Abstract
Excess reactive oxygen species production by mitochondria is a key mechanism of age-related vascular dysfunction. Our laboratory has shown that supplementation with the mitochondrial-targeted antioxidant MitoQ improves vascular endothelial function by reducing mitochondrial reactive oxygen species and ameliorates arterial stiffening in old mice, but the effects in humans are unknown. Here we sought to translate our preclinical findings to humans and determine the safety and efficacy of MitoQ. Twenty healthy older adults (60–79 years) with impaired endothelial function (brachial artery flow-mediated dilation <6%) underwent six weeks of oral supplementation with MitoQ (20 mg/day) or placebo in a randomized, placebo-controlled, double-blind, crossover design study. MitoQ was well tolerated and plasma MitoQ was higher after the treatment vs. placebo period (P<0.05). Brachial artery flow-mediated dilation was 42% higher after MitoQ vs. placebo (P<0.05); the improvement was associated with amelioration of mitochondrial reactive oxygen species-related suppression of endothelial function (assessed as the increase in flow-mediated dilation with acute, supra-therapeutic MitoQ [160 mg] administration, n=9, P<0.05). Aortic stiffness (carotid-femoral pulse wave velocity) was lower after MitoQ vs. placebo (P<0.05) in participants with elevated baseline levels (carotid-femoral pulse wave velocity >7.60 m/s, n=11). Plasma oxidized low-density lipoprotein, a marker of oxidative stress, also was lower after MitoQ vs. placebo (P<0.05). Participant characteristics, endothelium-independent dilation (sublingual nitroglycerin) and circulating markers of inflammation were not different (all P>0.1). These findings in humans extend earlier preclinical observations and suggest that MitoQ and other therapeutic strategies targeting mitochondrial reactive oxygen species may hold promise for treating age-related vascular dysfunction.
Keywords: aging, reactive oxygen species, endothelial function, arterial stiffness, mitochondria
Introduction
Cardiovascular diseases (CVD) remain the leading cause of morbidity and mortality in developed societies.1, 2 Advancing age is the primary risk factor for CVD, which is largely mediated by adverse changes to arteries.1, 3 Two features of vascular aging that are key antecedents to CVD are the development of endothelial dysfunction, as assessed by reduced endothelium-dependent dilation (EDD), and stiffening of the large elastic arteries (i.e., aortic stiffening).3, 4 Vascular dysfunction with age is a consequence of excessive superoxide-related oxidative stress, much of which is of mitochondrial origin.5–7 Given the projected increase in CVD prevalence in the coming decades, driven mainly by increases in the number of middle-aged and older (MA/O) adults,8, 9 identifying novel strategies that reduce excess mitochondrial reactive oxygen species (mtROS) to improve vascular function and reduce CVD risk in this population is a biomedical priority.
MitoQ is a mitochondria-targeted antioxidant consisting of the naturally occurring antioxidant ubiquinol attached to a lipophilic cation; the lipophilicity and positive charge of this compound enable it to cross cell membranes and accumulate on the matrix facing the surface of the mitochondrial inner membrane where it is optimally positioned to reduce mtROS.10, 11 Preclinical findings from our laboratory show that 4 weeks of oral supplementation of MitoQ (drinking water) completely restores EDD in old mice to levels observed in young mice.6 The improvement in EDD in old mice was mediated by a suppression of mtROS, as indicated by an increase in EDD with acute MitoQ administration in untreated, but not in MitoQ-treated, animals.6 More recently, we reported that 4 weeks of MitoQ treatment also ameliorated age-related increases in aortic stiffness in old mice, as indicated by a reduction in aortic pulse wave velocity (PWV).12 MitoQ supplementation did not impact endothelial function or aortic stiffness in young mice.6, 12
MitoQ has been used safely in Phase II clinical trials for Parkinson’s and liver disease.13, 14 Moreover, MitoQ is now available as a dietary supplement and recently was administered chronically (3 weeks) to healthy young adults without adverse effects.15 However, presently the efficacy of chronic MitoQ supplementation for improving vascular function in healthy MA/O adults is unknown. Accordingly, we sought to translate our preclinical findings to humans by conducting the first randomized, double-blind, placebo-controlled clinical trial with MitoQ in healthy late MA/O humans (crossover design with 6-week intervention arms). EDD, as measured by nitric oxide (NO)-dependent brachial artery flow-mediated dilation (BAFMD), was our primary outcome. To gain mechanistic insight into the possible role of reduced tonic suppression of EDD by mtROS in mediating improvements in endothelial function with MitoQ supplementation, we used a novel functional bioassay in which the acute change in BAFMD in response to a single supra-therapeutic dose of oral MitoQ was compared after both the active treatment and placebo phases of the trial. Based on our recent preclinical findings in mice,12 we also took the opportunity to evaluate the impact of MitoQ on aortic stiffness, assessed translationally using carotid-femoral PWV (CFPWV). Given that this trial was the first conducted in healthy MA/O adults, safety, tolerability and plasma levels of MitoQ with treatment also were evaluated. Lastly, we assessed changes in circulating markers of oxidative stress and inflammation for additional mechanistic insight.
Materials and Methods
All procedures were reviewed and approved by the Institutional Review Board at the University of Colorado Boulder. The nature, benefits, and risks of all study procedures were explained to volunteers and their written informed consent was obtained before participation in the study. This trial was registered on ClinicalTrials.gov (NCT02597023). All measurements were performed at the University of Colorado Boulder Clinical Translational Research Center (CTRC). The Food and Drug Administration categorized MitoQ as a dietary supplement for the manner utilized in this study and, as such, deemed that an IND was not needed. The data supporting the findings of this study are available from the corresponding author upon reasonable request.
Participants
Late MA/O men and postmenopausal women aged 60 to 79 years from Boulder County, Colorado and the surrounding areas were studied. All participants were non-smokers and free of clinical diseases, including peripheral arterial disease (ankle-brachial index >0.90) and overt CVD as determined by medical history, physical examination, blood chemistries, and blood pressure and electrocardiogram at rest and during incremental treadmill exercise. All participants demonstrated age-associated impairments in endothelial function at screening, defined as BAFMD <6%. Potential participants were excluded if they had abnormal blood chemistries, alcohol dependence, uncontrolled thyroid disease, severe obesity (body mass index [BMI] >40 kg/m2), or were not weight stable (defined as >2.5 kg change in body mass) for at least 3 months prior to enrolling in the study.
Study design, randomization, and intervention
The study design consisted of a 2 x 6-week randomized, double-blind, placebo-controlled, crossover clinical trial. To optimize the potential for observing effects of treatment, participants consumed MitoQ at the upper limit of the manufacturer recommended dose (20 mg, 1x/day; MitoQ Limited) or identical placebo capsules for 6 weeks before crossing over to the other treatment arm in a randomly determined order. Randomization was performed by a member of the study team not involved in the assessment of outcomes, and a block randomization scheme stratified for age and sex was used. MitoQ or placebo capsules were taken once each morning with breakfast. Every 2 weeks during the active treatment and placebo phases, in-person check-in visits were performed to exchange intervention capsules (a precise number of capsules were allocated until the participant’s next visit) and to assess participant adherence by survey and pill count. Tolerability and side effects were also evaluated during these check-in visits; treatment-emergent adverse events were documented throughout the study by the CTRC staff and reported to the CTRC Safety Monitoring Committee and the Institutional Review Board.
Measurements
All measurements were performed following a 12-hour fast from food (water allowed) and caffeine, a 24-hour abstention from alcohol, physical activity, prescription medications and the study compound (MitoQ and placebo) and a 48-hour abstention from over the counter medications and supplements.16 A single study investigator who was blinded to treatment condition of the subject performed all vascular data acquisition and analysis.
Participant characteristics and clinical blood assays
BMI was determined by anthropometry17 and arterial systolic and diastolic blood pressures were assessed in triplicate over the brachial artery at rest with a semi-automated device (Dinamap XL, Johnson & Johnson) at the end of each intervention phase and at check-in visits. Leisure time physical activity was determined by the Modifiable Activity Questionnaire at the end of each intervention phase.18
Blood samples were drawn from an intravenous catheter the cubital vein at screening and after each treatment arm. The Colorado Clinical and Translational Sciences Institute CTRC Core Laboratory and Boulder Community Hospital Clinical Laboratory performed the following blood assays as previously described.19 Fasting serum lipids were determined with standard assays. Fasting plasma glucose was measured by reflective spectrophotometry (Ortho Clinical Diagnostics). Plasma oxidized low-density lipoprotein (LDL) and serum interleukin (IL)-6 were assessed by ELISA (Mercodia). Serum high-sensitivity C-reactive protein was measured by immunoturbidimetry (Beckman Coulter).
Plasma MitoQ levels
EDTA-treated plasma collected 24–26 hours after capsule ingestion following each 6-week intervention period was obtained to determine the effects of chronic MitoQ supplementation on circulating MitoQ levels in plasma. Plasma samples were analyzed by reversed-phase liquid chromatography using gradient elution with acetonitile, water, and formic acid and the deuterated compound (d15-MitoQ) was used as an internal standard, as previously described.20
Vascular endothelial function
EDD was determined by NO-dependent BAFMD (using a five-minute forearm cuff occlusion) using high-resolution ultrasonography (Toshiba Xario XG), as described previously.16, 21 BAFMD was measured at screening to establish baseline endothelial dysfunction and after each 6-week supplementation period. BAFMD is reported as percentage and absolute change from baseline diameter.16 BAFMD shear rate was calculated as: [8 × mean velocity (m/s)]/occlusion diameter (m).16 Brachial artery dilation following 0.4 mg sublingual nitroglycerin was measured after each 6-week supplementation period to control for changes in smooth muscle sensitivity to NO. Dilation with nitroglycerin is reported as percent change from baseline diameter. Brachial artery diameters and blood velocities were captured and analyzed by Vascular Research Tools 5.10.9 (Medical Imaging Applications).
MtROS-mediated suppression of vascular endothelial function
After each 6-week treatment arm, BAFMD was measured before and 1 hour after an acute 160 mg oral dose of MitoQ, used to temporarily and reversibly reduce mtROS. The difference in FMD after vs. before acute oral MitoQ was taken as a measure of tonic suppression of EDD by mtROS.
Aortic stiffness
Aortic stiffness was assessed by CFPWV, as previously described.22 Briefly, CFPWV was determined by applanation tonometry with simultaneous electrocardiogram gating of the R-wave to measure the time delay between the foot of the carotid and femoral arterial pressure waves; PWV was calculated as the distance between arterial sites (m) divided by the arterial pressure wave transit time (s) at each site, with automated software (Non-Invasive Hemodynamics Workstation, Cardiovascular Engineering Inc.).
Data analysis
Statistical analyses were performed with G*Power 3.1 and GraphPad Prism version 7. Sample size was estimated based on an effect size to detect significant group differences in our primary outcome variable (FMD: 0.7) calculated from our laboratory’s previous intervention studies.23–26 Our sample size estimate indicated that n=18 participants would be adequate to detect a difference with >80% power at the α=0.05 level; to account for a potentially lower effect size and subject attrition, we enrolled 24 participants in the study. The impact of MitoQ on outcome variables was evaluated with paired t-tests. Given the crossover design, we also tested for the presence of a carryover effect for each of the outcomes using a mixed model ANOVA, with treatment order as a between group factor (no carryover effects were observed between conditions). The acute effect of supra-therapeutic MitoQ administration on FMD following chronic MitoQ and placebo supplementation was assessed with a 2-way repeated measures ANOVA and Least Significant Differences post-hoc analysis if a significant interaction was found. Data are expressed as mean ± standard error (SEM). Statistical significance was set a priori at α=0.05.
Results
Participants
Fifty-five individuals were consented for the study. Twenty-six did not meet inclusion criteria. Five individuals withdrew from the study prior to randomization due to the time commitment (n=4) or study restrictions (n=1). Of the remaining 24 participants, 13 participants were randomized to Group A, which received MitoQ first followed by placebo, and 11 subjects were randomized to Group B, which received placebo first followed by MitoQ. Three participants withdrew from Group A (procedure invasiveness, non-study related injury, and personal emergency). One participant was excluded from Group B because of an abnormal ECG (atrial fibrillation). The participant characteristics and clinical blood markers of the 20 participants who completed the trial are shown in Table 1. Anthropometrics, brachial artery blood pressure, fasting blood lipids, glucose and physical activity levels were unchanged with the intervention (Table 1).
Table 1.
Participant Characteristics
| Characteristics | Placebo | MitoQ |
|---|---|---|
| N, men/women | 9/11 | -- |
| Age, years | 68±1 | -- |
| Body mass index, kg/m2 | 23±1 | 23±1 |
| Systolic blood pressure, mmHg | 113±3 | 114±4 |
| Diastolic blood pressure, mmHg | 67±1 | 67±1 |
| Resting heart rate, beats/min | 62±2 | 61±1 |
| Physical activity caloric expenditure, kcal/wk | 5201±570 | 5326±726 |
| Total cholesterol, mmol/L | 4.9±0.2 | 4.9±0.2 |
| High-density lipoprotein cholesterol, mmol/L | 1.3±0.1 | 1.3±0.1 |
| Low-density lipoprotein cholesterol, mmol/L | 3.1±0.2 | 3.0±0.2 |
| Triglycerides, mmol/L | 1.2±0.2 | 1.2±0.1 |
| Glucose, mmol/L | 4.7±0.1 | 4.6±0.1 |
Data are mean±SEM
Safety and tolerability
Adherence to the intervention was excellent, with all participants consuming greater than 90% of all MitoQ and placebo capsules administered. The 20 mg/day dose of MitoQ was well tolerated, and no treatment-related serious adverse events occurred. A total of 7 treatment-emergent adverse events were reported by 7 of the 24 participants who were enrolled in the study. All self-reported adverse events were mild in severity and included gastrointestinal discomfort (n=1) during the MitoQ condition and gastrointestinal discomfort (n=2) and diarrhea (n=1) under the placebo condition; diarrhea (n=2) and vomiting (n=1) were reported following acute administration of 160 mg of MitoQ. None of the enrolled participants dropped out of the study because of side effects.
Plasma MitoQ
Plasma levels of MitoQ were higher after 6 weeks of MitoQ supplementation vs. placebo (Figure 1). The observed levels of MitoQ in plasma are consistent with the known pharmacokinetic profile of orally ingested MitoQ.10, 11 Moreover, the reported plasma MitoQ levels likely represent the lowest values achieved during the MitoQ supplementation phase because they were assessed 24–26 hours after the most recent dose (~1 hour prior to the subsequent dose).
Figure 1.
Plasma levels of MitoQ after 6 weeks of placebo or MitoQ supplementation, assessed ~24 hours after the last dose. Values are presented as mean±SEM. *P<0.05 vs. placebo.
Vascular endothelial function
BAFMD was 42% higher following chronic MitoQ supplementation vs. placebo (17/20 subjects had higher values after MitoQ vs. placebo, Figure 2). A post-hoc power analysis revealed an effect size of 0.66 and a statistical power of 80% for the observed difference in BAFMD (primary outcome). Other brachial artery parameters were not different between conditions (Supplementary Table 1). Smooth muscle sensitivity to NO (endothelium-independent dilation), assessed by brachial artery dilation with sublingual nitroglycerin, was not different between conditions (24±2% vs. 25±3%, for placebo and MitoQ, respectively, P>0.1).
Figure 2.
Brachial artery flow-mediated dilation (BAFMD) expressed as percent (left) and absolute (right) change after 6 weeks of placebo or MitoQ supplementation. Values are presented as mean±SEM, with individual responses below. *P<0.05 vs. placebo.
MtROS-mediated suppression of vascular endothelial function
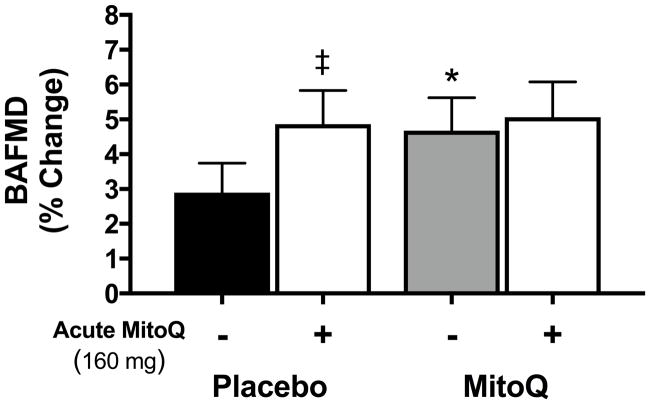
Oral administration of a single 160 mg dose of MitoQ increased BAFMD acutely (compared with the pre-administration baseline level) after the placebo supplementation phase, but not after the MitoQ supplementation phase (n=9; Figure 3). These results are consistent with the idea of reduced mtROS-mediated suppression of EDD following 6 weeks of chronic MitoQ supplementation.
Figure 3.
Brachial artery flow-mediated dilation (BAFMD) before (-) and 1 hour after (+) acute ingestion of 160 mg of MitoQ following 6 weeks of placebo or MitoQ supplementation assessed in a subset of n=9 participants. Values are presented as mean±SEM. ‡P<0.05 vs. before ingestion of 160 mg MitoQ; *P<0.05 vs. placebo.
Aortic stiffness
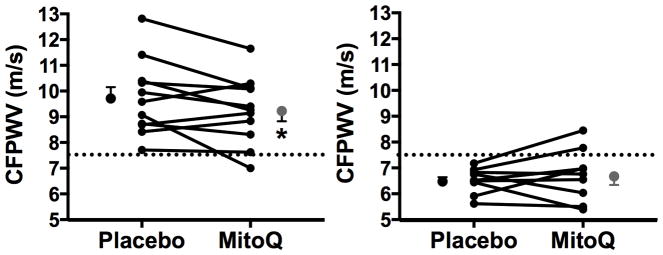
There were no differences between conditions in CFPWV in the group as a whole (placebo: 8.34±0.46 m/s vs. MitoQ: 8.28±0.41 m/s, P>0.1). However, the overall group consisted of a range of individual subject values in the untreated (placebo arm) condition, and our previous preclinical study12 found that MitoQ only reduced CFPWV in old mice (i.e., the group with elevated baseline aortic PWV); there was no effect in young animals. Accordingly, to determine if MitoQ treatment reduced CFPWV in subjects with elevated levels of CFPWV in the untreated state, we separated our subjects into 2 subgroups based on the recent Framingham Heart Study reported cutoff for “healthy vascular aging” of <7.60 m/s, which is the mean +2 standard deviations value from a reference sample of individuals <30 years old without CVD risk factors.27 The subgroup of our subjects with elevated aortic stiffness in the untreated state (n=11) was slightly older (69±1 vs. 65±1 years, P<0.05), and had higher systolic blood pressure (118±2 vs. 105±2 mmHg, P<0.05) and CFPWV (9.74±0.44 vs. 6.52±0.16 m/s, P<0.05) values, but the cohorts were otherwise not different. CFPWV was lower following MitoQ supplementation in the cohort with CFPWV values >7.60 m/s (9.25±0.39 m/s; P<0.05), but was unaltered in the cohort with CFPWV values <7.60 m/s (6.71±0.33 m/s; P>0.1) (Figure 4). MitoQ did not differentially affect blood pressure in the 2 subgroups.
Figure 4.
Carotid-femoral pulse wave velocity (CFPWV) after 6 weeks of placebo or MitoQ supplementation. Participants were separated into 2 subgroups, based on placebo (untreated) CFPWV values above and below 7.60 m/s (demarcated by the dashed line). Values are presented as mean±SEM and individual responses are depicted. *P<0.05 vs. placebo.
Circulating markers of oxidative stress and inflammation
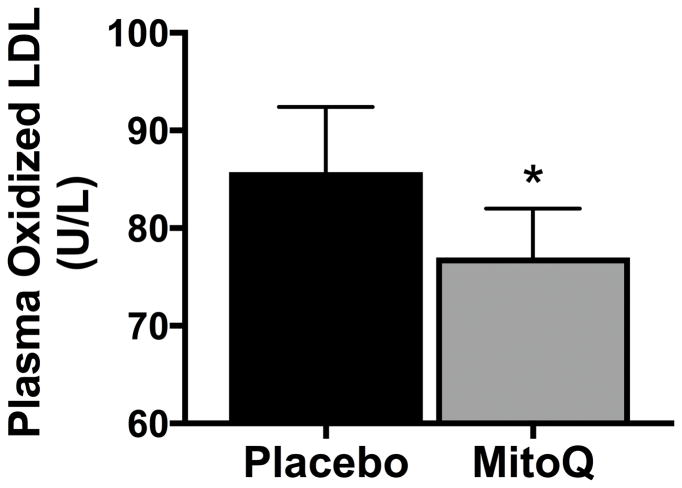
Plasma concentrations of oxidized LDL, a circulating marker of oxidative stress, were 13% lower following 6 weeks of MitoQ supplementation (Figure 5). There were no differences in circulating markers of inflammation, as assessed by C-reactive protein (placebo: 1.7±0.4 mg/L vs. MitoQ: 1.5±0.3 mg/L, P>0.1) and IL-6 (placebo:1.3±0.1 mg/L vs. MitoQ: 1.4±0.2 mg/L, P>0.1).
Figure 5.
Plasma levels of oxidized low-density lipoprotein (LDL), a circulating marker of oxidative stress, after 6 weeks of placebo or MitoQ supplementation. Values are presented as mean±SEM. *P<0.05 vs. placebo.
Discussion
In the current study, we translated our recent preclinical findings to humans using a rigorous randomized, placebo-controlled, double-blind crossover study design to evaluate the effects of the mitochondrial-targeted antioxidant MitoQ on vascular function in healthy late MA/O adults. We show that chronic administration of MitoQ increased plasma MitoQ levels and was safe and well-tolerated over the treatment duration (6 weeks) studied here. Most importantly, we found that supplementation with MitoQ improved our primary outcome, BAFMD (endothelial function), in this group with normal (modest) age-related impairments in baseline FMD, and present evidence that this improvement was mediated, at least in part, by a suppression of mtROS. MitoQ treatment also reduced aortic stiffness in participants exhibiting age-related aortic stiffening in the untreated state, and decreased plasma oxidized LDL, a marker of oxidative stress, without altering circulating markers of inflammation or traditional CVD risk factors. Collectively, these findings provide support for the concept that MitoQ, and perhaps other strategies targeting mtROS, may be novel therapeutic options for improving vascular function and reducing the risk of age-related CVD.
Preclinical findings from our laboratory show that oral MitoQ supplementation restores age-related decreases in EDD in old mice.6 To our knowledge, no prior studies have evaluated supplementation with MitoQ, or any other mitochondria-specific antioxidant, for improving vascular endothelial function in humans. In the present investigation, we extend our previous findings on aging in mice to healthy MA/O adults, and demonstrate for the first time that 6 weeks of MitoQ supplementation improves BAFMD, a measure of NO-mediated EDD and independent predictor of incident CVD risk.28, 29 The improvement in BAFMD with MitoQ occurred without a change in endothelium-independent dilation, indicating unaltered vascular smooth muscle sensitivity to NO and, therefore, that the increase in FMD with treatment reflects an improvement in endothelium-specific NO-mediated dilation. BAFMD was improved by 42% with MitoQ in the overall group, and increases were observed in both men and women. This magnitude of improvement in BAFMD is within the range produced by healthy lifestyle interventions known for exerting a strong physiological stimulus, such as caloric restriction-based weight loss (~30% improvement)23 and aerobic exercise (~50% improvement in men),30 when applied for longer durations (up to 3 months) than the present 6-week treatment period. Given that most MA/O adults do not meet current guidelines for healthy lifestyle behaviors,31–33 MitoQ supplementation may represent an alternative or complementary pharmacological strategy for enhancing vascular endothelial function in this group.
We found previously that acute ex vivo incubation with MitoQ increased EDD of isolated arterial segments from old mice,6 and others have reported similar results in skeletal muscle feed arteries biopsied from older adult humans,7 both of which indicate that age-related impairments in EDD are mediated by excess mtROS. Furthermore, in our preclinical study, chronic MitoQ supplementation ameliorated excess mtROS-mediated suppression of EDD in old mice, as shown by no change in EDD with acute ex vivo incubation of isolated arterial segments with MitoQ after 4 weeks of oral treatment with MitoQ.6 In the current study, we extend these findings to an in vivo setting in humans, as we observed an improvement in EDD in response to an acute supra-therapeutic dose of MitoQ following the placebo phase, which revealed the presence of tonic inhibition of EDD (BAFMD) by mtROS. The inhibition of EDD by mtROS was abolished following chronic MitoQ supplementation, as indicated by a lack of further increase in EDD with acute supra-therapeutic administration of MitoQ. These observations in older humans support our previous observations in old mice that MitoQ improves vascular endothelial function, at least in part, by reducing mitochondria-derived oxidative stress.
Our preclinical findings show that MitoQ treatment reduced aortic stiffness, as assessed by aortic PWV, in old mice.12 MitoQ did not alter aortic stiffness in young mice, suggesting favorable effects of MitoQ only in a setting of age-related aortic stiffening.12 The current study translates these findings to humans by showing that MitoQ lowers CFPWV in late MA/O adults exhibiting age-related aortic stiffening based on recently published cutoffs for CFPWV from the Framingham Heart Study.27 This finding has potentially important clinical implications in that CFPWV is the gold-standard in vivo measure of aortic stiffness and an independent predictor of CVD risk and other common disorders of aging, including cognitive impairment, chronic kidney disease and frailty.22, 34–37 MitoQ did not alter aortic stiffness in participants with CFPWV <7.60 m/s, consistent with our previous results in young mice.12
The mechanisms responsible for the de-stiffening effects of MitoQ in our subjects with elevated CFPWV are not obvious. Endothelial function affects arterial stiffness by influencing vascular smooth muscle tone22, 38 and average improvements in BAFMD tended to be greater (51% vs. 36% increase) in the subgroup with elevated initial CFPWV levels, which may have contributed to their reductions in CFPWV. Our recent findings in mice indicate that MitoQ treatment improved aortic stiffness in old animals by influencing the elastin component of intrinsic wall stiffness.12 However, the 6-week supplementation period in the present study in humans seems too brief to induce changes in structural proteins in the aortic wall and, instead, suggests functional influences on smooth muscle tone, perhaps linked to reductions in oxidative stress, as we have reported previously in postmenopausal estrogen-deficient women.39 Importantly, blood pressure was unaltered in the subgroup demonstrating reductions in CFPWV with MitoQ treatment, indicating that this was not a mechanism. Overall, although future studies are needed to elucidate responsible mechanisms, our findings provide the first evidence for chronic MitoQ supplementation lowering aortic stiffness in late MA/O adults with age-related aortic stiffening. These data have important implications for reducing the risk of CVD and numerous other age-related disorders linked with elevated aortic stiffness.
In our preclinical studies in mice, MitoQ supplementation normalized age-related increases in oxidative stress without affecting pro-inflammatory cytokine levels in old animals.6, 12 Consistent with this notion, in the present study MitoQ supplementation was associated with a decrease in plasma oxidized LDL, a circulating marker of oxidative stress, as well as with amelioration of mtROS-mediated suppression of EDD. MitoQ supplementation did not alter circulating markers of inflammation in the current study; moreover, BMI, blood lipids, glucose and blood pressure were unchanged with MitoQ. These data are in agreement with the lack of change in these potential modulatory factors observed in our preclinical studies6, 12 and suggest that improvements in vascular function observed with MitoQ were independent of overt changes in traditional CVD risk factors and inflammation. Taken together, these observations support a reduction in oxidative stress as an important mechanism underlying improvements in vascular function with MitoQ.
A limitation of the current study is that we did not directly assess the effect of MitoQ on ROS production by mitochondria. However, the ability of MitoQ to both chronically and acutely reduce mtROS in the vasculature is supported by: 1) our preclinical study showing that chronic MitoQ supplementation in old mice reduced aortic mitochondrial superoxide,6 assessed with a mitochondria-specific spin probe and electron paramagnetic resonance (EPR) spectroscopy - the gold-standard and most direct measure of ROS;40 and 2) ex vivo experiments in biopsied skeletal muscle feed arteries from humans demonstrating that 1 hour of MitoQ incubation improved EDD, which was associated with reduced EPR spectroscopy-based measures of mitochondrial superoxide levels.7 Regarding the latter assessments of mtROS in human tissue, we could not perform these invasive analyses in the current clinical intervention study; however, our innovative acute MitoQ administration paradigm is a direct translation of this experimental model to humans in vivo and provides evidence for reduced mtROS as a likely mechanism of action of chronic MitoQ supplementation.
Perspectives
Here, we demonstrate for the first time that supplementation with the mitochondria-targeted antioxidant MitoQ is safe and well tolerated in late MA/O adults, improves vascular endothelial function (likely by suppressing excess mtROS), reduces aortic stiffness in MA/O adults with elevated initial levels, and decreases oxidized LDL, a circulating marker of oxidative stress. Collectively, these findings establish the experimental basis for conducting a larger scale clinical trial in older adults or clinical populations, particularly those associated with endothelial dysfunction and/or elevated aortic stiffness. In the broadest terms, our results provide initial support for the idea that MitoQ and, potentially other mitochondria-targeted antioxidants, may be an effective treatment for improving vascular function and possibly decreasing the risk of CVD and other clinical disorders of aging, including cognitive dysfunction and chronic kidney disease.
Supplementary Material
Novelty and Significance.
What Is New?
This is the first trial in humans to show that 6 weeks of daily oral supplementation with the mitochondrial-targeted antioxidant MitoQ improves vascular endothelial function.
We provide the first in vivo evidence in humans that MitoQ improves endothelial function by suppressing mitochondrial-derived oxidative stress.
We provide the initial evidence in humans that MitoQ supplementation reduces aortic stiffness in adults with elevated baseline levels.
What Is Relevant?
Older individuals have a greater risk for cardiovascular diseases largely because of vascular dysfunction, including reduced endothelial function and aortic stiffening. Thus, it is important to establish evidence-based therapeutic options to improve vascular function in this group.
This study demonstrates that mitochondrial-targeted antioxidant MitoQ improves endothelial function and aortic stiffness in healthy late middle-aged and older adults with impaired baseline vascular function, thus establishing initial evidence for efficacy.
Summary
MitoQ supplementation improved vascular endothelial function in healthy late middle-aged and older adults by reducing the tonic suppressive effects of excessive mitochondrial-specific reactive oxygen species. MitoQ also reduced aortic stiffness in individuals with age-related arterial stiffening.
Acknowledgments
The authors thank the staff of the University of Colorado Boulder CTRC for their technical assistance.
Funding
This work was supported by NIH awards AG049451, AG000279, AG053009, Colorado CTSA UL1 TR001082 and an industry contract with MitoQ Limited. MPM is supported by UK MRC MC_U105663142 and as a Wellcome Trust Investigator (110159/Z/15/Z).
Contents are the authors’ sole responsibility and do not necessarily represent official NIH views.
Footnotes
Disclosures
MPM is on the scientific advisory board of Antipodean Pharmaceuticals. All other authors have no declarations of interest to disclose.
Author Contributions
Initial study design [MJR, JSP, RGR, MC, DRS], data acquisition/analysis [MJR, JSP, CCS, LMC, HLR, NZB, KW, MPM], data interpretation [MJR, MPM, DRS], creating tables/figures [MJR], writing initial draft [MJR], editing and approving final draft [MJR, JSP, CCS, NZB, LMC, HLR, RGR, MC, MPM, DRS].
References
- 1.Benjamin EJ, Blaha MJ, Chiuve SE, et al. Heart disease and stroke statistics-2017 update: A report from the american heart association. Circulation. 2017;135:e146–e603. doi: 10.1161/CIR.0000000000000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weintraub WS, Daniels SR, Burke LE, et al. Value of primordial and primary prevention for cardiovascular disease: A policy statement from the american heart association. Circulation. 2011;124:967–990. doi: 10.1161/CIR.0b013e3182285a81. [DOI] [PubMed] [Google Scholar]
- 3.Lakatta EG, Levy D. Arterial and cardiac aging: Major shareholders in cardiovascular disease enterprises: Part i: Aging arteries: A “set up” for vascular disease. Circulation. 2003;107:139–146. doi: 10.1161/01.cir.0000048892.83521.58. [DOI] [PubMed] [Google Scholar]
- 4.Widlansky ME, Gokce N, Keaney JF, Jr, Vita JA. The clinical implications of endothelial dysfunction. J Am Coll Cardiol. 2003;42:1149–1160. doi: 10.1016/s0735-1097(03)00994-x. [DOI] [PubMed] [Google Scholar]
- 5.Seo AY, Joseph AM, Dutta D, Hwang JC, Aris JP, Leeuwenburgh C. New insights into the role of mitochondria in aging: Mitochondrial dynamics and more. J Cell Sci. 2010;123:2533–2542. doi: 10.1242/jcs.070490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gioscia-Ryan RA, LaRocca TJ, Sindler AL, Zigler MC, Murphy MP, Seals DR. Mitochondria-targeted antioxidant (mitoq) ameliorates age-related arterial endothelial dysfunction in mice. J Physiol. 2014;592:2549–2561. doi: 10.1113/jphysiol.2013.268680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Park SY, Kwon OS, Andtbacka RHI, Hyngstrom JR, Reese V, Murphy MP, Richardson RS. Age-related endothelial dysfunction in human skeletal muscle feed arteries: The role of free radicals derived from mitochondria in the vasculature. Acta Physiol (Oxf) 2018:222. doi: 10.1111/apha.12893. [DOI] [PubMed] [Google Scholar]
- 8.Heidenreich PA, Trogdon JG, Khavjou OA, et al. Forecasting the future of cardiovascular disease in the united states: A policy statement from the american heart association. Circulation. 2011;123:933–944. doi: 10.1161/CIR.0b013e31820a55f5. [DOI] [PubMed] [Google Scholar]
- 9.Olshansky SJ, Goldman DP, Zheng Y, Rowe JW. Aging in america in the twenty-first century: Demographic forecasts from the macarthur foundation research network on an aging society. Milbank Q. 2009;87:842–862. doi: 10.1111/j.1468-0009.2009.00581.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Murphy MP, Smith RA. Targeting antioxidants to mitochondria by conjugation to lipophilic cations. Annu Rev Pharmacol Toxicol. 2007;47:629–656. doi: 10.1146/annurev.pharmtox.47.120505.105110. [DOI] [PubMed] [Google Scholar]
- 11.Smith RA, Murphy MP. Animal and human studies with the mitochondria-targeted antioxidant mitoq. Ann N Y Acad Sci. 2010;1201:96–103. doi: 10.1111/j.1749-6632.2010.05627.x. [DOI] [PubMed] [Google Scholar]
- 12.Gioscia-Ryan RA, Battson ML, Cuevas LM, Eng JS, Murphy MP, Seals DR. Mitochondria-targeted antioxidant therapy with mitoq ameliorates aortic stiffening in old mice. J Appl Physiol. 1985:2017. doi: 10.1152/japplphysiol.00670.2017. jap 00670 02017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gane EJ, Weilert F, Orr DW, Keogh GF, Gibson M, Lockhart MM, Frampton CM, Taylor KM, Smith RA, Murphy MP. The mitochondria-targeted anti-oxidant mitoquinone decreases liver damage in a phase ii study of hepatitis c patients. Liver Int. 2010;30:1019–1026. doi: 10.1111/j.1478-3231.2010.02250.x. [DOI] [PubMed] [Google Scholar]
- 14.Snow BJ, Rolfe FL, Lockhart MM, Frampton CM, O’Sullivan JD, Fung V, Smith RA, Murphy MP, Taylor KM Protect Study G. A double-blind, placebo-controlled study to assess the mitochondria-targeted antioxidant mitoq as a disease-modifying therapy in parkinson’s disease. Mov Disord. 2010;25:1670–1674. doi: 10.1002/mds.23148. [DOI] [PubMed] [Google Scholar]
- 15.Shill DD, Southern WM, Willingham TB, Lansford KA, McCully KK, Jenkins NT. Mitochondria-specific antioxidant supplementation does not influence endurance exercise training-induced adaptations in circulating angiogenic cells, skeletal muscle oxidative capacity or maximal oxygen uptake. J Physiol. 2016;594:7005–7014. doi: 10.1113/JP272491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harris RA, Nishiyama SK, Wray DW, Richardson RS. Ultrasound assessment of flow-mediated dilation. Hypertension. 2010;55:1075–1085. doi: 10.1161/HYPERTENSIONAHA.110.150821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lohman TG, RAaMR Anthropometric standardization reference manual. Human Kinetics. 1988 [Google Scholar]
- 18.Pereira MA, FitzerGerald SJ, Gregg EW, Joswiak ML, Ryan WJ, Suminski RR, Utter AC, Zmuda JM. A collection of physical activity questionnaires for health-related research. Med Sci Sport Exerc. 1997;29:S1–205. [PubMed] [Google Scholar]
- 19.DeVan AE, Johnson LC, Brooks FA, et al. Effects of sodium nitrite supplementation on vascular function and related small metabolite signatures in middle-aged and older adults. J Appl Physiol (1985) 2016;120:416–425. doi: 10.1152/japplphysiol.00879.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li Y, Zhang H, Fawcett JP, Tucker IG. Quantitation and metabolism of mitoquinone, a mitochondria-targeted antioxidant, in rat by liquid chromatography/tandem mass spectrometry. Rapid Commun Mass Spectrom. 2007;21:1958–1964. doi: 10.1002/rcm.3048. [DOI] [PubMed] [Google Scholar]
- 21.Eskurza I, Monahan KD, Robinson JA, Seals DR. Effect of acute and chronic ascorbic acid on flow-mediated dilatation with sedentary and physically active human ageing. J Physiol. 2004;556:315–324. doi: 10.1113/jphysiol.2003.057042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Townsend RR, Wilkinson IB, Schiffrin EL, et al. Recommendations for improving and standardizing vascular research on arterial stiffness: A scientific statement from the american heart association. Hypertension. 2015;66:698–722. doi: 10.1161/HYP.0000000000000033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pierce GL, Beske SD, Lawson BR, Southall KL, Benay FJ, Donato AJ, Seals DR. Weight loss alone improves conduit and resistance artery endothelial function in young and older overweight/obese adults. Hypertension. 2008;52:72–79. doi: 10.1161/HYPERTENSIONAHA.108.111427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Seals DR, Tanaka H, Clevenger CM, Monahan KD, Reiling MJ, Hiatt WR, Davy KP, DeSouza CA. Blood pressure reductions with exercise and sodium restriction in postmenopausal women with elevated systolic pressure: Role of arterial stiffness. J Am Coll Cardiol. 2001;38:506–513. doi: 10.1016/s0735-1097(01)01348-1. [DOI] [PubMed] [Google Scholar]
- 25.Jablonski KL, Racine ML, Geolfos CJ, Gates PE, Chonchol M, McQueen MB, Seals DR. Dietary sodium restriction reverses vascular endothelial dysfunction in middle-aged/older adults with moderately elevated systolic blood pressure. J Am Coll Cardiol. 2013;61:335–343. doi: 10.1016/j.jacc.2012.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Santos-Parker JR, Strahler TR, Bassett CJ, Bispham NZ, Chonchol MB, Seals DR. Curcumin supplementation improves vascular endothelial function in healthy middle-aged and older adults by increasing nitric oxide bioavailability and reducing oxidative stress. Aging (Albany NY) 2017;9:187–208. doi: 10.18632/aging.101149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Niiranen TJ, Lyass A, Larson MG, Hamburg NM, Benjamin EJ, Mitchell GF, Vasan RS. Prevalence, correlates, and prognosis of healthy vascular aging in a western community-dwelling cohort: The framingham heart study. Hypertension. 2017;70:267–274. doi: 10.1161/HYPERTENSIONAHA.117.09026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yeboah J, Crouse JR, Hsu FC, Burke GL, Herrington DM. Brachial flow-mediated dilation predicts incident cardiovascular events in older adults: The cardiovascular health study. Circulation. 2007;115:2390–2397. doi: 10.1161/CIRCULATIONAHA.106.678276. [DOI] [PubMed] [Google Scholar]
- 29.Yeboah J, Folsom AR, Burke GL, Johnson C, Polak JF, Post W, Lima JA, Crouse JR, Herrington DM. Predictive value of brachial flow-mediated dilation for incident cardiovascular events in a population-based study: The multi-ethnic study of atherosclerosis. Circulation. 2009;120:502–509. doi: 10.1161/CIRCULATIONAHA.109.864801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pierce GL, Eskurza I, Walker AE, Fay TN, Seals DR. Sex-specific effects of habitual aerobic exercise on brachial artery flow-mediated dilation in middle-aged and older adults. Clin Sci. 2010;120:13–23. doi: 10.1042/CS20100174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Douketis JD, Macie C, Thabane L, Williamson DF. Systematic review of long-term weight loss studies in obese adults: Clinical significance and applicability to clinical practice. Int J Obes (Lond) 2005;29:1153–1167. doi: 10.1038/sj.ijo.0802982. [DOI] [PubMed] [Google Scholar]
- 32.Saida T, Juul Sorensen T, Langberg H. Long-term exercise adherence after public health training in at-risk adults. Ann Phys Rehabil Med. 2017;60:237–243. doi: 10.1016/j.rehab.2017.02.006. [DOI] [PubMed] [Google Scholar]
- 33.Wing RR, Phelan S. Long-term weight loss maintenance. Am J Clin Nutri. 2005;82:222S–225S. doi: 10.1093/ajcn/82.1.222S. [DOI] [PubMed] [Google Scholar]
- 34.Ben-Shlomo Y, Spears M, Boustred C, et al. Aortic pulse wave velocity improves cardiovascular event prediction: An individual participant meta-analysis of prospective observational data from 17,635 subjects. J Am Coll Cardiol. 2014:636–646. doi: 10.1016/j.jacc.2013.09.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mitchell GF, Hwang SJ, Vasan RS, Larson MG, Pencina MJ, Hamburg NM, Vita JA, Levy D, Benjamin EJ. Arterial stiffness and cardiovascular events: The framingham heart study. Circulation. 2010;121:505–511. doi: 10.1161/CIRCULATIONAHA.109.886655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pase MP, Beiser A, Himali JJ, Tsao C, Satizabal CL, Vasan RS, Seshadri S, Mitchell GF. Aortic stiffness and the risk of incident mild cognitive impairment and dementia. Stroke. 2016;47:2256–2261. doi: 10.1161/STROKEAHA.116.013508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Abbatecola AM, Chiodini P, Gallo C, Lakatta E, Sutton-Tyrrell K, Tylavsky FA, Goodpaster B, de Rekeneire N, Schwartz AV, Paolisso G, Harris T Health ABCs. Pulse wave velocity is associated with muscle mass decline: Health abc study. Age (Dordr) 2012;34:469–478. doi: 10.1007/s11357-011-9238-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wilkinson IB, MacCallum H, Cockcroft JR, Webb DJ. Inhibition of basal nitric oxide synthesis increases aortic augmentation index and pulse wave velocity in vivo. Br J Clin Pharmacol. 2002;53:189–192. doi: 10.1046/j.1365-2125.2002.1528adoc.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moreau KL, Gavin KM, Plum AE, Seals DR. Ascorbic acid selectively improves large elastic artery compliance in postmenopausal women. Hypertension. 2005;45:1107–1112. doi: 10.1161/01.HYP.0000165678.63373.8c. [DOI] [PubMed] [Google Scholar]
- 40.Griendling KK, Touyz RM, Zweier JL, Dikalov S, Chilian W, Chen YR, Harrison DG, Bhatnagar A American Heart Association Council on Basic Cardiovascular S. Measurement of reactive oxygen species, reactive nitrogen species, and redox-dependent signaling in the cardiovascular system: A scientific statement from the american heart association. Circ Res. 2016;119:e39–75. doi: 10.1161/RES.0000000000000110. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.