Summary
Asthma is associated with higher rates of acute chest syndrome (ACS) and vaso-occlusive pain episodes among children with sickle cell anemia (SCA). Aeroallergen sensitization is a risk factor for asthma. We hypothesized that aeroallergen sensitization is associated with an increased incidence of hospitalizations for ACS and pain. Participants in a multicenter, longitudinal cohort study, ages 4–18 years with SCA, underwent skin prick testing to ten aeroallergens. ACS and pain episodes were collected from birth through the end of the follow-up period. The number of positive skin tests were tested for associations with prospective rates of ACS and pain. Multivariable models demonstrated additive effects of having positive skin tests on future rates of ACS (incidence rate ratio (IRR) for each positive test 1.23, 95% CI 1.11–1.36, p<0.001). Aeroallergen sensitization was not associated with future pain (IRR 1.14, 95%CI 0.97–1.33, p=0.11). Our study demonstrated that children with SCA and aeroallergen sensitization are at increased risk for future ACS. Future research is needed to determine whether identification of specific sensitizations and allergen avoidance and treatment reduce the risk of ACS for children with SCA.
Keywords: Allergy, Atopy, Sickle cell anemia, Acute chest syndrome, Asthma
Introduction
Sickle cell anemia (SCA) is the most common inherited blood disease in the United States. The most common complications for patients with SCA include acute vaso-occlusive pain (herein referred to as “pain”) and acute chest syndrome (ACS) (Platt et al, 1994; Castro et al, 1994). Previous studies have demonstrated that children with SCA and asthma have increased rates of ACS and pain and increased risk for premature death compared to those without asthma (Boyd et al, 2007; Bernaudin et al, 2008). Atopy is the most significant risk factor for the development of childhood asthma in the general population, with an increased risk of 10–20 fold (Burrows et al, 1989). Previous studies in children with SCA have demonstrated associations between markers of atopy (positive allergy skin tests (Knight-Madden, 2005; Strunk et al, 2014) and elevated total IgE (An et al, 2011)) and a history of prior ACS.
Given previous evidence suggesting that indicators of atopy may be associated with greater morbidity among children with SCA, we sought to further characterize this relationship. We hypothesize that aeroallergen sensitization is associated with an increased incidence of ACS episodes in children with SCA. The objectives of the current study were: 1) to determine the prevalence of aeroallergen sensitization among an unselected cohort of children with SCA using skin prick testing; 2) to examine associations between aeroallergen sensitization and prospective rates of pain and ACS; and 3) to explore whether scores on a composite index of atopy features (herein termed the “Atopy Index”) would be associated with prospective morbidity in children with SCA.
Methods
Study Design
Data were collected as part of the prospective, observational Sleep and Asthma Cohort (SAC) study. From 2005–2011, children ages 4 to 18 years with SCA (hemoglobin SS or Sβ0) were enrolled at three clinical centers (in St. Louis, MO, Cleveland, OH and London, UK) without regard to previous morbidity or diagnosis of asthma. Serum IgE and complete blood count were obtained at study entry. Percentage of blood eosinophils was determined using standard techniques at each clinical center. Medical records of participants were reviewed to obtain the number of hospitalizations for pain and ACS episodes both retrospectively (from birth whenever possible) and prospectively until the end of the study period in 2013 or until participants were lost to follow-up. Medical records of participants were also reviewed in order to determine asthma history. Asthma was defined in our cohort as a physician diagnosis of asthma in the medical record plus a prescription for an asthma medication such as short-acting beta agonist (SABA) and/or inhaled corticosteroid (ICS)(Strunk et al, 2014). Children were ineligible for the study if they were receiving chronic transfusion therapy or participating in a clinical trial evaluating hydroxyurea therapy at the time of recruitment. However, if participants were subsequently prescribed chronic transfusions or hydroxyurea therapy during the follow-up period, they were able to continue their participation. Participating sites had institutional approval. Written informed consent was obtained from parents and assent was obtained from children at enrollment according to institutional policies.
Allergy Skin Testing
Participants underwent allergy skin prick testing at study entry performed by SAC-certified technicians using the Multi-test II (Lincoln Diagnostics, Decatur, IL). Ten aeroallergens (Greer Laboratories, Lenoir, NC) were used for skin testing including: Aspergillus fumigatus, house dust mite (Dermatophagoides pteronyssinus and Dermatophagoides farinae), cockroach (American and German), cat (standardized), dog (mixed breeds), mouse, Alternaria alternata, grass (standardized southern mix), tree (eastern 8 tree mix), and weed (national mix). Skin tests were administered with histamine (positive) and saline (negative) controls. Tests were considered positive when the mean diameter of the wheal was ≥ 3 mm. Wheal reactions were traced onto translucent acetate paper and measured centrally by a single investigator (R.C.S.) for quality control of the results.
Definition of vaso-occlusive pain episode and acute chest syndrome
ACS was defined as a new radiographic pulmonary infiltrate in the context of an acute respiratory illness with symptoms such as cough, wheezing, rales, chest pain, decreased oxygen saturation >2% from baseline, use of accessory muscles of respiration, or increased respiratory rate, with or without fever. Pneumonia was considered an ACS episode. A pain episode was defined as a hospitalization for SCA-associated pain, excluding headaches, and requiring opioid treatment. Headaches were excluded from this definition because of the presumed difference in pathophysiology (Dowling et al, 2014). All ACS and pain episodes were reviewed by a single investigator at each participating site with over-reading by the principal investigator (M.R.D), to ensure uniform definitions of ACS and pain episodes in this multi-center study.
Atopy Index
The Atopy Index is a novel evaluation tool created for the purpose of this investigation and was based on 4 factors and their known associations with atopy in children as well as a potential association with SCA related morbidity and included: aeroallergen sensitization (Lipozenčić & Wolf, 2010; Strunk et al, 2014), serum IgE level (An et al, 2011), peripheral blood eosinophils (Canalli et al, 2004), and a history of asthma in either parent (Strunk et al, 2014). Z-scores for IgE levels were calculated for our cohort using normative data from race-, age-, and gender-matched controls from National Health and Nutrition Examination Survey (NHANES) III 2005–2006 participants (Hyattsville, 2005; Centers for Disease Control and Prevention (CDC), 2008). The Atopy Index score for each SAC participant ranged from 0–4: at least one positive aeroallergen skin test (+1), IgE Z score in the upper quartile (Z>0.67) (+1), peripheral blood eosinophils >4% (+ 1), and a history of asthma in either parent (+1).
Statistical Analysis
The number of positive skin tests (out of a possible 10) was used as a continuous variable; however, because of low numbers, individuals with 6 or more positive tests were grouped together. Associations between the number of positive skin tests and prospective rates of ACS and pain after the date of skin testing were tested with negative binomial regression models. Analyses were limited to participants with ACS and pain data from birth and a minimum of 12 months of prospective follow-up after skin testing. Multivariable models were built in two steps. Potential covariates were included in a screening model. In addition to the number of positive skin tests, covariates we considered to be potentially associated with prospective rates of ACS included sex, ACS prior to 4 years of age (DeBaun et al, 2014), white blood cell count, IgE level (An et al, 2011), fractional exhaled nitric oxide (FeNO), asthma diagnosis (Boyd et al, 2006), parental asthma (Strunk et al, 2014), and history of wheezing leading to shortness of breath (DeBaun et al, 2014). Covariates considered for inclusion in the model for prospective rates of pain were gender, age, white blood cell count, history of wheeze leading to shortness of breath (Glassberg et al, 2006), and hemoglobin. Following analyses with the number of positive skin tests, we constructed additional models to test associations between the score on the Atopy Index and prospective rates of ACS and pain. All covariates with p<0.20 in screening models were included in the final ACS and pain models. Models for the Atopy Index were compared to models for positive skin tests alone using the Likelihood test. Analyses were conducted using Stata statistical software (Version 12, College Station, TX: StataCorp LP) and IBM SPSS Statistics (Version 22, Chicago, IL, IBM).
Results
Recruitment and Demographics
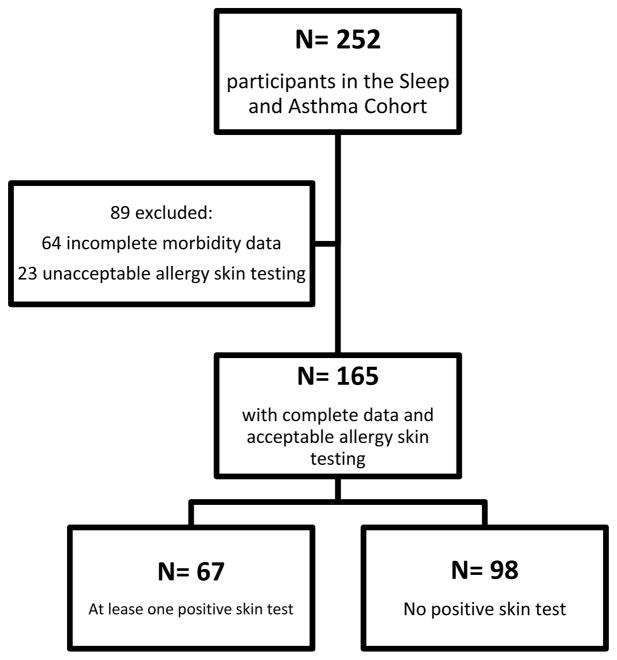
Of the 252 SAC participants, 188 had morbidity data available from birth and 165 had acceptable allergy skin test data, with a mean of 5.0 years (SD 1.2 years) of prospective follow-up after skin testing. Ninety eight participants (59%) did not have aeroallergen sensitization, while 67 participants (41%) had at least one positive skin test (Figure I). Clinical characteristics of the cohort are summarized in Table I. Children with at least one positive skin test had higher IgE levels than those who did not (p=0.01). Individuals were also more likely to have a positive skin test if they had a parent with asthma (p=0.01). The participants excluded from the analysis due to missing or invalid allergy skin test data were older (mean age 10.0 years compared to 13.8 years, p<0.01) and had a slightly higher IgE level (median 51.5 IU/ml compared to 27.0 IU/ml, p=0.05) compared to those who were included. These comparisons are summarized in Supplementary Table I. A total of 163 participants had complete data on all covariates used in our models and were therefore included in the analysis.
Figure I.
Selection of participants for evaluation and allergy skin testing
Table I.
Characteristics of the study population (N=165)*
| All patients N=165 | No positive skin tests N= 98 (59%) |
At least one positive skin test N= 67 (41%) |
P Value‡ | |
|---|---|---|---|---|
| Characteristic | ||||
| Age, years (mean, SD) | 10.0 (4.0) | 9.6 (4.2) | 10.7 (3.8) | 0.08 |
| Male (%) | 54.5 | 57.1 | 50.7 | 0.42 |
| Total follow up time from birth, years (mean, SD) | 14.9 (4.4) | 14.4 (4.4) | 15.7 (4.2) | 0.06 |
| Total follow-up time after study entry, years (mean, SD) | 4.9 (1.3) | 4.8 (1.2) | 5.0 (1.4) | 0.32 |
| Hemoglobin, g/l (mean, SD) | 83 (12) | 82 (11) | 84 (13) | 0.34 |
| White blood cell count, 109/L (mean, SD) | 12.1 (3.9) | 12.3 (3.8) | 11.9 (3.9) | 0.55 |
| Wheezing caused shortness of breath (%) | 24.2 | 22.4 | 26.9 | 0.52 |
| Eosinophils, × 109/l (median, IQR) (n=163) | 0.3 (0.4) | 0.3 (0.4) | 0.3 (0.4) | 0.75# |
| IgE, g/l (median, IQR) (n=156) | 51500 (13550) | 33000 (12590) | 91600 (13690) | 0.01# |
| Bronchodilator response ≥ 12% (%) (n=150) | 20.7 | 22.1 | 18.8 | 0.62 |
| Asthma diagnosis (%) (n=163) | 32.5 | 27.6 | 40.0 | 0.10 |
| Any parent has asthma (%) (n=140) | 24.3 | 17.2 | 35.8 | 0.01 |
| Retrospective rate of pain episodes per year (median, IQR) | 0.27 (0.70) | 0.26 (0.55) | 0.27 (0.99) | 0.30# |
| Retrospective rate of ACS episodes per year (median, IQR) | 0.13 (0.42) | 0.07 (0.39) | 0.24 (0.49) | 0.01# |
| ACS before age 4 (%) | 40.5 | 35.7 | 47.7 | 0.13 |
| Prospective rate of pain episodes per year (median, IQR) | 0.34 (1.13) | 0.26 (0.81) | 0.68 (1.15) | <0.01# |
| Prospective rate of ACS episodes per year (median, IQR) | 0.16 (0.35) | 0.0 (0.24) | 0.27 (0.61) | <0.01# |
Abbreviations: SD=standard deviation; IQR=interquartile range; ACS=acute chest syndrome
Means and SD are presented for normally distributed variables, Medians and IQR’s are presented for non-normally distributed variables
Mann-Whitney U test
T-test for means and chi-square test for percentages unless otherwise noted
Positive skin test is predictive of future ACS rate but not future pain
Multivariable models demonstrated additive significant effects of having positive skin tests on future rates of ACS such that each increase of 1 positive test was associated with a 23% increased rate of future ACS episodes (95% CI 11–36%, p<0.001) (Table II). In a multivariable model, aeroallergen sensitization was not a significant predictor of pain incidence rates (IRR 1.14, 95%CI 0.97–1.33, p=0.11).
Table II.
Final multivariable model of prospective rate of ACS in Children with SCAa (N= 163)
| Covariate | IRR | 95% CI | P value |
|---|---|---|---|
| Male gender | 0.70 | 0.46–1.05 | 0.080 |
| History of wheezing leading to shortness of breath | 1.95 | 1.22–3.13 | 0.006 |
| History of an ACS episode prior to 4 year of age | 2.41 | 1.62–3.59 | <0.001 |
| Number of positive aeroallergen skin tests | 1.23 | 1.11–1.36 | <0.001 |
Abbreviations: ACS=acute chest syndrome; SCA=sickle cell anemia; IRR=incidence rate ratio; CI=confidence interval
Negative binomial regression models with adjustment for over-dispersion, using robust standard errors. Two-tailed significance values.
Allergy skin testing provides additional information beyond asthma diagnosis
Aeroallergen sensitization does not appear to be simply a proxy for asthma status in our cohort. Although more children with asthma had at least one positive skin test compared to children without asthma, (50% vs. 36%), this difference was not statistically significant (p=0.09). Additionally, in analyses stratified by asthma status, among children without asthma the number of positive skin tests was significantly associated with increased rates of ACS, but not among children with a diagnosis of asthma (Table III).
Table III.
Final multivariable modela of prospective rate of ACS in Children with SCA, Stratified by Asthma Status
| No Asthma (N=110) | Asthma (N=53) | |||||
|---|---|---|---|---|---|---|
| Covariate | IRR | 95% CI | P value | IRR | 95% CI | P value |
| Male gender | 0.79 | 0.46–1.36 | 0.393 | 0.56 | 0.31–0.99 | 0.047 |
| Shortness of breath | 1.99 | 0.91–4.36 | 0.087 | 1.78 | 1.04–3.06 | 0.037 |
| History of an ACS episode prior to 4 year of age | 1.79 | 1.05–3.05 | 0.031 | 3.60 | 2.00–6.52 | <0.001 |
| Number of positive aeroallergen skin tests | 1.41 | 1.19–1.68 | <0.001 | 1.06 | 0.93–1.21 | 0.362 |
Negative binomial regression models with adjustment for over-dispersion, using robust standard errors. Two-tailed significance values
Atopy index is not more predictive of future ACS than allergy skin testing alone
Within our cohort, 132 participants had complete data for all four variables included in the Atopy Index. The Atopy Index was a significant predictor of future rates of ACS (IRR 1.44, 95% CI 1.17–1.76, p<0.001) (Table IV), but was not associated with future rates of pain (IRR 1.14, 95% CI 0.82–1.58, p=0.43). Using the likelihood test to compare models, the Atopy Index did not perform better than having at least one positive skin test in predicting future episodes of ACS (likelihood test p value=0.22), demonstrating that having a positive skin test was the critical covariate in the index.
Table IV.
Final model for ACS in Children with SCA with atopic index (N=163)
| Covariate | IRR | 95% CI | P value |
|---|---|---|---|
| Male gender | 0.78 | 0.49–1.23 | 0.286 |
| History of wheezing leading to shortness of breath | 1.81 | 1.11–2.93 | 0.017 |
| History of an ACS episode prior to 4 year of age | 2.53 | 1.64–3.90 | <0.001 |
| Atopic Index score | 1.44 | 1.17–1.76 | <0.001 |
Abbreviations: ACS=acute chest syndrome; SCA=sickle cell anemia; IRR=incidence rate ratio; CI=confidence interval
Negative binomial regression models with adjustment for over-dispersion, using robust standard errors. Two-tailed significance values.
Discussion
Asthma in SCA is a known risk factor for increased acute vaso-occlusive pain, ACS, and earlier death. However, the diagnosis of asthma is difficult in children with SCA, particularly because many features of asthma occur in individuals with SCA that do not have asthma, such as cough, wheezing, and airway hyperreactivity. Our study demonstrates for the first time that even a single positive skin test confers a significantly increased risk for future ACS, and more importantly, that increasing numbers of positive skin tests are associated with increased rates of prospective ACS. Furthermore, aeroallergen sensitization also seems to be the most significant indicator of atopy associated with SCA-related morbidity. The addition of other manifestations of atopy, including elevated IgE levels, eosinophilia, and parental asthma, to having a positive skin test did not improve our ability to predict ACS.
While there is strong evidence that a physician diagnosis of asthma is a risk factor for ACS (Boyd et al, 2007; Cohen et al, 2011; Knight-Madden et al, 2005; Sylvester et al, 2007), aeroallergen sensitization does not seem to be merely a marker of asthma status among children with SCA. In our cohort, we demonstrated a significant impact of aeroallergen sensitization on future ACS episodes in children without a doctor diagnosis of asthma. One potential explanation for this finding is that for children with SCD and asthma, the airways are already primed for exaggerated inflammatory response to a multitude of triggers – allergic, infectious, or other. In those children, a history of early life ACS is the best predictor of future ACS. In contrast, for non-asthmatic individuals whose airways aren’t as hyperresponsive to other triggers, exposure to an allergic trigger in the setting of aeroallergen sensitization is a significant factor associated with acute respiratory episodes including ACS(DeBaun & Strunk, 2016). Furthermore, our data demonstrates a nearly 2-fold increase in the risk of future ACS episodes with a history of ACS prior to age 4 years in those participants with asthma compared to those without an asthma diagnosis (Table III). In children with SCA without asthma, there may be more unexplained variation that allows detection by other covariates, such as the number of positive aeroallergen skin tests. Our results differ from a prior study of 340 children with SCA in the Silent Cerebral Infarct Transfusion (SIT) trial which found that having a positive serum allergen-specific IgE test to Alternaria, cockroach (B. germanica) or dust mite (D. pteronyssinus) was not associated with increased risk of ACS or pain episodes (An et al, 2011). However, the SIT Trial only included a limited panel of antibody testing to 3 aeroallergens. Serum aeroallergen-specific IgE testing to expanded panels of indoor (i.e., cat, dog, mouse, dust mite, cockroach, indoor molds) and outdoor (i.e., tree and grass pollens, ragweeds, outdoor molds) allergens are widely available, results are not invalidated when a patient is taking antihistamines, and only require a simple blood draw. Further research is needed to determine whether an expanded panel of aeroallergen-specific IgE testing, which is likely more feasible for providers caring for children with SCA, represents a satisfactory alternative to skin testing in this population.
Studies from transgenic sickle cell disease (SCD) mouse models offer a proposed mechanism for the connection between atopy and increased incidence of ACS episodes. Ovalbumin sensitized sickle mice have increased IL-5 levels and increased airway resistance compared to HbA mice (Pritchard et al, 2012), and when exposed to additional aerosolized ovalbumin, sickle mice have increased histologic evidence of airway and vascular inflammation, and increased risk of death compared to control mice (Nandedkar et al, 2008). Andemariam et al. has also demonstrated increased ovalbumin-induced allergic airway inflammation in sickle mice compared to wild type mice including increased BAL leukocytosis and eosinophilia, increased ovalbumin-specific serum IgE levels, and altered inflammatory cytokine profiles in SCD mice compared to control mice (Andemariam et al, 2015). These findings suggest that the pro-inflammatory state of SCD may prime the immune system to be hyper-responsive to inhaled allergens, and thus may help explain the high morbidity seen in patients with asthma and SCD. Mpollo et al. examined whether placental growth factor (PGF), a member of the vascular endothelial growth factor family known to be increased among patients with SCD, may be a potential mediator on the pathway between allergic sensitization and SCD morbidity (Eiymo Mwa Mpollo et al, 2016). In a series of experiments, they demonstrated that PGF knockout (Pgf−) mice have significantly less airway resistance, airway hyperresponsiveness, circulating IgE, airway eosinophilia, and goblet cell hyperplasia compared to wild type Pgf + mice. Furthermore, these investigators found that intratracheal exposure to house dust mite allergen extract was associated with upgregulation of plasma PGF levels in wild type mice. When the investigators then examined sickle mice, they noted exaggerated responses to house dust mite airway exposure in terms of airway hyperresponsiveness, and this finding was mediated by PGF through a leukotriene-dependent pathway. When sickle mice were treated with PGF antibodies and with zileuton (a leukotriene synthesis inhibitor) house dust mite-exposure-induced airway reactivity was significantly decreased (Eiymo Mwa Mpollo et al, 2016). Taken together, these data strongly suggest that individuals with SCD and asthma have airways that may be primed for an exaggerated response to a trigger, such as a viral respiratory infection in young children. This offers a mechanism for the high predictability of an ACS episode prior to age 4 years predicting future episodes of ACS. Additionally, the airways of children with SCD may have a predisposition to airway inflammation that is primed for aeroallergen sensitization leading to future ACS events. Furthermore, a single center study by Glassberg et al. demonstrated a benefit of daily inhaled corticosteroids in non-asthmatic adult patients with SCD (Glassberg et al, 2017).
Strengths of the current study include the rigorous data collection methods. All SCA-related morbidity data and allergy skin tests were reviewed by a study principal investigator to ensure a uniform definition across multiple sites. In contrast to the prior study about IgE levels in children with SCA in which IgE values from the SIT trial participants were stratified into elevated and non-elevated using comparison data from predominantly Caucasian cohorts (An et al, 2011), we standardized IgE levels into Z-scores using population-based data matched for age, gender, and race prior to incorporation into the Atopy Index. Limitations of the study are inclusion of only children with SCA; therefore our results cannot be generalized to children with other SCD genotypes (i.e. hemoglobin SC, Sβ+ thalassemia). The number of children with SCA and a positive allergy skin testing in our cohort is comparable to what has been demonstrated in other studies. NHANES III data from 5,640 participants show that 52.7% age 6–19 years had at least one positive skin test. The prevalence of having at least one positive skin test among children 6–19 years of age with asthma was 78% (Arbes et al, 2007). Furthermore, participants were from three distinct regions: St. Louis, Missouri; Cleveland, Ohio; and London, United Kingdom. Although this increased generalizability, regional differences and genetic admixture may have contributed to heterogeneity in our results. Our definition of asthma may have underestimated the number of children who actually had asthma because our definition required a physician of diagnosis of asthma with a prescription of asthma medication. A more liberal diagnosis of asthma commonly used in large epidemiologic studies is a physician only diagnosis of asthma (von Mutius et al, 2001; Subramanian & Kennedy, 2009; DeBaun & Strunk, 2016).
The results of the current study provide additional evidence that determination of aeroallergen sensitization, as detected by skin testing, is an objective strategy for identifying a subgroup of children at increased risk of developing ACS episodes. These results, coupled with other studies from the same cohort, demonstrate that aeroallergen sensitization, wheezing causing shortness of breath, and an ACS event prior to age 4 years, are easily identifiable risk factors for future ACS events in children with SCA (DeBaun et al, 2014). Additional studies are needed to determine if identifying children with SCA and allergic sensitization, along with strategies aimed at minimizing exposures to and maximizing treatment of the manifestations of aeroallergen sensitization, can decrease rates of future ACS episodes.
Supplementary Material
Acknowledgments
Supported in part by the National Heart, Lung, and Blood Institute: NIH 1R01HL079937 (DeBaun), UL1 RR024989 (CWRU CRU) and by Research and Development in the National Health Service (UK)
Sleep Asthma Cohort (SAC) investigative team Washington University, St. Louis, MO: Michael DeBaun, MD, MPH (Principal Investigator), Robert Strunk, MD (Co-investigator), Joshua Field, MD, Mario Castro, MD, MPH, Ping An, MD, Mark Johnson, MD, Michael Province, PhD, Lisa Garrett, RN, CCRP, Pamela Bates, CRT, RPFT, PRSGT, Rick Talbert, RPSGT, Sabrina Lockett, RPSGT, Valerie Morgan, RRT, Yan Yan, MD, PhD, Avril Adelman, PhD, Phillip Blanks, Tinishia Greene Case Western Reserve University, Cleveland, OH: Carol Rosen, MD (Principal Investigator), Susan Redline, MD, MPH, Heather Rogers, RPSGT, Susan Surovec, BA, Dan Craven, MD, Nancy Scott, BS, REEG/EPT, RPSGT, REDT, CNIM, Sinziana Seicean, MD, MPH, Mary DeBarr, RN, BSN, Brad Casucci, MA UCL Institute of Child Health and Great Ormond Street Hospital, London, UK: Fenella Kirkham, MD, FRCPCH (Principal Investigator), Janet Stocks, PhD, Jane Kirkby, PhD, Satwinder Sahota, BSc, Liam Welsh, PhD, Ursula Johnson, RN, Aidan Laverty, MSc, MBCS, Johanna Gavlak, BSc,, Anne Yardumian, MD, FRCP, Olu Wilkey, FRCPCH, Marilyn Roberts-Harewood, MRCPCH, Anne O’Reilly Imperial College, London, UK: Irene Roberts, MD, FRCPCH, John Warner, MD, FRCPCH Hull York Medical School, UK: Avijit Kumar Datta, MD, MRCP Medical College of Wisconsin, Milwaukee, WI: Kirk Pritchard, PhD (Principal Investigator), Thom Feroah, PhD, Cheryl Hillery, MD, Keith Oldham, MD Johns Hopkins University, Baltimore, MD: James Casella, MD (Principal Investigator)
Abbreviations
- SCA
sickle cell anemia
- SCD
sickle cell disease
- ACS
acute chest syndrome
- SAC
Sleep and Asthma Cohort
- SIT
Silent Cerebral Infarct Transfusion Trial
- HU
Hydroxyurea
Footnotes
Contributions: Dr. Willen and Dr. Cohen had full access to all of the data in the study and take responsibility for the integrity of the data, the accuracy of the data analysis, and the work as a whole. Dr. Willen (Clinical Fellow, Department of Pediatrics, Division of Hematology/Oncology, Vanderbilt University School of Medicine, Nashville, TN) interpreted the data, created the initial draft, and finalized the manuscript for submission; Dr. Rodeghier (Independent statistician, Chicago, Illinois) developed the statistical approach, performed those analyses, reviewed the integrity of those analyses, reviewed and revised the manuscript; Dr. Strunk (Division of Allergy, Immunology, and Pulmonary Medicine, Department of Pediatrics, Washington University School of Medicine, St Louis, MO) was the co-principal investigator for the SAC study, contributed to the development of the SAC project, study concepts, and procedures, and conceptualized the manuscript; Dr. Bacharier (Division of Allergy, Immunology, and Pulmonary Medicine, Department of Pediatrics, Washington University School of Medicine, St Louis, MO) interpreted the results, reviewed and revised the manuscript; Dr. Rosen (Department of Pediatrics, Case Western Reserve University School of Medicine, Rainbow Babies and Children’s Hospitals, University Hospitals Cleveland Medical Center, Cleveland, OH) contributed to the development of the SAC project, study concepts and procedures, and reviewed and helped revise the manuscript; Dr. Kirkham (UCL Great Ormond Street, London Institute of Child Health) was the site investigator for one site, helped develop the concepts for the SAC project and reviewed and revised the manuscript; Dr. DeBaun (Department of Pediatrics, Division of Hematology/Oncology, Vanderbilt and Meharry Center for Excellence in Sickle Cell Disease, Vanderbilt University Medical Center, Nashville, TN) was the principal investigator for the SAC project, helped design the concepts for SAC and this manuscript, interpreted the results, and reviewed and revised the manuscript; Dr. Cohen (Department of Pediatrics, Boston University School of Medicine, Boston, MA) interpreted the data, developed the initial draft, and reviewed and revised the manuscript; and all authors approved the final manuscript as submitted.
References
- An P, Barron-Casella EA, Strunk RC, Hamilton RG, Casella JF, DeBaun MR. Elevation of IgE in children with sickle cell disease is associated with doctor diagnosis of asthma and increased morbidity. Journal of Allergy and Clinical Immunology. 2011;127:1440–1446. doi: 10.1016/j.jaci.2010.12.1114. Available at: http://dx.doi.org/10.1016/j.jaci.2010.12.1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andemariam B, Adami AJ, Singh A, McNamara JT, Secor ER, Guernsey LA, Thrall RS. The sickle cell mouse lung: Proinflammatory and primed for allergic inflammation. Translational Research. 2015;166:254–268. doi: 10.1016/j.trsl.2015.03.001. Available at: http://dx.doi.org/10.1016/j.trsl.2015.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arbes SJ, Gergen PJ, Vaughn B, Zeldin DC. Asthma cases attributable to atopy: Results from the Third National Health and Nutrition Examination Survey. [Accessed October 14, 2017];Journal of Allergy and Clinical Immunology. 2007 120:1139–1145. doi: 10.1016/j.jaci.2007.07.056. Available at: http://www.ncbi.nlm.nih.gov/pubmed/17889931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernaudin F, Strunk RC, Kamdem A, Arnaud C, An P, Torres M, Delacourt C, DeBaun MR. Asthma is associated with acute chest syndrome, but not with an increased rate of hospitalization for pain among children in France with sickle cell anemia: A retrospective cohort study. [Accessed March 15, 2016];Haematologica. 2008 93:1917–1918. doi: 10.3324/haematol.13090. Available at: http://www.haematologica.org/cgi/doi/10.3324/haematol.13090. [DOI] [PubMed] [Google Scholar]
- Boyd JH, Macklin EA, Strunk RC, DeBaun MR. Asthma is associated with acute chest syndrome and pain in children with sickle cell anemia. [Accessed June 26, 2016];Blood. 2006 108:2923–7. doi: 10.1182/blood-2006-01-011072. Available at: http://www.bloodjournal.org/content/108/9/2923.abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd JH, Macklin EA, Strunk RC, DeBaun MR. Asthma is associated with increased mortality in individuals with sickle cell anemia. [Accessed March 15, 2016];Haematologica. 2007 92:1115–1118. doi: 10.3324/haematol.11213. Available at: http://www.haematologica.org/cgi/doi/10.3324/haematol.11213. [DOI] [PubMed] [Google Scholar]
- Burrows B, Martinez FD, Halonen M, Barbee RA, Cline MG. Association of asthma with serum IgE levels and skin-test reactivity to allergens. [Accessed April 6, 2016];The New England journal of medicine. 1989 320:271–7. doi: 10.1056/NEJM198902023200502. Available at: http://www.nejm.org/doi/full/10.1056/NEJM198902023200502. [DOI] [PubMed] [Google Scholar]
- Canalli AA, Conran N, Fattori A, Saad STO, Costa FF. Increased adhesive properties of eosinophils in sickle cell disease. Experimental Hematology. 2004;32:728–734. doi: 10.1016/j.exphem.2004.04.010. [DOI] [PubMed] [Google Scholar]
- Castro O, Brambilla DJ, Thorington B, Reindorf CA, Scott RB, Gillette P, Vera JC, Levy PS. The acute chest syndrome in sickle cell disease: incidence and risk factors. The Cooperative Study of Sickle Cell Disease. [Accessed May 9, 2016];Blood. 1994 84:643–9. Available at: http://www.bloodjournal.org/content/84/2/643.abstract. [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (CDC) Laboratory Procedure Manual for NHANES 2005–2006 data- Specific IgE/Total IgE Allergens in Serum. [Accessed January 23, 2017];National Center for Health Statistics. 2008 Available at: https://www.cdc.gov/nchs/data/nhanes/nhanes_05_06/al_ige_d_met_specific_ige_total_ige.pdf.
- Cohen RT, Madadi A, Blinder MA, DeBaun MR, Strunk RC, Field JJ. Recurrent, severe wheezing is associated with morbidity and mortality in adults with sickle cell disease. American Journal of Hematology. 2011;44:756–761. doi: 10.1002/ajh.22098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeBaun MR, Rodeghier M, Cohen R, Kirkham FJ, Rosen CL, Roberts I, Cooper B, Stocks J, Wilkey O, Inusa B, Warner JO, Strunk RC. Factors predicting future ACS episodes in children with sickle cell anemia. [Accessed December 16, 2015];American journal of hematology. 2014 89:E212–7. doi: 10.1002/ajh.23819. Available at: http://www.ncbi.nlm.nih.gov/pubmed/25088663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeBaun MR, Strunk RC. The intersection between asthma and acute chest syndrome in children with sickle-cell anaemia. The Lancet. 2016;387:2545–2553. doi: 10.1016/S0140-6736(16)00145-8. Available at: http://dx.doi.org/10.1016/S0140-6736(16)00145-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowling MM, Noetzel MJ, Rodeghier MJ, Quinn CT, Hirtz DG, Ichord RN, Kwiatkowski JL, Roach ES, Kirkham FJ, Casella JF, DeBaun MR. Headache and Migraine in Children with Sickle Cell Disease Are Associated with Lower Hemoglobin and Higher Pain Event Rates But Not Silent Cerebral Infarction. [Accessed May 24, 2017];The Journal of Pediatrics. 2014 164:1175–1180.e1. doi: 10.1016/j.jpeds.2014.01.001. Available at: http://www.ncbi.nlm.nih.gov/pubmed/24529619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eiymo Mwa Mpollo MS, Brandt EB, Shanmukhappa SK, Arumugam PI, Tiwari S, Loberg A, Pillis D, Rizvi T, Lindsey M, Jonck B, Carmeliet P, Kalra VK, Le Cras TD, Ratner N, Wills-Karp M, Hershey GKK, Malik P. Placenta growth factor augments airway hyperresponsiveness via leukotrienes and IL-13. The Journal of clinical investigation. 2016;126:571–84. doi: 10.1172/JCI77250. Available at: http://www.ncbi.nlm.nih.gov/pubmed/26690703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glassberg J, Minnitti C, Cromwell C, Cytryn L, Kraus T, Skloot GS, Connor JT, Rahman AH, Meurer WJ. Inhaled Steroids Reduce Pain and sVCAM Levels in Individuals with Sickle Cell Disease: A Triple-Blind, Randomized Trial. [Accessed April 28, 2017];American Journal of Hematology. 2017 doi: 10.1002/ajh.24742. Available at: http://www.ncbi.nlm.nih.gov/pubmed/28370266. [DOI] [PMC free article] [PubMed]
- Glassberg J, Spivey JF, Strunk R, Boslaugh S, DeBaun MR. Painful Episodes in Children With Sickle Cell Disease and Asthma are Temporally Associated With Respiratory Symptoms. [Accessed March 15, 2016];Journal of Pediatric Hematology/Oncology. 2006 28:481–485. doi: 10.1097/01.mph.0000212968.98501.2b. Available at: http://content.wkhealth.com/linkback/openurl?sid=WKPTLP:landingpage&an=00043426-200608000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyattsville M. National Center for Health Statistics (NCHS). National Health and Nutrition Examination Survey Data. [Accessed January 7, 2017];US Department of Health and Human Services, Centers for Disease Control and Prevention. 2005 Public data general release file documentation Available at: https://www.cdc.gov/nchs/nhanes/nhanes3/data_files.htm.
- Knight-Madden JM. Asthma in children with sickle cell disease and its association with acute chest syndrome. [Accessed June 26, 2016];Thorax. 2005 60:206–210. doi: 10.1136/thx.2004.029165. Available at: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=1747351&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight-Madden JM, Forrester TS, Lewis Na, Greenough A. Asthma in children with sickle cell disease and its association with acute chest syndrome. [Accessed March 15, 2016];Thorax. 2005 60:206–210. doi: 10.1136/thx.2004.029165. Available at: http://thorax.bmj.com/cgi/doi/10.1136/thx.2004.029165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipozenčić J, Wolf R. The diagnostic value of atopy patch testing and prick testing in atopic dermatitis: facts and controversies. [Accessed December 21, 2016];Clinics in Dermatology. 2010 28:38–44. doi: 10.1016/j.clindermatol.2009.03.008. Available at: http://www.ncbi.nlm.nih.gov/pubmed/20082949. [DOI] [PubMed] [Google Scholar]
- von Mutius E, Schwartz J, Neas LM, Dockery D, Weiss ST. Relation of body mass index to asthma and atopy in children: the National Health and Nutrition Examination Study III. [Accessed October 14, 2017];Thorax. 2001 56:835–8. doi: 10.1136/thorax.56.11.835. Available at: http://www.ncbi.nlm.nih.gov/pubmed/11641506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nandedkar SD, Feroah TR, Hutchins W, Weihrauch D, Konduri KS, Wang J, Strunk RC, DeBaun MR, Hillery CA, Pritchard KA. Histopathology of experimentally induced asthma in a murine model of sickle cell disease. [Accessed June 26, 2016];Blood. 2008 112:2529–38. doi: 10.1182/blood-2008-01-132506. Available at: http://www.bloodjournal.org/content/112/6/2529.abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platt OS, Brambilla DJ, Rosse WF, Milner PF, Castro O, Steinberg MH, Klug PP. Mortality In Sickle Cell Disease -- Life Expectancy and Risk Factors for Early Death. [Accessed March 15, 2016];New England Journal of Medicine. 1994 330:1639–1644. doi: 10.1056/NEJM199406093302303. Available at: http://www.nejm.org/doi/abs/10.1056/NEJM199406093302303. [DOI] [PubMed] [Google Scholar]
- Pritchard KA, Feroah TR, Nandedkar SD, Holzhauer SL, Hutchins W, Schulte ML, Strunk RC, DeBaun MR, Hillery CA. Effects of Experimental Asthma on Inflammation and Lung Mechanics in Sickle Cell Mice. [Accessed March 15, 2016];American Journal of Respiratory Cell and Molecular Biology. 2012 46:389–396. doi: 10.1165/rcmb.2011-0097OC. Available at: http://www.atsjournals.org/doi/abs/10.1165/rcmb.2011-0097OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strunk RC, Cohen RT, Cooper BP, Rodeghier M, Kirkham FJ, Warner JO, Stocks J, Kirkby J, Roberts I, Rosen CL, Craven DI, DeBaun MR. Wheezing Symptoms and Parental Asthma Are Associated with a Physician Diagnosis of Asthma in Children with Sickle Cell Anemia. [Accessed March 17, 2016];The Journal of Pediatrics. 2014 164:821–826.e1. doi: 10.1016/j.jpeds.2013.11.034. Available at: http://linkinghub.elsevier.com/retrieve/pii/S0022347613014698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian SV, Kennedy MH. Perception of neighborhood safety and reported childhood lifetime asthma in the United States (U.S.): a study based on a national survey. [Accessed October 14, 2017];PloS one. 2009 4:e6091. doi: 10.1371/journal.pone.0006091. Available at: http://dx.plos.org/10.1371/journal.pone.0006091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sylvester KP, Patey RA, Broughton S, Rafferty GF, Rees D, Thein SL, Greenough A. Temporal relationship of asthma to acute chest syndrome in sickle cell disease. [Accessed March 15, 2016];Pediatric Pulmonology. 2007 42:103–106. doi: 10.1002/ppul.20430. Available at: http://doi.wiley.com/10.1002/ppul.20430. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.