish Tele-Savvy’s efficacy in mitigating the affective impact on dementia caregivers compared to caregivers in an attention control or usual care condition.
Aim 2. Establish Tele-Savvy’s efficacy in promoting PLWDs’ quality of life (e.g., reducing BPSD frequency and severity) compared to PLWD whose caregivers are in an attention control or usual care condition.
Aim 3. Establish Tele-Savvy’s efficacy in enhancing caregiver mastery and test the mediating effect of mastery and enactment on Aim 1 & 2 outcomes.
Exploratory aim
Examine the comparability of Tele-Savvy’s efficacy across the three racial/ethnic groups: African Americans, whites, and Latinos/Hispanics.
Hypotheses are not stated, but the three aims’ phrasing indicates an expectation that Tele-Savvy participation will benefit caregivers and PLWDs over time compared to those in the attention control or usual care condition. We do not posit a hypothesis regarding the exploratory aim.
Study Design
This is a prospective, longitudinal, randomized three-group controlled trial conducted by a consortium of four National Institute on Aging (NIA)-supported Alzheimer’s Disease Centers (ADCs). The co-principal investigators (co-PIs) provide administrative leadership, and co-investigators (co-Is) collaborate in subject recruitment and project management. One PI and all co-Is lead the Outreach, Recruitment, and Education Cores of the ADCs affiliated with respective institutions. The collaborating sites provide access either to rural or urban ethnically diverse population of dementia caregivers. Each of these three sites has a co-investigator and part-time study coordinator. These sites refer potentially eligible participants to the head site for screening and eligibility. The study was approved by each site’s institutional review board (IRB).
Recruitment began in May 2017. We aim to enroll 270 participants (18 cohorts of 15 subjects) at a rate of two cohorts (15 participants each) every six weeks through the project’s year 2.5. Allocation uses a 2:2:1 post-baseline randomization scheme: Tele-Savvy intervention (N=108); Healthy Living attention control (N=108); and usual care (N=54). Caregivers randomized to Tele-Savvy participate immediately; those randomized to attention control and usual care will participate in Tele-Savvy six months post-baseline. The lead institution coordinates recruitment, consent, data gathering and analysis, and intervention and attention control activities.
Recruitment is multifaceted. The principal route is through registries and recruitment activities at the collaborating ADCs. Other recruitment methods include ClinicalTrials.gov, the Alzheimer’s Association’s TrialMatch@alz, and networking with local Alzheimer’s Association chapters and the SCP implementation programs across the country. All recruitment activities flow to the central study coordinator at the lead institution who is responsible for follow-up screening and the conduct of an IRB-approved phone consent procedure. All consented subjects are grouped by their availability for videoconference participation either during business hours, evenings, or weekends. When a cohort of 15 participants is formed around a common time slot, participants are contacted by the project research interviewer who collects baseline data via a videoconference or telephone. During the baseline interview, participants self-report on their residence type: urban, rural, or suburban. They also report on their person’s dementia diagnosis and other sociodemographic variables. We rely on caregivers’ self-report of dementia; no verifications of care recipients’ diagnoses or dyads’ residence types are performed. At this point, subjects are randomized into the three study arms and informed of their assignment. Study navigators connect with Tele-Savvy and Healthy Living participants to orient them to videoconferencing and web-site with daily videos. If caregivers do not have a webcam, they are provided with one. The navigators provide technical assistance to caregivers for the duration of their participation.
Tele-Savvy intervention condition
A description of Tele-Savvy intervention is provided above. The 75-minute videoconferences enable debriefing caregivers’ home application of program materials, reviewing content from video lessons, promoting connectedness among caregivers, engaging in interactive exercises, and covering new material (Figure 2). Videoconferences are hosted on Vidyo (Vidyo, n. d.), a service for which the lead institution has a license. Videoconferences are not recorded, and access to them is available only to participants and study staff; thus, videoconferences pose no privacy concerns. Participants join the videoconferences on their computers or mobile devices to create a virtual classroom environment. Caregivers unable to join via a videoconference can join via telephone, in which case they can hear others and speak without video participation. This second option is not encouraged (i.e., everyone is expected to join via a videoconference), but it is provided as an alternative to those who may have technical difficulties. Daily video lessons are stored on a secure version of Canvas (Canvas, 2017), a course management web-site. Participants get daily emails from Canvas that provide links to the video lessons.
Figure 2.
Tele-Savvy program structure.
Attention control condition
The attention control condition, Healthy Living, mirrors Tele-Savvy in the amount of intervention exposure delivered synchronously and asynchronously (see Figure 2). Developed by one PI and co-I (PG, JN), the content focuses on healthy living: nutrition, exercise, fall prevention, and general safety. Dementia caregiving is not discussed. It uses materials from the National Institute on Aging Go4Life program (Go4Life® from the National Institute on Aging at NIH, n. d.) adapted for videoconferences and on-line videos.
Usual care
Participants randomized to usual care are asked to continue with their established care scheme until after six months post-baseline, when they are invited to complete Tele-Savvy, which is considered delayed study participation
Facilitator training
Facilitators for the Tele-Savvy sessions were recruited from among SCP instructors with substantial experience teaching in-person SCP and working with dementia caregivers. The Healthy Living videoconferences are led by an experienced health educator. All facilitators were trained by the co-PIs to acquaint them with the curricula and familiarize them with techniques for engaging on-line participation. Tele-Savvy facilitators completed a dry run with caregivers not included in the study. The PIs observed these dry runs and provided additional coaching. The PIs periodically observe videoconferences and rate facilitators’ adherence to checklist-based fidelity criteria. Sub-threshold adherence prompts additional training.
Coordination between study sites
The four study sites collaborate in participant recruitment. A full-time study director, experienced in RCT management, manages key project activities at the head site in collaboration with investigators and staff: recruitment; screening; consent; randomization of participants to study arms; forming cohorts of participants; data collection; data analysis; IRB and data safety and monitoring board (DSMB) liaison; and identification of opportunities to disseminate study results. The head site assures delivery of the experimental and control conditions. Monthly videoconferences are held between all co-Is during which site, adherence the schedule, challenges at the sites, and solutions for them are discussed. These meetings augment weekly project management videoconferences at the head site. The study director is responsible for communication between sites (co-Is and staff) and sites’ staff report directly to the study director who coordinates communication with the PIs as needed.
Sample
The study is targeted to non-paid family or non-relative dementia caregivers; at least 18 years of age; who are cognitively competent; are able to read, speak, and understand English; and who provide on average at least four hours of assistance to a non-institutionalized PLWD daily. Candidates cannot be participating in another caregiver training study or have completed SCP or Tele-Savvy previously. They need access to a computer or mobile device, speakers, internet, and email. Uncorrectable hearing or vision impairments prevent study participation. The study coordinator screens participants for these criteria prior to consent.
Sample Size Justification and Power
With an expected 20% attrition, based on analogous values in previous trials with dementia caregivers (Belle et al., 2006; Boots, de Vugt, Kempen, & Verhey, 2016; Ostwald et al., 1999), we aim for a final sample N=215: 86 (Tele-Savvy), 86 (attention control), and 43 (usual care). Power analysis was conducted with PASS 14 Power Analysis and Sample Size Software (NCSS Statistical Software, 2015) (α=5% and β=80%). Primary analysis will compare outcomes of the three group changes, T1 to T2 to T3 (3-by-3 repeated measures design of three groups). The sample N=215 will allow detection of small-to-moderate effect sizes, Cohen’s f=0.21–0.24, for the group, time, and group-by-time interaction effects, given moderate correlation between measurement pairs, r=0.3–0.5 After adjusting for potential group differences, the overall Tele-Savvy intervention effect for the combined T1-to-T3 pre-post effects for the immediate Tele-Savvy plus T3-T5 pre-post effects for the attention control and usual care will allow detection of a small effect size, Cohen’s d=0.19. Pooled sample will allow testing of the mediating effect of caregiver mastery on Aims 1 and 2 outcomes. For the exploratory aim, comparing the program’s efficacy across ethnic groups (expected estimated proportions: African Americans – 20%, Whites – 65%, and Hispanic/Latino – 15%), detection of moderate effect sizes is expected: Cohen’s f2= 0.19–0.23 (Cohen, 1988).
Data Collection and Management
Quantitative data are collected by an interviewer blinded to participants’ study condition. Post-baseline, data are collected at three, six, nine, and 12 months, yielding a total of five data collection time points. Participants are reimbursed with $25 gift cards for each interview and receive a monthly newsletter. Quantitative data are securely stored in RedCap (Harris et al., 1999). The interviewer and a second staff member enter the data independently into RedCap. A PI or the study coordinator reviews the doubly entered records, identifying and correcting discordant entries.
Qualitative results supplement and expand quantitative findings. A sub-sample of participants will be interviewed about their experience in Tele-Savvy. Maximum variation sampling will be used to select subjects to allow exploration of variations in experiences, while establishing common patterns (Guest, 2012). Variation will be derived from participants’ socio-demographic characteristics (race/ethnicity, education, and relationship to the PLWD). Interviews will be conducted at T2 and T3 (among Tele-Savvy participants) and at T4 and T5 (among attention control and usual care participants). Interview questions will center on caregivers’ experience with Tele-Savvy; their view on the strongest and weakest elements; opinions on on-line participation and preference of on-line versus in-person class; sense of connectedness to other participants and facilitator; potential improvements for Tele-Savvy; and ways Tele-Savvy may better serve caregivers of their ethnicity/race and residence type (urban vs. rural). Several investigators will interview caregivers via videoconference or telephone; interviews will be audiotaped and transcribed. Reflexive statements (Barry, Britten, Barber, Bradley, & Stevenson, 1999; Malterud, 2001) and analytic memos (Birks, Chapman, & Francis, 2008) will be created for interviews.
Measures
Table 1 delineates the battery of instruments employed in the study. With the exception of demographic information and baseline assessments of care recipient function and cognitive capacity, the full battery is administered at all five data collection points.
Table 1.
Caregiver- and Care Recipient-Centered Measures
| Category | Instrument | Instrument Description | Psychometrics |
|---|---|---|---|
| Baseline Assessment Only | |||
| PLWD cognitive status | Informant rating of Cognitive Decline in the Elderlya,b,c | A 16-item Likert-type scale that can be completed by a caregiver; is not subject to contamination by the person’s education level | Correlates well with dementia diagnosis. |
| Demographic information | Demographic information sheet developed for the study | Age, race, ethnicity, gender, employment, education, residence type (urban, suburban, or rural), relationship to PLWD, age of PLWD, residence with the PLWD, time since PLWD’s dementia diagnosis, duration of caregiving, caregiver receives help in caregiving. | N/A |
| PLWD functional status | Lawton: Activities and Instrumental Activities of Daily Livingd | Fourteen-item Likert-type scale elicits caregivers’ assessment of their persons’ function in activities of daily living and instrumental activities of daily living | Inter-rater reliability 0.87–0.91d |
| Variables Assessed at Each Data Collection Event: Caregiver-Centered Variables | |||
| Stress | Perceived Stress Scalee | Fourteen-item Likert-type scale. Assesses the degree to which an individual finds events in his/her life stressful. | Cronbach’ α = 0.84–0.86; Established validity.f |
| Depressive symptoms | Center for Epidemiologic Studies - Depressiong | Twenty-item Likert-type scale. Measures depressive symptoms, covers components related to sleep disturbance, depressed mood, psychomotor retardation, and hopelessness.h | Cronbach’s α=0.91. intraclass correlation coefficient 0.87. Established validity.h |
| Anxiety | State-Trait Anxiety Inventoryi,j | Twenty-item Likert-type scale. Evaluates transitory anxiety. | State-sensitive reliability α=0.94.i |
| Burden | Zarit Burden Interviewk | Twenty-two-item Likert-type scale. Assesses CG burden in the according to the domains of personal strain and role strain.l,m | Good reliability and validity.n |
| Dyadic relationship | Dyadic Relationship Scaleo | Eleven-item Likert-type scale. Assesses dyadic strain and dyadic interaction perceived by caregiver. | Cronbach’s α=0.84–0.85.o |
| Mastery | Pearlin’s Caregiver Mastery, Loss, and Competence Scalesp | Three sub-scales (designed to be used independently: Relational Deprivation (six items), Caregiver Competence (four items), and Management of Situation (four items). Likert-type scales. | Cronbach’s α = 0.77, 0.74, and 0.67, respectively.p |
| Skill | Caregiver Assessment of Behavioral Skill – Self-Reportq | Seventeen-item Likert-type scale. Elicits caregivers’ self-report of behavioral management skills. | Cronbach’s α = 0.75–0.94. Good validity.q |
| Reward | Positive Appraisal of Care Scaler | One subscale is used: Consequential Gain (six items, Likert-type scale). Evaluates caregiver’s degree of positive appraisal they associate with their caregiving. | Cronbach’s α=0.84. Established validity. Test-retest reliability 0.86 (p<0.01).r |
| Self-care | Mindful Self-Care Scale Sub-Scaless | Sub-scales assessing self-compassion and purpose (six items), mindful relaxation (six items), and other ways of self-care (clinical and general, total of nine items). Likert-type scales. | Cronbach’s α = 0.89 for total scale, 0.83 for Self-Compassion and Purpose; 0.77 for Mindful Relaxation.s |
| Health | Self-rated healtht,u,v,w,x | One-item survey | Reliability 0.92.t |
| Variables Assessed at Each Data Collection Event: Patient-Centered Variables | |||
| BPSD | Revised Memory and Behavior Checklisty | Twenty-two-item Likert-type scale. Assesses presence of a variety of behavioral and psychological symptoms of dementia, their frequency (never to daily) and caregiver’s associated distress (not at all to extreme). Has three sub-scales: memory, depression, and disruption. | Cronbach’s α = 0.84–0.90.y |
| Mood | Neuropsychiatric Inventoryz | Twelve-item scale. Assesses presence of twelve behaviors or symptoms (yes/no answer). If a behavior or a symptom is present, assesses severity of this behavior (Likert-type scale). | Test-retest correlations between total symptom and distress scores: 0.90 and 0.94 (p<0.00001 for both). |
Teri, Truax, Logsdon, Uomoto, Zarit, & Vitaliano (1992);
Statistical Analysis
Analyses will be run in SPSS (IBM, Version 22.0, 2016). Multi-level mixed effects models with random (participant) and fixed (cohort) effects will be used to test linear and non-linear trajectories of change for the intervention groups and model group by time interactions. To compare the Tele-Savvy intervention to the attention control and usual care groups, the first three time points will be used to test for differences between the three groups’ longitudinal trajectories. We will combine the intervention effects for the three groups across their main time points from before intervention receipt to immediately after the intervention to the first follow-up time point (T1-to-T-2-to-T3 for the immediate Tele-Savvy group and T3-to-T4-to-T5 for the attention control and usual care, respectively). These combined time points are designated cT1, cT2, and cT3 (“c” denotes “combined”). If significant differences between or among the three groups at the time point immediately prior to intervention (cT1) are noted, these baseline effects will be included and adjusted for in the final model. Overall time effects will be examined. Planned post hoc comparisons for immediate effects (cT1 to cT2), longer term effects (cT1 to cT3) and sustained effects (cT2 to cT3) will be assessed. Sidak Type I error rate adjustment will be used. Longer term sustained effects will be captured for the immediate Tele-Savvy group using additional follow-up times at T4 and T5. Evaluation will be conducted for placebo effects that may arise from being enrolled in the study (Benedetti, 2014) since three baseline time points for the attention control and usual care are captured. Data captured at T1-T2-T3 for the attention control and usual care will be used to evaluate for potential decline. All model assumptions will be tested. Grand mean centering to help combat multicollinearity will be used. Standard diagnostic tests and influence statistics will be used to test the distributions of the residuals. Covariate predictors of missingness over time will be included (Hedeker & Gibbons, 2006). Ninety-five percent confidence intervals will be used.
To test mastery as a mediator on Aims 1 and 2 outcomes, PROCESS module for SPSS will be employed (Hayes, 2013). The focus will be on the immediate (T2 for immediate Tele-Savvy and T4 for pooled attention control and usual care) and sustained (T3 for immediate Tele-Savvy and T5 for pooled attention control and usual care) effects of Tele-Savvy on the mediating measure of mastery on the Aims 1 and 2 outcomes. These mediation effects will be tested using structural equation modeling (AMOS v. 22) (Arbuckle, 2013).
Analysis of covariance will allow evaluation of the program’s effect on participants of three ethnicities/races. Race/ethnicity and caregivers’ residence (urban/suburban vs. rural) will be covariates. The expected participant distribution is 20:65:15 for African Americans: Whites: Hispanics/Latinos.
Qualitative Analysis
Qualitative descriptive methods (Sandelowski, 2000, 2009) will be used to formatively evaluate Tele-Savvy and to augment qualitative analysis of the exploratory aim. Proofed transcripts will be analyzed using descriptive content analysis, allowing pattern discovery (Sandelowski, Holditch Davis, & Harris, 1989). Following classical guidelines, analysis will include: (1) data coding from notes and interviews; (2) recording insights and reflections on the data; (3) sorting through the data to identify similar phrases, patterns, themes, sequences and key features; (4) seeking commonalities and differences in the data and extracting them for further consideration and analysis; (5) gradually deciding on a small group of generalizations that hold true; and (6) examining these generalizations in light of knowledge that is known (Miles, Huberman, & Saldaña, 2014). The analysis will lead to the formulation of themes – conceptually related units of meaning. Repeating ideas will drive themes’ and sub-themes’ identification (Ryan & Bernard, 2003). Two investigators will read the raw data and the emerging interpretations; unresolvable disagreements will be adjudicated by a third investigator. NVivo (2012) will be used to aid in qualitative data analysis.
Rigor will be maintained by ensuring participants freedom to speak and ensuring participants’ voices are heard and their perceptions are accurately represented (Milne & Oberle, 2005). To ensure freedom to speak, a purposive sampling plan will be used, allowing the investigator to choose participants who can provide in-depth information relevant to the formative evaluation and the exploratory aim. To ensure participants’ voices are heard, attention will be paid to verbal and non-verbal cues suggesting a participant might have more to share and using probes to clarify and increase descriptive detail of their narratives. Timely transcription, re-reading and comparing the transcribed data and tapes will ensure that participants’ perceptions are accurately represented, enhancing rigor.
Discussion
To date (16 months post-award and 7 months post-DSMB clearance and 25% of the way into the total time allocated for recruitment), 102 participants have been recruited, 37.8% of the goal of N = 270. The consented caregivers reside in 15 states. DSMB convened its first meeting in April 2017. In response to its recommendation, a protocol was developed to address instances in which caregivers’ or PLWDs’ safety and psychological well-being appeared to be seriously compromised. All project staff have been trained on the protocol; to date, it has been invoked in only one situation.
The first three cohorts have completed Tele-Savvy and Healthy Living conditions. We did not record the timing of each videoconference, but the navigators noted that Tele-Savvy videoconferences last at least 75 minutes each and occasionally longer. Occasionally, participants need to leave earlier, but this occurs very infrequently. Of the 36 participants in the first three cohorts (six in Tele-Savvy and six in Healthy living per cohort), two were lost to follow-up (consented but did not complete baseline interviews), and two, because of changes in work schedules, were rescheduled to future cohorts that can fit with their new schedules. No unsolvable technical difficulties were reported in the first cohort; however, two participants (one in the second and one in the third cohort) missed four and three videoconference sessions, respectively, because of bandwidth or connectivity problems. In one of these cases, the rural participant used the phone option to continue in the program. This experience has alerted us that at least two to three videoconference practices are required, especially with rural caregivers, before participants begin the program. These may be brief (e.g., five minute) check-ins, when the navigator checks that the audio and video transmission are intact. Additionally, we now conduct internet speed tests (e.g., sending caregivers a link for such test freely available on-line) to develop work-arounds, if needed. So far, caregivers have experienced no problems accessing video lessons housed on the Canvas platform.
Challenges and Limitations
The study faces four not-unexpected challenges that will limit the generalizability of its results. We face two main technological challenges: bandwidth and internet access. As reported, several caregivers have experienced difficulties joining or remaining connected to the videoconferences. If participants have sufficiently strong connections, the study navigators have been able to help them to initiate and maintain connection. Without strong enough bandwidth, less than optimal alternatives (i.e., smartphone connections) are available. Our experience to date mirrors that in the pilot: bandwidth problems do occur, but only in a small percentage of cases. Because the connectedness of participants with each other and with the facilitator is so integral to the program and so reliant on face-to-face videoconferencing, and because the problems are relatively infrequent, we plan no changes in our videoconference delivery strategy.
A number of factors affect potential participants’ internet access. Older adults make up a large segment of dementia family caregivers, and these adults are among the slowest cohort of internet adopters. Some areas, particularly in rural America, do not have access to internet coverage. And for some, the cost of internet coverage is beyond their means. We acknowledge that the Tele-Savvy trial will not include caregivers who fall into any of these categories, so that creates a constricted and somewhat biased sample, thereby limiting the generalizability of our results. We believe that trends in age-based internet use and the universality of internet coverage will continue to reduce these limitations, though likely not in time to have a serious effect on our sample.
Although the study is ahead of schedule in meeting its enrollment goals, attaining the study’s goal to recruit a diverse sample (65% Caucasian; 20% African American; 15% Latino) remains a challenge. Each of the participating ADCs focuses some part of its recruiting activities on diverse communities; nevertheless, recruitment among African Americans and Latinos is below targeted levels. Several strategies have been implemented to increase diversity of caregiver participants. We are deliberately seeking to ensure racial concordance between potential participants and study recruiters, a method shown to be effective in increasing research study participation among ethnic minorities (Carroll et al., 2011). In addition to listing the project through ClinicalTrials.gov and TrialMatch@alz.org, we have moved recruitment activities beyond the ADCs, focusing on recruitment through each site’s local research and community outreach events. We appreciate, however, that some caregivers may be more difficult to reach due to dementia-related cultural context.
The complexity of caregivers’ lives and the geographical reach of the project comprise our fourth challenge: structuring study cohorts around the time availability of caregivers from across the U.S (from Maine to Hawai’i and Alaska). We build toward cohorts of 15 caregivers who can all meet at the same time; once a cohort is formed, its members can be randomly assigned to one of the three study groups. Logistically, this means accumulating consented caregivers into groups sorted by availability to take part in videoconferences either (1) during weekday daytime hours, (2) only on weekends, or (3) only in the evening. Building cohorts to fit availability results in delays between consent and participation for some caregivers. To ensure that consented caregivers do not lose interest and become lost to follow-up at the time when their program begins, the study coordinator maintains contact, through calls and email, with caregivers who may experience a delay between their consent and beginning of the program. We also use monthly project newsletters, and birthday and seasonal cards as means to maintain contact with those awaiting assignment and to update them on the study progress. Although we have had a low dropout rate, we monitor this closely.
Although this is an inevitable consequence of any randomized trial, we note that some participants report disappointment with being randomly assigned to the attention control or usual care condition; quite naturally, they were hoping to learn caregiving strategies immediately. We reinforce the importance of these caregivers’ participation in this trial as a way to contribute to dementia caregiving research and thus help other patients and families affected by dementia, but we also emphasize that Tele-Savvy will be offered to them following the six-month data collection point.
We aimed to make the program transportable. Its curriculum and components – videoconferences, daily videos, and caregiver manual and workbook – can be used by healthcare and community agencies. Widely available technology (videoconferencing platforms (e.g., Google Hangouts) and course management programs (e.g., Canvas)) provide mechanisms that would enable organizations to offer Tele-Savvy in a manner faithful to the program’s psychoeducational intent.
To our best knowledge, none of the numerous on-line interventions for dementia caregivers has transformed an efficacious evidence-based in-person psychoeducational program into a version in which caregivers can partake from their homes and which seeks to provide an intact virtual version of the original. Other programs use a web-site as a “hub” of the intervention (Chiu et al., 2009; Cristancho-Lacroix et al., 2015; Hayden et al., 2012), yet do not include the same degree of structured, curriculum-guided communication between facilitators (experts) and participants, as does Tele-Savvy. Other programs likewise differ in their flexible agendas, based on caregivers’ requests, as opposed to providing a set, theoretically-based curriculum (O’Connell et al., 2014). Few of these on-line interventions have been conducted as RCTs.
Conclusion
Tele-Savvy will be tested in a longitudinal three-armed randomized controlled trial with a targeted sample of 270 dementia family caregivers. The study will test the effect on caregivers and care recipients of an adaptation of an in-person evidence-based psychoeducational program to an entirely on-line version of itself that caregivers can join from their homes and that maintains the elements that made it successful, including live interaction between participants and facilitator, a factor considered key in ensuring program’s success (Boots et al., 2014). The program is well-aligned with the expanding use of telehealth in clinical practice (Austrom et al., 2015; Dowling et al., 2014; Merrell, 2015) and mirrors analogous programs’ reports that support the feasibility of such on-line interventions (Ho, Mak, Kwok, Au, & Ho, 2015; Lewis, Hobday, & Hepburn, 2010; Marziali & Donahue, 2006).
Results of this trial will provide data on feasibility and efficacy of an on-line psychoeducational intervention for dementia caregivers of various races/ethnicities who live in diverse settings. We anticipate that this study will enable healthcare and community agencies to use Tele-Savvy to expand caregivers’ access to needed education.
References
- Algase DL, Beattie ER, Antonakos C, Beel-Bates CA, Yao L. Wandering and the physical environment. American Journal of Alzheimer’s Disease and Other Dementias. 2010;25(4):340–346. doi: 10.1177/1533317510365342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alzheimer’s Association. 2017 Alzheimer’s disease facts and figures. Alzheimers & Dementia. 2017;13(4):325–373. https://doi.org/10.1016/j.jalz.2017.02.001. [Google Scholar]
- Alzheimer’s Association. The Savvy Caregiver. n. d Retrieved from http://www.alz.org/nca/in_my_community_103874.asp.
- Anderson RA, Bailey DE, Jr, Wu B, Corazzini K, McConnell ES, Thygeson NM, Docherty SL. Adaptive leadership framework for chronic illness: Framing a research agenda for transforming care delivery. ANS Advances in Nursing Science. 2015;38(2):83–95. doi: 10.1097/ANS.0000000000000063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arbuckle J. AMOS. New York, NY: IBM Corporation; 2013. [Google Scholar]
- Association of State and Territorial Health Officials. Maine: Improving dementia care through the Savvy Caregiver Program. 2014 Retrieved from http://www.astho.org/Healthy-Aging/ME-Caregiving-Case-Study/
- Austrom MG, Geros KN, Hemmerlein K, McGuire SM, Gao S, Brown SA, … Clark DO. Use of a multiparty web based videoconference support group for family caregivers: Innovative practice. Dementia (London) 2015;14(5):682–690. doi: 10.1177/1471301214544338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball L, Jansen S, Desbrow B, Morgan K, Moyle W, Hughes R. Experiences and nutrition support strategies in dementia care: Lessons from famiy carers. Nutrition & Dietetics. 2015;72:22–29. doi: 10.1111/1747-0080.12107. [DOI] [Google Scholar]
- Bandura A. Self-efficacy: Toward a unifying theory of behavioral change. Psychological Review. 1977;84(2):191–215. doi: 10.1037/0033-295x.84.2.191. [DOI] [PubMed] [Google Scholar]
- Bandura A. Human agency in social cognitive theory. The American Psychologist. 1989;44(9):1175–1184. doi: 10.1037/0003-066x.44.9.1175. [DOI] [PubMed] [Google Scholar]
- Barry CA, Britten N, Barber N, Bradley C, Stevenson F. Using reflexivity to optimize teamwork in qualitative research. Qualitative Health Research. 1999;9(1):26–44. doi: 10.1177/104973299129121677. [DOI] [PubMed] [Google Scholar]
- Beck CK, Vogelpohl TS, Rasin JH, Uriri JT, O’Sullivan P, Walls R, … Baldwin B. Effects of behavioral interventions on disruptive behavior and affect in demented nursing home residents. Nursing Research. 2002;51(4):219–228. doi: 10.1097/00006199-200207000-00002. [DOI] [PubMed] [Google Scholar]
- Belle SH, Burgio L, Burns R, Coon D, Czaja SJ, Gallagher-Thompson D … Resources for Enhancing Alzheimer’s Caregiver Health (REACH) II Investigators. Enhancing the quality of life of dementia caregivers from different ethnic or racial groups: A randomized, controlled trial. Annals of Internal Medicine. 2006;145(10):727–738. doi: 10.7326/0003-4819-145-10-200611210-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedetti F. Placebo effects: Understanding the mechanisms in health and disease. Oxford, UK: Oxford University Press; 2014. [Google Scholar]
- Bennett JM, Fagundes CP, Kiecolt-Glaser J. The chronic stress of caregiving accelerates the natural aging of the immune system. In: Bosch J, Philips A, Lord J, editors. Immunosenescence. New York, NY: Springer; 2012. [Google Scholar]
- Birks M, Chapman Y, Francis K. Memoing in qualitative research: Probing data and processes. Journal of Research in Nursing. 2008;13:68. doi: 10.1177/1744987107081254. [DOI] [Google Scholar]
- Boots LM, de Vugt ME, van Knippenberg RJ, Kempen GI, Verhey FR. A systematic review of Internet-based supportive interventions for caregivers of patients with dementia. International Journal of Geriatric Psychiatry. 2014;29(4):331–344. doi: 10.1002/gps.4016. [DOI] [PubMed] [Google Scholar]
- Boots LM, de Vugt ME, Kempen GI, Verhey FR. Effectiveness of the blended care self-management program “Partner in Balance” for early-stage dementia caregivers: Study protocol for a randomized controlled trial. Trials. 2016;17(1):231. doi: 10.1186/s13063-016-1351-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown JW, Alligood MR. Realizing wrongness: Stories of older wife caregivers. Journal of Applied Gerontology. 2004;23:104–119. doi: 10.1177/0733464804265609. [DOI] [Google Scholar]
- Burgio L, Stevens A, Guy D, Roth DL, Haley WE. Impact of two psychosocial interventions on white and African American family caregivers of individuals with dementia. The Gerontologist. 2003;43(4):568–579. doi: 10.1093/geront/43.4.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canvas. Canvas. 2017 Retrieved from https://www.canvaslms.com/?lead_source_description=instructure.com.
- Carroll J, Yancey A, Spring B, Figueroa-Moseley C, Mohr D, Mustian K, Fiscella K. What are successful recruitment and retention strategies for underserved populations? Examining physical activity interventions in primary care and community settings. Translational Behavioral Medicine. 2011;1:234–251. doi: 10.1007/s13142-011-0034-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu T, Marziali E, Colantonio A, Carswell A, Gruneir M, Tang M, Eysenbach G. Internet-based caregiver support for Chinese Canadians taking care of a family member with alzheimer disease and related dementia. Canadian Journal on Aging. 2009;28(4):323–336. doi: 10.1017/S0714980809990158. [DOI] [PubMed] [Google Scholar]
- Cohen J. Statisical Analysis for the Behavioral Sciences. 2. New York, NY: Psychology Press; 1988. [Google Scholar]
- Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. Journal of Health and Social Behavior. 1983;24:385–396. [PubMed] [Google Scholar]
- Cohen S, Willimson G. Perceived stress in a probability sample of the United States. In: Spacapam S, Oskamp S, editors. The social psychology of health: Claremont Symposium on applied social psychology. Newbury Park, CA: Sage; 1988. [Google Scholar]
- Coffman I, Resnick HE, Lathan CE. Behavioral health characteristics of a technology-enabled sample of Alzheimer’s caregivers with high caregiver burden. Mhealth. 2017;3:36. doi: 10.21037/mhealth.2017.08.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook-Cottone CP, Guyker WM. The development and validation of the Mindful Self-Care Scale (MSCS): An assessment of practices that support positive embodiment. Mindfulness. 2017:1–15. doi: 10.1007/s12671-017-0759-1. [DOI] [Google Scholar]
- Coon DW, Thompson L, Steffen A, Sorocco K, Gallagher-Thompson D. Anger and depression management: Psychoeducational skill training interventions for women caregivers of a relative with dementia. The Gerontologist. 2003;43(5):678–689. doi: 10.1093/geront/43.5.678. https://doi-org.proxy.library.emory.edu/10.1093/gerona/56.11.M707. [DOI] [PubMed] [Google Scholar]
- Cristancho-Lacroix V, Wrobel J, Cantegreil-Kallen I, Dub T, Rouquette A, Rigaud AS. A web-based psychoeducational program for informal caregivers of patients with Alzheimer’s disease: A pilot randomized controlled trial. Journal of Medical Internet Research. 2015;17(5):e117. doi: 10.2196/jmir.3717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai AK, Schwartz L, Grossberg GT. Behavioral disturbance in dementia. Current Psychiatry Reports. 2012;14(4):298–309. doi: 10.1007/s11920-012-0288-5. [DOI] [PubMed] [Google Scholar]
- Dowling GA, Merrilees J, Mastick J, Chang VY, Hubbard E, Moskowitz JT. Life enhancing activities for family caregivers of people with frontotemporal dementia. Alzheimer Disease and Associated Disorders. 2014;28(2):175–181. doi: 10.1097/WAD.0b013e3182a6b905. [DOI] [PubMed] [Google Scholar]
- Eisdorfer C, Czaja SJ, Loewenstein DA, Rubert MP, Arguelles S, Mitrani VB, Szapocznik J. The effect of a family therapy and technology-based intervention on caregiver depression. The Gerontologist. 2003;43(4):521–531. doi: 10.1093/geront/43.4.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farran CJ, Fogg LG, McCann JJ, Etkin C, Dong X, Barnes LL. Assessing family caregiver skill in managing behavioral symptoms of Alzheimer’s disease. Aging & Mental Health. 2011;15(4):510–521. doi: 10.1080/13607863.2010.536140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folkman S, Lazarus RS. Coping as a mediator of emotion. Journal of Personality and Social Psychology. 1988;54(3):466–475. [PubMed] [Google Scholar]
- Folkman S, Lazarus RS, Gruen RJ, DeLongis A. Appraisal, coping, health status, and psychological symptoms. Journal of Personality and Social Psychology. 1986;50(3):571–579. doi: 10.1037//0022-3514.50.3.571. [DOI] [PubMed] [Google Scholar]
- Fonareva I, Oken BS. Physiological and functional consequences of caregiving for relatives with dementia. International Psychogeriatrics. 2014;26(5):725–747. doi: 10.1017/S1041610214000039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher-Thompson D, Gray HL, Dupart T, Jimenez D, Thompson LW. Effectiveness of cognitive/behavioral small group intervention for reduction of depression and stress in non-Hispanic White and Hispanic/Latino women dementia family caregivers: Outcomes and mediators of change. Journal of Rational-Emotive and Cognitive-Behavior Therapy. 2008;26(4):286–303. doi: 10.1007/s10942-008-0087-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher-Thompson D, Steffen AM. Comparative effects of cognitive-behavioral and brief psychodynamic psychotherapies for depressed family caregivers. Journal of Consulting & Clinical Psychology. 1994;62(3):543–549. doi: 10.1037//0022-006x.62.3.543. [DOI] [PubMed] [Google Scholar]
- Gaugler JE, Zarit SH, Pearlin LI. Caregiving and institutionalization: Perceptions of family conflict and socioemotional support. International Journal of Aging & Human Development. 1999;49(1):1–25. doi: 10.2190/91A8-XCE1-3NGX-X2M7. [DOI] [PubMed] [Google Scholar]
- Gillespie R, Mullan J, Harrison L. Managing medications: The role of informal caregivers of older adults and people living with dementia. A review of the literature. Journal of Clinical Nursing. 2014;23(23–24):3296–3308. doi: 10.1111/jocn.12519. [DOI] [PubMed] [Google Scholar]
- Gitlin LN, Corcoran M, Winter L, Boyce A, Hauck WW. A randomized, controlled trial of a home environmental intervention: Effect on efficacy and upset in caregivers and on daily function of persons with dementia. The Gerontologist. 2001;41(1):4–14. doi: 10.1093/geront/41.1.4. [DOI] [PubMed] [Google Scholar]
- Gitlin LN, Hauck WW, Dennis MP, Winter L. Maintenance of effects of the home environmental skill-building program for family caregivers and individuals with Alzheimer’s disease and related disorders. The Journals of Gerontology. Series A, Biological Sciences and Medical Sciences. 2005;60(3):368–374. doi: 10.1093/gerona/60.3.368. [DOI] [PubMed] [Google Scholar]
- Gitlin LN, Kales HC, Lyketsos CG. Nonpharmacologic management of behavioral symptoms in dementia. JAMA. 2012;308(19):2020–2029. doi: 10.1001/jama.2012.36918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gitlin LN, Marx K, Stanley IH, Hodgson N. Translating evidence-based dementia caregiving interventions into practice: State-of-the-science and next steps. The Gerontologist. 2015;55(2):210–226. doi: 10.1093/geront/gnu123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gitlin LN, Winter L, Burke J, Chernett N, Dennis MP, Hauck WW. Tailored activities to manage neuropsychiatric behaviors in persons with dementia and reduce caregiver burden: a randomized pilot study. American Journal of Psychiatry. 2008;16(3):229–239. doi: 10.1097/JGP.0b013e318160da72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gitlin LN, Winter L, Corcoran M, Dennis MP, Schinfeld S, Hauck WW. Effects of the home environmental skill-building program on the caregiver-care recipient dyad: 6-month outcomes from the Philadelphia REACH initiative. The Gerontologist. 2003;43(4):532–546. doi: 10.1093/geront/43.4.532. [DOI] [PubMed] [Google Scholar]
- Go4Life ® from the National Institute on Aging at NIH. Go4Life ® from the National Institute on Aging at NIH. n. d Retrieved from https://go4life.nia.nih.gov/
- Griffiths PC, Whitney MK, Kovaleva M, Hepburn K. Development and implementation of Tele-Savvy for dementia caregivers: A department of Veterans Affairs clinical demonstration project. The Gerontologist. 2016;56(1):145–154. doi: 10.1093/geront/gnv123. [DOI] [PubMed] [Google Scholar]
- Guest G. Describing mixed methods research: An alternative to typologies. Journal of Mixed Methods Research. 2012;7(2):141–151. https://doi.org/10.1177/1558689812461179. [Google Scholar]
- Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap) -- a metadata-driven methodology and workflow process for providing translational research informatics support. Journal of Biomedical Informatics. 2009;42(2):377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayden LJ, Glynn SM, Hahn TJ, Randall F, Randolph E. The use of Internet technology for psychoeducation and support with dementia caregivers. Psychological Services. 2012;9(2):215–218. doi: 10.1037/a0027056. [DOI] [PubMed] [Google Scholar]
- Hayes AF. Introduction to mediation, moderation, and conditional process analysis: A regression-based approach. New York, NY: Guilford Press; (2103) [Google Scholar]
- Hébert R, Bravo G, Préville M. Reliability, validity and reference values of the Zarit Burden Interview for assessing informal caregivers of community-dwelilng odler persons with dementia. Canadian Journal on Aging. 2000;19(4):494–507. [Google Scholar]
- Hedecker D, Gibbons RD. Longitudinal data analysis. Hoboken, NJ: John Wiley & Sons; 2006. [Google Scholar]
- Hepburn K, Lewis M, Narayan S, Center B, Tornatore J, Bremer KL, Kirk LN. Partners in caregiving: A psychoeducation program affecting dementia family caregivers’ distress and caregiver outlook. Clinical Gerontologist. 2005;29(1):53–69. doi: 10.1300/J018v29n01_05. [DOI] [Google Scholar]
- Hepburn K, Lewis M, Sherman CW, Tornatore J. The Savvy Caregiver Program: Developing and testing a transportable dementia family caregiver training program. The Gerontologist. 2003;43(6):908–915. doi: 10.1093/geront/43.6.908. [DOI] [PubMed] [Google Scholar]
- Hepburn K, Lewis M, Tornatore J, Sherman CW, Bremer KL. The Savvy Caregiver program: The demonstrated effectiveness of a transportable dementia caregiver psychoeducation program. Journal of Gerontological Nursing. 2007;33(3):30–36. doi: 10.1093/geront/43.6.908. [DOI] [PubMed] [Google Scholar]
- Ho DWH, Mak V, Kwok TCY, Au A, Ho FKY. Development of a web-based training programs for dementia caregivers in Hong Kong. Clinical Gerontologist. 2015;38(3):211–233. doi: http://dx.doi.org/10.1080/07317115.2015.1008115. [Google Scholar]
- Hughes S, Shuman SB, Wiener JM, Gould E. Research on supportive approaches for family and other caregivers. 2017 Retrieved from https://aspe.hhs.gov/pdf-report/research-supportive-approaches-family-and-other-caregivers.
- IBM Corp. IBM SPSS Statistics for Windows (Version 22.0) [Software] Armonk, NY: IBM Corp; 2016. [Google Scholar]
- Idler EL, Angel RJ. Self-rated health and mortality in the NHANES-I epidemiologic follow-up study. American Journal of Public Health. 1990;80:446–452. doi: 10.2105/ajph.80.4.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorm A. The Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE): A review. International Psychogeriatrics. 2004;16(3):275–293. doi: 10.1017/s1041610204000390. [DOI] [PubMed] [Google Scholar]
- Jorm AF, Jacomb PA. The Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE): Socio-demographic correlates, reliability, validity, and some norms. Psychological Medicine. 1989;19(4):1015–1022. doi: 10.1017/s0033291700005742. [DOI] [PubMed] [Google Scholar]
- Jorm AF, Scott R, Cullen JS, MacKinnon AJ. Performance of the Informant Questionnarie on Cognitive Decline in the Elderly (IQCODE) as a screening test for dementia. Psychological Medicine. 1991;21(3):785–790. doi: 10.1017/s0033291700022418. [DOI] [PubMed] [Google Scholar]
- Julian L. Measures of anxiety. State-Trait Anxiety Inventory (STAI), Beck Anxiety Inventory (BAI), and Hospital Anxiety and Depression Scale-Anxiety (HADS-A) Arthritis Care Research (Hoboken) 2011;63(Suppl 11):S467–72. doi: 10.1002/acr.20561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajiyama B, Thompson LW, Eto-Iwase T, Yamashita M, Di Mario J, Marian Tzuang Y, Gallagher-Thompson D. Exploring the effectiveness of an internet-based program for reducing caregiver distress using the iCare Stress Management e-Training Program. Aging & Mental Health. 2013;17(5):544–554. doi: 10.1080/13607863.2013.775641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kally Z, Cote SD, Gonzalez J, Villarruel M, Cherry DL, Howland S, … Hepburn K. The Savvy Caregiver Program: Impact of an evidence-based intervention on the well-being of ethnically diverse caregivers. Journal of Gerontological Social Work. 2014;57(6–7):681–693. doi: 10.1080/01634372.2013.850584. [DOI] [PubMed] [Google Scholar]
- Karon S, Gould E, Hughes S, Verrier Piersol C, Maier J, Leopold J, Wiener J. Training family caregivers on skills for behavioral symptoms. 2015 Retrieved from https://nadrc.acl.gov/sites/default/files/uploads/docs/Behavioral_Training_Paper_FINAL%20Oct%202015.pdf.
- Kaufer DL, Cummings JL, Ketchel P, Smith V, MacMillan A, Shelley T, … DeKosky T. Validation of the NPI-Q, a brief form of the Neuropsychiatric Inventory. Journal of Neuropsychiatry and Clinical Neuroscience. 2000;12(2):233–239. doi: 10.1176/jnp.12.2.233. [DOI] [PubMed] [Google Scholar]
- Kovach CR, Noonan PE, Schlidt AM, Wells T. A model of consequences of need-driven, dementia-compromised behavior. Journal of Nursing Scholarship. 2005;37(2):134–140. doi: 10.1111/j.1547-5069.2005.00025_1.x. discussion 140. [DOI] [PubMed] [Google Scholar]
- Kovaleva M, Blevins L, Griffiths PC, Hepburn K. An online program for caregivers of persons living with dementia: Lessons learned. Journal of Applied Gerontology. 2017 doi: 10.1177/0733464817705958. Advance on-line publication. [DOI] [PubMed] [Google Scholar]
- Lawton MP, Brody EM. Assessment of older people: Self-maintaining and instrumental activities of daily living. The Gerontologist. 1969;9(3):179–186. [PubMed] [Google Scholar]
- Lewis ML, Hobday JV, Hepburn K. Internet-based program for dementia caregivers. American Journal of Alzheimer’s Disease and Other Dementias. 2010;25(8):674–679. doi: 10.1177/1533317510385812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Link G. The Administration for Community Living: Programs and initiatives providing family caregiver support. 2015–2016 Retrieved from http://fsrtc.ahslabs.uic.edu/wp-content/uploads/sites/9/2016/03/Generations-ACL-Caregiver-Programs.pdf.
- Lloyd J, Patterson T, Muers J. The positive aspects of caregiving in dementia: A critical review of the qualitative literature. Dementia (London) 2016;15(6):1534–1561. doi: 10.1177/1471301214564792. [DOI] [PubMed] [Google Scholar]
- Long E, Gould E, Hughes S, O’Keefe C, Wiener J. The Alzheimer’s disease supportive services program. Washington, DC: RTI International; 2014. [Google Scholar]
- Lorig K, Steward A, Ritter P, Gonzales V, Laurent D, Lynch J. Outcome measures for health education and other health care interventions. Thousand Oaks, CA: Sage; 1996. [Google Scholar]
- Losada A, Marquez-Gonzalez M, Romero-Moreno R, Mausbach BT, Lopez J, Fernandez-Fernandez V, Nogales-Gonzalez C. Cognitive-behavioral therapy (CBT) versus acceptance and commitment therapy (ACT) for dementia family caregivers with significant depressive symptoms: Results of a randomized clinical trial. Journal of Consulting & Clinical Psychology. 2015;83(4):760–772. doi: 10.1037/ccp0000028. [DOI] [PubMed] [Google Scholar]
- Malterud K. Qualitative research: Standards, challenges, and guidelines. Lancet. 2001;358(9280):483–488. doi: 10.1016/s0140-6736(01)05627-6. [DOI] [PubMed] [Google Scholar]
- McCabe M, You E, Tatangelo G. Hearing their voice: A systematic review of dementia family caregivers’ needs. The Gerontologist. 2016;55(5):e70–88. doi: 10.1093/geront/gnw078. [DOI] [PubMed] [Google Scholar]
- Merrell RC. Geriatric telemedicine: Background and evidence for telemedicine as a way to address the challenges of geriatrics. Healthcare Informatics Research. 2015;21(4):223–229. doi: 10.4258/hir.2015.21.4.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles MB, Huberman AM, Saldaña J. Qualitative data analysis: A methods sourcebook. Thousand Oaks, CA: SAGE; 2014. [Google Scholar]
- Miller WC, Anton HA, Townson AF. Measurement properties of the CESD scale among individuals with spinal cord injury. Spinal Cord. 2008;46(4):287–292. doi: 10.1038/sj.sc.3102127. [DOI] [PubMed] [Google Scholar]
- Miller EA, Schneider LS, Rosenheck RA. Predictors of nursing home admission among Alzheimer’s disease patients with psychosis and/or agitation. International Psychogeriatrics. 2011;23(1):44–53. doi: 10.1017/S1041610210000244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milne J, Oberle K. Enhancing rigor in qualitative description: A case study. Journal of Wound, Ostomy, and Continence Nursing. 2005;32(6):413–420. doi: 10.1097/00152192-200511000-00014. [DOI] [PubMed] [Google Scholar]
- Mittelman MS, Ferris SH, Shulman E, Steinberg G, Levin B. A family intervention to delay nursing home placement of patients with Alzheimer disease. A randomized controlled trial. JAMA. 1996;276(21):1725–1731. [PubMed] [Google Scholar]
- Mittelman MS, Roth DL, Coon DW, Haley WE. Sustained benefit of supportive intervention for depressive symptoms in caregivers of patients with Alzheimer’s disease. American Journal of Psychiatry. 2004;161(5):850–856. doi: 10.1176/appi.ajp.161.5.850. [DOI] [PubMed] [Google Scholar]
- Munro Cullum C, Hynan LS, Grosch M, Parikh M, Weiner MF. Teleneuropscychology: Evidence for video and teleconference-based neuropsychological assessment. Journal of the International Neuropsychological Society. 2014;20(10):1028–1033. doi: 10.1017/S1355617714000873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navaie M. Accessibilty of caregiver education and support prograqms: Reacing hard-to-reach caregivers. In: Toseland RW, Haigler DH, Monahan DJ, editors. Education and Support Programs for Caregivers. New York, NY: Springer; 2011. pp. 13–28. [Google Scholar]
- NCSS Statistical Software. PASS 14 Power Analysis and Sample Size Software. Kaysville, UT: NCSS, LCC; 2015. [Google Scholar]
- NVivo. NVivo qualitative data software (version 10) QSR International PTy Ltd; 2012. Available from http://www.qsrinternational.com/nvivo/nvivo-products. [Google Scholar]
- O’Connell ME, Crossley M, Cammer A, Morgan D, Allingham W, Cheavins B, … Morgan E. Development and evaluation of a telehealth videoconferenced support group for rural spouses of individuals diagnosed with atypical early-onset dementias. Dementia (London) 2014;13(3):382–395. doi: 10.1177/1471301212474143. [DOI] [PubMed] [Google Scholar]
- Ostwald SK, Hepburn KW, Caron W, Burns T, Mantell R. Reducing caregiver burden: A randomized psychoeducational intervention for caregivers of persons with dementia. The Gerontologist. 1999;39(3):299–309. doi: 10.1093/geront/39.3.299. [DOI] [PubMed] [Google Scholar]
- Parra-Vidales E, Soto-Pérez F, Perea-Bartolomé MV, Franco-Martin MA, Muňoz-Sánchez JL. Online interventions for caregivers of people with dementia: A systematic review. Actas Espaniolas de Psiquiatria. 2017;45(3):116–126. [PubMed] [Google Scholar]
- Pearlin LI, Mullan JT, Semple SJ, Skaff MM. Caregiving and the stress process: An overview of concepts and their measures. The Gerontologist. 1990;30(5):583–594. doi: 10.1093/geront/30.5.583. [DOI] [PubMed] [Google Scholar]
- Pew Research Center. Internet/broad band fact sheet. 2017 Retrieved from http://www.pewinternet.org/fact-sheet/internet-broadband/
- Pinquart M, Sörensen S. Correlates of physical health of informal caregivers: A meta-analysis. Journals of Gerontology, Series B. Psychological Sciences and Social Sciences. 2007;62(2):126–137. doi: 10.1093/geronb/62.2.p126. [DOI] [PubMed] [Google Scholar]
- Radloff LS. The CES-D scale: A self report depression scale for research in the general population. Applied Psychological Measurements. 1977;1:385–401. [Google Scholar]
- Roth DL, Fredman L, Haley WE. Informal caregiving and its impact on health: A reappraisal from population-based studies. The Gerontologist. 2015;55(2):309–319. doi: 10.1093/geront/gnu177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan GW, Bernard HR. Techniques to identify themes. Field Methods. 2003;15(1):85–109. doi: 10.1177/1525822x02239569. [DOI] [Google Scholar]
- Ryan P, Sawin KJ. The individual and family self-management theory: Background and perspectives on context, process, and outcomes. Nursing Outlook. 2009;57(4):217–225. e216. doi: 10.1016/j.outlook.2008.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandelowski M, Holditch Davis D, Harris BG. Artful design: Writing the proposal for research in the naturalist paradigm. Research in Nursing & Health. 1989;12:77–84. doi: 10.1002/nur.4770120204. [DOI] [PubMed] [Google Scholar]
- Sandelowski M. Whatever happened to qualitative description? Research in Nursing and Health. 2000;23(4):334–340. doi: 10.1002/1098-240X(200008)23:4<334::AID-NUR9>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Sandelowski M. What’s in a name? Qualitative description revisited. Research in Nursing & Health. 2009;33(1):77–84. doi: 10.1002/nur.20362. [DOI] [PubMed] [Google Scholar]
- Schoenfeld DE, Malmrose LC, Blazer DG, Gold DT, Seeman TE. Self-rated health and mortality in the high-functioning elderly -- a closer look at healthy individuals: McArhtur field study of successful aging. Journal of Gerontology. 1994;49(3):M109–115. doi: 10.1093/geronj/49.3.m109. [DOI] [PubMed] [Google Scholar]
- Schulz R, Martire LM. Family caregiving of persons with dementia: Prevalence, health effects, and support strategies. American Journal of Geriatric Psychiatry. 2004;12(3):240–249. doi: 10.1097/00019442-200405000-00002. [DOI] [PubMed] [Google Scholar]
- Sebern MD, Whitlatch CJ. Dyadic Relationship Scale: A measure of the impact of the provision and receipt of family care. The Gerontologist. 2007;47(6):741–751. doi: 10.1093/geront/47.6.741. [DOI] [PubMed] [Google Scholar]
- Sepe-Monti M, Vanacore N, Bartorelli L, Tognetti A, Giubilei F Caregiver Study Group S. The Savvy Caregiver Program: A probe multicenter randomized controlled pilot trial in caregivers of patients affected by Alzheimer’s disease. Journal of Alzheimer’s Disease. 2016;54(3):1235–1246. doi: 10.3233/JAD-160235. [DOI] [PubMed] [Google Scholar]
- Spielberger CD, Vagg PR. Psychometric properties of the STAI: A reply to Ramanaiah, Franzen, and Schill. Journal of Personality Assessment. 1984;48:95–97. doi: 10.1207/s15327752jpa4801_16. [DOI] [PubMed] [Google Scholar]
- Spillman B, Wolff J, Freedman VA, Kasper JD. Informal caregiving for older Americans: An analysis of the 2011 National Health and Aging Trends Study. 2011 Retrieved from https://aspe.hhs.gov/report/informal-caregiving-older-americans-analysis-2011-national-study-caregiving.
- Teri L, Logsdon RG, Uomoto J, McCurry SM. Behavioral treatment of depression in dementia patients: A controlled clinical trial. Journals of Gerontology, Series B. Psychological Sciences and Social Sciences. 1997;52(4):P159–166. doi: 10.1093/geronb/52b.4.p159. [DOI] [PubMed] [Google Scholar]
- Tremont G, Davis JD, Bishop DS. Unique contribution of family functioning in caregivers of patients with mild to moderate dementia. Dementia and Geriatric and Cognitive Disorders. 2006;21(3):170–174. doi: 10.1159/000090699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Berg N, Schumann M, Kraft K, Hoffman W. Telemedicine and telecare for older adults -- a systematic review. Maturitas. 2012;73(2):94–114. doi: 10.1016/j.maturitas.2012.06.010. [DOI] [PubMed] [Google Scholar]
- Vidyo. Vidyo. n.d Retrived from https://www.vidyo.com/
- Ware JE, Jr, Nelson EC, Sherbourne CD, Stewart AL. Preliminary tests of a 6-item general health survey: A patient application. In: Stewart AL, Ware JE Jr, editors. Measuring functioning and well-being: The medical outcomes study approach. Durham, NC: Duke University Press; 1992. [Google Scholar]
- Whitlatch CJ, Zarit SH, von Eye A. Efficacy of interventions with caregivers: A reanalysis. The Gerontologist. 1991;31(1):9–14. doi: 10.1093/geront/31.1.9. [DOI] [PubMed] [Google Scholar]
- Wisniewski SR, Belle SH, Coon DW, Marcus SM, Ory MG, Burgio LD … REACH Investigators. The Resources for Enhancing Alzheimer’s Caregiver Health (REACH): Project design and baseline characteristics. Psychology & Aging. 2003;18(3):375–384. doi: 10.1037/0882-7974.18.3.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolinsky FD, Johnson RJ. Perceived health status and mortality among older men and women. Journal of Gerontology. 1992;47(6):S304–S312. doi: 10.1093/geronj/47.6.s304. [DOI] [PubMed] [Google Scholar]
- Yamamoto-Mitani N, Sugishita C, Ishigaki K, Hasegawa K, Maekawa N, Kuniyoshi M, Hayashi K. Development of instruments to measure appraisal fo care among Japanese family caregivers of the elderly. Scholarly Inquiry in Nursing Practice. 2001;15(2):113–135. discussion 137–141. [PubMed] [Google Scholar]
- Zarit SH, Anthony CR, Boutselis M. Interventions with care givers of dementia patients: Comparison of two approaches. Psychology and Aging. 1987;2(3):225–232. doi: 10.1037//0882-7974.2.3.225. [DOI] [PubMed] [Google Scholar]
- Zarit SH, Reever KE, Bach-Peterson J. Relatives of the impaired elderly: Correlates of feelings of burden. The Gerontologist. 1980;20(6):649–655. doi: 10.1093/geront/20.6.649. [DOI] [PubMed] [Google Scholar]
- Zhu CW, Scarmeas N, Torgan R, Albert M, Brandt J, Blacker D, … Stern Y. Clinical characteristics and longitudinal changes of informal cost of Alzheimer’s disease in the community. Journal of the American Geriatrics Society. 2006;54(10):1596–1602. doi: 10.1111/j.1532-5415.2006.00871.x. [DOI] [PMC free article] [PubMed] [Google Scholar]