Abstract
Background
Cardiovascular implantable electronic devices (CIEDs) are common in patients undergoing heart transplantation (HT), and complete removal is not always possible at the time of transplantation.
Methods
We retrospectively assessed the frequency of retained CIED leads and clinical consequences in consecutive HT patients from 2013 to 2016. Clinical outcomes included bacteremia, upper-extremity deep venous thrombosis (UEDVT), lead migration, and inability to perform magnetic resonance imaging (MRI).
Results
A total of 138 patients (55 ± 11 years of age, 76% male) were identified; 37 (27%) had retained lead fragments (RLFs) at discharge. Patients with RLFs were older, had longer lead implantation time before HT, and a higher prevalence of dual-coil CIED leads compared with those without RLFs (P < .05 for all). Lead implantation time was identified as an independent predictor for RLFs (P < .05). Patients with RLFs had a higher frequency of DVT compared with the non-RLF group during the 1-year study period (42% vs 21%; P < .04). There was no difference in bacteremia. Fourteen patients (38%) could not undergo clinically indicated MRI.
Conclusion
RLFs after HT occur commonly and are associated with a higher rate of UEDVT and limit the use of MRI. Although no significant difference was found in the rates of bacteremia between the groups, this finding might be explained by the overall low incidence. Patients with risk factors for RLFs should be identified before transplantation, and complete lead removal should be considered with a multidisciplinary approach.
Keywords: Heart transplantation, retained ICD leads, deep venous thrombosis (DVT), magnetic resonance imaging (MRI)
The rate of cardiovascular implantable electronic device (CIED) insertion has significantly increased over the past 2 decades. Implantable cardioverter-defibrillator (ICD) implantation as primary prevention is a class 1 indication for patients with a left ventricular ejection fraction (LVEF) <35% and New York Heart Association functional class II or III chronic systolic heart failure and is a class 2a indication for those awaiting heart transplantation (HT).1 Patients with heart failure and a wide QRS duration also are recommended to have implantation of cardiac resynchronization therapy (CRT).2 As a result, most patients undergoing HT have an implanted device, often with multiple leads in the vascular space. At the time of HT surgery, leads within the recipient’s heart are cut, usually at the level of the superior vena cava (SVC), and the generator is removed from the pectoral pocket. Lysis of adhesions within the vascular bed is performed, especially within the SVC, but excessive manual traction needs to be avoided to prevent vascular injury. Consequently, complete lead removal by means of manual extraction is not always possible, especially in the setting of heavy adhesion of leads to the vessel wall or heavy calcification. To date, there is no consensus on the management of retained lead fragments (RLFs) after HT.
We aimed to assess the frequency of RLFs and their impact on 3 clinical consequences: risk of upper-extremity deep vein thrombosis (UEDVT), rate of infection defined by bacteremia, and lead migration. Because the presence of RLFs is a contraindication for magnetic resonance imaging (MRI), the frequency that clinically indicated MRI could not be performed was also assessed.1
Methods
In this retrospective study, a total of 165 consecutive patients underwent HT at the University of Chicago from April 2011 to October 2016. Of these, 147 (90%) had CIEDs at the time of HT. We excluded 3 patients with incomplete documentation and 6 patients with survival <1 month after HT. A total of 138 patients composed the study cohort. Chest radiographs performed at time of discharge were reviewed to identify RLFs. The last available chest radiograph for each patient with RLFs was reviewed to assess for lead migration. RLFs were characterized by type (pacemaker or ICD), location (pocket, subclavian vein, innominate vein, SVC, or right atrium), and number (single or multiple). We evaluated the percentage of patients with RLFs at the time of hospital discharge after HT. Clinical data, including UEDVT (diagnosed by vascular ultrasound) and bacteremia, were collected from the electronic medical record. Furthermore, we evaluated instances when MRI was indicated but could not be performed during follow-up after HT. The University of Chicago Institutional Review Board approved this retrospective chart review.
Statistical Analysis
Continuous variables were expressed as mean ± SD and compared by means of unpaired t test or Mann-Whitney U test as appropriate. Categoric variables were compared by means of chi-square test or Fisher exact test as appropriate.
Logistic regression analysis was performed to identify risk factors for RLFs after HT among all baseline characteristics and device parameters. Variables with P < .05 in the univariate analyses were included in the multivariate analyses. Cutoff level of preoperative lead duration for the event of retained lead after HT was assessed by means of receiver operating characteristic analysis. Clinical events rates after HT, including DVT and bacteremia, stratified by the existence of RLFs were assessed with the use of Kaplan-Meier analyses and compared by means of log-rank tests. Cox proportional regression analysis was performed to assess significance of RLFs as a risk factor for the development of UEDVT. All statistical analyses were performed with the use of SPSS Statistics 22 (Chicago, Illinois).
Results
Patient Characteristics
A total of 138 consecutive patients composed the study cohort. The mean age was 55 ± 11 years old, and 105 (76%) were male. Patients with RLFs were significantly older than those without RLFs (57.8 ± 7.4 vs 53.5 ± 12.0 years; P = .012) and had a higher incidence of hypertension (65 vs 47%; P = .043). The remaining patient characteristics were similar and are summarized in Table 1.
Table 1.
Baseline Characteristics
| Characteristic | All Patients (n = 138) |
Retained ICD Lead (n = 37) |
No Retained ICD Lead (n = 101) |
P Value |
|---|---|---|---|---|
| Age (y) | 54.6 ± 11.1 | 57.8 ± 7.4 | 53.5 ± 12.0 | .012* |
| Male sex | 105 (76) | 26 (70) | 79 (78) | .226 |
| Ischemic cardiomyopathy | 44 (32) | 15 (41) | 29 (29) | .314 |
| DM | 48 (35) | 16 (43) | 32 (32) | .144 |
| HTN | 71 (51) | 24 (65) | 47 (47) | .043† |
| AFIB | 47 (34) | 15 (41) | 32 (32) | .22 |
| VT | 69 (50) | 23 (62) | 46 (46) | .062 |
| COPD | 6 (4) | 1 (3) | 5 (5) | .566 |
| BSA (m2) | 2.06 ± 0.26 | 2.09 ± 0.31 | 2.04 ± 0.24 | .310 |
| BMI (kg/m2) | 29.2 ± 5.3 | 30.1 ± 6.3 | 28.8 ± 4.9 | .227 |
| IABP | 73 (53) | 20 (54) | 53 (52) | .512 |
| VAD | 35 (25) | 6 (16) | 29 (29) | .099 |
Results are presented as mean ± SD or n (%). AFIB, atrial fibrillation; BMI, body mass index; BSA, body surface area; COPD, chronic obstructive pulmonary disease; DM, diabetes mellitus; HTN, hypertension; IABP, intra aortic balloon pump; ICD, implantable cardioverter-defibrillator; VAD, ventricular assist device; VT, ventricular tachycardia.
P < .05 by unpaired t test or Mann-Whitney U test as appropriate.
P < .05 by chi-square test or Fisher exact test as appropriate.
Frequency of RLFs
Removal of RLFs was successfully performed in 101 cases (72.3%) by cardiothoracic surgery before discharge from the index hospitalization. In the residual cases, excessive calcifications or adhesions to the vessel wall prevented complete removal with the use of manual traction at the time of HT. In 2 cases of failed surgical removal, RLF extraction was successfully performed via percutaneous approach (laser lead extraction) by means of electrophysiology before discharge. There were no adverse events associated with either surgical or percutaneous lead removal. A total of 37 patients (27%) had RLFs identified on chest x-ray at the time of discharge. In 3 patients, RLFs were removed via a percutaneous approach after discharge. For the purpose of the present analysis, these patients were included in the RLF group (Fig. 1).
Fig. 1.

Patient cohort algorithm: 165 patients underwent heart transplantation (HT) at the University of Chicago from April 2011 to October 2016. Of these, 147 (90%) had cardiovascular implantable electronic devices at the time of HT. Of these, 138 patients met the inclusion criteria and were included in the study. Complete lead removal was performed in 101 patients (72.3%) before discharge from the index hospitalization, including 2 cases in whom retained lead fragment (RLF) extraction was successfully performed via percutaneous approach (laser lead extraction) before discharge after surgical removal was unsuccessful. At the time of discharge, a total of 37 patients (27%) had RLFs. In 3 cases, RLFs were removed via percutaneous approach after discharge.
Device Characteristics and Risk Factors for RLFs
Patients in the RLF group were more likely to have a dual-coil lead (89% vs 67%; P = .004) as well as a greater number of overall leads (2.8 ± 1.0 vs 2.4 ± 1.0; P = .028). Median time from lead implantation to HT was significantly longer in the RLF group (77 ± 47.5 vs 41.6 ± 38.6 months; P < .001; Table 2). Univariate and multivariate logistic regression analyses demonstrated that duration between lead implantation and HT was an independent predictor for RLFs (P < .05; Table 3; Fig. 2) with an odds ratio of 1.015 (95% confidence interval [CI] 1.005–1.026) and an ideal cutoff of 38 months based on ROC analysis (sensitivity 0.778 and specificity 0.608).
Table 2.
Device Characteristics
| Characteristic | Retained ICD Lead (n = 37) |
No Retained ICD Lead (n = 101) |
P Value |
|---|---|---|---|
| CRT-P | 1 (3) | 0 (0) | .097 |
| CRT-D | 24 (65) | 54 (53) | .237 |
| ICD single-chamber | 3 (8) | 25 (25) | .023† |
| ICD dual-chamber | 9 (24) | 18 (18) | .266 |
| PM dual-chamber | 0 (0) | 4 (4) | .219 |
| Single coil | 3 (8) | 31 (31) | .004† |
| Dual coil | 33 (89) | 68 (67) | .004† |
| Number of leads | 2.8 ± 1.0 | 2.4 ± 1.0 | .028* |
| Age of device (mo) | 77.2 ± 47.5 | 41.6 ± 38.6 | <.001* |
Results are presented as n (%) or mean ± SD. CRT-D, cardiac resynchronization therapy defibrillator; CRT-P, cardiac resynchronization therapy pacemaker; ICD, implantable cardioverter-defibrillator; PM, pacemaker.
P < .05 by unpaired t test or Mann-Whitney U test as appropriate.
P < .05 by chi-square test or Fisher exact test as appropriate.
Table 3.
Risk Factors for Retained Lead Fragments Per Univariate and Multivariate Analysis
| Univariate Analysis
|
Multivariate Analysis
|
|||||
|---|---|---|---|---|---|---|
| Factor | P Value | Odds Ratio | 95% CI | P Value | Odds Ratio | 95% CI |
| Dual coil | .012* | 5.015 | 1.428–17.61 | .075 | 2.937 | 0.982–1.077 |
| Age of device | <.001* | 1.019 | 1.009–1.029 | .004* | 1.015 | 1.005–1.026 |
| Number of leads | .033* | 1.523 | 1.036–2.240 | .339 | 1.252 | 0.790–1.984 |
| Patient’s age | .045* | 1.042 | 1.001–1.085 | .231 | 1.029 | 0.982–1.077 |
CI, confidence interval.
P < .05 by unpaired t test or Mann-Whitney U test as appropriate.
Fig. 2.
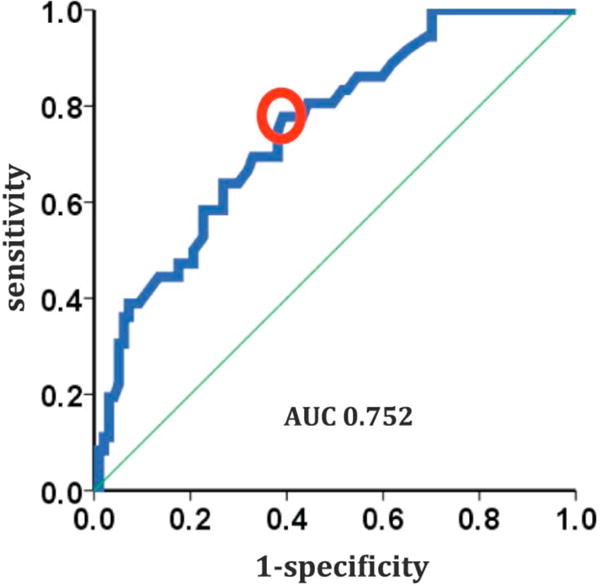
Device implantation duration as risk factor for retained leads. Univariate and multivariate logistic regression analyses identified the duration between implantable cardioverter-defibrillator implantation and heart transplantation as an independent predictor for retained lead fragments (P < .05), with a cutoff of 38 months (sensitivity 0.778 and specificity 0.608). AUC, area under the receiver operating characteristic curve.
Description of RLFs
The majority of RLFs originated in the device pocket and traversed the subclavian vein close to SVC–right atrium (RA) junction (Fig. 3A). In 30%, RLFs were exclusively located within the intravascular space, which precludes later percutaneous removal because they are not accessible from the device pocket (Fig. 3B). The most commonly identified RLF was an ICD lead with an intact remnant of a proximal defibrillator coil (65%). The remainder of the RLF characteristics are summarized in Table 4.
Fig. 3.

Retained lead fragments. (A) Implantable cardioverter-defibrillator lead with remnant of intact proximal coil reaching from the device pocket to the superior vena cava–right atrium junction. (B) Remnant of intact proximal coil with pure intravascular location.
Table 4.
Characteristics of Retained Lead Fragments
| Characteristic | n | % |
|---|---|---|
| Lead fragment location | ||
| 1 | 2.7 | |
| Pocket to subclavian vein—right atrium | 25 | 67.6 |
| Subclavian vein | 7 | 18.9 |
| Innominate vein | 4 | 10.8 |
| Type of lead fragment | ||
| ICD lead with coil remnant intact | 28 | 64.9 |
| ICD lead with coil remnant unreaveled | 6 | 16.2 |
| Remnant of proximal coil | 2 | 5.4 |
| ICD lead, no coil | 2 | 5.4 |
| PM lead | 17 | 45.9 |
| Epicardial LV lead | 1 | 2.7 |
| Generator | 1 | 2.7 |
| Number of lead fragments | ||
| 1 | 24 | 64.9 |
| 2 | 9 | 24.3 |
| 3 | 4 | 10.8 |
ICD, implantable cardioverter-defibrillator; LV, left ventricular; PM, pacemaker.
Clinical End Points
Cox regression analysis established RLFs as a risk factor for the development of UEDVT with a hazard ratio of 2.17 (95% CI 1.12–4.22; P = .022). Patients with RLFs had a significantly higher rate of DVT compared with the non-RLF group during the 1-year study period (42% vs 21%; P = .018; Fig. 4A), including both contralateral and ipsilateral UEDVTS with reference to the previous CIED implantation side. For the entire study cohort, 34.09% of UEDVTs were found ipsilateral. There was no significant difference between RLF and non-RLF patients regarding UEDVT site localization (ipsilateral 37.5 vs 34.61%, contralateral 37.5 vs 46.15%, and bilateral 25.0 vs 19.23 %, RLF vs non-RLF respectively; P = .84).
Fig. 4.

Clinical end points. A. Upper extremity deep venous thrombosis (DVT): the RLF group had a significantly lower event-free DVT rate compared with the non-RLF group during the 1-year study period (58% vs 79%; P = .018). (B) Infections: log-rank test did not identify a significant difference in rate of infection, defined as sepsis with positive blood cultures, between the RLF and non-RLF groups. RLF, retained lead fragment.
There was no difference in the rate of bacteremia between the groups (P = .81; Fig. 4B). No lead migration was observed. In the group with RLFs, 14 (38%) were unable to undergo a clinically indicated MRI. MRI was needed for 4 cardiac indications (2 rejection monitoring, 1 assessment of perfusion, and 1 evaluation of a depressed ejection fraction), 8 neurologic indications, and 2 orthopedic indications. One patient with a neurologic indication for MRI underwent successful percutaneous removal of RLFs before the study.
Discussion
In this study we evaluated the prevalence and clinical consequences of RLFs in HT patients. The main findings are: (1) RLFs are a common occurrence in a modern cohort of HT recipients; (2) longer time interval between lead implantation and HT is an independent predictor for RLFs; (3) the presence of RLFs is significantly associated with the development of UEDVT; and (4) RLFs prevent the use of clinically indicated MRI in a significant percentage of patients.
The prevalence of RLFs after HT has been reported previously.2,3 Martin et al reported that 39% of patients (22/59) had RLFs and only 2 had their lead fragments removed before discharge.2 In their study, a CIED implanted >18 months before HT increased the risk of RLFs. Here we report a slightly lower event rate of RLFs in a large contemporary cohort and additional risk factors, such as the presence of dual leads and the number of total leads. The identified risk factors increase the chance of developing significant adhesions that may preclude manual extraction. The SVC-RA junction is thin-walled, and the presence of the proximal coil increases fibrosis and adhesions to the vascular wall. The amount of calcification, especially under the clavicle, also increases with lead implantation time. Both of these factors may preclude successful lead removal at the time of HT.
Previously, RLFs were related to mechanical complications. Martin et al reported 1 case of erosion into the mediastinum, 1 embolization into the right pulmonary artery, and 1 migration of the inner conductor into the right ventricle. All of these were complications of stretched defibrillator leads involving the SVC coil.2 In our cohort, we did not observe direct RLF mechanical complications, such as lead migration, embolization, or perforation. However, we had a low number of stretched or uncoiled ICD lead remnants, which could explain those differences.
Risk of RLF Extraction
Previous reports have shown that abandonment of nontransected leads was not associated with adverse outcomes.3,4 However, transected leads post HT may lead to substantially increased risk of adverse events. In the general population, there are multiple reports of mechanical complications of transected abandoned pacemaker and ICD leads, including right ventricle perforation, perforation of the atrial septum, and subsequent cerebral embolization as well as paradoxic embolization of lead fragments.5–7
In the nontransplant population, there are limited data available on the ideal management of sterile abandoned leads, and decisions for or against extraction are made on a case-to-case basis.4,8,9 A recent study in a nontransplant population showed that extraction of noninfected superfluous leads is feasible and successful and associated with fewer complications than extraction in the setting of lead infection.10 In a different registry study evaluating 1-year outcomes following ICD lead abandonment versus lead extraction, patients with extraction had higher in-hospital complications and deaths.11
Kusmierski et al described an algorithm to percutaneously remove RLF after HT in 7 patients.12 All patients underwent percutaneous lead extraction 10–27 days after HT, and there were no procedural complications noted.12 In our cohort, 5 patients had retained leads extracted percutaneously after HT (2 before and 3 after discharge), also without any complications.
Risk of UEDVT in Patients With RLFs
We identified increased risk for UEDVT in patients with RLFs. The majority of UEDVTs were diagnosed within the 1st 12 weeks after HT. Early after HT, patients are in a proinflammatory and prothrombotic state characterized by platelet activation and decreased fibrinolysis possibly resulting in increased risk for UEDVT. The UEDVT risk is then further aggravated by repeated endothelial injury in the setting of frequent endomyocardial biopsies. Furthermore, immunosuppression can lead to prothrombotic effects.13,14 And the combination of mycophenolate mofetil and cyclosporine, cyclosporine monotherapy, and mammalian target of rapamycin inhibitors have been associated with increased risk of thrombotic events.15–17
Recently, Elboudwarej et al described 117 cases (9.3%) of DVT in a study cohort of 1258 patients after HT, and Alvarez-Alvarez et al reported a similar rate (8.5%) in 635 patients.15,18–21 Fortunately, UEDVTs are associated with a low incidence of pulmonary embolism both in the general population (6%)22,23 and after HT (8%–9%).15,23
Pacemaker and defibrillator leads are well established risk factors for the development of UEDVT24–26 in non-HT patients; this is the 1st report to link them in the HT population. The presence of UEDVT is especially problematic in HT patients owing to the need for repeated central venous access for endomyocardial biopsy.26–28 In our analysis, most UEDVTs occurred early in the post-transplantation course, mostly in the 1st 12 weeks.
A potential explanation for the higher incidence of UEDVTs in the early post-transplantation course could be the higher rate of invasive procedures with frequent right heart catheterizations and endomyocardial biopsies, as well as the presence of central lines, in addition to recent major surgery.
No complications, such as vascular injuries, have been documented in operative reports in cases of failed lead removal at the time of transplantation. It is, however, possible that additional or more forceful traction attempts could have been made, leading to microtrauma and further enhancing the risk of DVT. Nevertheless, for the entire study cohort, 78% of UEDVTs were noted in the 1st 12 weeks. Interestingly, the percentage of UEDVs within this time frame was 68% for the RLF group and 82% for the non-RLF group, making increased thrombotic risk from failed intraoperative extraction less likely.
It is important to note that the early occurrence of UEDVT after transplantation is especially challenging when managing anticoagulation, not only with drug-drug interactions, but also with frequent need for interruption when undergoing biopsies with potentially increased procedural risk. Currently, no guidelines are available to manage venous thromboembolism after HT, and the optimal duration of therapy is unknown.29
Inability to Undergo MRI Scans
Cardiac MRI has evolved as a noninvasive radiation-free tool for rejection monitoring in recent years. It is especially promising because it allows evaluation of the entire myocardium for inflammatory changes and at the same time allows correlation with cardiac dimensions and function.30 In addition, MRI is the preferred imaging modality in disorders of the brain, spine, and joints. In the present study, none of the patients with RLFs could undergo MRI and 38% had a clinical indication for imaging that could not be completed.
Retained leads are more prone to current induction by MRI and heat generation than leads attached to a pulse generator.31 Thus, lead fragments are open circuits that can cause thermic injury to the vessel. Although MRIs have been performed in the presence of RLFs in a few small case series, the risk to these patients has not been well studied. A recent study demonstrating the safety of pacemakers or ICD generators in patients undergoing thoracic MRI excluded patients with retained leads.32,33
Therefore, to date the use of MRI in patients with RLFs remains contraindicated in clinical practice, leading to the loss of a valuable diagnostic tool.1
Infection
The presence of RLFs complicates the management of CIED infection, including higher 1-month mortality, longer procedure time, and worse clinical outcome, in non-HT patients.34 This type of infection would be especially challenging in immunocompromised HT recipients. One patient with RLFs developed endocarditis with Staphylococcus hominis bacteremia. The RLFs could not be removed because they were entirely located within the vasculature. This case highlights the importance of assuring access to lead fragments from the device pocket in cases when complete intraoperative removal of lead fragments is not possible (discussed in subsequent section).
Surprisingly, we did not identify a statistically significant difference in bacteremia between the RLF and non-RLF groups. One explanation for the lack of significant differences in the rate of infection could be the overall low rate of bacteremia, too low to allow for valid associations between severity of infection and the presence of RLFs.
Suggested Management of Retained Lead Fragments
We think that the presence of RLFs after HT poses significant risks, based on the incidence of UEDVT and the inability to undergo MRI scanning. Importantly, we found that most leads could be successfully, safely, and completely removed during the index hospitalization of HT. Based on these findings, we recommend complete CIED system removal at the time of HT whenever possible. We propose an approach that first identifies the patients at risk for RLF based on lead dwell time.
If there are vascular adhesions or heavy calcification precluding complete lead removal at the time of HT, certain surgical maneuvers at the time of HT can significantly enhance later percutaneous RLF removal: leads should be cut in the SVC-RA region as proximal as possible, and mechanical lysis of adhesions can be attempted to free up the lead remnants. Leads should then be capped and left intact in the pectoral pocket and not cut short or allowed to retract into the vascular space. Percutaneous extraction should be considered based on a number of factors, including stability of anastomoses, clinical stability, and risk of extraction (Fig. 5).
Fig. 5.

Proposed management algorithm. Patients should be evaluated with a multidisciplinary approach to achieve complete removal of all retained lead fragments (RLFs) whenever possible. If complete surgical removal of all lead fragments is not possible at the time of orthotopic heart transplantation (OHT), then lead access from the pectoral pocket for percutaneous removal should be secured. Patients undergoing implantable cardioverter-defibrillator (ICD) implantation while awaiting OHT should receive single-lead, single-coil, or subcutaneous ICDs. PM, pacemaker
Because patients with RLFs had a higher prevalence of dual-coil leads and multiple leads in our study, implanters may wish to consider using single- as opposed to dual-lead devices and single-coil ICD leads in patients awaiting heart transplantation. The subcutaneous ICD, which has no intravascular leads, is an attractive option because it avoids the issue of RLFs altogether.
Study Limitations
The study is limited by its retrospective design. There is no protocol in place to obtain venous duplex in all patients after HT. Because UEDVTs are frequently asymptomatic, the number of cases could be underrecognized. The exact ICD lead implantation time is not known in all cases.
Conclusion
The presence of RLFs after HT is an underrecognized and common problem and is associated with a significantly higher risk of developing UEDVT. Although no significant difference was found in the rates of bacteremia between the groups, this finding might be explained by the overall low incidence. RLFs also severely limit the use of MRI scanning. Patients with risk factors for lead retention should be identified before transplantation, and complete lead removal should be considered with an interdisciplinary approach.
Footnotes
Disclosures
None.
References
- 1.Nazarian S, Beinart R, Halperin HR. Magnetic resonance imaging and implantable devices. Circ Arrhythm Electrophysiol. 2013;6:419–28. doi: 10.1161/CIRCEP.113.000116. [DOI] [PubMed] [Google Scholar]
- 2.Martin A, Voss J, Shannon D, Ruygrok P, Lever N. Frequency and sequelae of retained implanted cardiac device material post heart transplantation. Pacing Clin Electrophysiol. 2014;37:242–8. doi: 10.1111/pace.12274. [DOI] [PubMed] [Google Scholar]
- 3.Glikson M, Suleiman M, Luria DM, Martin ML, Hodge DO, Shen WK, et al. Do abandoned leads pose risk to implantable cardioverter-defibrillator patients? Heart Rhythm. 2009;6:65–8. doi: 10.1016/j.hrthm.2008.10.012. [DOI] [PubMed] [Google Scholar]
- 4.Bohm A, Pinter A, Duray G, Lehoczky D, Dudás G, Tomcsányi I, et al. Complications due to abandoned noninfected pacemaker leads. Pacing Clin Electrophysiol. 2001;24:1721–4. doi: 10.1046/j.1460-9592.2001.01721.x. [DOI] [PubMed] [Google Scholar]
- 5.Dalal JJ, Robinson CJ, Henderson AH. An unusual complication of the unremoved unwanted pacing wire. Pacing Clin Electrophysiol. 1981;4:14–6. doi: 10.1111/j.1540-8159.1981.tb03669.x. [DOI] [PubMed] [Google Scholar]
- 6.Bohm A, Banyai F, Komaromy K, Pinter A, Preda I. Cerebral embolism due to a retained pacemaker lead: a case report. Pacing Clin Electrophysiol. 1998;21:629–30. doi: 10.1111/j.1540-8159.1998.tb00111.x. [DOI] [PubMed] [Google Scholar]
- 7.Dieuzaide P, Savon N, Chalvidan T, LeTallec L, DeHaro JC, Djiane P. A complication of pacemaker lead extraction: paradoxical embolism of a lead fragment in a leg artery. Pacing Clin Electrophysiol. 1998;21:2699–700. doi: 10.1111/j.1540-8159.1998.tb00053.x. [DOI] [PubMed] [Google Scholar]
- 8.Silvetti MS, Drago F. Outcome of young patients with abandoned, nonfunctional endocardial leads. Pacing Clin Electrophysiol. 2008;31:473–9. doi: 10.1111/j.1540-8159.2008.01017.x. [DOI] [PubMed] [Google Scholar]
- 9.Wollmann CG, Bocker D, Loher A, Köbe J, Scheld HH, Breithardt GE, et al. Incidence of complications in patients with implantable cardioverter/defibrillator who receive additional transvenous pace/sense leads. Pacing Clin Electrophysiol. 2005;28:795–800. doi: 10.1111/j.1540-8159.2005.00169.x. [DOI] [PubMed] [Google Scholar]
- 10.Huang XM, Fu H, Osborn MJ, Asirvatham SJ, McLeod CJ, Glickson M, et al. Extraction of superfluous device leads: a comparison with removal of infected leads. Heart Rhythm. 2015;12:1177–82. doi: 10.1016/j.hrthm.2015.02.005. [DOI] [PubMed] [Google Scholar]
- 11.Zeitler EP, Wang Y, Dharmarajan K, Anstrom KJ, Peterson ED, Daubert JP, et al. Outcomes 1 year after implantable cardioverter-defibrillator lead abandonment versus explantation for unused or malfunctioning leads: a report from the National Cardiovascular Data Registry. Circ Arrhythm Electrophysiol. 2016;9 doi: 10.1161/CIRCEP.116.003953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kusmierski K, Przybylski A, Oreziak A, Sobieszczanska-Malek M, Kolsut P, Rozanski J. Post heart transplant extraction of the abandoned fragments of pacing and defibrillation leads: proposed management algorithm. Kardiol Pol. 2013;71:159–63. doi: 10.5603/KP.2013.0009. [DOI] [PubMed] [Google Scholar]
- 13.Saikali JA, Truong LD, Suki WN. Sirolimus may promote thrombotic microangiopathy. Am J Transplant. 2003;3:229–30. doi: 10.1034/j.1600-6143.2003.00018.x. [DOI] [PubMed] [Google Scholar]
- 14.Puschel A, Lindenblatt N, Katzfuss J, Vollmar B, Klar E. Immunosuppressants accelerate microvascular thrombus formation in vivo: role of endothelial cell activation. Surgery. 2012;151:26–36. doi: 10.1016/j.surg.2011.06.026. [DOI] [PubMed] [Google Scholar]
- 15.Elboudwarej O, Patel JK, Liou F, Rafiei M, Osborne A, Chai W, et al. Risk of deep vein thrombosis and pulmonary embolism after heart transplantation: clinical outcomes comparing upper extremity deep vein thrombosis and lower extremity deep vein thrombosis. Clin Transplant. 2015;29:629–35. doi: 10.1111/ctr.12566. [DOI] [PubMed] [Google Scholar]
- 16.Thibodeau JT, Mishkin JD, Patel PC, Kaiser PA, Ayers CR, Mammen PP, et al. Sirolimus use and incidence of venous thromboembolism in cardiac transplant recipients. Clin Transplant. 2012;26:953–9. doi: 10.1111/j.1399-0012.2012.01677.x. [DOI] [PubMed] [Google Scholar]
- 17.Baas MC, Gerdes VE, ten Berge IJ, Heutinck KM, Florquin S, Meijers JC, et al. Treatment with everolimus is associated with a procoagulant state. Thromb Res. 2013;132:307–11. doi: 10.1016/j.thromres.2013.07.004. [DOI] [PubMed] [Google Scholar]
- 18.Alvarez-Alvarez RJ, Barge-Caballero E, Chavez-Leal SA, Paniagua-Martin MJ, Marzoa-Rivas R, Caamaño CB, et al. Venous thromboembolism in heart transplant recipients: incidence, recurrence and predisposing factors. J Heart Lung Transplant. 2015;34:167–74. doi: 10.1016/j.healun.2014.09.039. [DOI] [PubMed] [Google Scholar]
- 19.Benza RL, Cavender MA, Barchue J, Tallaj JA, Bourge RC, Kirklin JK, et al. Alterations in the fibrinolytic cascade post-transplant: evidence of a bimodal expression pattern. J Heart Lung Transplant. 2007;26:494–7. doi: 10.1016/j.healun.2007.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hognestad A, Michelsen A, Brosstad F, Damås JK, Holm T, Simonsen S, et al. Platelet activation in heart transplant recipients. Clin Transplant. 2004;18:142–7. doi: 10.1046/j.1399-0012.2003.00137.x. [DOI] [PubMed] [Google Scholar]
- 21.Holm T, Aukrust P, Andreassen AK, Ueland T, Brosstad F, Frøland SS, et al. Peripheral endothelial dysfunction in heart transplant recipients: possible role of proinflammatory cytokines. Clin Transplant. 2000;14:218–25. doi: 10.1034/j.1399-0012.2000.140307.x. [DOI] [PubMed] [Google Scholar]
- 22.Owens CA, Bui JT, Knuttinen MG, Gaba RC, Carrillo TC. Pulmonary embolism from upper extremity deep vein thrombosis and the role of superior vena cava filters: a review of the literature. J Vasc Interv Radiol. 2010;21:779–87. doi: 10.1016/j.jvir.2010.02.021. [DOI] [PubMed] [Google Scholar]
- 23.Munoz FJ, Mismetti P, Poggio R, Valle R, Barrón M, Guil M, et al. Clinical outcome of patients with upper-extremity deep vein thrombosis: results from the RIETE registry. Chest. 2008;133:143–8. doi: 10.1378/chest.07-1432. [DOI] [PubMed] [Google Scholar]
- 24.Mandal S, Pande A, Mandal D, Kumar A, Sarkar A, Kahali D, et al. Permanent pacemaker-related upper extremity deep vein thrombosis: a series of 20 cases. Pacing Clin Electrophysiol. 2012;35:1194–8. doi: 10.1111/j.1540-8159.2012.03467.x. [DOI] [PubMed] [Google Scholar]
- 25.van Rooden CJ, Molhoek SG, Rosendaal FR, Schalij MJ, Meinders AE, Huisman MV. Incidence and risk factors of early venous thrombosis associated with permanent pacemaker leads. J Cardiovasc Electrophysiol. 2004;15:1258–62. doi: 10.1046/j.1540-8167.2004.04081.x. [DOI] [PubMed] [Google Scholar]
- 26.Kucher N. Clinical practice. Deep-vein thrombosis of the upper extremities. N Engl J Med. 2011;364:861–9. doi: 10.1056/NEJMcp1008740. [DOI] [PubMed] [Google Scholar]
- 27.Elman EE, Kahn SR. The post-thrombotic syndrome after upper extremity deep venous thrombosis in adults: a systematic review. Thromb Res. 2006;117:609–14. doi: 10.1016/j.thromres.2005.05.029. [DOI] [PubMed] [Google Scholar]
- 28.Schulman S, Lindmarker P, Holmstrom M, Lärfars G, Carlsson A, Nicol P, et al. Post-thrombotic syndrome, recurrence, and death 10 years after the first episode of venous thromboembolism treated with warfarin for 6 weeks or 6 months. J Thromb Haemost. 2006;4:734–42. doi: 10.1111/j.1538-7836.2006.01795.x. [DOI] [PubMed] [Google Scholar]
- 29.Costanzo MR, Dipchand A, Starling R, Anderson A, Chan M, Desai S, et al. The International Society of Heart and Lung Transplantation guidelines for the care of heart transplant recipients. J Heart Lung Transplant. 2010;29:914–56. doi: 10.1016/j.healun.2010.05.034. [DOI] [PubMed] [Google Scholar]
- 30.Butler CR, Kumar A, Toma M, Thompson R, Chow K, Isaac D, et al. Late gadolinium enhancement in cardiac transplant patients is associated with adverse ventricular functional parameters and clinical outcomes. Can J Cardiol. 2013;29:1076–83. doi: 10.1016/j.cjca.2012.10.021. [DOI] [PubMed] [Google Scholar]
- 31.Langman DA, Goldberg IB, Finn JP, Ennis DB. Pacemaker lead tip heating in abandoned and pacemaker-attached leads at 1.5 Tesla MRI. J Magn Reson Imaging. 2011;33:426–31. doi: 10.1002/jmri.22463. [DOI] [PubMed] [Google Scholar]
- 32.Russo RJ, Costa HS, Silva PD, Anderson JL, Arshad A, Biederman RW, et al. Assessing the risks associated with MRI in patients with a pacemaker or defibrillator. N Engl J Med. 2017;376:755–64. doi: 10.1056/NEJMoa1603265. [DOI] [PubMed] [Google Scholar]
- 33.Higgins JV, Gard JJ, Sheldon SH, Espinosa RE, Wood CP, Felmlee JP, et al. Safety and outcomes of magnetic resonance imaging in patients with abandoned pacemaker and defibrillator leads. Pacing Clin Electrophysiol. 2014;37:1284–90. doi: 10.1111/pace.12419. [DOI] [PubMed] [Google Scholar]
- 34.Hussein AA, Tarakji KG, Martin DO, Gadre A, Fraser T, Kim A, et al. Cardiac implantable electronic device infections. Added complexity and suboptimal outcomes with previously abandoned leads. JACC Clin Electrophysiol. 2017;3:1–9. doi: 10.1016/j.jacep.2016.06.009. January 2017, NI.1, 2017. [DOI] [PubMed] [Google Scholar]


