Abstract
Objective
We assessed the relationship of self-reported adherence versus antiretroviral therapy (ART) concentrations in hair with virologic outcomes among young people living with HIV.
Design
This was a cross-sectional study that enrolled young people living with HIV age 11–24 years, who attended a youth HIV clinic in Moshi, Tanzania.
Methods
ART adherence was assessed by self-report, drug concentration in hair samples, and plasma HIV-1 RNA measurements. Those with virologic failure, defined as plasma HIV-1 RNA > 400 copies/mL, had genotypic resistance assessed. Receiver operating characteristic (ROC) curves were used to evaluate ART-concentration threshold cut-offs for virologic suppression, after excluding those with known high-level resistance mutations.
Results
Among 280 young people enrolled, 227 were included in the final analysis. Seventy-two (32%) self-reported inadequate adherence and 91 (40%) had virologic failure. Hair ART-concentration (p<0.001), but not self-reported adherence (p=0.53), was associated with virologic outcome. Sixty-seven (74%) of those with virologic failure had resistance testing performed, of whom60% had high-level resistance. ROC curves demonstrated moderate or high classification performance for association with virologic suppression with specific hair ART-concentration cut-offs for lopinavir (1.8ng/mg), efavirenz (1.04 ng/mg), and nevirapine (33.2 ng/mg).
Conclusions
Hair ART-concentrations were significantly associated with virologic outcomes among young people living with HIV. ART-concentration thresholds associated with virologic suppression are proposed. Hair analysis may provide a non-invasive, cost-effective adherence assessment tool in settings with limited 2nd and 3rd-line treatment options.
Keywords: adherence, HIV, hair analysis, resource-limited setting, young people, adolescents, youth
Introduction
AIDS is the leading cause of death for adolescents in Africa and the second cause globally[1]. Adequate adherence to antiretroviral therapy (ART) is key to remaining healthy, preventing opportunistic infections, maintaining sustained virologic suppression, and averting viral resistance [2]; however, young people have particular difficulty adhering to ART with worse virologic outcomes compared to both children [3] and adults[4]. Poor adherence among young people could be due to a number of biologic and psychosocial factors, such as mode of HIV acquisition (perinatally-acquired versus behaviorally-acquired HIV)[5]; perceived difficulty of regimen and forgetfulness [6]; stigma and/or mental health issues[7]; and, medication side effects and pill burden [2].
Measuring ART adherence remains a challenge. Self-report from caregivers or patients is common, low-cost, and easily implemented, but is highly subjective with known limitations of overestimating adherence compared to objective measures [8]. More objective measures like pill counts, pharmacy refills, and electronic monitoring systems—pill bottles which digitally record bottle openings—have shown stronger association with virologic outcomes, yet are limited by expense[9]and an inability to reflect actual medication consumption[10]. Plasma and urine levels are more reliable quantitative measures of drug adherence; however, both are limited by reflecting only short windows (one to two days) of adherence and require specimen collection using biohazardous precautions and cold storage[11, 12]. Compared to these other measures, direct measurement of HIV-1 RNA levels may afford greater insight into adherence; however, frequent viral load testing may be limited by expense in less resourced settings and interpretation might be complicated in the setting of drug resistance.
Analyzing drug concentration in hair as a metric of adherence affords some advantages. Hair measures are objective and provide a long-term window of adherence (weeks to months)[13]. Collection is logistically simpler than for blood because phlebotomy skills are not required, collection is non-invasive, and samples can be stored at room temperature[14], avoiding cold-storage challenges in settings with unpredictable power outages. When partnered with viral load testing, ART concentrations in hair can provide further insight into the possibility of drug resistance (e.g., likely if both hair ART-concentration andHIV-1 RNA level are high). In adults, a number of studies have shown the relationship between ART levels in hair and virologic outcomes [12, 15–18], but little research exists to assess the validity of this measure among young people. ART concentrations in the pediatric population can offer not only information on adherence, but also provide pharmacokinetic information in the setting of dosing changes and maturing metabolizing and clearance mechanisms. Identifying drug concentrations at which virologic suppression is expected may provide insight into dosing and help unravel whether failure is due to adherence versus resistance.
The aim of this study was to determine the association between self-reported adherence and ART concentration in hair with virologic outcomes and to define ART-level thresholds expected to predict virologic suppression in a cohort of young people living with HIV in Tanzania.
Methods
This was a cross-sectional study that enrolled HIV-positive young people between 11 and 24 years of age who knew their HIV status and attended a monthly youth-focused HIV clinic called “Teen Club”at either Kilimanjaro Christian Medical Centre (KCMC) (December 2013 – December 2014) or Mawenzi Regional Referral Hospital (MRRH) (February – July 2015) in Moshi, Tanzania. Teen Club is a unique Saturday HIV clinic for young people (11–24 years of age). Young people who know their HIV status are referred to Teen Club from the standard care and treatment clinic. All young people attending Teen Club receive education on topics such as stigma, adherence, and sexual reproductive health, in addition to monthly doctor visit and ART prescription refill.
A structured questionnaire was administered to study participants in Swahili. Four trained, native Swahili-speaking, female research assistants aged 25–35 years administered the questionnaire to study participants, which included queries on self-reported adherence by asking dichotomously, “Have you missed any doses of your medication in the last two weeks, yes or no?” and categorically, “Think about the past week (7 days); on average, how often did you miss a dose of medication?” Response options included, “(1) once a day; (2) more than once a week, but not every day; (3) once a week; or (4) I don’t miss my medicine.” Inadequate adherence by self-report was defined as reporting any missed ART doses on either of the survey items. Participant ART regimen was extracted from the clinical file. First-line regimens included two nucleoside reverse transcriptase inhibitors (NRTI) plus a non-nucleoside reverse transcriptase (NNRTI) of either nevirapine (NVP) or efavirenz (EFV); second-line regimens included two NRTIs plus a ritonavir (RTV) boosted protease inhibitor (PI) of either lopinavir (LPV) or atazanavir (ATV).
HIV measures
The participants’ most recent CD4 result and current ART regimen at the time of the questionnaire were extracted from the clinical file. Using previously described methods[15], hair samples of 20–30 strands were cut from the occipital scalp close to the follicle, enclosed in foil, sealed in a plastic bag at room temperature, labeled with a participant identifier number, and stored in a dark locked drawer. If braided, hair was collected between braids. Blood for HIV-1 RNA measurements was collected concurrently with hair. ART concentrations in hair were measured for EFV, NVP, LPV, ATV, and RTV at the University of California, San Francisco Hair Analytical Laboratory (HAL). The methods for analyzing ART concentrations in hair are validated, reproducible liquid chromatography-tandem mass spectrometry-based methods that are peer-reviewed and approved by the National Institutes of Health-support Division of AIDS Clinical Pharmacology and Quality Assurance Program[19, 20]. HIV-1 RNA analysis was performed at the Kilimanjaro Clinical Research Institute (KCRI) Biotechnology Laboratory, which participates in international external quality assurance programs, using the Abbott m2000 (Des Plaines, IL). Virologic failure was defined as plasma HIV-1 RNA > 400 copies/mL. For those with virologic failure, plasma samples were sent to the Duke Human Vaccine Institute (DHVI) where reverse transcriptase resistance testing was performed using SuperScript® III Reverse Transcriptase (Invitrogen; Carlsbad, CA) following the manufacture’s instruction. Sequencing results were edited using Geneious R8 (Auckland 1010, New Zealand). Sequences were aligned and the phylogenetic tree was drawn by using SeaView version 4 [21], with sequence interpretation performed by the Stanford University HIVdb program[22].
Statistical analyses
Descriptive statistics were used to summarize demographic, adherence, and virologic outcomes. Statistical analyses to compare demographic characteristics between participants with virologic suppression versus failure were performed using the chi-square test of independence or Wilcoxon rank sum test for categorical and continuous variables, respectively. Drug-concentration threshold values were derived with receiver operating characteristic (ROC) curves, excluding those with known high-level resistance mutations. All statistics were generated using Stata/SE 14.1 (StataCorp, College Station, Texas).
Ethical review and informed consent
Written informed consent was provided by participants 18 years or older. A parent or guardian provided consent for participants younger than 18 years of age, along with youth assent. The Duke University Medical Center Institutional Review Board, the KCMC Research Ethics Committee, and the Tanzanian National Institute of Medical Research approved the study protocol.
Results
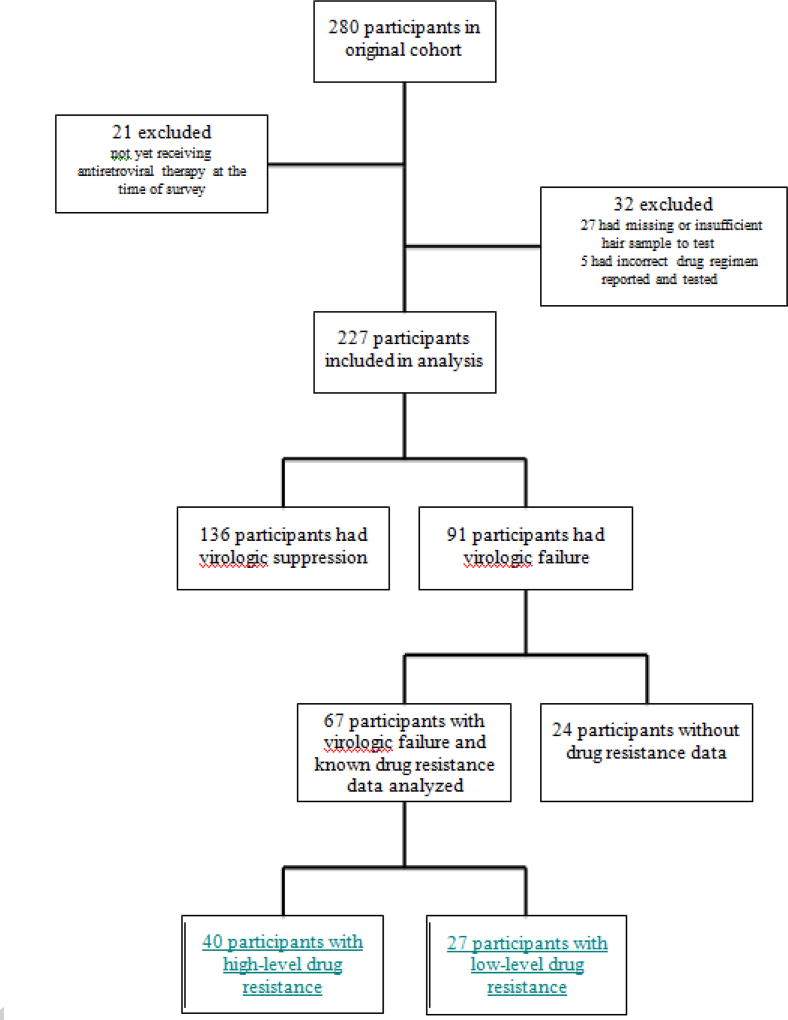
Of 280 participants enrolled, we excluded 21 who had not yet started ART, 27 who had insufficient hair for sampling, and five who had an incorrect ART regimen reported and tested, leaving 227 in this analysis (Figure 1). Excluded participants were similar to included participants in demographics and self-reported adherence, with the exception of more participants being excluded from KCMC and experiencing virologic failure. Individuals with and without virologic failure had similar demographics (Table 1). Median age of participants was 16 years and nearly half were female;61% attended KCMC. The median time receiving ART was six years and the majority (72%) were still receiving first-line ART. Approximately 30% of participants self-reported missing ART medication overall; 30% of those with virologic suppression self-reported missing ART and were defined as having inadequate adherence. Virologic failure was demonstrated in 40% overall. Absolute CD4 cell counts were significantly higher in those with virologic suppression compared to those with virologic failure (p<0.001).
Figure 1.
Table 1.
Demographic Characteristics by Virologic Outcome (N=227)
| Characteristics | Viral Suppression (N=136) |
Viral Failure (N=91) |
pa | ||
|---|---|---|---|---|---|
| No. | % | No. | % | ||
|
| |||||
| Hospital Site | |||||
|
| |||||
| Kilimanjaro Christian Medical Centre | 81 | 59.6 | 58 | 63.7 | 0.527 |
| Mawenzi Hospital | 55 | 40.4 | 33 | 36.3 | |
|
| |||||
| Age, y | |||||
|
| |||||
| Median (IQR) | 16 | (14–18) | 16 | (14–18) | 0.679 |
|
| |||||
| Gender | |||||
|
| |||||
| Female | 66 | 48.5 | 36 | 39.6 | 0.183 |
| Male | 70 | 51.5 | 55 | 60.4 | |
|
| |||||
| Time receiving ART, y | |||||
|
| |||||
| Median (IQR) | 6 | (4–9) | 6 | (4–8) | 0.381 |
|
| |||||
| Current ART | |||||
|
| |||||
| NNRTI-based | 103 | 75.7 | 61 | 67.0 | 0.151 |
| PI-based | 33 | 24.3 | 30 | 33.0 | |
|
| |||||
| CD4, cells/mm3 | |||||
|
| |||||
| < 350 | 28 | 20.6 | 41 | 45.1 | < 0.001 |
| >/= 350 | 108 | 79.4 | 50 | 54.9 | |
|
| |||||
| Self-Reported Adherent | |||||
|
| |||||
| Adherent | 95 | 69.9 | 60 | 65.9 | 0.534 |
| Non-Adherent | 41 | 30.1 | 31 | 34.1 | |
Viral Failure: HIV-1 RNA >400 copies/mL; IQR, interquartile range; ART, antiretroviral therapy; NNRTI, non-nucleoside reverse transcriptase inhibitor; PI, protease inhibitor;
Chi-Square test of independence or Wilcoxon rank sum test.
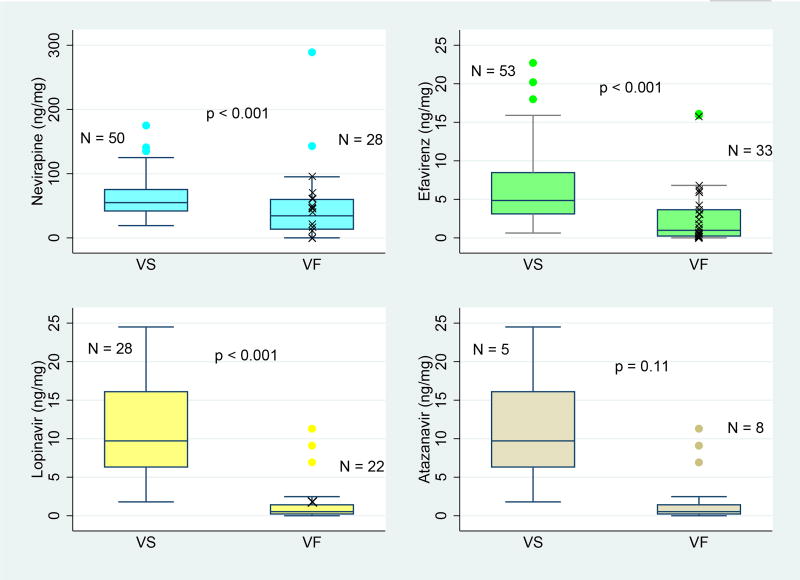
Virologic outcomes were not significantly associated with self-reported adherence (p=0.53; Table 1), but were significantly associated with ART concentration in hair (p<0.001) for all drugs except ATV (p=0.11; Table 2; Figure 2). Of 91 participants with virologic failure, 67(74%) underwent ART resistance testing, 40 (60%) of whom had high-level resistance (Figure 1). For participants receiving first-line therapy, high-level resistance was identified for 15/16 (94%) participants failing NVP and 24/26(92%) failing EFV. The one participant failing therapy and without resistance to NVP had a hair drug-level below the limit of detection (<0.50 ng/mg). Similarly, the two youth without resistance to EFV had low hair drug-levels of <0.05 ng/mg and 0.62 ng/mg. For participants receiving second-line therapy,1/18 (6%) had high-level resistance to LPV and no youth (0/7) had resistance to ATV.
Table 2.
Hair Drug-Concentration by Virologic Outcome (N=227)
| Viral Suppression (N = 136) |
Viral Failure (N = 91) |
||||||
|---|---|---|---|---|---|---|---|
|
|
|||||||
| ART Analyzed |
Na | Median concentration (ng/mg) |
IQR | Na | Median concentration (ng/mg) |
IQR | pb |
| ATV | 5 | 7.09 | (2.30–7.12) | 8 | 2.06 | (0.75–3.22) | 0.11 |
| LPV | 28 | 9.72 | (6.32–16.10) | 22 | 0.53 | (0.23–1.42) | < 0.001 |
| RTVc | 33 | 0.84 | (0.61–1.27) | 30 | 0.14 | (0.03–0.51) | < 0.001 |
| EFV | 53 | 4.85 | (3.11–8.47) | 33 | 0.98 | (0.24–3.65) | < 0.001 |
| NVP | 50 | 54.85 | (41.90–75.30) | 28 | 34.35 | (13.55–59.80) | < 0.001 |
Viral Failure: HIV-1 RNA >400 copies/mL; ART, antiretroviral therapy; IQR, interquartile range; ATV, Atazanavir; LPV, Lopinavir; RTV, Ritonavir; EFV, Efavirenz; NVP, Nevirapine;
The total exceeds 136 and 91 for viral suppression and viral failure, respectively, due to the use of a combination pill of RTV with either ATV (ATV/r) or LPV (LPV/r);
Wilcoxon rank sum test;
RTV is the booster drug administered as a combination pill for both ATZ/r and LPV/r.
Figure 2. Hair ART-concentration by virologic outcome.
VS, virologic suppression; VF, virologic failure: HIV-1 RNA >400 copies/mL; p-value for Wilcoxon rank sum test; participants with VF and high-level resistance mutations shown (X), if known.
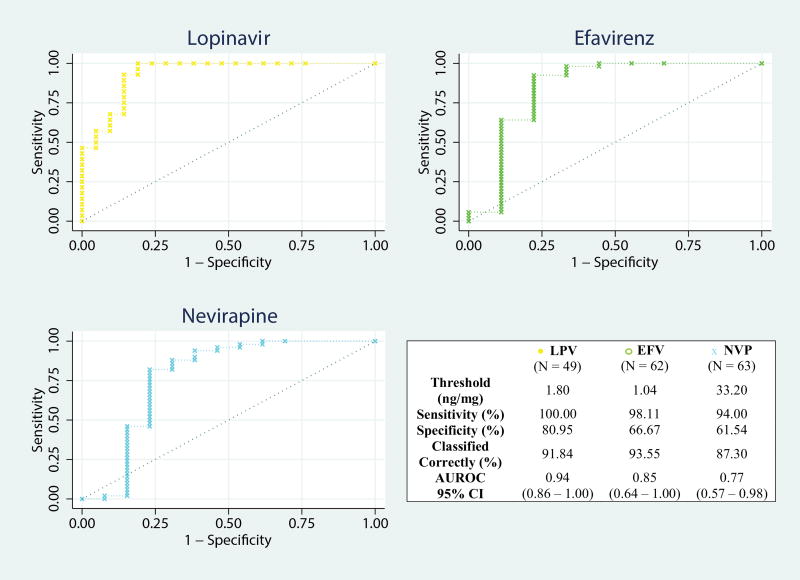
After excluding those with high-level resistance mutations, the ROC curve demonstrated high classification performance in predicting virologic outcome for LPV concentrations in hair (area under the curve [AUC]= 0.94, 95% confidence interval [CI], 0.86–1.00;Figure 3) and moderate classification performance for EFV hair-levels (AUC = 0.85 [95% CI, 0.64–1.00]), as well as NVP hair-levels (AUC = 0.77 [95% CI, 0.57–0.98]). Using the hair ART-concentration with the highest percent of correctly classified samples, we determined hair ART-concentration thresholds predicting virologic suppression for LPV (1.80 ng/mg, sensitivity 100.00%, specificity 80.95%), EFV (1.04 ng/mg, sensitivity 98.11%, specificity 66.67%), and NVP (33.20 ng/mg, sensitivity 94.00%, specificity 61.54%; Figure 3).
Figure 3. Hair ART-concentration receiver operating characteristic curves.
ART, antiretroviral therapy; LPV, Lopinavir; EFV, Efavirenz; NVP, Nevirapine; AUROC, area under receiver operating characteristic curve; CI, confidence interval.
Discussion
Our study of young people living with HIV in Tanzania found high rates of virologic failure (40%) and high rates of viral resistance (60%). The study also demonstrated a strong relationship between an objective measure of adherence to ART using hair levels (but not self-reported adherence) with virologic suppression. This study is among the first to investigate the association of hair ART-concentrations with virologic outcomes among young people and is the first to determine thresholds of hair concentrations of various ARTs (NVP, EFV, and LPV) associated with virologic suppression. The only other pediatric study evaluating hair NNRTI-levels demonstrated a non-significant association of hair metrics with other adherence measures, but did not examine the association between hair levels and virologic outcomes [23]. Three other studies in the pediatric population have reported that increased PI levels in hair are significantly associated with virologic suppression [14, 24, 25]. The age range of children in two of these studies [14, 24] was 7 to 13 years, representing a younger developmental phase, likely with different pharmacokinetic characteristics[26] than participants in our study. In a third study, ATV levels in hair were significantly associated with virologic outcomes in adolescents[25]; however, our study had only a few participants receiving ATV-based regimens (N=13), limiting our ability to identify concentration thresholds for this drug.
Self-reported adherence was not associated with virologic outcomes, a finding consistent with other studies demonstrating the poor utility of self-report as an adherence measure [8]. Within this finding, 30% of participants who self-reported missing doses, therefore defined as inadequate adherence over the past 2 weeks, actually had virologic suppression. In the present age of more robust ART, missing one or two doses a week may be adequate to maintain virologic suppression with some regimens[27]. Based on the self-report adherence questions used in this study, we were unable to assess the relationship between longer self-reported periods of non-adherence and virologic outcomes.
Virologic failure rates among the Tanzanian young people in this cohort were staggeringly high (40%), higher than youth studies elsewhere in Africa[28–30] and Asia[31, 32]. With limited access to viral load testing, one reason might be the challenge for physicians of knowing when to switch a patient from first-line to second-line therapies, leading to conditions in which viral resistance mutations can accumulate on a failing regimen. Self-report poorly assesses adherence, and while the WHO has established clinical criteria to help physicians judge when to switch regimens, in resource-limited settings these criteria have also been documented as poor predictors of failure[33–35].
The high rate of virologic failure observed in this study underscores the vulnerability of this population and represents a major public health concern. Sub-optimal adherence can lead to virologic failure which increases the risk of forward transmission and the development of additional resistance mutations over time, limiting future treatment options[36]. The longer virologic failure persists, and at a higher viral load, the greater the number of resistance mutations that accumulate [37].
A large proportion (74%) of participants with virologic failure (HIV-1 RNA > 400 copies/mL) in our study underwent resistance testing. The majority of young people failing first-line therapy had high-level resistance mutations to NVP and EFV. As NNRTI-based regimens are considered first-line treatment for children over 3 years old in developing settings, resistance to these backbone medications poses a significant public health challenge. As these settings have limited access to second- and third-line treatments, it is necessary that children and adolescents be maintained on first-line medications as long as possible; however, a major limitation to durability is inadequate adherence. The WHO global 90-90-90 targets (90% of people with HIV diagnosed, 90% of diagnosed receiving treatment, and 90% of treated with virologic suppression) will be significantly challenged with high rates of virologic failure and ART resistance[38].
Determining ART-concentration thresholds in hair above which young people are likely to suppress virologically is clinically useful. If plasmaHIV-1 RNA is elevated, one must determine whether the problem is poor adherence, a resistant virus, or both. Through identifying a low drug concentration in hair, practitioners can intervene with adherence-focused counseling with the goal to reach an adequate drug level and suppress viral loads without the need to switch regimens. Chawana et al.[25] were able to identify a threshold level of ATV in hair (>2.35 ng/mg) associated with virologic suppression, although 38% of those above the cut-off still exhibited virologic failure. Using ROC curves, we identified a threshold with high classification performance for LPV (1.80 ng/mg) and thresholds with moderate performance for EFV (1.04 ng/mg) and NVP (33.20 ng/mg). All three had high sensitivities, but with reduced specificities, especially for EFV and NVP, possibly due to a quarter of the samples lacking resistance data.
Although hair collection is inexpensive and easy, liquid chromatography/tandem mass spectrometry-based methods of analyses are expensive[16]. However, lower-cost methods using thin-layer chromatography are currently in development[39]. As data on the promise of this adherence metric burgeons, the benefit to reducing the risk of high-level HIV resistance mutations for both adults and young people by objective adherence measurement might prove cost-effective[40]. Hair analysis coupled with viral load measurement has the added benefit of allowing the clinician to infer information regarding resistance. Knowing the viral load alone cannot provide the distinction between failure due to poor adherence or secondary to viral resistance.
Our study has limitations. First, this is a cross-sectional study and therefore cannot assess the development of resistance mutations and their association with adherence overtime. Second, since resistance testing was not available for a quarter of those with virologic failure, inclusion of these samples in the ROC analysis might have skewed the results if high-level resistance was indeed present. Lastly, we have not rigorously tested threshold ART levels in hair. Future research with a larger sample size and more complete resistance data is needed to validate the sensitivity and specificity of ART-specific threshold levels associated with virologic suppression to enable implementation of these thresholds into clinical care.
Conclusion
Finding low-cost, effective methods to assess adherence remain critical to managing HIV treatment outcomes of young people. In this study, ART concentrations in hair were significantly associated with virologic outcomes and represented a more accurate adherence measure compared to self-report. Identifying an ART-concentration threshold associated with virologic suppression may provide a non-invasive, cost-effective, clinically-useful tool for young people living with HIV in settings with limited second-and third-line treatment options.
Acknowledgments
Source of Funding: This work was supported by Duke University Center for AIDS Research (CFAR), an NIH funded program [P30 AI064518 to DED]; International Research Scientist Development Award funded by the Fogarty International Center and the National Institute of Mental Health [K01 TW-009985 to DED]; the Global Health Fellows Program of the National Institutes of Health funded by the Fogarty International Center and the National Institute of Mental Health [R25 TW009337 to ZJT]; the Infectious Diseases Society of America Medical Scholars Program [to ZJT]. The National Institute of Allergy and Infectious Diseases at the National Institutes of Health funded analyses of hair samples [2RO1AI098472 to MG] in the UCSF Hair Analytical Laboratory (HAL).
The authors would like to thank the clinical, research, and laboratory staff and patients at the Kilimanjaro Christian Medical Centre Child Centered Family Care Clinic and Mawenzi Regional Care and Treatment Center. We especially thank Elizabeth Amos, HedwigaMrema, ElissianaNdossa, Eunice Chibanda, and Gabriel Karia for their hard work in consenting, enrolling, obtaining hair samples and questionnaire responses, phlebotomy, and providing medical records during the duration of this project. We also thank the Kilimanjaro Christian Research Institute Biotechnology Laboratory staff who ran the HIV-1 RNA and the DHVI laboratory, and Drs. FengGao and FangpingCai for their work on resistance sequencing. We want to thank the other members of the UCSF Hair Analytical Laboratory for their time and effort, and to thank John Gallis of the Research Design and Analysis Core of the Duke Global Health Institute for his input on the ROC analysis. Finally, we extend a sincere thanks to the passionate and energetic youth who gave their time, hair, and blood.
Footnotes
Conflicts of Interest All remaining authors report no conflicts.
Author contributions: D.E.D., C.K.C., B.T.M., and A.S.M. designed the trial. D.E.D., B.T.M, A.S.M. oversaw data collection. Z.J.T., M.G., A.L., K.K., H.O., E.L.T., and D.E.D. analyzed the data. Z.J.T., E.L.T., and D.E.D. interpreted the data. Z.J.T., E.L.T., M.G., C.K.C., and D.E.D. wrote the manuscript. All authors reviewed and edited draft versions of the manuscript and contributed to the intellectual content of the article.
References
- 1.United Nations Children's Emergency Fund (UNICEF) Children & AIDS: 2015 statistical update. 2015 [Google Scholar]
- 2.Nabukeera-Barungi N, Elyanu P, Asire B, Katureebe C, Lukabwe I, Namusoke E, et al. Adherence to antiretroviral therapy and retention in care for adolescents living with HIV from 10 districts in Uganda. BMC Infect Dis. 2015;15:520. doi: 10.1186/s12879-015-1265-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dow DE, Shayo AM, Cunningham CK, Reddy EA. Durability of antiretroviral therapy and predictors of virologic failure among perinatally HIV-infected children in Tanzania: A four-year follow-up. BMC Infect Dis. 2014;14:567. doi: 10.1186/s12879-014-0567-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nachega JB, Hislop M, Nguyen H, Dowdy DW, Chaisson RE, Regensberg L, et al. Antiretroviral therapy adherence, virologic and immunologic outcomes in adolescents compared with adults in southern Africa. J Acquir Immune Defic Syndr. 2009;51(1):65–71. doi: 10.1097/QAI.0b013e318199072e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chandwani S, Koenig LJ, Sill AM, Abramowitz S, Conner LC, D'Angelo L. Predictors of antiretroviral medication adherence among a diverse cohort of adolescents with HIV. J Adolesc Health. 2012;51(3):242–251. doi: 10.1016/j.jadohealth.2011.12.013. [DOI] [PubMed] [Google Scholar]
- 6.Ankrah DN, Koster ES, Mantel-Teeuwisse AK, Arhinful DK, Agyepong IA, Lartey M. Facilitators and barriers to antiretroviral therapy adherence among adolescents in Ghana. Patient preference and adherence. 2016;10:329–337. doi: 10.2147/PPA.S96691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dow DE, Turner EL, Shayo AM, Mmbaga B, Cunningham CK, O'Donnell K. Evaluating mental health difficulties and associated outcomes among HIV-positive adolescents in Tanzania. AIDS Care. 2016;28(7):825–833. doi: 10.1080/09540121.2016.1139043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vreeman RC, Nyandiko WM, Liu H, Tu W, Scanlon ML, Slaven JE, et al. Measuring adherence to antiretroviral therapy in children and adolescents in western Kenya. Journal of the International AIDS Society. 2014;17:19227. doi: 10.7448/IAS.17.1.19227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Muller AD, Jaspan HB, Myer L, Hunter AL, Harling G, Bekker LG, et al. Standard measures are inadequate to monitor pediatric adherence in a resource-limited setting. AIDS Behav. 2011;15(2):422–431. doi: 10.1007/s10461-010-9825-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kagee A, Nel A. Assessing the association between self-report items for HIV pill adherence and biological measures. AIDS Care. 2012;24(11):1448–1452. doi: 10.1080/09540121.2012.687816. [DOI] [PubMed] [Google Scholar]
- 11.Duong M, Piroth L, Peytavin G, Forte F, Kohli E, Grappin M, et al. Value of patient self-report and plasma human immunodeficiency virus protease inhibitor level as markers of adherence to antiretroviral therapy: Relationship to virologic response. Clin Infect Dis. 2001;33(3):386–392. doi: 10.1086/321876. [DOI] [PubMed] [Google Scholar]
- 12.Yan J, Liu J, Su B, Pan X, Wang Z, Wu J, et al. Lamivudine concentration in hair and prediction of virologic failure and drug resistance among HIV patients receiving free art in China. PLoS One. 2016;11(4):e0154421. doi: 10.1371/journal.pone.0154421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gandhi M, Greenblatt RM. Hair it is: The long and short of monitoring antiretroviral treatment. Ann Intern Med. 2002;137(8):696–697. doi: 10.7326/0003-4819-137-8-200210150-00016. [DOI] [PubMed] [Google Scholar]
- 14.Pintye J, Bacchetti P, Teeraananchai S, Kerr S, Prasitsuebsai W, Singtoroj T, et al. Brief report: Lopinavir hair concentrations are the strongest predictor of viremia in HIV-infected Asian children and adolescents on second-line antiretroviral therapy. J Acquired Immune Defic Syndromes. 2017;76(4):367–371. doi: 10.1097/QAI.0000000000001527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hickey MD, Salmen CR, Tessler RA, Omollo D, Bacchetti P, Magerenge R, et al. Antiretroviral concentrations in small hair samples as a feasible marker of adherence in rural Kenya. J Acquir Immune Defic Syndr. 2014;66(3):311–315. doi: 10.1097/QAI.0000000000000154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baxi SM, Greenblatt RM, Bacchetti P, Jin C, French AL, Keller MJ, et al. Nevirapine concentration in hair samples is a strong predictor of virologic suppression in a prospective cohort of HIV-infected patients. PLoS One. 2015;10(6):e0129100. doi: 10.1371/journal.pone.0129100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gandhi M, Ameli N, Bacchetti P, Anastos K, Gange SJ, Minkoff H, et al. Atazanavir concentration in hair is the strongest predictor of outcomes on antiretroviral therapy. Clin Infect Dis. 2011;52(10):1267–1275. doi: 10.1093/cid/cir131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koss CA, Natureeba P, Mwesigwa J, Cohan D, Nzarubara B, Bacchetti P, et al. Hair concentrations of antiretrovirals predict viral suppression in HIV-infected pregnant and breastfeeding Ugandan women. AIDS. 2015;29(7):825–830. doi: 10.1097/QAD.0000000000000619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang Y, Gandhi M, Greenblatt RM, Gee W, Lin ET, Messenkoff N. Sensitive analysis of anti-HIV drugs, efavirenz, lopinavir and ritonavir, in human hair by liquid chromatography coupled with tandem mass spectrometry. Rapid Commun Mass Spectrom. 2008;22(21):3401–3409. doi: 10.1002/rcm.3750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.DiFrancesco R, Tooley K, Rosenkranz S, Siminski S, Taylor CR, Pande P, et al. Clinical pharmacology quality assurance for hiv and related infectious diseases research. Clin Pharmacol Ther. 2013;93(6):479–482. doi: 10.1038/clpt.2013.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gouy M, Guindon S, Gascuel O. Seaview version 4: A multiplatform graphical user interface for sequence alignment and phylogenetic tree building. Mol Biol Evol. 2009;27(2):221–224. doi: 10.1093/molbev/msp259. [DOI] [PubMed] [Google Scholar]
- 22.Liu TF, Shafer RW. Web resources for HIV type 1 genotypic-resistance test interpretation. Clin Infect Dis. 2006;42(11):1608–1618. doi: 10.1086/503914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Olds PK, Kiwanuka JP, Nansera D, Huang Y, Bacchetti P, Jin C, et al. Assessment of HIV antiretroviral therapy adherence by measuring drug concentrations in hair among children in rural Uganda. AIDS Care. 2015;27(3):327–332. doi: 10.1080/09540121.2014.983452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Prasitsuebsai W, Kerr SJ, Truong KH, Ananworanich J, Do VC, Nguyen LV, et al. Using lopinavir concentrations in hair samples to assess treatment outcomes on second-line regimens among Asian children. AIDS Res Hum Retroviruses. 2015;31(10):1009–1014. doi: 10.1089/aid.2015.0111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chawana TD, Gandhi M, Nathoo K, Ngara B, Louie A, Horng H, et al. Defining a cut-off for atazanavir in hair samples associated with virological failure among adolescents failing second-line antiretroviral treatment. J Acquir Immune Defic Syndr. 2017;76(1):55–59. doi: 10.1097/QAI.0000000000001452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lu H, Rosenbaum S. Developmental pharmacokinetics in pediatric populations. The Journal of Pediatric Pharmacology and Therapeutics : JPPT. 2014;19(4):262–276. doi: 10.5863/1551-6776-19.4.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Butler K, Turkova A, Inshaw J, Compagnucci A, Kenny J, Saidi Y, et al. Weekends-off efavirenz-based antiretroviral therapy in HIV-infected children, adolescents, and young adults (BREATHER): A randomised, open-label, non-inferiority, phase 2/3 trial. The Lancet HIV. 2016;3(9):e421–e430. doi: 10.1016/S2352-3018(16)30054-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Makadzange AT, Higgins-Biddle M, Chimukangara B, Birri R, Gordon M, Mahlanza T, et al. Clinical, virologic, immunologic outcomes and emerging HIV drug resistance patterns in children and adolescents in public ART care in Zimbabwe. PLoS One. 2015;10(12):e0144057. doi: 10.1371/journal.pone.0144057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Davies MA, Moultrie H, Eley B, Rabie H, Van Cutsem G, Giddy J, et al. Virologic failure and second-line antiretroviral therapy in children in south Africa-The IeDEA southern Africa collaboration. Journal of acquired immune deficiency syndromes (1999) 2011;56(3):270. doi: 10.1097/QAI.0b013e3182060610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kekitiinwa A, Asiimwe AR, Kasirye P, Korutaro V, Kitaka S, Maganda A, et al. Prospective long-term outcomes of a cohort of Ugandan children with laboratory monitoring during antiretroviral therapy. The Pediatric infectious disease journal. 2012;31(8):e117–e125. doi: 10.1097/INF.0b013e31825cb9d6. [DOI] [PubMed] [Google Scholar]
- 31.Kyaw NTT, Harries AD, Kumar AM, Oo MM, Kyaw KWY, Win T, et al. High rate of virological failure and low rate of switching to second-line treatment among adolescents and adults living with HIV on first-line ART in Myanmar, 2005–2015. PLoS One. 2017;12(2):e0171780. doi: 10.1371/journal.pone.0171780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bunupuradah T, Sricharoenchai S, Hansudewechakul R, Klinbuayaem V, Teeraananchai S, Wittawatmongkol O, et al. Risk of first-line antiretroviral therapy failure in HIV-infected Thai children and adolescents. The Pediatric infectious disease journal. 2015;34(3):e58–e62. doi: 10.1097/INF.0000000000000584. [DOI] [PubMed] [Google Scholar]
- 33.Rawizza HE, Chaplin B, Meloni ST, Eisen G, Rao T, Sankalé JL, et al. Immunologic criteria are poor predictors of virologic outcome: Implications for HIV treatment monitoring in resource-limited settings. Clin Infect Dis. 2011;53(12):1283–1290. doi: 10.1093/cid/cir729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reynolds SJ, Nakigozi G, Newell K, Ndyanabo A, Galiwongo R, Boaz I, et al. Failure of immunologic criteria to appropriately identify antiretroviral treatment failure in Uganda. AIDS (London, England) 2009;23(6):697. doi: 10.1097/QAD.0b013e3283262a78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Emmett SD, Cunningham CK, Mmbaga BT, Kinabo GD, Schimana W, Swai ME, et al. Predicting virologic failure among HIV-1-infected children receiving antiretroviral therapy in Tanzania: A cross-sectional study. Journal of acquired immune deficiency syndromes (1999) 2010;54(4):368. doi: 10.1097/QAI.0b013e3181cf4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim SH, Gerver SM, Fidler S, Ward H. Adherence to antiretroviral therapy in adolescents living with HIV: Systematic review and meta-analysis. AIDS (London, England) 2014;28(13):1945. doi: 10.1097/QAD.0000000000000316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Guichet E, Aghokeng A, Serrano L, Bado G, Toure-Kane C, Eymard-Duvernay S, et al. High viral load and multidrug resistance due to late switch to second-line regimens could be a major obstacle to reach the 90-90-90 UNAIDS objectives in sub-Saharan Africa. AIDS Res Hum Retroviruses. 2016;32(12):1159–1162. doi: 10.1089/AID.2016.0010. [DOI] [PubMed] [Google Scholar]
- 38.World Health Organization (WHO), United States Centers for Disease Control and Prevention (CDC), The Global Fund. HIV drug resistance report 2017. Geneva, Switzerland: WHO; 2017. [Google Scholar]
- 39.Gandhi M, Yang Q, Bacchetti P, Huang Y. A low-cost method for analyzing nevirapine levels in hair as a marker of adherence in resource-limited settings. AIDS Res Hum Retroviruses. 2014;30(1):25–28. doi: 10.1089/aid.2013.0239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Beyrer C, Pozniak A. HIV drug resistance—an emerging threat to epidemic control. N Engl J Med. 2017;377(17):1605–1607. doi: 10.1056/NEJMp1710608. [DOI] [PubMed] [Google Scholar]