Abstract
The activity of the renin-angiotensin-aldosterone-system is triggered by the release of the protease renin from the kidneys, which in turn is controlled in the sense of negative feedback loops. It is widely assumed that angiotensin II (Ang II) directly inhibits renin expression and secretion via a short loop feedback by an effect on renin-producing cells (RPCs) mediated by Ang II type 1 (AT1) receptors. Since the concept of such a direct short loop negative feedback control which originates mostly from in vitro experiments has not yet been systematically proven in vivo, we aimed to test the validity of this concept by studying the regulation of renin synthesis and secretion in mice lacking Ang II-AT1 receptors on RPCs. We found that RPCs of the kidney express Ang II-AT1 receptors. Mice with conditional deletion of Ang II-AT1 receptors in RPCs were normal with regard to number of renin cells, renal renin mRNA and plasma renin concentrations. Renin expression and secretion of these mice responded to angiotensin I-converting enzyme inhibition and to Ang II-infusion like in wild type controls. In summary, we did not obtain evidence that Ang II-AT1 receptors on RPCs are of major relevance for the normal regulation of renin expression and secretion in mice. Therefore, we doubt the existence of a direct negative feedback function of Ang II on RPCs.
Keywords: Renin, Angiotensin II type 1 (AT1) receptor, kidney, conditional AT1a knockout mouse, negative feedback, renin producing cells
INTRODUCTION
The protease renin is the key regulator of the renin-angiotensin-aldosterone system (RAAS) which essentially controls blood pressure and fluid homeostasis of the organism1, 2. Renin expression has been demonstrated for a variety of organs with the highest expression level in the kidney3. Therefore the activity of the circulating RAAS is considered to be triggered by renin originating in the kidney4, 5. There renin expression and secretion is regulated by negative feedback loops involving angiotensin II6. At the cellular level, responsiveness to Ang II is conferred by expression of angiotensin (AT) receptors which belongs to the large family of seven transmembrane G protein-coupled receptors7. To date virtually all classical physiological actions caused by Ang II have been attributed to the Ang II type 1 (AT1) receptor8. The AT1a receptor is present in all organs which have a potential to affect blood pressure including the vasculature, immune system, heart, nervous system, adrenal gland, and the kidney9. The effects of AT1a receptors lead to vasoconstriction and hence to an increase in blood pressure. Furthermore extracellular fluid volume and renal tubular sodium reabsorption increase and aldosterone release are stimulated10–13.
Renin is mainly produced and secreted by pericyte like cells14 associated with renal preglomerular arteries15. Even in the adult kidney the number of renin-producing cells (RPCs) shows a high degree of plasticity16. In states of a challenged RAAS such as salt deficiency or hypotension the number of RPCs increases17, 18 whilst in states with less demand for the RAAS such as during salt overload or hypertension the number of RPCs decreases2, 19. In states of genetic loss of functions of the RAAS a marked hyperplasia of RPCs develops in humans and rodents16, 20–25. Hyperplasia and concomitant hypersecretion of renin in humans and laboratory animals can also be induced pharmacologically by RAAS inhibitors such as direct renin inhibitors26, angiotensin I converting enzyme inhibitors27–29 or Ang II-AT1 receptor blockers30–32.
In vitro studies have suggested the expression of Ang II-AT1 receptors by juxtaglomerular cells33, 34. In addition Ang II has been repeatedly shown to rapidly inhibit renin secretion from isolated perfused kidneys35–37 or kidney slices6, 38. The combination of these in vivo and in vitro findings has led to the concept that renin synthesis and secretion might be directly controlled by Ang II in the sense of a negative feedback at the level of potential renin producers39–41. Although intriguing, the relevance of such a short loop feedback regulation of renin synthesis and secretion in vivo has not yet really been proven. In vivo experiments with pharmacological manipulations of ANGII generation or action are not suitable to prove the concept of a direct negative feedback, because they are always confounded by side effects relevant for renin secretion such as changes of blood pressure and or sodium balance. In contrast, in vivo studies addressing the role AT1 on renin cells already raised first doubts on the relevance of a direct negative feedback of renin by ANGII42, 43. In any case there exists yet no systematic analysis of the potential role of Ang II-AT1 receptors on RPCs for the control of renin synthesis and secretion in vivo. We therefore have addressed this topic by analyzing the regulation of renin expression and secretion in mice lacking Ang II-AT1 receptors on RPCs.
CONCISE METHODS
The data that support the findings of this study are available from the corresponding author upon reasonable request. For detailed descriptions of experimental procedures please see the online supplement.
Animals and experimental protocols
All procedures performed on animals were done in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals and were approved by the German government (approval number 54-2532.1-29/13).
Both male and female 6- to 9-weeks-old modified mice and wild type (WT) littermates derived from heterozygous breeding pairs were used in this study. Renin cell-specific AT1a-deficient (Ren1d+/Cre x AT1afl/fl) mice were generated by crossbreeding mice with loxP flanked AT1a alleles10, and mice with targeted insertion of Cre recombinase into the renin-1d locus (Ren1d)16. The renin cell-specific double knockout mice for AT1a and AT1b (Ren1d+/Cre x AT1afl/fl x AT1b−/−) were generated by crossing the aforementioned mouse lines with mice with a systemic deletion of AT1b44. Unless otherwise noted, the animals had free access to standard rodent chow (0.6% NaCl; Ssniff, Germany) and tap water.
In addition to baseline measurements, the following experimental protocols were used:
To stimulate renin production, animals received the loop diuretic furosemide (2.28 mmol/l) in the tap water for 7 days or low salt diet (0.02% NaCl, Sniff, Germany) combined with the angiotensin converting enzyme (ACE) inhibitor enalapril in the tap water (10 mg/kgBW*d−1) for 14 days (n=10 per group). Except sodium chloride content low salt diet and standard chow were identical.
To suppress renin production Angiotensin II was applied by osmotic minipumps (2.5 μg/kgBW*min−1 in isotonic saline, Alzet model 1002) for 10 days. In a control group, osmotic minipumps were filled with vehicle (n=5 per group). Pumps were implanted dorsal-subcutaneously under sevoflurane anesthesia.
Immunohistochemistry
Perfusion-fixed paraffin-embedded kidneys were costained for renin and anti-α-smooth muscle actin (α-SMA). Micrographs were obtained using a AxioObserver Z1 Microscope (Carl Zeiss, Germany).
Chromogenic In situ hybridization (ISH)
Localization of AT1a and AT1b receptor mRNA was performed on 3% PFA perfusion-fixed, paraffin-embedded kidney tissue using the RNAscope® 2.5 HD Brown Reagent Kit (Advanced Cell Diagnostics ACD, USA), following manufacturer’s instructions. Probes for AT1a and AT1b were obtained from ACD (Cat No. 404001, 406231-C2). The detailed procedure is described elsewhere45. After the ISH-procedure we performed immunohistochemical stainings on the same slice as described above. Brightfield-imaging was done with an AxioObserver Z1 microscope.
Determination of mRNA expression by Real time RT-PCR
For quantification of mRNA expression, real-time PCR was performed using a Light Cycler 480 Instrument and the LightCycler 480 SYBR Green I Master Kit (Roche Diagnostics, Germany). mRNA expression data were normalized to glyceraldehyde-3-phosphate dehydrogenase (GAPDH).
Determination of plasma renin concentration
Blood samples were taken from the tail vein into EDTA-coated capillary tubes to prevent clotting. Plasma was separated by centrifugation (8 min, 8,000 rpm) and 5 μl of the plasma (diluted 1:20 in 100 μl volume) were incubated for 90 min at 37 °C with plasma from bilaterally nephrectomized male rats which served as substrate for renin. Plasma renin concentration (PRC) was determined by measuring the capacity of plasma samples to generate Ang I in the presence of excess renin substrate. The generated Ang I (in ng/ml*h−1) was determined by ELISA (IBL, Germany).
Blood pressure measurement by tail-cuff method
Systolic blood pressure (BP) of conscious mice was determined by tail-cuff manometry (TSE Systems, Germany). Mice were conditioned by placing them into the holding devices on 5 consecutive days before the first measurement was performed. Blood pressure was measured 8x per mouse daily for 10 days. The daily average values per mouse were used for analysis. (n=5 per group).
Ca2+-imaging
For intracellular Ca2+-imaging, we crossed Ren1d+/Cre x AT1afl/fl x AT1b−/− with mice harboring a BAC-GFP reporter construct under the control of the Ren1 gene promotor46. A single cell suspension of kidney cells was incubated with fura-2-AM and 10nM Ang II or 10μM ATP were applied. Fura-2 emission ratios of the ROIs were detected at 340nm and 380nm excitation.
Statistical analysis
Student’s t-test was used to test significance of difference between two groups. Otherwise, analysis of variance (ANOVA), followed by Bonferroni post hoc test, was used to test significance of differences between groups. The data were analyzed using Graph Pad Prism 6 software to determine their significant differences. Values were presented as means ±SEM. A p-value of less than 0.05 was considered statistically significant.
RESULTS
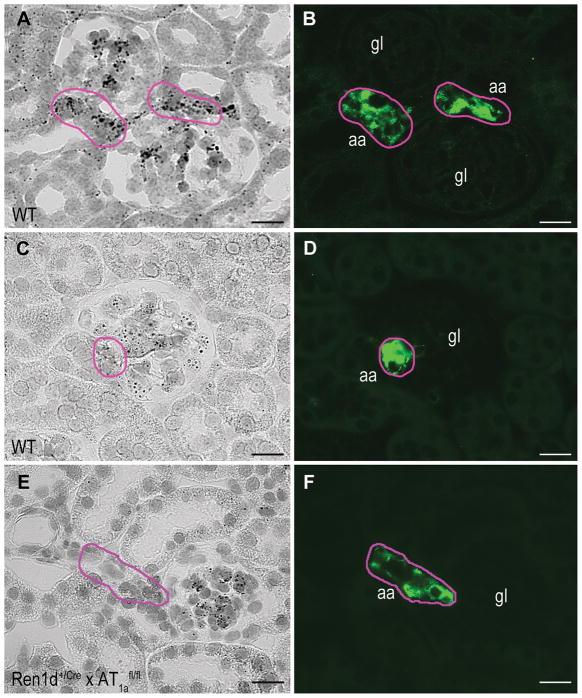
Intrarenal localization of Ang II-AT1 receptors was performed by in situ hybridization via RNA scope. As shown in Fig. 1 clear hybridization signals were obtained for both AT1a receptor-mRNA (Fig.1A) and AT1b receptor-mRNA (Fig.1C) in preglomerular renin-producing cells (RPCs). Immunohistochemical studies of the same slice for renin exhibit the typical position of RPCs in the media layer of afferent arterioles at the juxtaglomerular poles of the glomeruli (Fig.1B, D). Outside RPCs prominent signals for AT1a and AT1b receptor-mRNA were seen within the glomerulus and in the extraglomerular mesangium. Significant but less prominent signals for AT1a and AT1b receptor-mRNA were obtained for smooth muscle cells of afferent arterioles and for proximal tubules.
Figure 1.
Intrarenal distribution of AT1a receptor-mRNA expression (A) and AT1b receptor-mRNA expression (C) in WT kidneys can be observed as black dots. AT1a and AT1b receptor-mRNA was visualized by in situ hybridization with the RNAscope method. To identify renin-producing cells (RPCs) immunostaining of renin (green) was performed on the same slices (B, D). Pink labels indicate renin positive areas. Conditional renin-cell specific AT1a-deficient mice (Ren1d+/Cre x AT1afl/fl) show intraglomerular and tubular AT1a receptor distribution but no expression in RPCs (E, F). Magnification 400x, Scale bars = 20 μm, gl indicates glomerulus; aa, afferent arteriole.
To determine the in vivo relevance of AT1a receptors on RPCs for the regulation of renin expression and secretion we generated mice with a renin cell-specific deletion of AT1a receptors (Ren1d+/Cre x AT1afl/fl) using the Cre/loxP system. In the kidneys of these mice signals for AT1a receptor mRNA were absent from RPCs but still visible within glomeruli indicating effective Cre-lox recombination in RPCs (Fig.1E, F).
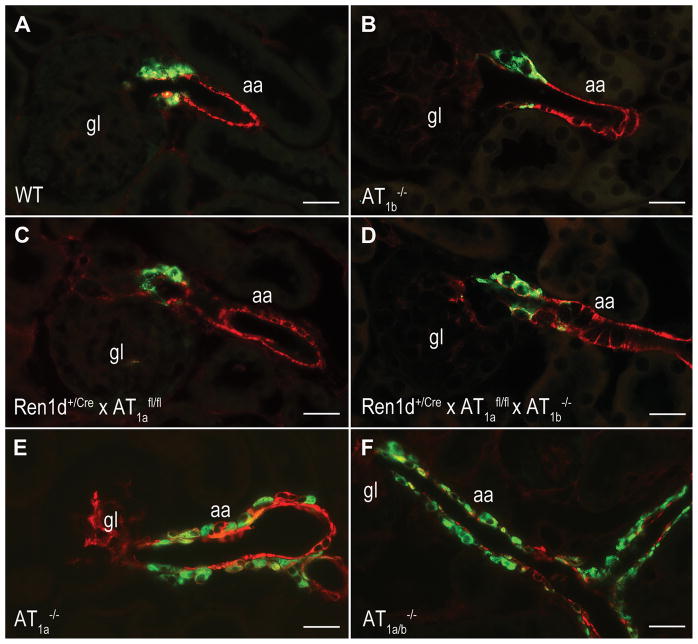
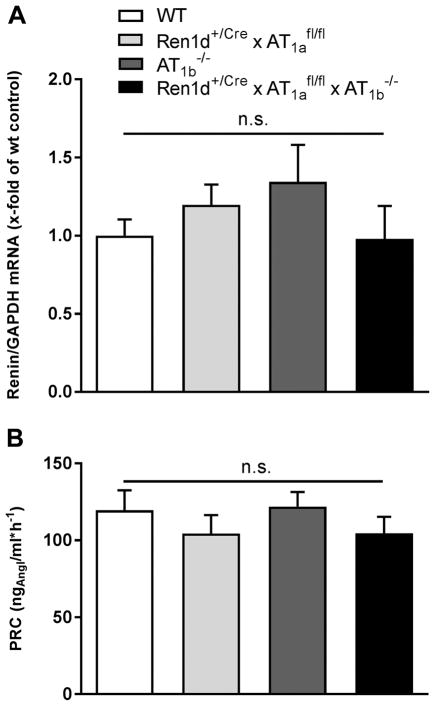
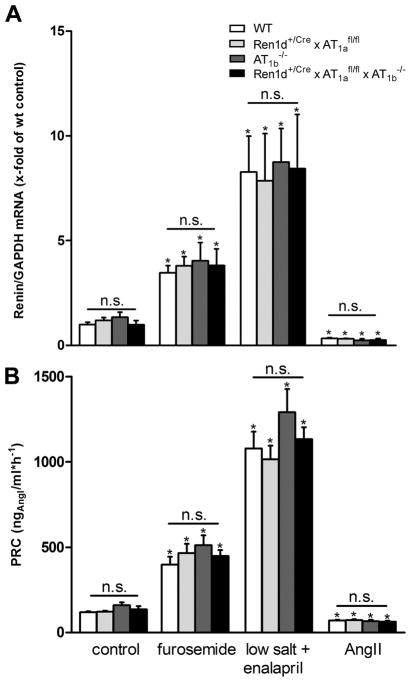
The number and distribution of RPCs in the kidneys of Ren1d+/Cre x AT1afl/fl mice was rather similar to wild type controls (Fig. 2A, C). In wild type kidneys, RPCs were typically located in the media layer of afferent arterioles at the juxtaglomerular poles of the glomeruli, identified by smooth muscle actin (Fig. 2A). The cuboid RPCs are few in number. In contrast, both in systemic AT1a-deficient mice and systemic AT1a x AT1b double knockout mice, the number of RPCs was drastically increased (Fig. 2E, F). Renin cells were associated with the juxtaglomerular apparatus, afferent and efferent arterioles, and to a minor extent also with bigger vessels like interlobular and arcuate side arteries. RPCs were found within the vessel walls but also in association with the outer circumference of the vessels. Renin-cell specific AT1a-deficient mice (Ren1d+/Cre x AT1afl/fl) featured RPCs only at the afferent arterioles in the juxtaglomerular area at the entrance into the glomerular tuft (Fig. 2C). In accordance also renal renin mRNA abundance and plasma renin concentrations were normal in these animals (Fig. 3).
Figure 2.
Immunostainings of renin (green) and α-SMA (red) in WT(A), AT1b−/−(B), conditional renin-cell specific AT1a-deficient mice (Ren1d+/Cre x AT1afl/fl)(C) and conditional renin-cell specific AT1a-deficient mice in an AT1b-deficient background (Ren1d+/Cre x AT1afl/fl x AT1b−/−)(D). The kidneys reveal normal number and distribution of RPCs. In contrast systemic AT1a−/− (E) and AT1a−/− x AT1b−/− mice (F) show massive recruitment of RPCs. Magnification 400x, Scale bars = 20 μm, gl indicates glomerulus; aa, afferent arteriole.
Figure 3.
Renin mRNA abundance (A) and plasma renin concentration (PRC) (B) under control conditions in WT, Ren1d+/Cre x AT1afl/fl, AT1b−/− and Ren1d+/Cre x AT1afl/fl x AT1b−/− mice. Renin mRNA expression level of WT control mice are set to 1 and serve as a reference. Data are means ±SEM. n=10 kidneys per group.
To not ignore a potential contribution of Ang II-AT1b receptors in the control of the renin system, we also studied mice with a global deletion of Ang II-AT1b receptors (AT1b−/−). AT1b−/− mice had normal RPCs, renin mRNA abundance, and plasma renin concentrations as a measure for in vivo renin secretion (Fig. 2B, Fig. 3).
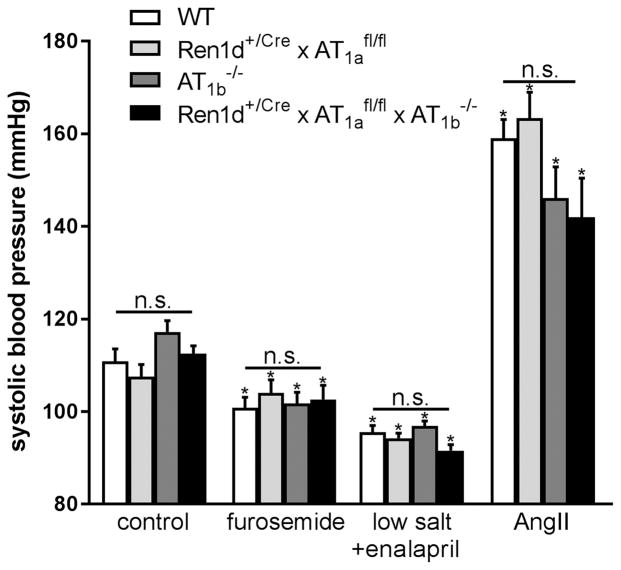
We next generated mice with conditional deletion of AT1a receptors on RPCs and a global deletion of AT1b receptors (Ren1d+/Cre x AT1afl/fl x AT1b−/−). Again, also these mice appeared normal with regard to RPCs (Fig. 2D), renin mRNA and plasma renin concentrations (Fig. 3). In accordance all genotypes analyzed in this context had normal blood pressure values determined by tail-cuff measurements (on average 112 mmHg) (Fig. 6 control).
Figure 6.
Systolic blood pressure (BP) under control conditions, after furosemide or low salt diet plus enalapril administration. Chronic Ang II infusion led to a clear increase in BP which was not diverging between the four genotypes. Data are means ±SEM. n=5 mice per group. * indicates P<0.05 vs untreated group of the same genotype n.s. = not significant.
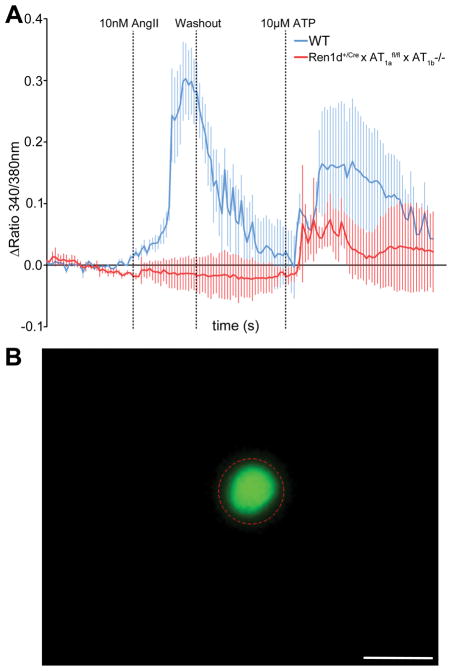
Additional evidence for a functional deletion of AT1 receptors was obtained from intracellular Ca2+-imaging in isolated single RPCs using Fura-2. For identification of the cells we crossed Ren1d+/Cre x AT1afl/fl x AT1b−/− and wild type with mice harboring GFP-expression in all active RPCs46. After Ang II-administration we could detect a marked increase in intracellular Ca2+ in wild type (0.30±0.06 Δ ratio 340/380 ±SEM) which was completely blunted in AT1-deficient cells (−0.01±0.02 Δ ratio 340/380 ±SEM) (Fig. 4).
Figure 4.
A) After Ang II administration the increase in intracellular free Ca2+ is completely abolished in Ren1d+/Cre x AT1afl/fl x AT1b−/− cells. However all cells responded to ATP in a control-setup. n=10 cells per group. Scale bar = 10 μm. B) Representative image of a GFP-positive RPC and the related ROI.
Next we examined the renin system in states of challenges of the RAAS which were induced by a combination treatment with low salt diet (0.02% NaCl) and with an ACE-inhibitor (enalapril), and by treatment with the loop diuretic furosemide. Combination treatment with low salt diet plus enalapril led to a strong increase of renin mRNA (8-fold) and plasma renin concentrations (9-fold) in wild types (Fig. 5). Furosemide also provoked a clear stimulation of the renin system. It is of note that all of these changes of the renin system found for wild types were seen in the same way and to the same extent in Ren1d+/Cre x AT1afl/fl, AT1b−/−, and Ren1d+/Cre x AT1afl/fl x AT1b−/− (Fig. 5).
Figure 5.
Renin mRNA abundance (A) and plasma renin concentration (PRC) (B) after furosemide treatment, low salt diet + enalapril or Ang II infusion in the different genotypes.
Data are means ±SEM. n = 5–10 kidneys per group. * indicates P<0.05 vs untreated group of the same genotype.
Combination treatment with low salt diet and ACE-inhibitor led to fall of blood pressure to 95 mmHg which however was not different between the mouse genotypes under investigation (Fig. 6). Furosemide treatment resulted in a statistically relevant decrease of blood pressure to on average 105 mmHg in all experimental groups (Fig. 6).
Subcutaneous infusion of angiotensin II (Ang II) lowered renin mRNA abundance by 70% and plasma renin concentration by 40% (Fig. 5). Very similar changes of renin mRNA and of plasma renin concentration upon infusion of Ang II were also seen for Ren1d+/Cre x AT1afl/fl, AT1b−/−, and Ren1d+/Cre x AT1afl/fl x AT1b−/− (Fig. 5). Ang II increased systolic blood pressure to similar levels (about 150 mmHg) in all genotypes (Fig. 6).
DISCUSSION
Our study aimed to directly test the concept of a negative feedback regulation of the renin system by Ang II mediated via Ang II-AT1 receptors in vivo directly on the level of RPCs.
The results of the present study strongly suggest the existence of functional Ang II-AT1 receptors on RPCs. This finding confirms previous studies33, 34. Effective deletion of AT1 receptors from RPCs was achieved by Cre-lox recombination of AT1a receptors (Fig.1) using Cre-recombinase driven by the mouse ren-1 promotor16 in combination with global constitutive deletion of AT1b receptors44. Strikingly RPCs in these mice developed normal and also basal renin expression and renin secretion were normal. This observation is quite different from the effects of global constitutive deletions of AT1a receptors or combined deletions of AT1a and AT1b receptors which show a 5-fold or 10-fold increase in basal renin expression versus WT47, 48. Constitutive deletion of AT1a receptors is associated with renin cell hyperplasia20, 22, 49 and this hyperplasia is further exaggerated by co-deletion of AT1a and AT1b receptors44. Notably, renin expression in mice with preferential deletion of AT1 receptors from renin cells strongly increased in response to low salt diet if the RAAS was generally inhibited by an angiotensin I-converting enzyme (ACE)-inhibitor, suggesting that Ang II indirectly affects renin expression. Again, the well-known strong stimulation of renin expression and secretion induced by ACE-inhibition in combination with low salt diet50 was not different between controls and mice with preferential deletion of AT1 receptors on renin cells. In accordance with previous reports40, 41 we also found that infusion of Ang II lowered renin expression and secretion in control mice. Notably, the suppression of the renin system by Ang II infusion occurred in a very similar fashion in Ren1d+/Cre x AT1afl/fl and controls. These findings doubting the existence of an Ang II short-loop feedback are in agreement with conclusions drawn from two previous studies. These studies found either no association between juxtaglomerular AT1a-chimerism and renin expression42 or reported normal renal renin expression when kidneys lacking AT1a receptors were transplanted into mice with intact systemic AT1a receptors43. In addition an in vitro single cell study reported that supraphysiological concentrations of Ang II caused inhibition of renin secretion in only less than 40% of renin cells isolated from adult kidneys. This finding therefore would already suggest that renin secretion from the majority of renin cells does not directly respond to Ang II and that the potential responding cells require high concentrations of Ang II that are probably not reached in vivo38. The question arises about the pathways along which Ang II could influence the renin system if there is in vivo no apparent direct effect on the level of RPCs. One common regulator of renin expression and secretion in normal mice and in mice lacking AT1 receptors on RPCs could be the blood pressure, which is known to influence the renin system in a reciprocal fashion1, 47, 51. ACE-inhibition in combination with low salt diet caused similar falls of systolic blood pressure in control mice and in mice lacking AT1 receptors on RPCs. Conversely, infusion of Ang II led to similar increases of blood pressure in both mouse genotypes.
A certain limitation of our study is that during development the mouse ren-1 promotor is active also in cells other than cells becoming finally renin producers in the kidney16. Therefore, it has to be taken into account, that the AT1a receptor was predominantly but not exclusively deleted from RPCs in Ren1d+/Cre x AT1afl/fl mice investigated in this study. Nonetheless, in these mice the renin system was normal and therefore clearly different from mice with global deletion of AT1a receptors indicating that the Ang II-AT1a receptors which are relevant for regulation of renin expression and secretion are still active in Ren1d+/Cre x AT1afl/fl mice. Possible candidates for such Ang II-AT1 receptors could be receptors on vascular smooth muscle cells, on tubular cells or on adrenal gland cells, all of which contribute to blood pressure10–13. It should be added in this context that mice with global deletion have low blood pressure52 whilst Ren1d+/Cre x AT1afl/fl mice as investigated in this study have normal blood pressure.
PERSPECTIVES
In summary, the results of the present study provide no evidence for a direct negative feedback control of renin production and secretion by Ang II in vivo. Since Ang II still influences renin expression in the absence of AT1 receptors in the renin cell lineage, AT1 receptors on other cells likely influence the renin system in a more indirect fashion. To identify the localization of AT1 receptors relevant for renin regulation remains a future and solvable task.
Supplementary Material
NOVELITY AND SIGNIFICANCE.
What is new
This study provides direct in vivo evidence that ANGII-ATR1-signaling in renin producing cells is dispensable for the control of renal renin synthesis and secretion.
What is relevant
Therapeutic inhibition of the renin-angiotensin-aldosterone system (RAAS) is confounded by a compensatory increase of renin synthesis and secretion. Although the mechanisms underlying this phenomenon have not yet been unraveled, it is commonly assumed that it reflects the removal of a direct and tonic inhibition of renin synthesis and secretion by ANGII via ANGII-AT1 receptors. This assumption forms the concept of a direct negative feedback of ANGII on renin.
Summary
We obtained no in vivo evidence for the concept of a direct negative feedback of ANGII mechanism of the renin system by ANG II on the level of renin producing cells. Thus, the well known disinhibition of renin synthesis and secretion by RAAS-inhibitors is more likely due to indirect effects of ANGII on renin secreting cells.
Acknowledgments
We gratefully acknowledge the expert technical assistance of Anita Zügner.
SOURCES OF FUNDING
This work was supported by Roswell Park Cancer Institute and National Cancer Institute (NCI) grant P30CA016056 and the German Research Council SFB 699/B2 and B6.
Footnotes
DISCLOSURE / (CONFLICT OF INTEREST)
All authors declared no competing interests.
References
- 1.Hackenthal E, Paul M, Ganten D, Taugner R. Morphology, physiology, and molecular biology of renin secretion. Physiological reviews. 1990;70:1067–1116. doi: 10.1152/physrev.1990.70.4.1067. [DOI] [PubMed] [Google Scholar]
- 2.Wagner C, Kurtz A. Regulation of renal renin release. Current opinion in nephrology and hypertension. 1998;7:437–441. doi: 10.1097/00041552-199807000-00015. [DOI] [PubMed] [Google Scholar]
- 3.Ekker M, Tronik D, Rougeon F. Extra-renal transcription of the renin genes in multiple tissues of mice and rats. Proc Natl Acad Sci U S A. 1989;86:5155–5158. doi: 10.1073/pnas.86.13.5155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Campbell DJ, Kladis A, Duncan AM. Nephrectomy, converting enzyme inhibition, and angiotensin peptides. Hypertension. 1993;22:513–522. doi: 10.1161/01.hyp.22.4.513. [DOI] [PubMed] [Google Scholar]
- 5.Peters J, Obermuller N, Woyth A, Peters B, Maser-Gluth C, Kranzlin B, Gretz N. Losartan and angiotensin ii inhibit aldosterone production in anephric rats via different actions on the intraadrenal renin-angiotensin system. Endocrinology. 1999;140:675–682. doi: 10.1210/endo.140.2.6489. [DOI] [PubMed] [Google Scholar]
- 6.Naftilan AJ, Oparil S. Inhibition of renin release from rat kidney slices by the angiotensins. Am J Physiol. 1978;235:F62–68. doi: 10.1152/ajprenal.1978.235.1.F62. [DOI] [PubMed] [Google Scholar]
- 7.Timmermans PB, Wong PC, Chiu AT, Herblin WF, Benfield P, Carini DJ, Lee RJ, Wexler RR, Saye JA, Smith RD. Angiotensin ii receptors and angiotensin ii receptor antagonists. Pharmacol Rev. 1993;45:205–251. [PubMed] [Google Scholar]
- 8.Berry C, Touyz R, Dominiczak AF, Webb RC, Johns DG. Angiotensin receptors: Signaling, vascular pathophysiology, and interactions with ceramide. American journal of physiology. Heart and circulatory physiology. 2001;281:H2337–2365. doi: 10.1152/ajpheart.2001.281.6.H2337. [DOI] [PubMed] [Google Scholar]
- 9.Crowley SD, Tharaux PL, Audoly LP, Coffman TM. Exploring type i angiotensin (at1) receptor functions through gene targeting. Acta physiologica Scandinavica. 2004;181:561–570. doi: 10.1111/j.1365-201X.2004.01331.x. [DOI] [PubMed] [Google Scholar]
- 10.Gurley SB, Riquier-Brison AD, Schnermann J, Sparks MA, Allen AM, Haase VH, Snouwaert JN, Le TH, McDonough AA, Koller BH, Coffman TM. At1a angiotensin receptors in the renal proximal tubule regulate blood pressure. Cell metabolism. 2011;13:469–475. doi: 10.1016/j.cmet.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Griendling KK, Ushio-Fukai M, Lassegue B, Alexander RW. Angiotensin ii signaling in vascular smooth muscle. New concepts. Hypertension. 1997;29:366–373. doi: 10.1161/01.hyp.29.1.366. [DOI] [PubMed] [Google Scholar]
- 12.Aguilera G. Role of angiotensin ii receptor subtypes on the regulation of aldosterone secretion in the adrenal glomerulosa zone in the rat. Molecular and cellular endocrinology. 1992;90:53–60. doi: 10.1016/0303-7207(92)90101-b. [DOI] [PubMed] [Google Scholar]
- 13.Masilamani S, Kim GH, Mitchell C, Wade JB, Knepper MA. Aldosterone-mediated regulation of enac alpha, beta, and gamma subunit proteins in rat kidney. The Journal of clinical investigation. 1999;104:R19–23. doi: 10.1172/JCI7840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sequeira-Lopez ML, Lin EE, Li M, Hu Y, Sigmund CD, Gomez RA. The earliest metanephric arteriolar progenitors and their role in kidney vascular development. American journal of physiology. Regulatory, integrative and comparative physiology. 2015;308:R138–149. doi: 10.1152/ajpregu.00428.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sauter A, Machura K, Neubauer B, Kurtz A, Wagner C. Development of renin expression in the mouse kidney. Kidney international. 2008;73:43–51. doi: 10.1038/sj.ki.5002571. [DOI] [PubMed] [Google Scholar]
- 16.Sequeira Lopez ML, Pentz ES, Nomasa T, Smithies O, Gomez RA. Renin cells are precursors for multiple cell types that switch to the renin phenotype when homeostasis is threatened. Dev Cell. 2004;6:719–728. doi: 10.1016/s1534-5807(04)00134-0. [DOI] [PubMed] [Google Scholar]
- 17.Spangler WL. Pathophysiologic response of the juxtaglomerular apparatus to dietary sodium restriction in the dog. Am J Vet Res. 1979;40:809–809. [PubMed] [Google Scholar]
- 18.Graham PC, Stewart HV, Downie I, Lindop GB. The distribution of renin-containing cells in kidneys with renal artery stenosis--an immunocytochemical study. Histopathology. 1990;16:347–355. doi: 10.1111/j.1365-2559.1990.tb01138.x. [DOI] [PubMed] [Google Scholar]
- 19.Schweda F. Salt feedback on the renin-angiotensin-aldosterone system. Pflugers Archiv : European journal of physiology. 2015;467:565–576. doi: 10.1007/s00424-014-1668-y. [DOI] [PubMed] [Google Scholar]
- 20.Machura K, Steppan D, Neubauer B, Alenina N, Coffman TM, Facemire CS, Hilgers KF, Eckardt KU, Wagner C, Kurtz A. Developmental renin expression in mice with a defective renin-angiotensin system. American journal of physiology. Renal physiology. 2009;297:F1371–1380. doi: 10.1152/ajprenal.00378.2009. [DOI] [PubMed] [Google Scholar]
- 21.Makhanova N, Lee G, Takahashi N, Sequeira Lopez ML, Gomez RA, Kim HS, Smithies O. Kidney function in mice lacking aldosterone. Am J Physiol Renal Physiol. 2006;290:F61–69. doi: 10.1152/ajprenal.00257.2005. [DOI] [PubMed] [Google Scholar]
- 22.Oliverio MI, Madsen K, Best CF, Ito M, Maeda N, Smithies O, Coffman TM. Renal growth and development in mice lacking at1a receptors for angiotensin ii. Am J Physiol. 1998;274:F43–50. doi: 10.1152/ajprenal.1998.274.1.F43. [DOI] [PubMed] [Google Scholar]
- 23.Kayes-Wandover KM, Tannin GM, Shulman D, Peled D, Jones KL, Karaviti L, White PC. Congenital hyperreninemic hypoaldosteronism unlinked to the aldosterone synthase (cyp11b2) gene. The Journal of clinical endocrinology and metabolism. 2001;86:5379–5382. doi: 10.1210/jcem.86.11.8005. [DOI] [PubMed] [Google Scholar]
- 24.Rosler A. The natural history of salt-wasting disorders of adrenal and renal origin. The Journal of clinical endocrinology and metabolism. 1984;59:689–700. doi: 10.1210/jcem-59-4-689. [DOI] [PubMed] [Google Scholar]
- 25.Kim HS, Maeda N, Oh GT, Fernandez LG, Gomez RA, Smithies O. Homeostasis in mice with genetically decreased angiotensinogen is primarily by an increased number of renin-producing cells. The Journal of biological chemistry. 1999;274:14210–14217. doi: 10.1074/jbc.274.20.14210. [DOI] [PubMed] [Google Scholar]
- 26.Wood JM, Maibaum J, Rahuel J, Grutter MG, Cohen NC, Rasetti V, Ruger H, Goschke R, Stutz S, Fuhrer W, Schilling W, Rigollier P, Yamaguchi Y, Cumin F, Baum HP, Schnell CR, Herold P, Mah R, Jensen C, O’Brien E, Stanton A, Bedigian MP. Structure-based design of aliskiren, a novel orally effective renin inhibitor. Biochemical and biophysical research communications. 2003;308:698–705. doi: 10.1016/s0006-291x(03)01451-7. [DOI] [PubMed] [Google Scholar]
- 27.Gomez RA, Chevalier RL, Everett AD, Elwood JP, Peach MJ, Lynch KR, Carey RM. Recruitment of renin gene-expressing cells in adult rat kidneys. The American journal of physiology. 1990;259:F660–665. doi: 10.1152/ajprenal.1990.259.4.F660. [DOI] [PubMed] [Google Scholar]
- 28.Gomez RA, Lynch KR, Chevalier RL, Everett AD, Johns DW, Wilfong N, Peach MJ, Carey RM. Renin and angiotensinogen gene expression and intrarenal renin distribution during ace inhibition. The American journal of physiology. 1988;254:F900–906. doi: 10.1152/ajprenal.1988.254.6.F900. [DOI] [PubMed] [Google Scholar]
- 29.Mooser V, Nussberger J, Juillerat L, Burnier M, Waeber B, Bidiville J, Pauly N, Brunner HR. Reactive hyperreninemia is a major determinant of plasma angiotensin ii during ace inhibition. Journal of cardiovascular pharmacology. 1990;15:276–282. doi: 10.1097/00005344-199002000-00015. [DOI] [PubMed] [Google Scholar]
- 30.Wagner C, Kees F, Kramer BK, Kurtz A. Role of sympathetic nerves for the stimulation of the renin system by angiotensin ii receptor blockade. Journal of hypertension. 1997;15:1463–1469. doi: 10.1097/00004872-199715120-00014. [DOI] [PubMed] [Google Scholar]
- 31.Case DB, Laragh JH. Reactive hyperreninemia in renovascular hypertension after angiotensin blockage with saralasin or converting enzyme inhibitor. Annals of internal medicine. 1979;91:153–160. doi: 10.7326/0003-4819-91-2-153. [DOI] [PubMed] [Google Scholar]
- 32.Owen RA, Molon-Noblot S, Hubert MF, Kindt MV, Keenan KP, Eydelloth RS. The morphology of juxtaglomerular cell hyperplasia and hypertrophy in normotensive rats and monkeys given an angiotensin ii receptor antagonist. Toxicol Pathol. 1995;23:606–619. doi: 10.1177/019262339502300319. [DOI] [PubMed] [Google Scholar]
- 33.Kakinuma Y, Fogo A, Inagami T, Ichikawa I. Intrarenal localization of angiotensin ii type 1 receptor mrna in the rat. Kidney Int. 1993;43:1229–1235. doi: 10.1038/ki.1993.174. [DOI] [PubMed] [Google Scholar]
- 34.Gasc JM, Shanmugam S, Sibony M, Corvol P. Tissue-specific expression of type 1 angiotensin ii receptor subtypes. An in situ hybridization study. Hypertension. 1994;24:531–537. doi: 10.1161/01.hyp.24.5.531. [DOI] [PubMed] [Google Scholar]
- 35.Scholz H, Kaissling B, Inagami T, Kurtz A. Differential response of renin secretion to vasoconstrictors in the isolated perfused rat kidney. J Physiol. 1991;441:453–468. doi: 10.1113/jphysiol.1991.sp018761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schweda F, Segerer F, Castrop H, Schnermann J, Kurtz A. Blood pressure-dependent inhibition of renin secretion requires a1 adenosine receptors. Hypertension. 2005;46:780–786. doi: 10.1161/01.HYP.0000183963.07801.65. [DOI] [PubMed] [Google Scholar]
- 37.Aldehni F, Tang T, Madsen K, Plattner M, Schreiber A, Friis UG, Hammond HK, Han PL, Schweda F. Stimulation of renin secretion by catecholamines is dependent on adenylyl cyclases 5 and 6. Hypertension. 2011;57:460–468. doi: 10.1161/HYPERTENSIONAHA.110.167130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tufro-McReddie A, Johns DW, Geary KM, Dagli H, Everett AD, Chevalier RL, Carey RM, Gomez RA. Angiotensin ii type 1 receptor: Role in renal growth and gene expression during normal development. Am J Physiol. 1994;266:F911–918. doi: 10.1152/ajprenal.1994.266.6.F911. [DOI] [PubMed] [Google Scholar]
- 39.Davis JO, Freeman RH. Mechanisms regulating renin release. Physiol Rev. 1976;56:1–56. doi: 10.1152/physrev.1976.56.1.1. [DOI] [PubMed] [Google Scholar]
- 40.Johns DW, Peach MJ, Gomez RA, Inagami T, Carey RM. Angiotensin ii regulates renin gene expression. Am J Physiol. 1990;259:F882–887. doi: 10.1152/ajprenal.1990.259.6.F882. [DOI] [PubMed] [Google Scholar]
- 41.Vander AJ, Geelhoed GW. Inhibition of renin secretion by angiotensin. Ii. Proc Soc Exp Biol Med. 1965;120:399–403. doi: 10.3181/00379727-120-30547. [DOI] [PubMed] [Google Scholar]
- 42.Matsusaka T, Nishimura H, Utsunomiya H, Kakuchi J, Niimura F, Inagami T, Fogo A, Ichikawa I. Chimeric mice carrying ‘regional’ targeted deletion of the angiotensin type 1a receptor gene. Evidence against the role for local angiotensin in the in vivo feedback regulation of renin synthesis in juxtaglomerular cells. J Clin Invest. 1996;98:1867–1877. doi: 10.1172/JCI118988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Crowley SD, Gurley SB, Oliverio MI, Pazmino AK, Griffiths R, Flannery PJ, Spurney RF, Kim HS, Smithies O, Le TH, Coffman TM. Distinct roles for the kidney and systemic tissues in blood pressure regulation by the renin-angiotensin system. J Clin Invest. 2005;115:1092–1099. doi: 10.1172/JCI200523378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Oliverio MI, Kim HS, Ito M, Le T, Audoly L, Best CF, Hiller S, Kluckman K, Maeda N, Smithies O, Coffman TM. Reduced growth, abnormal kidney structure, and type 2 (at2) angiotensin receptor-mediated blood pressure regulation in mice lacking both at1a and at1b receptors for angiotensin ii. Proc Natl Acad Sci U S A. 1998;95:15496–15501. doi: 10.1073/pnas.95.26.15496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gerl K, Nolan KA, Karger C, Fuchs M, Wenger RH, Stolt CC, Willam C, Kurtz A, Kurt B. Erythropoietin production by pdgfr-beta(+) cells. Pflugers Archiv : European journal of physiology. 2016;468:1479–1487. doi: 10.1007/s00424-016-1829-2. [DOI] [PubMed] [Google Scholar]
- 46.Glenn ST, Jones CA, Pan L, Gross KW. In vivo analysis of key elements within the renin regulatory region. Physiol Genomics. 2008;35:243–253. doi: 10.1152/physiolgenomics.00017.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Machura K, Neubauer B, Steppan D, Kettl R, Grobeta A, Kurtz A. Role of blood pressure in mediating the influence of salt intake on renin expression in the kidney. American journal of physiology. Renal physiology. 2012;302:F1278–1285. doi: 10.1152/ajprenal.00688.2011. [DOI] [PubMed] [Google Scholar]
- 48.Le TH, Oliverio MI, Kim HS, Salzler H, Dash RC, Howell DN, Smithies O, Bronson S, Coffman TM. A gammagt-at1a receptor transgene protects renal cortical structure in at1 receptor-deficient mice. Physiol Genomics. 2004;18:290–298. doi: 10.1152/physiolgenomics.00120.2003. [DOI] [PubMed] [Google Scholar]
- 49.Sugaya T, Nishimatsu S, Tanimoto K, Takimoto E, Yamagishi T, Imamura K, Goto S, Imaizumi K, Hisada Y, Otsuka A, et al. Angiotensin ii type 1a receptor-deficient mice with hypotension and hyperreninemia. J Biol Chem. 1995;270:18719–18722. doi: 10.1074/jbc.270.32.18719. [DOI] [PubMed] [Google Scholar]
- 50.Neubauer B, Machura K, Kettl R, Lopez ML, Friebe A, Kurtz A. Endothelium-derived nitric oxide supports renin cell recruitment through the nitric oxide-sensitive guanylate cyclase pathway. Hypertension. 2013;61:400–407. doi: 10.1161/HYPERTENSIONAHA.111.00221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nafz B, Berthold H, Ehmke H, Hackenthal E, Kirchheim HR, Persson PB. Flow versus pressure in the control of renin release in conscious dogs. Am J Physiol. 1997;273:F200–205. doi: 10.1152/ajprenal.1997.273.2.F200. [DOI] [PubMed] [Google Scholar]
- 52.Ito M, Oliverio MI, Mannon PJ, Best CF, Maeda N, Smithies O, Coffman TM. Regulation of blood pressure by the type 1a angiotensin ii receptor gene. Proc Natl Acad Sci U S A. 1995;92:3521–3525. doi: 10.1073/pnas.92.8.3521. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.